Incorporation of Functionalized Halloysite Nanotubes (HNTs) into Thin-Film Nanocomposite (TFN) Nanofiltration Membranes for Water Softening
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
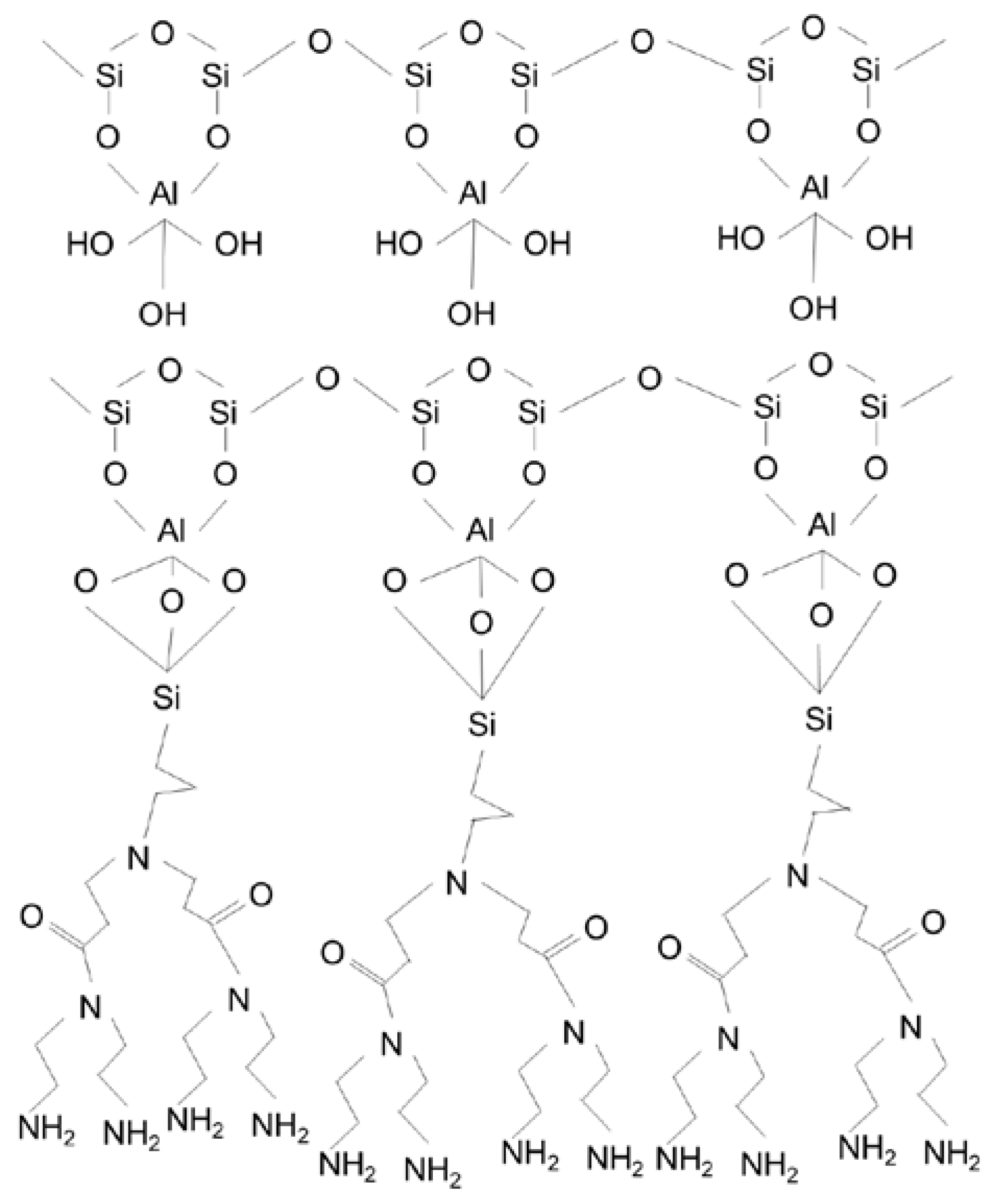
2.2. Functionalization of HNTs
2.3. Synthesis of TFC and TFN Membranes
2.4. Nanofiltration Performance
2.5. Membrane and Nanoparticle Physical Characterization
3. Results and Discussion
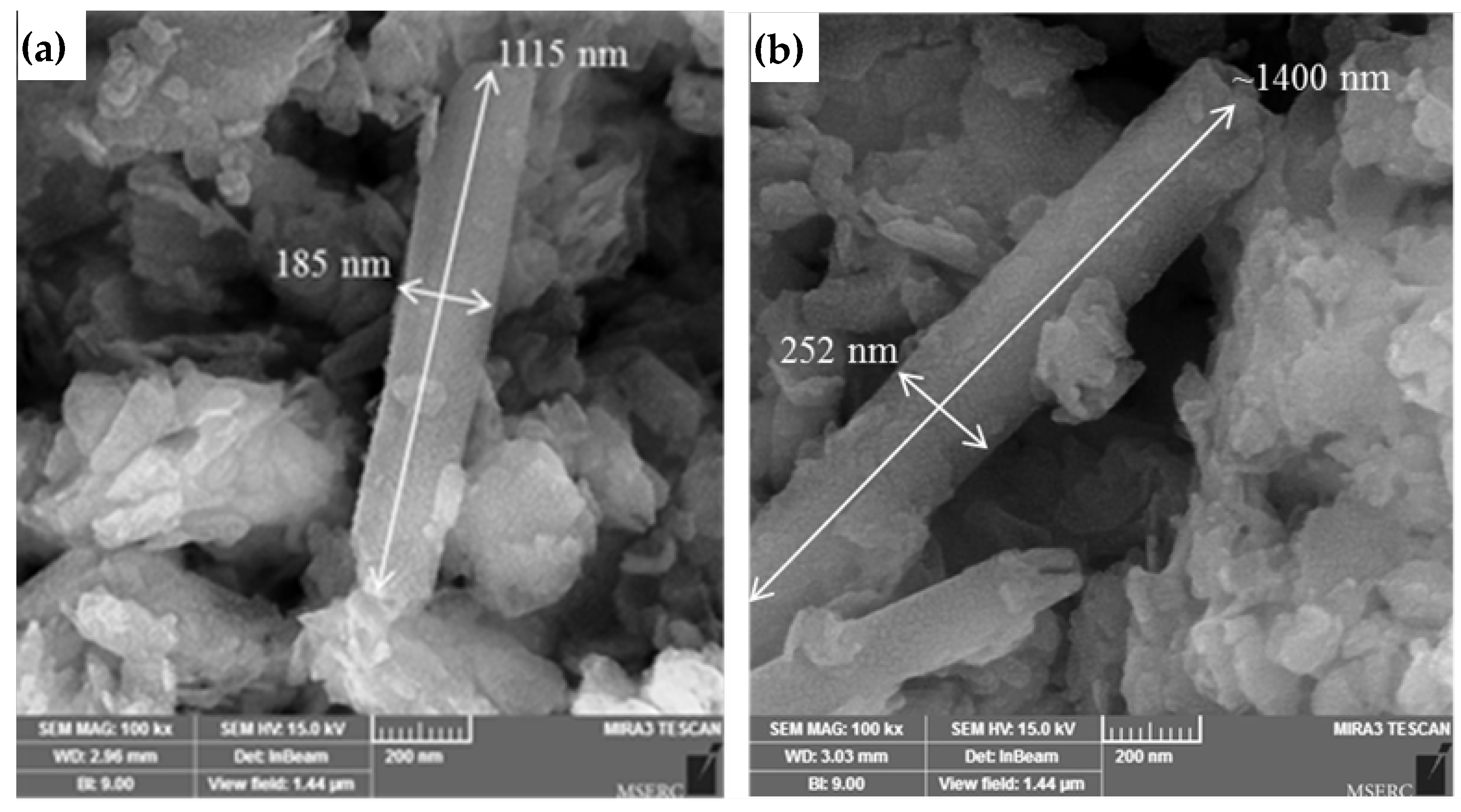
3.1. Characterization of the Nanoparticles
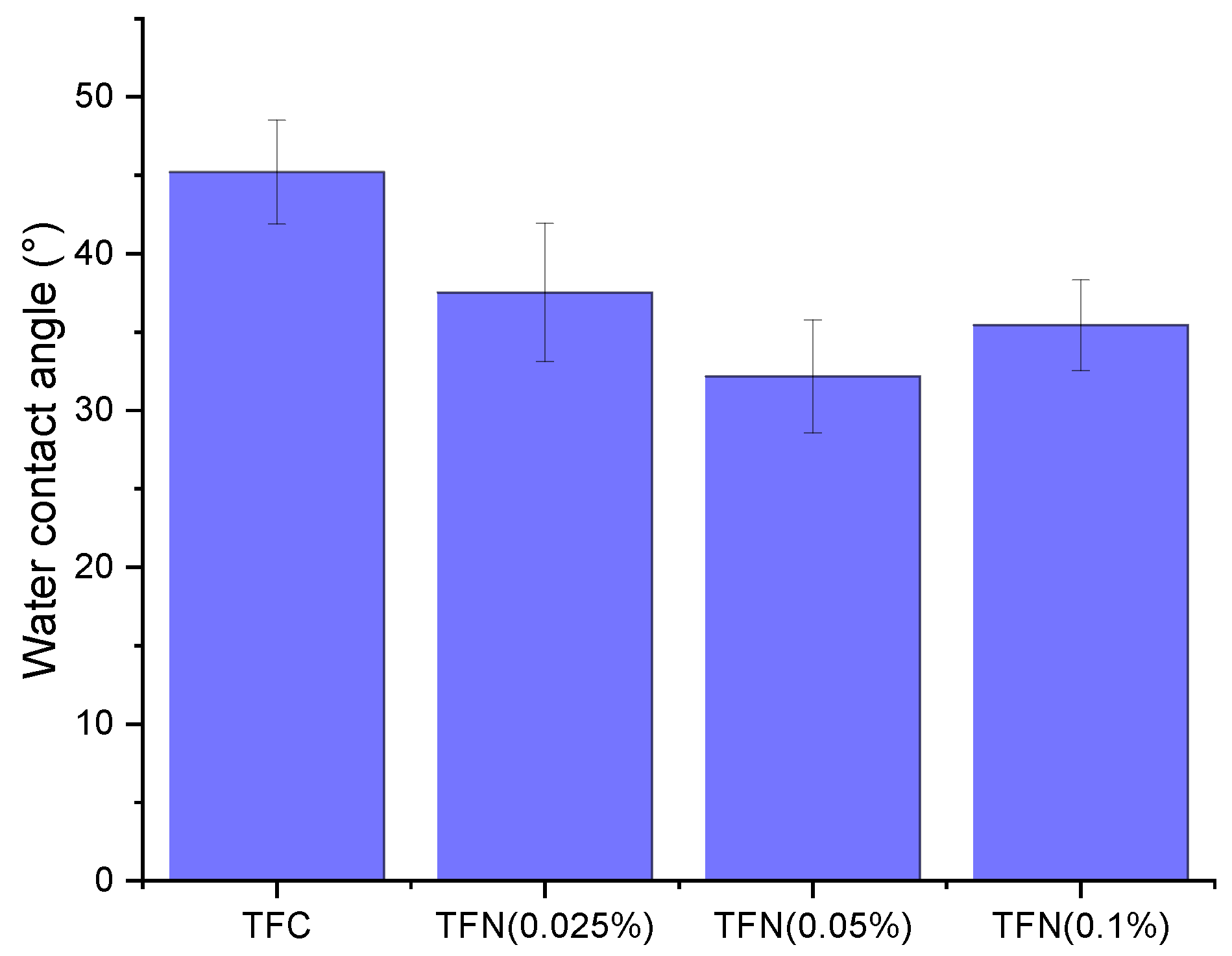
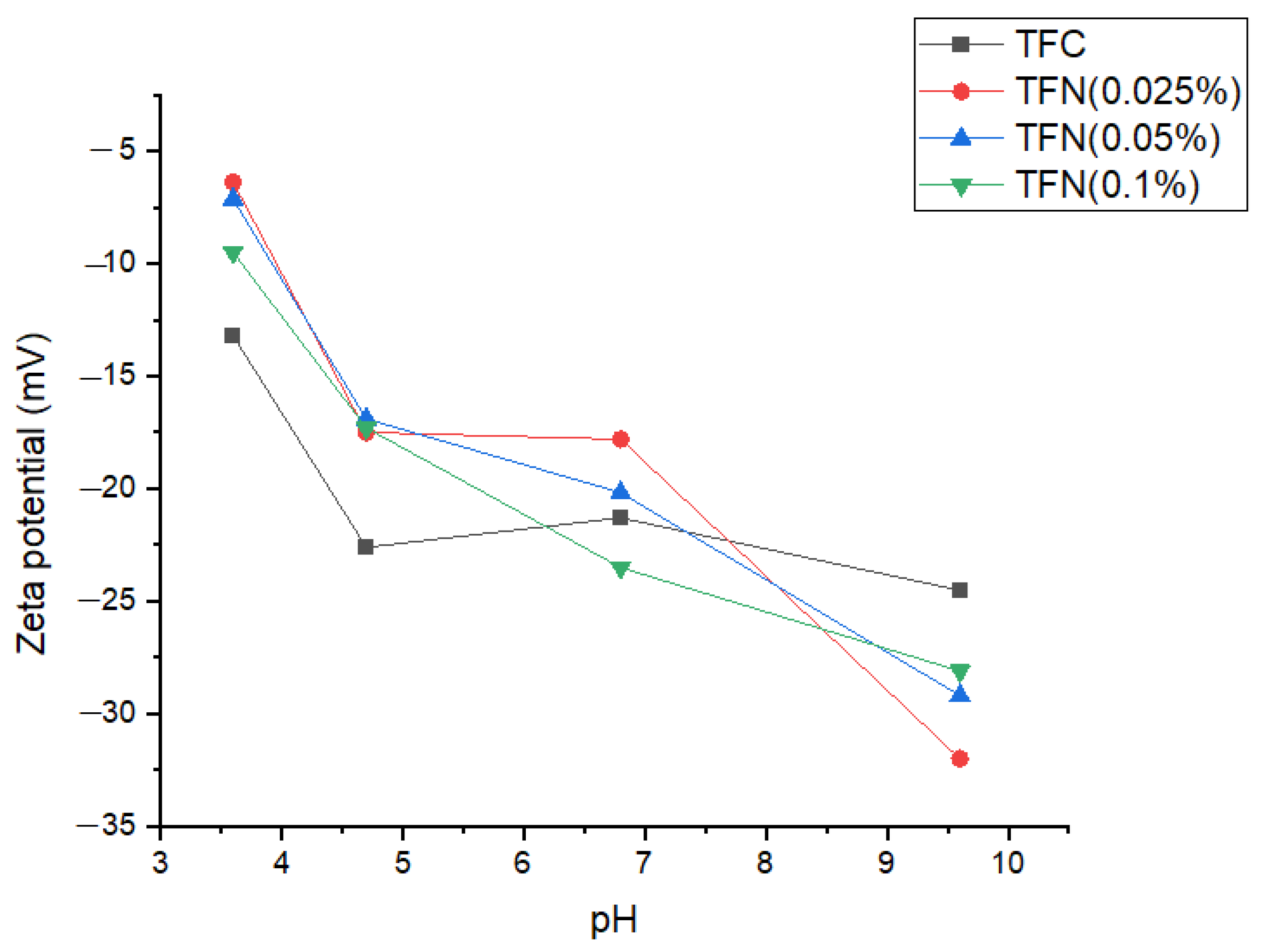
3.2. Characterization of the Membranes
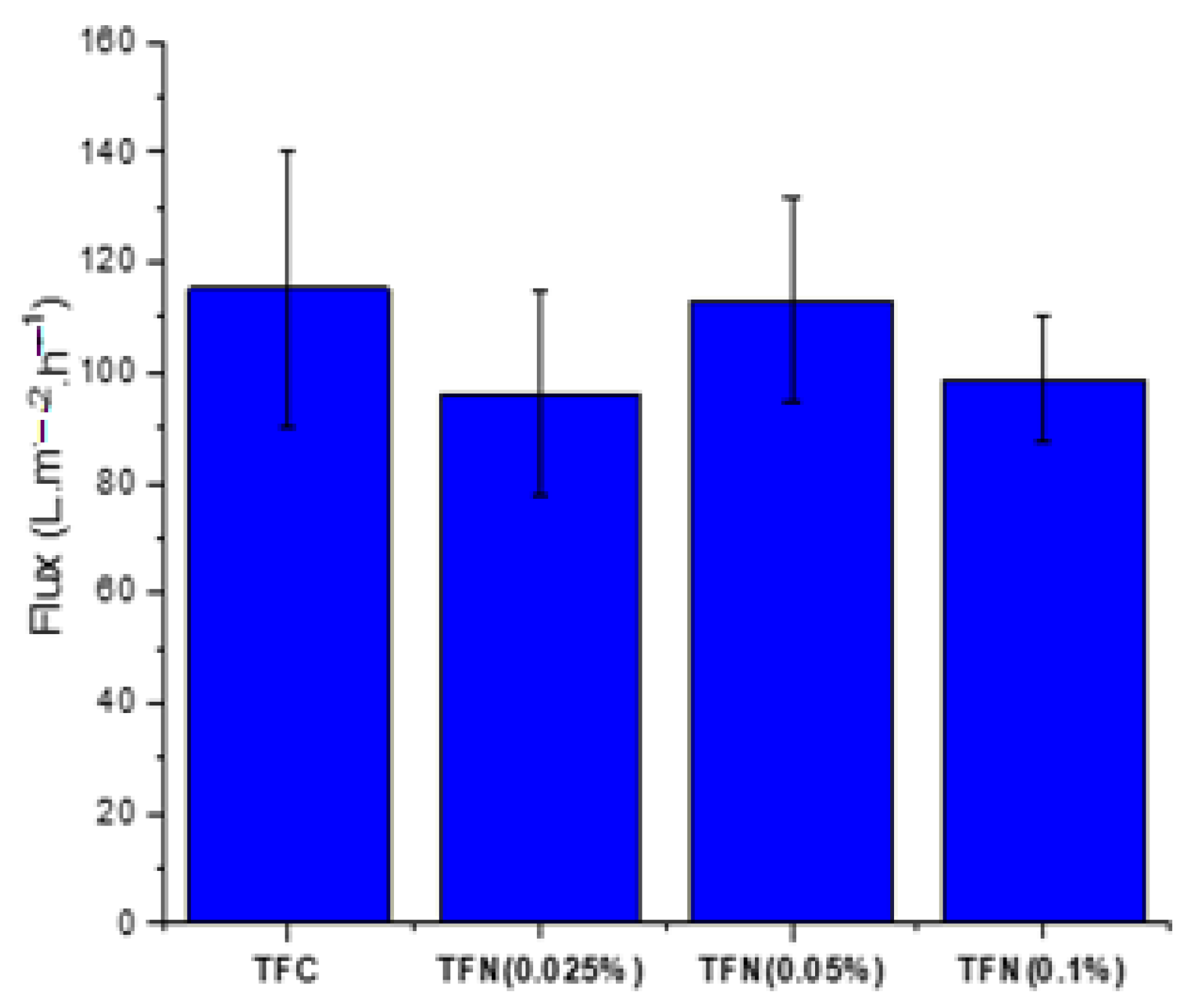
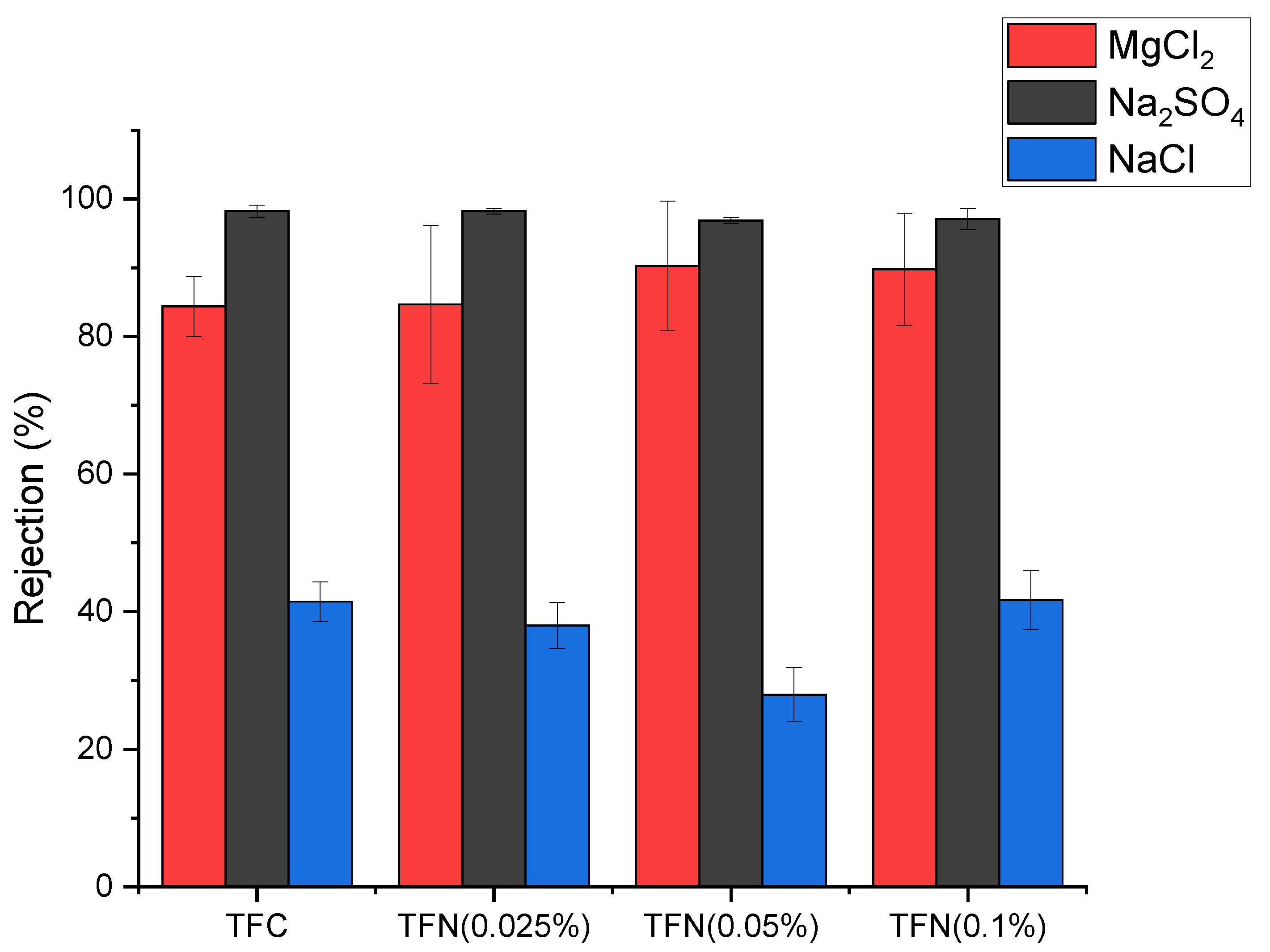
3.3. NF Performance of the Membranes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conidi, C.; Cassano, A.; Caiazzo, F.; Drioli, E. Separation and purification of phenolic compounds from pomegranate juice by ultrafiltration and nanofiltration membranes. J. Food Eng. 2017, 195, 1–13. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Fountoulis, G.; Gekas, V. Nanofiltration of brackish groundwater by using a polypiperazine membrane. Desalination 2012, 286, 277–284. [Google Scholar] [CrossRef]
- Bes-Piá, A.; Mendoza-Roca, J.A.; Roig-Alcover, L.; Iborra-Clar, A.; Iborra-Clar, M.I.; Alcaina-Miranda, M.I. Comparison between nanofiltration and ozonation of biologically treated textile wastewater for its reuse in the industry. Desalination 2003, 157, 81–86. [Google Scholar] [CrossRef]
- Chandrapala, J.; Duke, M.C.; Gray, S.R.; Weeks, M.; Palmer, M.; Vasiljevic, T. Nanofiltration and nanodiafiltration of acid whey as a function of pH and temperature. Sep. Purif. Technol. 2016, 160, 18–27. [Google Scholar] [CrossRef]
