Fabrication of Unidirectional Water Permeable PS/PET Composite Nanofibers Modified with Silver Nanoparticles via Electrospinning
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrolysis of PET Membranes
2.3. Preparation of AgNPs-PS Solution
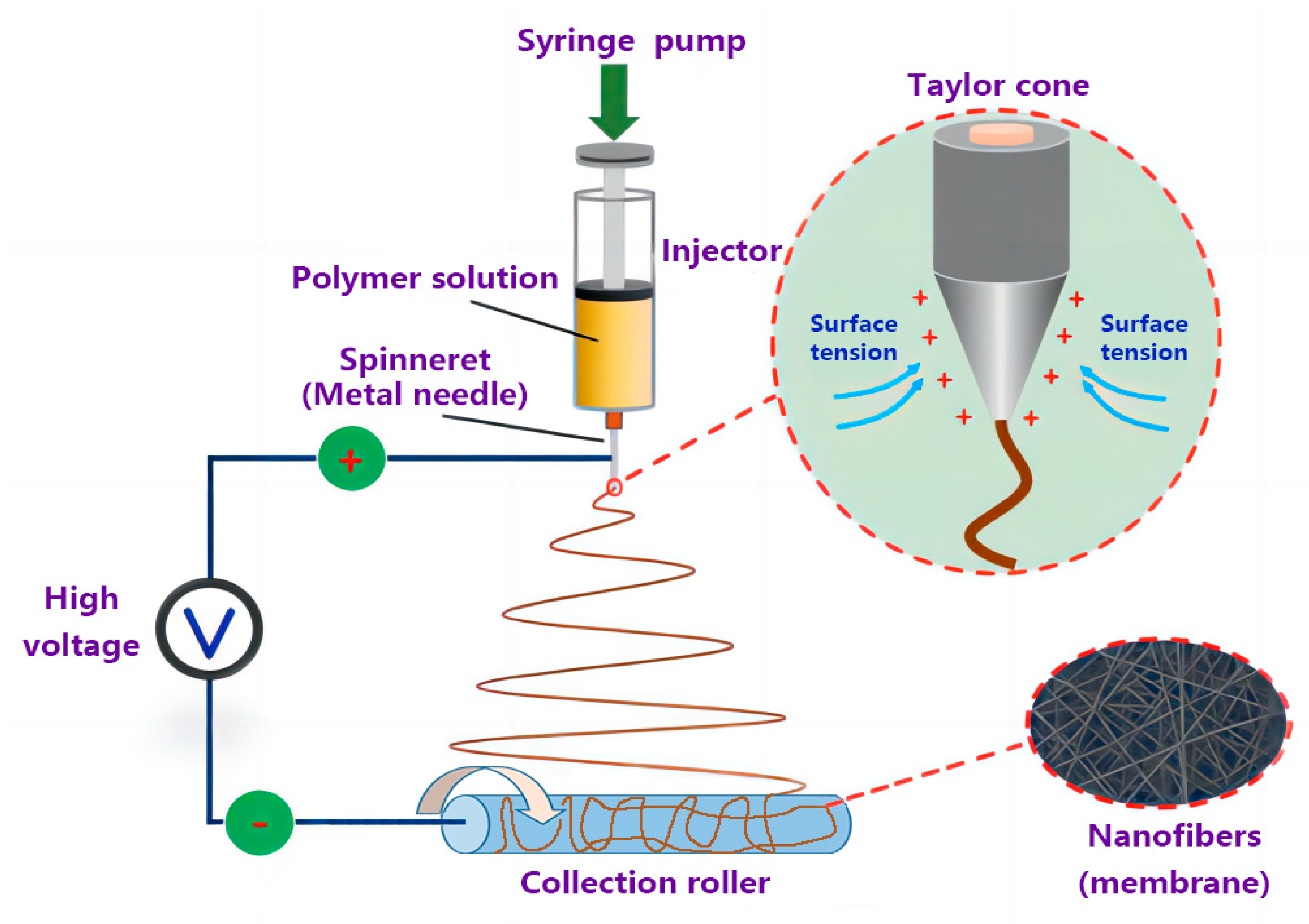
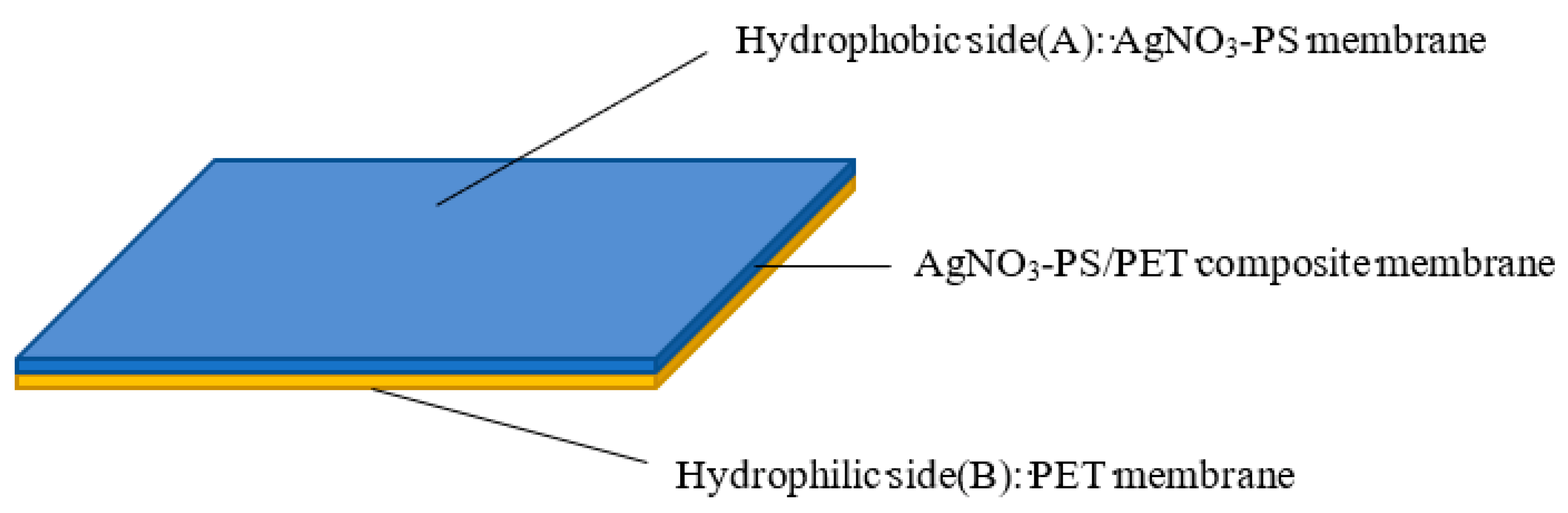
2.4. Electrospinning of AgNPs-PS/PET Composite Membranes
2.5. Material Characterizations
3. Results and Discussion
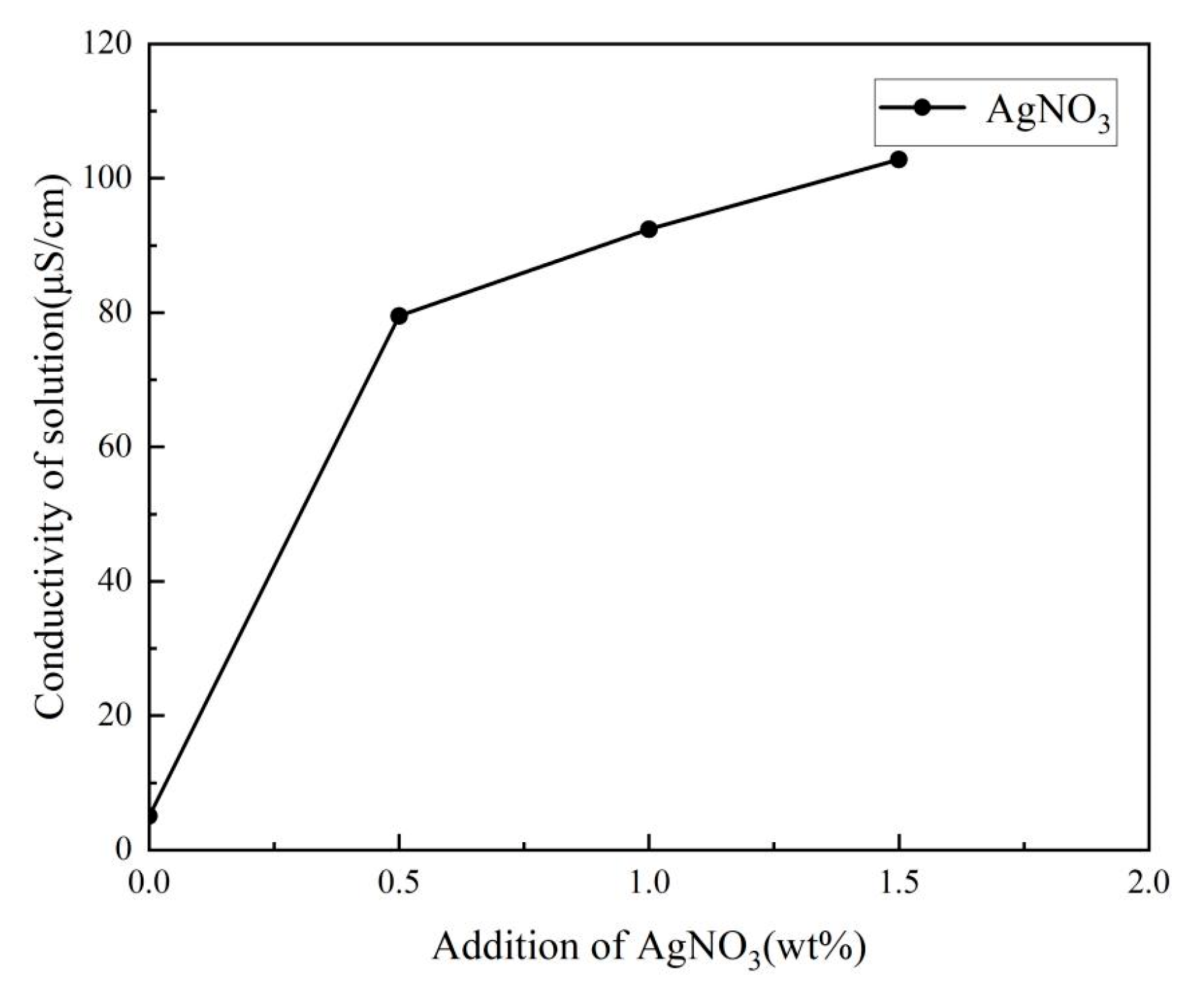
3.1. Viscosity and Conductivity of Electrospinning Solution
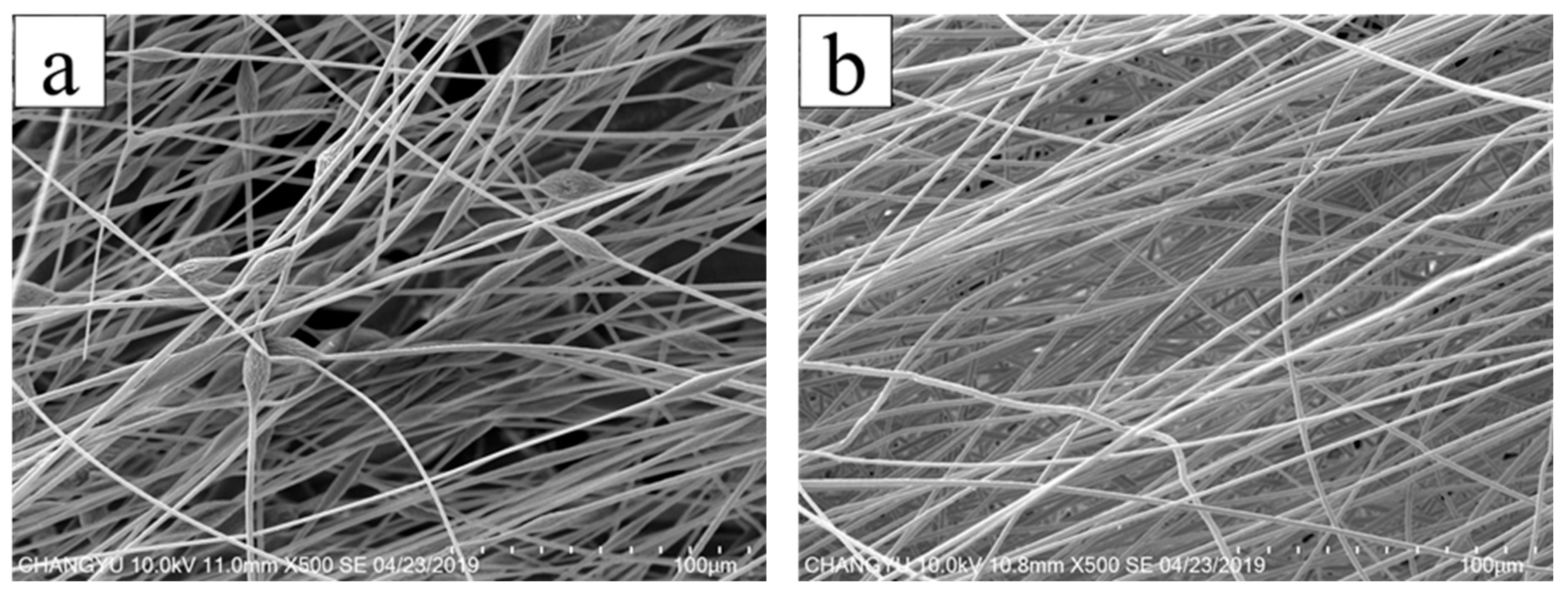
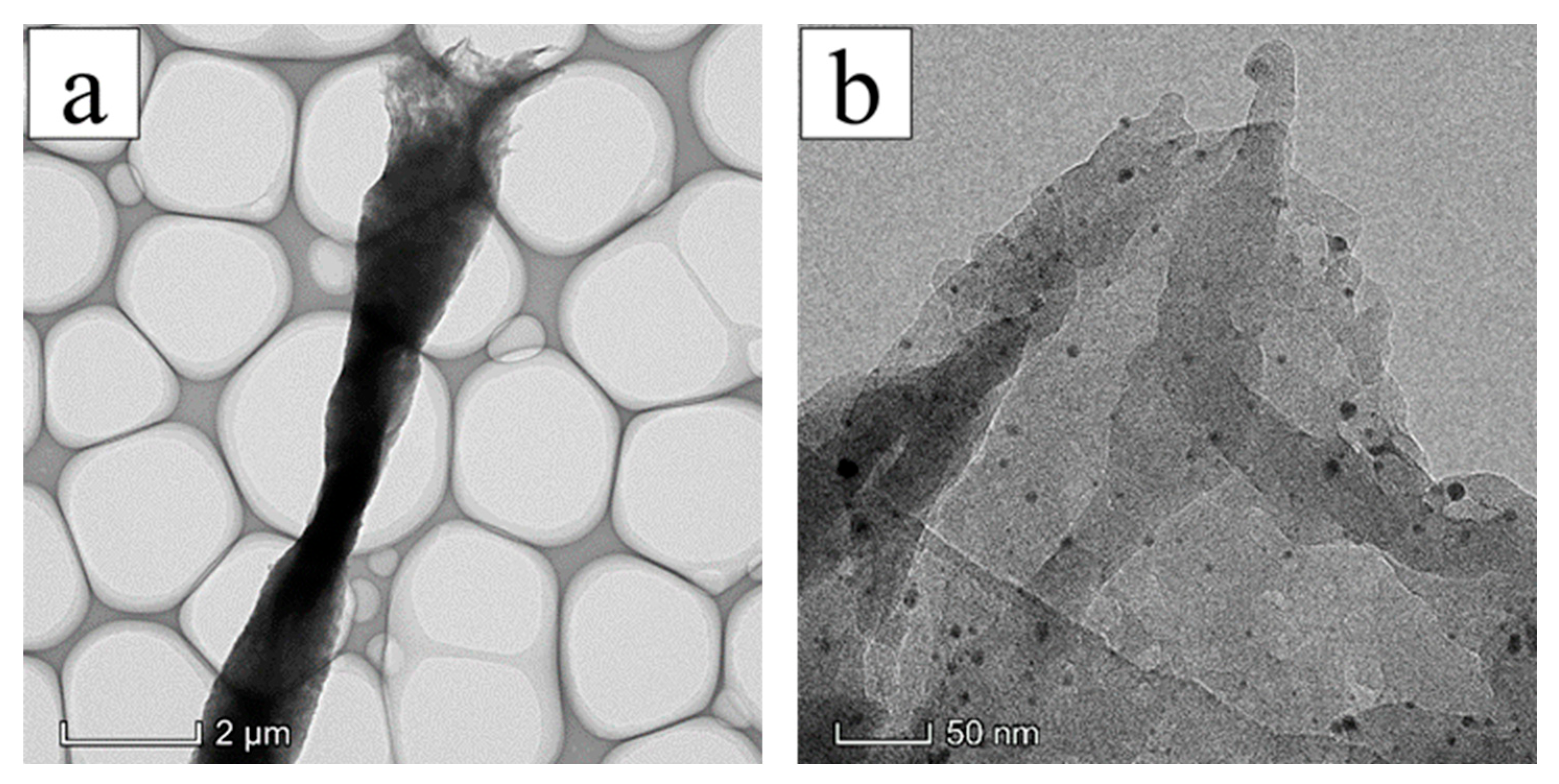
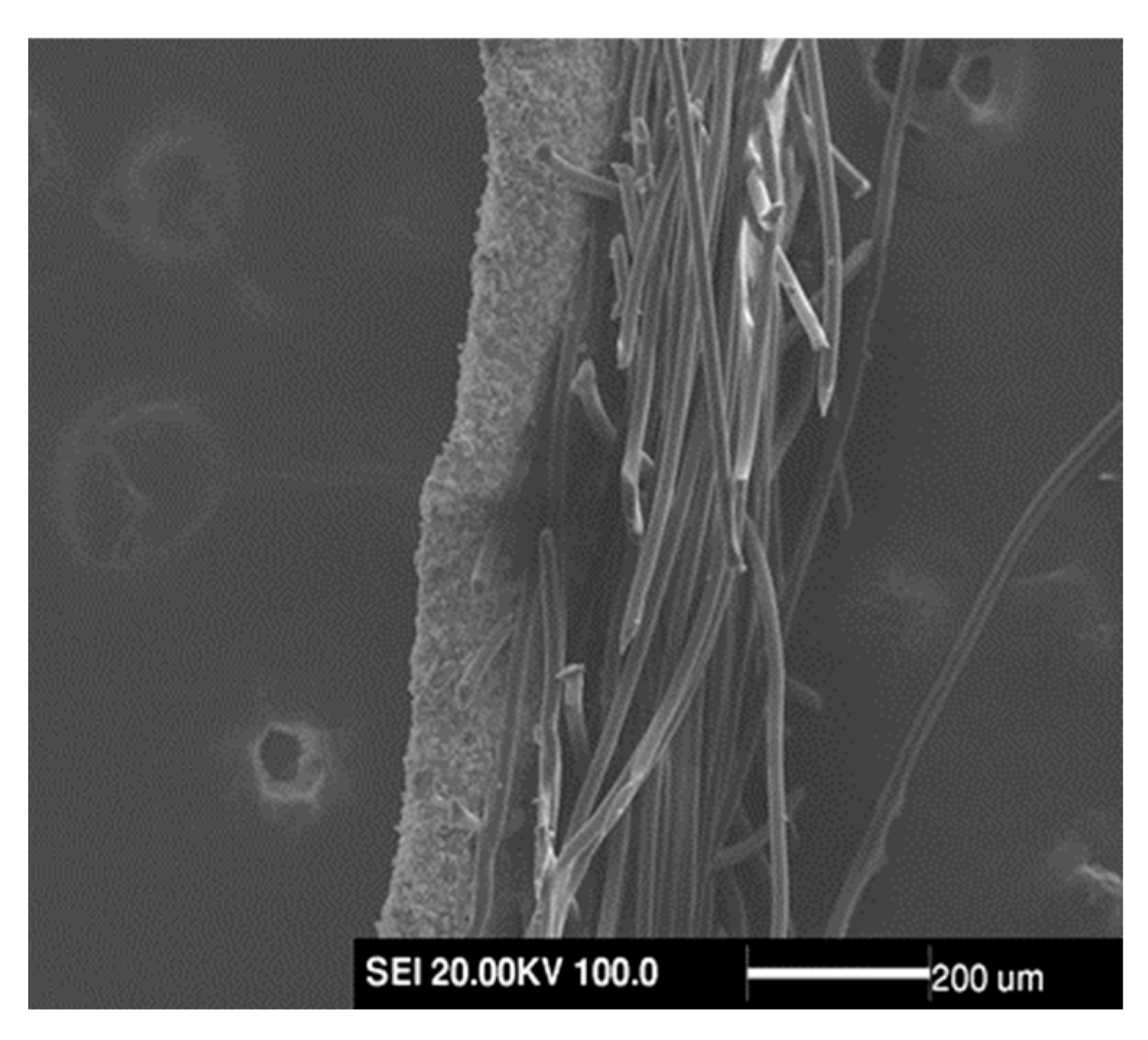
3.2. Surface Morphology of AgNPs-PS Side in the Composite Membranes
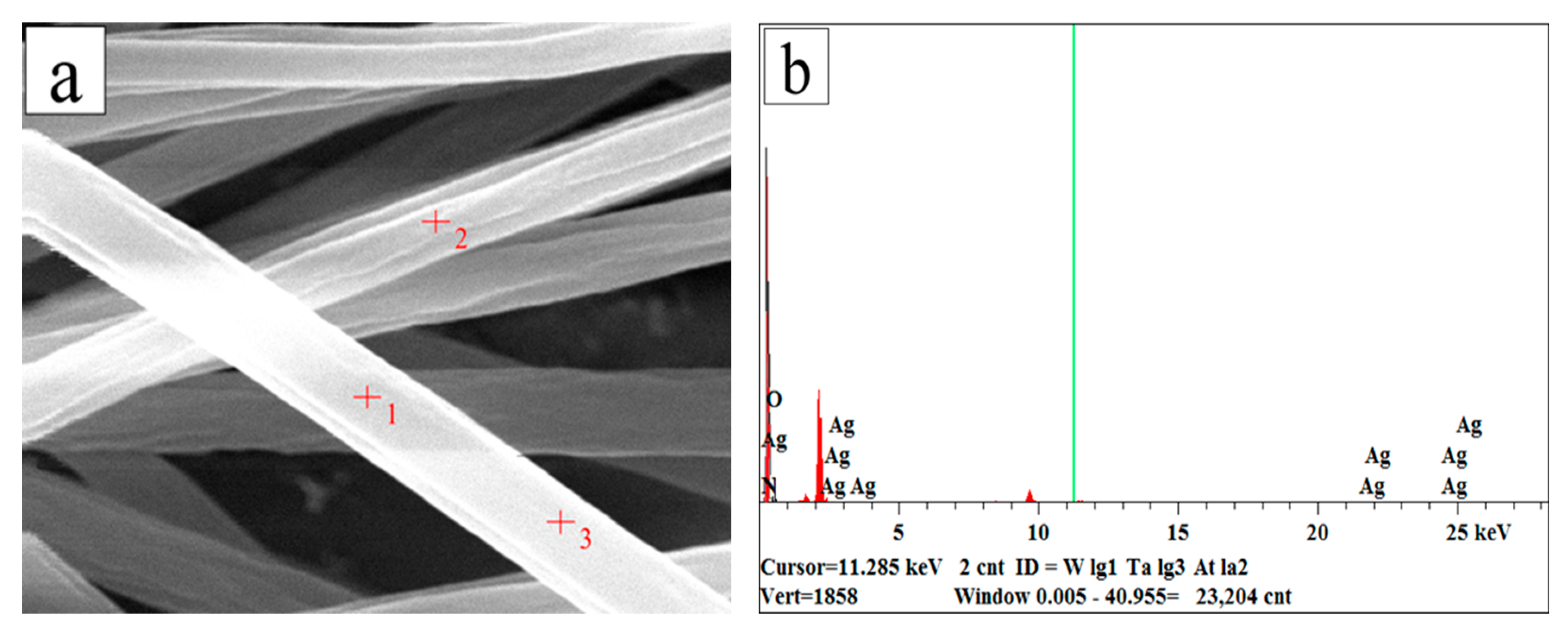
3.3. Composition of AgNPs-PS Side in the Composite Membrane
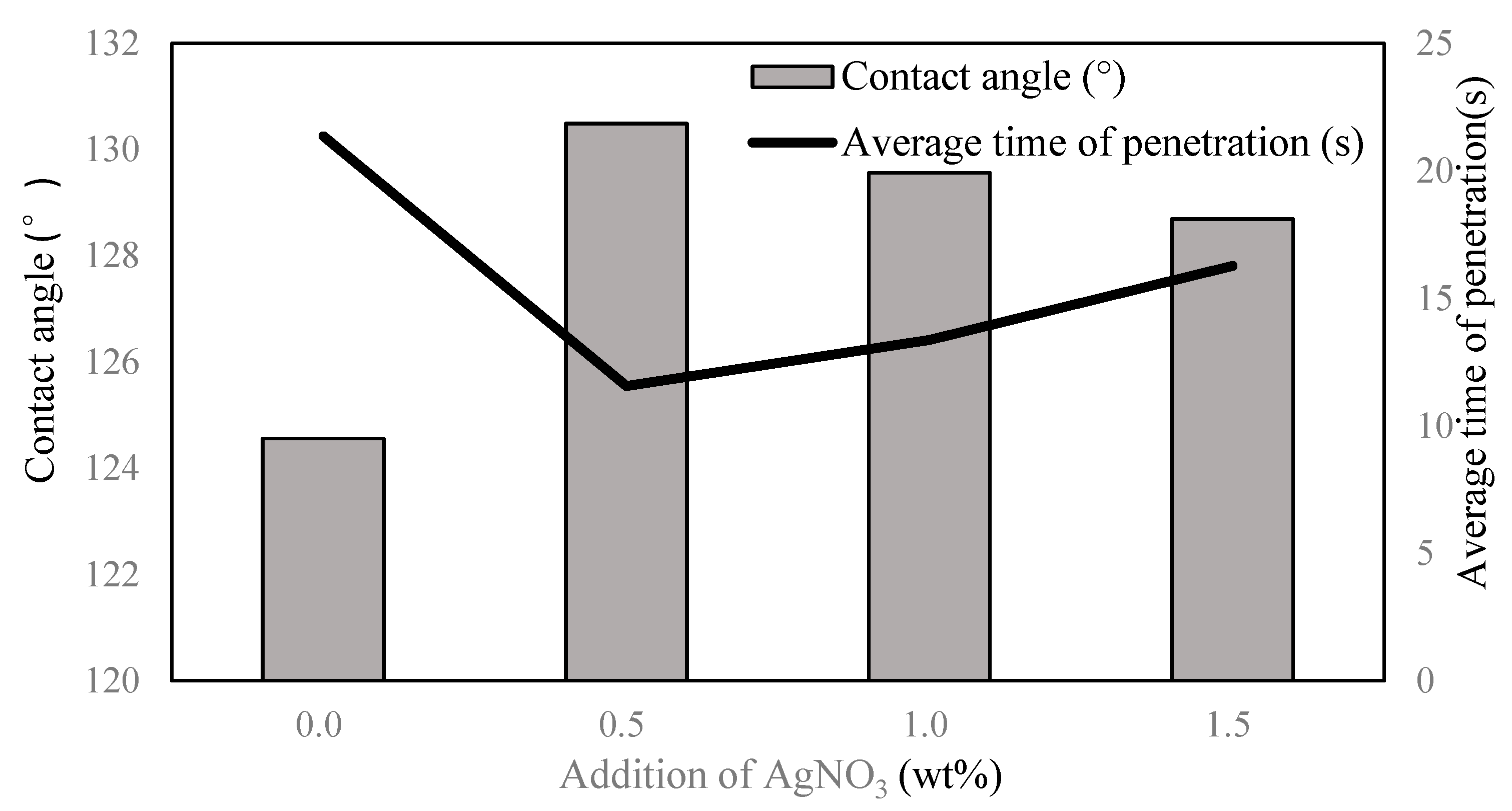
3.4. Contact Angle and Average Time of Penetration
3.5. Mechanism of Unidirectional Water Penetration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, H.; Li, L.; Hu, L.; Cui, X. Continuous aligned polymer fibers produced by a modified electrospinning method. Ploymer 2006, 47, 4901–4904. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Fujihara, K.; Teo, W.E.; Lim, T.C.; Ma, Z. An Introduction to Electrospinning and Nanofibers. Characterization 2005, 46, 192–246. [Google Scholar]
- Khan, M.Q.; Kharaghani, D.; Nishat, N.; Ishikawa, T.; Ullah, S.; Lee, H.; Khatri, Z.; Kim, I.S. The development of nanofiber tubes based on nanocomposites of polyvinylpyrrolidone incorporated gold nanoparticles as scaffolds for neuroscience application in axons. Text. Res. J. 2018, 89, 2713–2720. [Google Scholar] [CrossRef]
