Green Method for the Selective Electromembrane Extraction of Parabens and Fluoroquinolones in the Presence of NSAIDs by Using Biopolymeric Chitosan Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. EME Procedure
2.3. HPLC-DAD Chromatographic Determination
2.4. Box–Behnken Experimental Design for EME
2.5. Greenness Assessment
2.6. Water Samples
3. Results and Discussion
3.1. Chromatographic Conditions
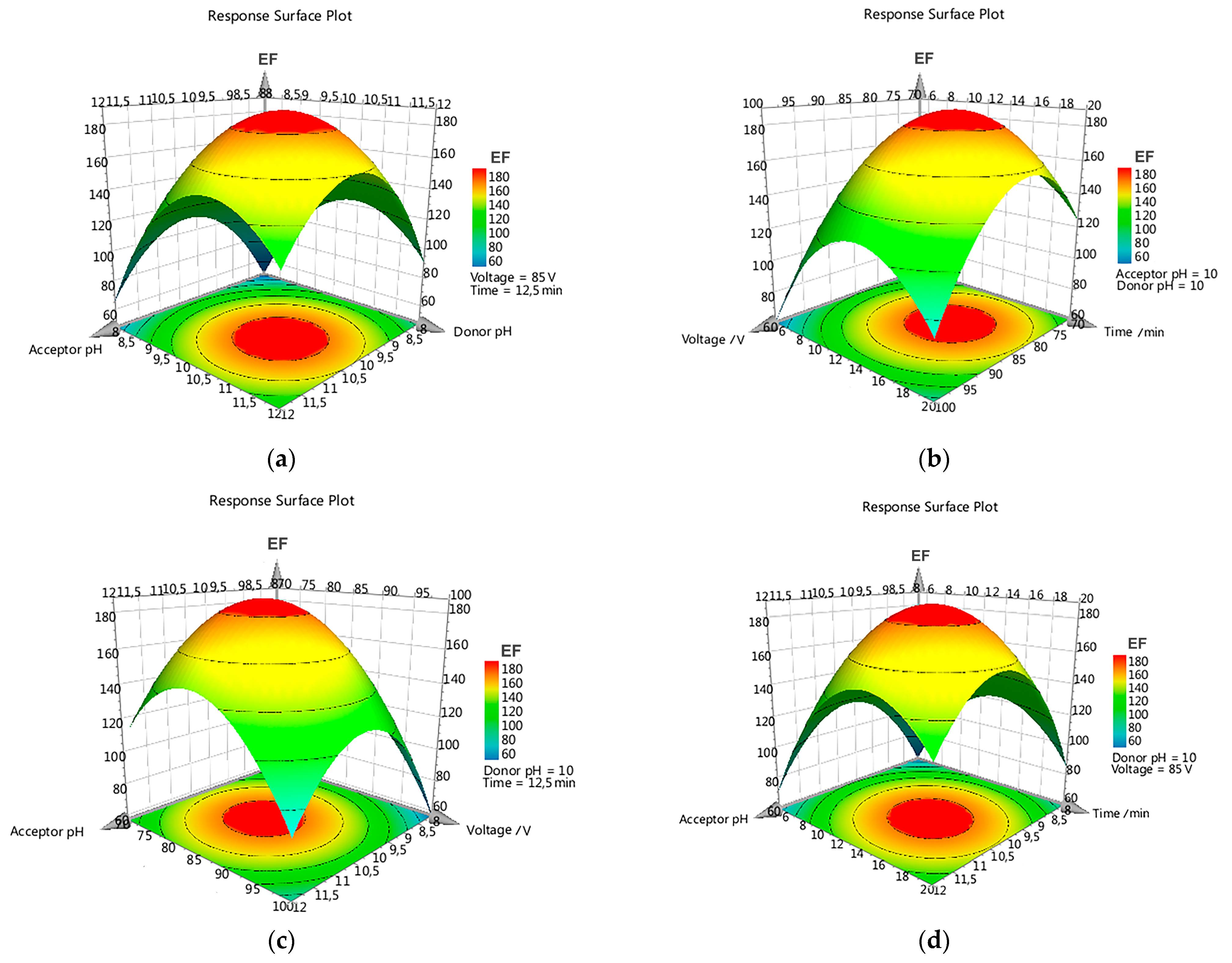
3.2. Optimization of EME Experimental Conditions
3.2.1. Preliminary Assays
3.2.2. Box–Behnken Design
3.2.3. Selectivity Assays
3.3. Analytical Performance of the Proposed EME Method
3.4. Application to Water Samples
3.5. Comparative Assesment with Other Miniaturized Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barceló, D. Emerging pollutants in Water Analysis. TrAC Trends Anal. Chem. 2003, 22, XIV–XVI. [Google Scholar] [CrossRef]
- White paper aquatic life criteria for contaminants of emerging concern—Part I—General Challenges and Recommendations. Prepared by the OW/ORD Emerging Contaminants Workgroup 3 June 2008. Available online: https://www.epa.gov/wqc/contaminants-emerging-concern-including-pharmaceuticals-and-personal-care-products (accessed on 3 February 2023).