- Zhang, W.; He, G.; Gao, P.; Chen, G. Development and characterization of composite nanofiltration membranes and their application in concentration of antibiotics. Sep. Purif. Technol. 2003, 30, 27–35. [Google Scholar] [CrossRef]
- Siddique, A.; Mirza, M.A.; Kanwal, A. Potential use of ultrafiltration (UF) membrane for remediation of metal contaminants. In Emerging Techniques for Treatment of Toxic Metals from Wastewater; Ahmad, A., Kumar, R., Jawaid, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 341–364. [Google Scholar]
- Tang, C.; Chen, V. Nanofiltration of textile wastewater for water reuse. Desalination 2002, 143, 11–20. [Google Scholar] [CrossRef]
- Ahmad, A. Application and fabrication of nanofiltration membrane for separation of metal ions from wastewater. In Emerging Techniques for Treatment of Toxic Metals from Wastewater; Ahmad, A., Kumar, R., Jawaid, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 365–398. [Google Scholar]
- Mondal, S.; Wickramasinghe, S.R. Produced water treatment by nanofiltration and reverse osmosis membranes. J. Memb. Sci. 2008, 322, 162–170. [Google Scholar] [CrossRef]
- Juang, L.C.; Tseng, D.H.; Lin, H.Y. Membrane processes for water reuse from the effluent of industrial park wastewater treatment plant: A study on flux and fouling of membrane. Desalination 2007, 202, 302–309. [Google Scholar] [CrossRef]
- Koyuncu, I.; Topacik, D.; Yuksel, E. Reuse of reactive dyehouse wastewater by nanofiltration: Process water quality and economical implications. Sep. Purif. Technol. 2004, 36, 77–85. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Mänttäri, M.; Nyström, M. Drawbacks of applying nanofiltration and how to avoid them: A review. Sep. Purif. Technol. 2008, 63, 251–263. [Google Scholar] [CrossRef]