- Yu, D.G.; Chatterton, N.P.; Yang, J.H.; Wang, X.; Liao, Y.Z. Coaxial Electrospinning with Triton X-100 Solutions as Sheath Fluids for Preparing PAN Nanofibers. Macromol. Mater. Eng. 2011, 297, 395–401. [Google Scholar] [CrossRef]
- Bd, A.; Xy, A.; Yi, Z.A.; Yz, A.; Xl, B.; Yang, Z.B.; Ky, A. A nanofibrous composite membrane of PLGA–chitosan/PVA prepared by electrospinning. Eur. Polym. J. 2006, 42, 2013–2022. [Google Scholar]
- Xu, F.; Cui, F.Z.; Jiao, Y.P.; Meng, Q.Y.; Wang, X.P.; Cui, X.Y. Improvement of cytocompatibility of electrospinning PLLA microfibers by blending PVP. J. Mater. Sci. Mater. Med. 2009, 20, 1331–1338. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, Y.; Wang, C.; Wei, Y. Fabrication of CdS Nanorods in PVP Fiber Matrices by Electrospinning. Macromol. Rapid Commun. 2005, 26, 1325–1329. [Google Scholar] [CrossRef]
- Yan, C.; Yu, H.; Dong, F.; Jia, Y.; Chen, L. One-step fabrication of ammonia sensor by electrospinning PS-b-PMA nanofibers on quartz crystal microbalance. Sens. Actuators B. Chem. 2014, 203, 459–464. [Google Scholar]
- Baker, S.C.; Southgate, J. Towards control of smooth muscle cell differentiation in synthetic 3D scaffolds. Biomaterials 2008, 29, 3357–3366. [Google Scholar] [CrossRef]
- Shin, C. Filtration application from recycled expanded polystyrene. Colloid Interface 2006, 302, 267–271. [Google Scholar] [CrossRef]
- An, H.; Shin, C.; Chase, G.G. Ion exchanger using electrospun polystyrene nanofibers. J. Membr. Sci. 2006, 283, 84–87. [Google Scholar] [CrossRef]