- Morales-Paredes, C.; Rodríguez-Díaz, J.; Boluda-Botella, N. Pharmaceutical compounds used in the COVID-19 pandemic: A review of their presence in water and treatment techniques for their elimination. Sci. Total Environ. 2022, 814, 152691. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Y.; Sun, Y.; Wang, X.; Ma, P.; Song, D.; Fei, Q. Extraction of parabens by melamine sponge with determination by high-performance liquid chromatography. J. Sep. Sci. 2022, 45, 697–705. [Google Scholar] [CrossRef]
- Shishov, A.; Gerasimov, A.; Nechaeva, D.; Volodina, N.; Bessonova, E.; Bulatov, A. An effervescence-assisted dispersive liquid–liquid microextraction based on deep eutectic solvent decomposition: Determination of ketoprofen and diclofenac in liver. Microchem. J. 2020, 156, 104837. [Google Scholar] [CrossRef]
- Chen, J.; Deng, W.; Li, X.; Wang, X.; Xiao, Y. Hexafluoroisopropanol/Brij-35 based supramolecular solvent for liquid-phase microextraction of parabens in different matrix samples. J. Chromatogr. A 2019, 1591, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Barahona, F.; Albero, B.; Tadeo, J.; Martín-Esteban, A. Molecularly imprinted polymer-hollow fiber microextraction of hydrophilic fluoroquinolone antibiotics in environmental waters and urine samples. J. Chromatogr. A 2019, 1587, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Drouin, N.; Kubáň, P.; Rudaz, S.; Pedersen-Bjergaard, S.; Schappler, J. Electromembrane extraction: Overview of the last decade. TrAC Trends Anal. Chem. 2019, 113, 357–363. [Google Scholar] [CrossRef]
- Aranda-Merino, N.; Ramos-Payán, M.; Callejón-Mochón, M.; Villar-Navarro, M.; Fernández-Torres, R. Comparison of three electromembrane-based extraction systems for NSAIDs analysis in human urine samples. Anal. Bioanal. Chem. 2020, 412, 6811–6822. [Google Scholar] [CrossRef] [PubMed]
- Román-Hidalgo, C.; Aranda-Merino, N.; López-Pérez, G.; Sánchez-Coronilla, A.; Villar-Navarro, M.; Martín-Valero, M. Chitosan biofilms: Insights for the selective electromembrane extraction of fluoroquinolones from biological samples. Anal. Chim. Acta 2021, 1179, 338832. [Google Scholar] [CrossRef]
- Villar-Navarro, M.; Moreno-Carballo, M.C.; Fernández-Torres, R.; Callejón-Mochón, M.; Bello-López, M. Electromembrane extraction for the determination of parabens in water simples. Anal. Bioanal. Chem. 2016, 408, 1615–1621. [Google Scholar] [CrossRef]
- Li, J.; Zhu, R.; Shen, X.; Huang, C. Functional materials and chemicals in electromembrane extraction. TrAC Trends Anal. Chem. 2022, 150, 116574. [Google Scholar] [CrossRef]
- Santos, L.B.; Assis, R.S.; Barreto, J.A.; Bezerra, M.A.; Novaes, C.G.; Lemos, V.A. Deep eutectic solvents in liquid-phase microextraction: Contribution to green chemistry. TrAC Trends Anal. Chem. 2022, 146, 116478. [Google Scholar] [CrossRef]
- Eie, L.V.; Pedersen-Bjergaard, S.; Hansen, F.A. Electromembrane extraction of polar substances—Status and perspectives. J. Pharm. Biomed. Anal. 2022, 207, 114407. [Google Scholar] [CrossRef]