- Liu, L.F.; Huang, X.; Zhang, X.; Li, K.; Ji, Y.L.; Yu, C.Y.; Gao, C.J. Modification of polyamide TFC nanofiltration membrane for improving separation and antifouling properties. RSC Adv. 2008, 8, 15102–15110. [Google Scholar] [CrossRef]
- Rajaeian, B.; Rahimpour, A.; Tade, M.O.; Liu, S. Fabrication and characterization of polyamide thin film nanocomposite (TFN) nanofiltration membrane impregnated with TiO2 nanoparticles. Desalination 2013, 313, 176–188. [Google Scholar] [CrossRef]
- McGinnis, R.L.; Elimelech, M. Global challenges in energy and water supply: The promise of engineered osmosis. Environ. Sci. Technol. 2008, 42, 8625–8629. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Shintani, T.; Tsuru, T. “Pre-seeding”-assisted synthesis of a high performance polyamide-zeolite nanocomposite membrane for water purification. N. J. Chem. 2010, 34, 2101–2104. [Google Scholar] [CrossRef]
- Emadzadeh, D.; Lau, W.J.; Rahbari-Sisakht, M.; Daneshfar, A.; Ghanbari, M.; Mayahi, A.; Matsuura, T.; Ismail, A.F. A novel thin film nanocomposite reverse osmosis membrane with superior anti-organic fouling affinity for water desalination. Desalination 2015, 368, 106–113. [Google Scholar] [CrossRef]
- Shah, M.S.A.S.; Nag, M.; Kalagara, T.; Singh, S.; Manorama, S.V. Silver on PEG-PU-TiO2 polymer nanocomposite films: An excellent system for antibacterial applications. Chem. Mater. 2008, 20, 2455–2460. [Google Scholar] [CrossRef]
- Wong, K.C.; Goh, P.S.; Ng, B.C.; Ismail, A.F. Thin film nanocomposite embedded with polymethyl methacrylate modified multi-walled carbon nanotubes for CO2 removal. RSC Adv. 2015, 5, 31683–31690. [Google Scholar] [CrossRef]