- Tu, C.W.; Tsai, C.H.; Wang, C.F.; Kuo, S.W.; Chang, F.C. Fabrication of Superhydrophobic and Superoleophilic Polystyrene Surfaces by a Facile One-Step Method. Macromol. Rapid Commun. 2007, 28, 2262–2266. [Google Scholar] [CrossRef]
- Kang, M.; Jung, R.; Kim, H.S.; Jin, H.J. Preparation of superhydrophobic polystyrene membranes by electrospinning. Colloids Surf. A Phys. Chem. Eng. Asp. 2008, 313, 411–414. [Google Scholar] [CrossRef]
- Ogawa, T.; Ding, B.; Sone, Y.; Shiratori, S. Super-hydrophobic surfaces of layer-by-layer structured film-coated electrospun nanofibrous membranes. Nanotechnology 2007, 18, 695–700. [Google Scholar] [CrossRef]
- Sun, T.; Wang, G.; Feng, L.; Liu, B.; Ma, Y.; Jiang, L.; Zhu, D. Titelbild: Reversible Switching between Superhydrophilicity and Superhydrophobicity. Angew. Chem. 2004, 116, 263. [Google Scholar] [CrossRef]
- Wu, J.; Wang, N.; Wang, L.; Dong, H.; Zhao, Y.; Jiang, L. Unidirectional water-penetration composite fibrous film via electrospinning. Soft Matter 2012, 8, 5996–5999. [Google Scholar] [CrossRef]
- Allcock, H.R.; Steely, L.B.; Singh, A. Hydrophobic and superhydrophobic surfaces from polyphosphazenes. Polym. Int. 2010, 55, 621–625. [Google Scholar] [CrossRef]
- Ju, J.; Shi, Z.; Deng, N.; Liang, Y.; Kang, W.; Cheng, B. Designing waterproof breathable material with moisture unidirectional transport characteristics based on a TPU/TBAC tree-like and TPU nanofiber double-layer membrane fabricated by electrospinning. RSC Adv. 2017, 7, 32155–32163. [Google Scholar] [CrossRef]
- Cho, J.Y.; Hong, C.J.; Choi, H.M. Microwave-Assisted Glycolysis for PET with Highly Hydrophilic Surface. Industrial 2013, 52, 2309–2315. [Google Scholar] [CrossRef]