- Sedehi, S.; Tabani, H.; Nojavan, S. Electro-driven extraction of polar compounds using agarose gel as a new membrane: Determination of amino acids in fruit juice and human plasma samples. Talanta 2018, 179, 318–325. [Google Scholar] [CrossRef]
- Román-Hidalgo, C.; Ramos-Payán, M.; Ocaña-González, J.; Martín-Valero, M.; Bello-López, M. Agar films containing silver nanoparticles as new support for electromembrane extraction. Anal. Bioanal. Chem. 2015, 407, 1519–1525. [Google Scholar] [CrossRef]
- Asadi, S.; Tabani, H.; Nojavan, S. Application of polyacrylamide gel as a new membrane in electromembrane extraction for the quantification of basic drugs in breast milk and wastewater samples. J. Pharm. Biomed. Anal. 2018, 151, 178–185. [Google Scholar] [CrossRef]
- Hajizadeh, S.; Farhadi, K.; Molaei, R.; Forough, M. Silver nanoparticles-tragacanth gel as a green membrane for effective extraction and determination of capecitabine. J. Separ. Sci. 2020, 43, 2666–2674. [Google Scholar] [CrossRef]
- Román-Hidalgo, C.; López-Pérez, G.; Martín-Valero, M.J.; Bello-López, M. Chitosan tailor-made membranes as biopolymeric support for electromembrane extraction. Talanta 2019, 199, 290–295. [Google Scholar] [CrossRef]
- Gholami, H.; Ghaedi, M.; Arabi, M.; Ostovan, A.; Bagheri, A.; Mohamedian, H. Application of Molecularly Imprinted Biomembrane for Advancement of Matrix Solid-Phase Dispersion for Clean Enrichment of Parabens from Powder Sunscreen Samples: Optimization of Chromatographic Conditions and Green Approach. ACS Omega 2019, 4, 3839–3849. [Google Scholar] [CrossRef]
- Román-Hidalgo, C.; López-Pérez, G.; Villar-Navarro, M.; Martín-Valero, M. Green electromembrane extraction procedure based on biodegradable chitosan films for determination of polyphenolic compounds in food samples: Greenness assessment of the sample preparation approach. Talanta 2023, 253, 124034. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J. A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Wojnowski, W.; Tobiszewski, M.; Pena-Pereira, F.; Psillakis, E. AGREEprep—Analytical greenness metric for sample preparation. TrAC Trends Anal. Chem. 2022, 149, 116553. [Google Scholar] [CrossRef]
- Katakam, L.N.R.; Ettaboina, S.K.; Dongala, T. A simple and rapid HPLC method for determination of parabens and their degradation products in pharmaceutical dosage forms. Biomed. Chromatogr. 2021, 35, e5152. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, A.; Kabir, A.; Ulusoy, S.; Sperandio, E.; Piccolantonio, S.; Ulusoy, H.I.; Furton, K.G.; Locatelli, M. FPSE-HPLC-PDA analysis of seven paraben residues in human whole blood, plasma, and urine. J. Chromatogr. B 2019, 1125, 121707. [Google Scholar] [CrossRef]
- Yamini, Y.; Seidi, S.; Rezazadeh, M. Electrical field-induced extraction and separation techniques: Promising trends in analytical chemistry—A review. Anal. Chim. Acta 2014, 814, 1–22. [Google Scholar] [CrossRef]
- Tabani, H.; Fakhari, A.R.; Shahsavani, A. Simultaneous determination of acidic and basic drugs using dual hollow fibre electromembrane extraction combined with CE. Electrophoresis 2013, 34, 269–276. [Google Scholar] [CrossRef]