- Ghanbari, M.; Emadzadeh, D.; Lau, W.J.; Lai, S.O.; Matsuura, T.; Ismail, A.F. Synthesis and characterization of novel thin film nanocomposite (TFN) membranes embedded with halloysite nanotubes (HNTs) for water desalination. Desalination 2015, 358, 33–41. [Google Scholar] [CrossRef]
- Emadzadeh, D.; Lau, W.J.; Matsuura, T.; Rahbari-Sisakht, M.; Ismail, A.F. A novel thin film composite forward osmosis membrane prepared from PSf-TiO2 nanocomposite substrate for water desalination. Chem. Eng. J. 2014, 237, 70–80. [Google Scholar] [CrossRef]
- Asempour, F.; Akbari, S.; Bai, D.; Emadzadeh, D.; Matsuura, T.; Kruczek, B. Improvement of stability and performance of functionalized halloysite nano tubes-based thin film nanocomposite membranes. J. Memb. Sci. 2018, 563, 470–480. [Google Scholar] [CrossRef]
- Asempour, F.; Akbari, S.; Kanani-Jazi, M.H.; Atashgar, A.; Matsuura, T.; Kruczek, B. Chlorine-resistant TFN RO membranes containing modified poly(amidoamine) dendrimer-functionalized halloysite nanotubes. J. Memb. Sci. 2021, 623, 119039. [Google Scholar] [CrossRef]
- Moslehyani, A.; Mobaraki, M.; Ismail, A.F.; Matsuura, T.; Hashemifard, S.A.; Othman, M.H.D.; Mayahi, A.; Rezaei Dashtarzhandi, M.; Soheilmoghaddam, M.; Shamsaei, E. Effect of HNTs modification in nanocomposite membrane enhancement for bacterial removal by cross-flow ultrafiltration system. React. Funct. Polym. 2015, 95, 80–87. [Google Scholar] [CrossRef]
- Ormanci-Acar, T.; Celebi, F.; Keskin, B.; Mutlu-Salmanlı, O.; Agtas, M.; Turken, T.; Tufani, A.; Imer, D.Y.; Ince, G.O.; Demir, T.U.; et al. Fabrication and characterization of temperature and pH resistant thin film nanocomposite membranes embedded with halloysite nanotubes for dye rejection. Desalination 2018, 429, 20–32. [Google Scholar] [CrossRef]