- Gao, Z.; Dong, Q.; Shang, M.; Shentu, B.; Wu, C. Microstructure and properties of poly(butylene terephthalate)/poly(ethylene terephthalate) composites based on carbon nanotubes/graphene nanoplatelets hybrid filler systems. J. Appl. Polym. Sci. 2022, 139, 51733. [Google Scholar] [CrossRef]
- Pingale, N.D.; Shukla, S.R. Microwave-assisted aminolytic depolymerization of PET waste. Eur. Polym. J. 2009, 45, 2695. [Google Scholar] [CrossRef]
- Parab, Y.S.; Shah, R.V.; Shukla, S.R. Microwave irradiated synthesis and characterization of 1, 4-phenylene bis-oxazoline form bis-(2-hydroxyethyl) terephthalamide obtained by depolymerization of poly (ethylene terephthalate) (PET) bottle wastes. Curr. Chem. Lett. 2012, 1, 81–90. [Google Scholar] [CrossRef]
- Sammon, C.; Yarwood, J.; Everall, N. An FT–IR study of the effect of hydrolytic degradation on the structure of thin PET films. Polym. Degrad. Stab. 2000, 67, 149–158. [Google Scholar] [CrossRef]
- Korniienko, V.; Husak, Y.; Yanovska, A.; Banasiuk, R.; Yusupova, A.; Savchenko, A.; Holubnycha, V.; Pogorielov, M. Functional and biological characterization of chitosan electrospun nanofibrous membrane nucleated with silver nanoparticles. Appl. Nanosci. 2022, 12, 1061–1070. [Google Scholar] [CrossRef]
- Phan, D.-N.; Dorjjugder, N.; Khan, M.Q.; Saito, Y.; Taguchi, G.; Lee, H.; Mukai, Y.; Kim, I.-S. Synthesis and attachment of silver and copper nanoparticles on cellulose nanofibers and comparative antibacterial study. Cellulose 2019, 26, 6629–6640. [Google Scholar] [CrossRef]