- Cuadros, L.; García, A.; Bosque, J. Statistical estimation of linear calibration range. Anal. Lett. 1996, 29, 1231–1239. [Google Scholar] [CrossRef]
- Miller, J.; Miller, J. Statistics and Chemometrics for Analytical Chemistry, 4th ed.; Prentice Hall: London, UK, 2000. [Google Scholar]
- Santigosa-Murillo, E.; Muñoz-Berbel, X.; Maspoch, S.; Muñoz, M.; Ramos-Payán, M. Impedance model for voltage optimization of parabens extraction in an electromembrane millifluidic device. J. Chromatogr. A 2020, 1625, 461270. [Google Scholar] [CrossRef]
- Werner, J.; Zgoła-Grześkowiak, A.; Grześkowiak, T. Development of novel thin-film solid-phase microextraction materials based on deep eutectic solvents for preconcentration of trace amounts of parabens in surface waters. J. Sep. Sci. 2022, 45, 1374–1384. [Google Scholar] [CrossRef]
- Tao, Y.; Jia, L.; Qin, H.; Niu, R.; Qiao, L. A new magnetic ionic liquid based salting-out assisted dispersive liquid–liquid microextraction for the determination of parabens in environmental water samples. Anal. Methods 2022, 14, 4775–4783. [Google Scholar] [CrossRef]
- Ramos-Payán, M.; Villar-Navarro, M.; Fernández-Torres, R.; Callejón-Mochón, M.; Bello-López, M.Á. Electromembrane extraction (EME)—An easy, novel and rapid extraction procedure for the HPLC determination of fluoroquinolones in wastewater samples. Anal. Bioanal. Chem. 2013, 405, 2575–2584. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Tao, Y.; Yao, W.; Zhao, J.; Yan, Y. A magnetic ionic liquid based vortex-assisted dispersive liquid-liquid microextraction coupled with back-extraction for the enrichment of fluoroquinolone antibiotics. J. Pharm. Biomed. Anal. 2022, 219, 114903. [Google Scholar] [CrossRef] [PubMed]


| Exp Number | Run Order | Acceptor pH | Donor pH | Voltage (V) | Time (min) |
|---|---|---|---|---|---|
| 1 | 7 | 8 | 8 | 85 | 12.5 |
| 2 | 27 | 12 | 8 | 85 | 12.5 |
| 3 | 24 | 8 | 12 | 85 | 12.5 |
| 4 | 10 | 12 | 12 | 85 | 12.5 |
| 5 | 22 | 10 | 10 | 70 | 5 |
| 6 | 16 | 10 | 10 | 100 | 5 |
| 7 | 4 | 10 | 10 | 70 | 20 |
| 8 | 25 | 10 | 10 | 100 | 20 |
| 9 | 2 | 8 | 10 | 85 | 5 |
| 10 | 20 | 12 | 10 | 85 | 5 |
| 11 | 15 | 8 | 10 | 85 | 20 |
| 12 | 5 | 12 | 10 | 85 | 20 |
| 13 | 8 | 10 | 8 | 70 | 12.5 |
| 14 | 3 | 10 | 12 | 70 | 12.5 |
| 15 | 6 | 10 | 8 | 100 | 12.5 |
| 16 | 12 | 10 | 12 | 100 | 12.5 |
| 17 | 18 | 8 | 10 | 70 | 12.5 |
| 18 | 26 | 12 | 10 | 70 | 12.5 |
| 19 | 23 | 8 | 10 | 100 | 12.5 |
| 20 | 19 | 12 | 10 | 100 | 12.5 |
| 21 | 11 | 10 | 8 | 85 | 5 |
| 22 | 21 | 10 | 12 | 85 | 5 |
| 23 | 1 | 10 | 8 | 85 | 20 |
| 24 | 13 | 10 | 12 | 85 | 20 |
| 25 | 14 | 10 | 10 | 85 | 12.5 |
| 26 | 17 | 10 | 10 | 85 | 12.5 |
| 27 | 9 | 10 | 10 | 85 | 12.5 |
| Analyte | Linear Range (µg L−1) | Linearity (%) | MLOD (µg L−1) | MLOQ (µg L−1) | EF |
|---|---|---|---|---|---|
| MeP | 0.5–500 | 98.6 | 0.2 | 0.5 | 189 |
| EtP | 0.6–500 | 97.5 | 0.2 | 0.6 | 168 |
| PrP | 0.5–500 | 98.3 | 0.2 | 0.5 | 195 |
| iPrP | 0.5–500 | 98.1 | 0.2 | 0.5 | 194 |
| BuP | 3.6–500 | 97.6 | 1.1 | 3.6 | 28 |