- Ghanbari, M.; Emadzadeh, D.; Lau, W.J.; Matsuura, T.; Davoody, M.; Ismail, A.F. Super hydrophilic TiO2/HNT nanocomposites as a new approach for fabrication of high performance thin film nanocomposite membranes for FO application. Desalination 2015, 371, 104–114. [Google Scholar] [CrossRef]
- Esfand, R.; Tomalia, D.A. Poly (amidoamine) (PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–436. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Safian, M.T.; Rashid, M.; Parveen, T.; Umar, K.; Ibrahim, M.N.M. Introduction of smart polymer nanocomposites. In Smart Polymer Nanocomposites; Bhawani, S.A., Khan, A., Jawaid, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–25. [Google Scholar]
- Shahamati Fard, F.; Akbari, S.; Pajootan, E.; Arami, M. Enhanced acidic dye adsorption onto the dendrimer-based modified halloysite nanotubes. Desalin. Water Treat. 2016, 57, 26222–26239. [Google Scholar] [CrossRef]
- Asempour, F.; Emadzadeh, D.; Matsuura, T.; Kruczek, B. Synthesis and characterization of novel Cellulose Nanocrystals-based Thin Film Nanocomposite membranes for reverse osmosis applications. Desalination 2018, 439, 179–187. [Google Scholar] [CrossRef]
- Wu, C.; Xie, Q.; Hong, Z.; Shen, L.; Yu, T.; Guo, H.; Xiong, Y.; Zhang, G.; Lu, Y.; Shao, W. Thin-film nanocomposite nanofiltration membrane with enhanced desalination and antifouling performance via incorporating L-aspartic acid functionalized graphene quantum dots. Desalination 2021, 498, 114811. [Google Scholar] [CrossRef]
- Joo, Y.; Jeon, Y.; Lee, S.U.; Sim, J.H.; Ryu, J.; Lee, S.; Lee, H.; Sohn, D. Aggregation and stabilization of carboxylic acid functionalized halloysite nanotubes (HNT-COOH). J. Phys. Chem. C. 2012, 116, 18230–18235. [Google Scholar] [CrossRef]