- Ali, A.A.; Al-Asmari, A.K. Wet-electrospun CuNP/carbon nanofibril composites: Potential application for micro surface-mounted components. Appl. Nanosci. 2012, 2, 55–61. [Google Scholar] [CrossRef]
- Nguyen Dang, L.; Oh, J.; Lee, Y.; Yeon, J.H.; Hur, J.; Park, J.J.; Kim, J.M.; Nam, J.-D. Immobilization of gold nanoparticles on poly(methyl methacrylate) electrospun fibers exhibiting solid-state surface plasmon effect. Surf. Interface Anal. 2012, 44, 318–321. [Google Scholar] [CrossRef]
- Kang, P.H.; Gohs, U.; Richter, M.; Wolz, D.S.J.; Richter, B.; Cherif, C.; Boehm, R.; Jaeger, H. Fabrication and Characterization of Titanium Dioxide Nanoparticle Filled Polyacrylonitrile Fiber for Photocatalytic Application by Wet Spinning. Fibers Polym. 2021, 22, 2995–3002. [Google Scholar] [CrossRef]
- Rokbani, H.; Daigle, F.; Ajji, A. Combined Effect of Ultrasound Stimulations and Autoclaving on the Enhancement of Antibacterial Activity of ZnO and SiO2/ZnO Nanoparticles. Nanomaterials 2018, 8, 129. [Google Scholar] [CrossRef]
- Rodriguez-Tobias, H.; Morales, G.; Ledezma, A.; Romero, J.; Grande, D. Novel antibacterial electrospun mats based on poly(d,l-lactide) nanofibers and zinc oxide nanoparticles. J. Mater. Sci. 2014, 49, 8373–8385. [Google Scholar] [CrossRef]
- Ferreira, C.A.M.; Guerreiro, S.F.C.; Valente, J.F.A.; Patricio, T.M.F.; Alves, N.; Mateus, A.; Dias, J.R. Advanced Face Mask Filters Based on PCL Electrospun Meshes Dopped with Antimicrobial MgO and CuO Nanoparticles. Polymers 2022, 14, 3329. [Google Scholar] [CrossRef]