| iBuP | 1.1–500 | 99.1 | 0.3 | 1.1 | 94 |
| BzP | 0.9–500 | 97.8 | 0.2 | 0.9 | 111 |
| MRB | 1.3–500 | 98.5 | 0.4 | 1.3 | 75 |
| ENR | 1.4–500 | 97.3 | 0.4 | 1.4 | 72 |
| FLM | 1.9–500 | 97.0 | 0.6 | 1.9 | 52 |
| Target Analytes | Solid Support/Membrane | Solvent/SLM | Extraction Method and Conditions | Extraction Efficiency | MLOD (µgL−1) | MLOQ (µgL−1) | Matrix | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| EF | R (%) | ||||||||
| MeP, EtP, PrP | Stainless steel mesh | DES | TF-SPME Sample: pH 5 (0.06 g mL−1 NaCl) 40min, 120 rpm (adsorption), 10min, 200 rpm (desorption) | 166–183 | − | 0.018–0.055 | 0.06–0.182 | Lake and river waters | [31] |
| MeP, EtP, PrP, BuP | None | MIL (extraction), ACN (dispersion) | SA-DLLME 1min, vortex | − | 82.0–114.6 | 0.6–0.84 | 2.0–2.8 | Tap, lake, and river waters | [32] |
| EtP, PrP, BuP, iBuP, BzP | PP Accurel® S6/2 hollow fiber | 1-octanol | HF-EME DP: pH 4 AP: pH 12 30V, 40min, 300 rpm | 30–49 | − | 0.98–1.43 | − | Surface environmental water | [11] |
| EtP, BuP, iBuP | PP flat membrane | 1-octanol | Microfluidic-EME DP: pH 3 AP: pH 11.5 4V, 10min-SLM, 7min-dynamic mode | − | 100.6–104.2 | 70–120 | 240–380 | Urine | [30] |
| MRB, NRF, CPR, DNF, ENR, GTF, GRP | PP Accurel® S6/2 hollow fiber | 1-octanol | HF-EME DP: pH 5 AP: pH 2 50V, 15min, 750 rpm | 40–85 | − | 0.005–0.07 | 0.007–0.15 | Urban wastewaters | [33] |
| ENO, LEV, NRF, CPR, ENR | None | MIL (extraction) | VA-DLLME | 19–25 | − | 0.75–1.5 | 2.5–5.0 | Tap water, milk, honey, chicken | [34] |
| MeP, EtP, PrP, iPrP, BuP, iBuP, BzP, MRB, ENR, FLM | Chitosan flat membrane | SLM-free | EME DP: pH 10 AP: pH 10 80V, 15min, 600 rpm | 28–195 | − | 0.2–1.1 | 0.5–3.6 | Environmental water (in the presence of NSAIDs) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Román-Hidalgo, C.; Martín-Valero, M.J.; López-Pérez, G.; Villar-Navarro, M. Green Method for the Selective Electromembrane Extraction of Parabens and Fluoroquinolones in the Presence of NSAIDs by Using Biopolymeric Chitosan Films. Membranes 2023, 13, 326. https://doi.org/10.3390/membranes13030326
Román-Hidalgo C, Martín-Valero MJ, López-Pérez G, Villar-Navarro M. Green Method for the Selective Electromembrane Extraction of Parabens and Fluoroquinolones in the Presence of NSAIDs by Using Biopolymeric Chitosan Films. Membranes. 2023; 13(3):326. https://doi.org/10.3390/membranes13030326
Chicago/Turabian StyleRomán-Hidalgo, Cristina, María Jesús Martín-Valero, Germán López-Pérez, and Mercedes Villar-Navarro. 2023. "Green Method for the Selective Electromembrane Extraction of Parabens and Fluoroquinolones in the Presence of NSAIDs by Using Biopolymeric Chitosan Films" Membranes 13, no. 3: 326. https://doi.org/10.3390/membranes13030326
APA StyleRomán-Hidalgo, C., Martín-Valero, M. J., López-Pérez, G., & Villar-Navarro, M. (2023). Green Method for the Selective Electromembrane Extraction of Parabens and Fluoroquinolones in the Presence of NSAIDs by Using Biopolymeric Chitosan Films. Membranes, 13(3), 326. https://doi.org/10.3390/membranes13030326