- Bai, L.; Liu, Y.; Bossa, N.; Ding, A.; Ren, N.; Li, G.; Liang, H.; Wiesner, M.R. Incorporation of Cellulose Nanocrystals (CNCs) into the Polyamide Layer of Thin-Film Composite (TFC) Nanofiltration Membranes for Enhanced Separation Performance and Antifouling Properties. Environ. Sci. Technol. 2018, 52, 11178–11187. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.Q.; Shao, L.; Lau, C.H. High flux polyethylene glycol based nanofiltration membranes for water environmental remediation. J. Memb. Sci. 2015, 476, 95–104. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, X.; Wang, J.; Tang, C.Y. Novel polyethyleneimine/TMC-based nanofiltration membrane prepared on a polydopamine coated substrate. Front. Chem. Sci. Eng. 2018, 12, 273–282. [Google Scholar] [CrossRef]
- Nightingale, E.R. Phenomenological theory of ion solvation. Effective radii of hydrated ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Wu, M.; Ma, T.; Su, Y.; Wu, H.; You, X.; Jiang, Z.; Kasher, R. Fabrication of composite nanofiltration membrane by incorporating attapulgite nanorods during interfacial polymerization for high water flux and antifouling property. J. Memb. Sci. 2017, 544, 79–87. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, H.; Meng, N.; Jian, M.; Wang, H.; Zhang, X. Graphene oxide incorporated thin film nanocomposite membrane at low concentration monomers. J. Memb. Sci. 2018, 565, 380–389. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zhu, J.; Zhang, Y.; Liu, J.; Van der Bruggen, B. Construction of TiO2@graphene oxide incorporated antifouling nanofiltration membrane with elevated filtration performance. J. Memb. Sci. 2017, 533, 279–288. [Google Scholar] [CrossRef]