- Liang, G.D.; Bao, S.P.; Tjong, S.C. Microstructure and properties of polypropylene composites filled with silver and carbon nanotube nanoparticles prepared by melt-compounding. Mater. Sci. Eng. B 2007, 142, 55–61. [Google Scholar] [CrossRef]
- Rastogi, L.; Kora, A.J.; Sashidhar, R.B. Antibacterial effects of gum kondagogu reduced/stabilized silver nanoparticles in combination with various antibiotics: A mechanistic approach. Appl. Nanosci. 2015, 5, 535–543. [Google Scholar] [CrossRef]
- Hong, K.H. Preparation and properties of electrospun poly(vinyl alcohol)/silver fiber web as wound dressings. Polym. Eng. Sci. 2010, 47, 43–49. [Google Scholar] [CrossRef]
- Wei, L. Flexible Tactile Sensor Based on Patterned Ag-Nanofiber Electrodes through Electrospinning. Sens. Actuators B. Chem. 2021, 21, 2413. [Google Scholar]
- Mai, Z.; Fan, S.; Wang, Y.; Chen, J.; Chen, Y.; Bai, K.; Deng, L.; Xiao, Z. Catalytic nanofiber composite membrane by combining electrospinning precursor seeding and flowing synthesis for immobilizing ZIF-8 derived Ag nanoparticles. J. Membr. Sci. 2022, 643, 120045. [Google Scholar] [CrossRef]
- Fong, H.; Chun, I.; Reneker, D.H. Beaded nanofibers formed during electrospinning. Polym. Compos. 1999, 40, 4585–4592. [Google Scholar] [CrossRef]
- Koombhongse, S.; Reneker, D.H. Flat, Branched and Split Electrospun Fibers; American Physical Society: Washington, DC, USA, 2001. [Google Scholar]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Tan, N.C.B. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Sichani, G.N.; Morshed, M.; Amirnasr, M.; Abedi, D. In situ preparation, electrospinning, and characterization of polyacrylonitrile nanofibers containing silver nanoparticles. J. Appl. Polym. Sci. 2010, 116, 1021–1029. [Google Scholar] [CrossRef]
- Mostafa, M.; Kandile, N.G.; Mahmoud, M.K.; Ibrahim, H.M. Synthesis and characterization of polystyrene with embedded silver nanoparticle nanofibers to utilize as antibacterial and wound healing biomaterial. Heliyon 2022, 8, e08772. [Google Scholar] [CrossRef]
- Li, Z.; Huang, H.; Shang, T.; Yang, F.; Manohar, S.K. Facile synthesis of single-crystal and controllable sized silver nanoparticles on the surfaces of polyacrylonitrile nanofibres. Nanotechnology 2006, 17, 917. [Google Scholar] [CrossRef]
- Hong, K.H.; Park, J.L.; Sul, I.H.; Youk, J.H.; Kang, T.J. Preparation of antimicrobial poly(vinyl alcohol) nanofibers containing silver nanoparticles. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 2468–2474. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Arbatan, A.T. Preparation and Characterization of Microfiltration Membrane Embedded with Silver Nano-Particles. Iran. J. Chem. 2010, 29, 105–111. [Google Scholar]
- Xie, J.; Wang, C.H. Electrospun Micro- and Nanofibers for Sustained Delivery of Paclitaxel to Treat C6 Glioma in Vitro. Pharm. Res. 2006, 23, 1817–1826. [Google Scholar] [CrossRef]
- Baji, A.; Abtahi, M.; Ramakrishna, S. Bio-Inspired Electrospun Micro/Nanofibers with Special Wettability. J. Nanosci. Nanotechnol. 2014, 14, 4781–4798. [Google Scholar] [CrossRef]


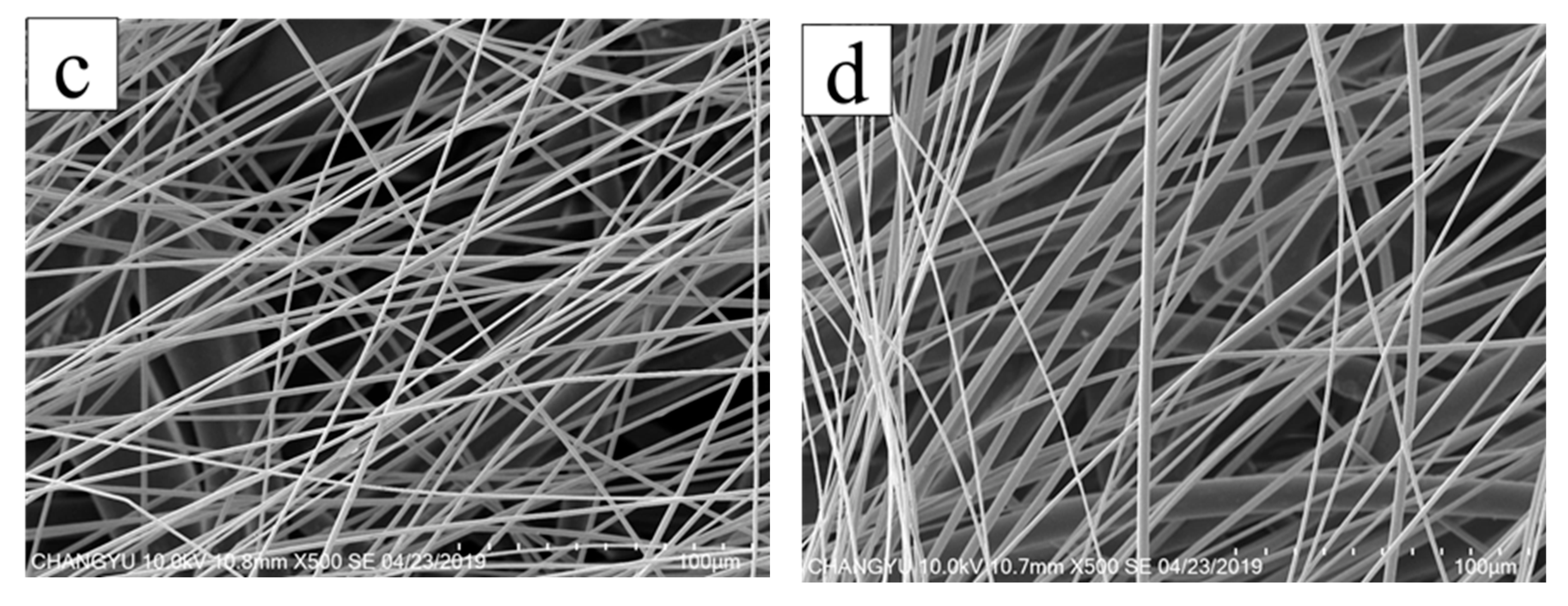

| NO. | AgNO3 Concentration (wt%) | Average Diameter (μm) | Variance | Minimum Diameter (μm) | Maximum DIAMETER (μm) |
|---|---|---|---|---|---|
| a | 0 | 2.82 | 2.47 | 2.79 | 7.68 |