- Wen, P.; Chen, Y.; Hu, X.; Cheng, B.; Liu, D.; Zhang, Y.; Nair, S. Polyamide thin film composite nanofiltration membrane modified with acyl chlorided graphene oxide. J. Memb. Sci. 2017, 535, 208–220. [Google Scholar] [CrossRef]
- Ji, Y.L.; An, Q.F.; Weng, X.D.; Hung, W.S.; Lee, K.R.; Gao, C.J. Microstructure and performance of zwitterionic polymeric nanoparticle/polyamide thin-film nanocomposite membranes for salts/organics separation. J. Memb. Sci. 2018, 548, 559–571. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Chen, J.; Li, Z.; Yin, Y.; Cao, L.; Zhong, Y.; Wu, H. Covalent organic framework modified polyamide nanofiltration membrane with enhanced performance for desalination. J. Memb. Sci. 2017, 523, 273–281. [Google Scholar] [CrossRef]
- Kang, Y.; Obaid, M.; Jang, J.; Kim, I.S. Sulfonated graphene oxide incorporated thin film nanocomposite nanofiltration membrane to enhance permeation and antifouling properties. Desalination 2019, 470, 114125. [Google Scholar] [CrossRef]
- Belle, M.; Yap, M.; Trilles, C.A.; Reyes, M.; Guzman, D. Improved performance of thin- fi lm nanocomposite nano filtration membranes as induced by embedded polydopamine-coated silica nanoparticles. Sep. Purif. Technol. 2019, 224, 113–120. [Google Scholar] [CrossRef]
- Kong, Q.; Xu, H.; Liu, C.; Yang, G.; Ding, M.; Yang, W.; Lin, T.; Chen, W.; Gray, S.; Xie, Z. Fabrication of high performance TFN membrane containing NH2-SWCNTs: Via interfacial regulation. RSC Adv. 2020, 10, 25186–25199. [Google Scholar] [CrossRef] [PubMed]