| b | 0.5 | 3.38 | 1.23 | 2.27 | 6.42 |
| c | 1 | 2.08 | 0.77 | 1.02 | 4.04 |
| d | 1.5 | 2.89 | 2.05 | 2.32 | 9.29 |
| Element Type | Element Content % | Unit | Error |
|---|---|---|---|
| C | 94.644 | wt% | 1.988 |
| N | 0.000 | wt% | 0.000 |
| O | 4.205 | wt% | 3.513 |
| Ag | 1.151 | wt% | 1.310 |
| Addition of AgNO3 (wt%) | Contact Angle (°) | Average Time of Penetration (s) |
|---|---|---|
| 0 | 124.56 | 21.36 |
| 0.5 | 130.49 | 11.56 |
| 1.0 | 129.56 | 13.36 |
| 1.5 | 128.69 | 16.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Wang, H.; Zhao, X.; Yang, K.; Meng, Q.; Zhang, L. Fabrication of Unidirectional Water Permeable PS/PET Composite Nanofibers Modified with Silver Nanoparticles via Electrospinning. Membranes 2023, 13, 257. https://doi.org/10.3390/membranes13030257
Li C, Wang H, Zhao X, Yang K, Meng Q, Zhang L. Fabrication of Unidirectional Water Permeable PS/PET Composite Nanofibers Modified with Silver Nanoparticles via Electrospinning. Membranes. 2023; 13(3):257. https://doi.org/10.3390/membranes13030257
Chicago/Turabian StyleLi, Chong, Haoyu Wang, Xiaolei Zhao, Kaihua Yang, Qinhua Meng, and Longwang Zhang. 2023. "Fabrication of Unidirectional Water Permeable PS/PET Composite Nanofibers Modified with Silver Nanoparticles via Electrospinning" Membranes 13, no. 3: 257. https://doi.org/10.3390/membranes13030257
APA StyleLi, C., Wang, H., Zhao, X., Yang, K., Meng, Q., & Zhang, L. (2023). Fabrication of Unidirectional Water Permeable PS/PET Composite Nanofibers Modified with Silver Nanoparticles via Electrospinning. Membranes, 13(3), 257. https://doi.org/10.3390/membranes13030257