- Baroña, G.N.B.; Choi, M.; Jung, B. High permeate flux of PVA/PSf thin film composite nanofiltration membrane with aluminosilicate single-walled nanotubes. J. Colloid Interface Sci. 2012, 386, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.N.; Yu, C.C.; Ruan, H.M.; Gao, C.J.; Van der Bruggen, B. Preparation and characterization of thin-film nanocomposite membranes embedded with poly(methyl methacrylate) hydrophobic modified multiwalled carbon nanotubes by interfacial polymerization. J. Memb. Sci. 2013, 442, 18–26. [Google Scholar] [CrossRef]
- Zhao, F.Y.; Ji, Y.L.; Weng, X.D.; Mi, Y.F.; Ye, C.C.; An, Q.F.; Gao, C.J. High-Flux Positively Charged Nanocomposite Nanofiltration Membranes Filled with Poly(dopamine) Modified Multiwall Carbon Nanotubes. ACS Appl. Mater. Interfaces. 2016, 8, 6693–6700. [Google Scholar] [CrossRef]
- Abedi, F.; Dubé, M.A.; Emadzadeh, D.; Kruczek, B. Improving nanofiltration performance using modified cellulose nanocrystal-based TFN membranes. J. Memb. Sci. 2023, 670, 12369. [Google Scholar] [CrossRef]


| Membrane | PIP in H2O (w/v) % | TMC in n-Hexane (w/v) % | HNTs-G1 in TMC/n-Hexane (w/v) % |
|---|---|---|---|
| TFC | 2 | 0.05 | 0 |
| TFN (0.025%) | 2 | 0.05 | 0.025 |
| TFN (0.05%) | 2 | 0.05 | 0.05 |
| TFN (0.1%) | 2 | 0.05 | 0.1 |
| Ion | Hydrated Radius (Å) |
|---|---|
| Cl− | 3.32 |
| SO42− | 3.79 |
| Na+ | 3.58 |
| Mg2+ | 4.28 |
| NPs | Weight Loss at 800 °C (%) | Zeta Potential * (mV) |
|---|---|---|
| HNTs | 17.65 | −34.5 |
| HNTs-G1 | 22.94 | +2.2 |
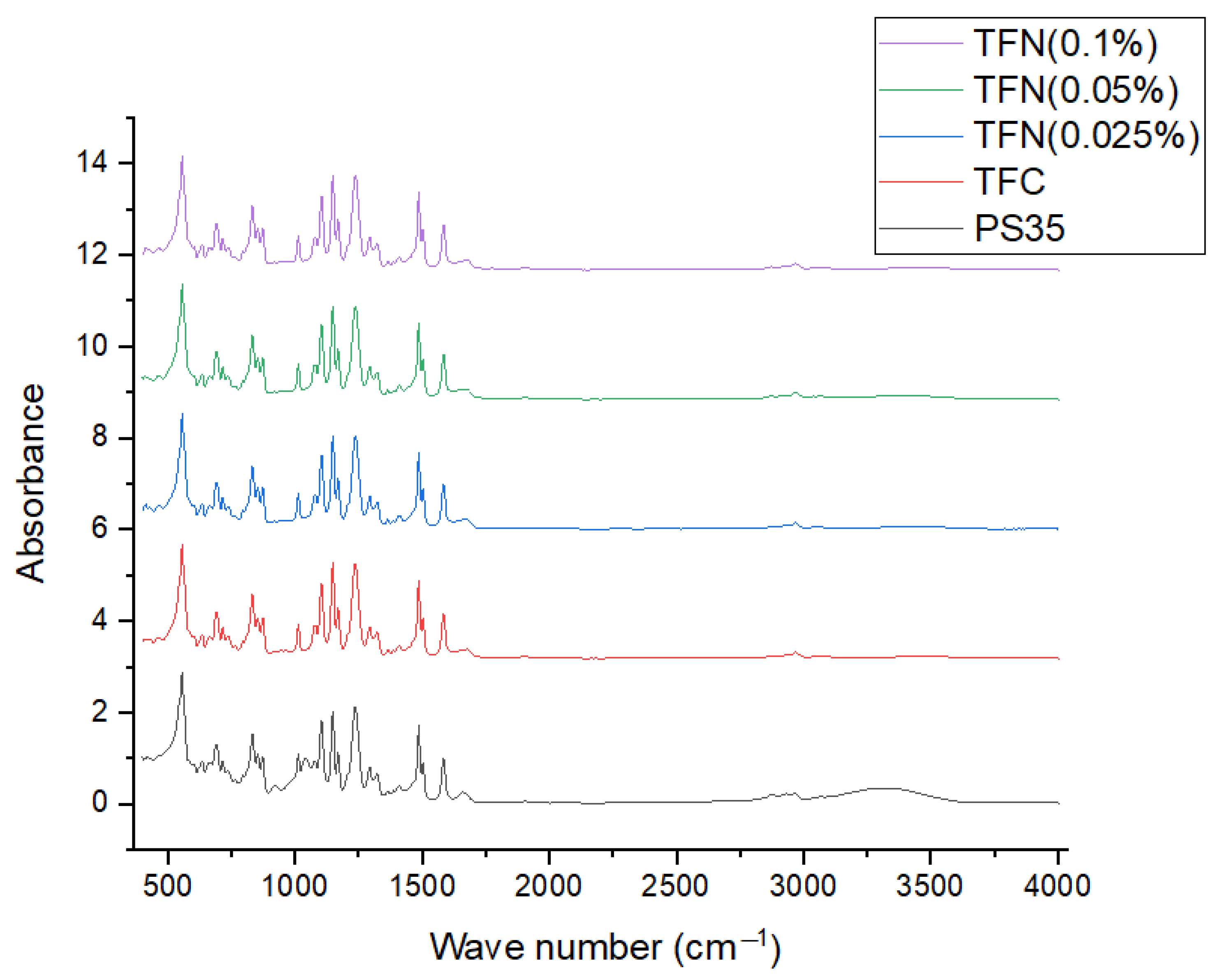
| Membrane | Primary Amide (1618–1720 cm−1) | Aromatic Amide (1600–1618 cm−1) | 3120–3706 cm−1 |
|---|---|---|---|
| PS35 | 15.6 | 2.6 | 123.2 |
| TFC | 16.7 | 3.2 | 29.1 |
| TFN (0.025%) | 18.4 | 3.5 | 36.5 |
| TFN (0.05%) | 18.8 | 3.7 | 44.3 |
| TFN (0.1%) | 17.3 | 3.3 | 30.6 |
| Nanomaterial | MgCl2 Rejection (%) | Na2SO4 Rejection (%) | Water Permeance (L m−2 h−1 bar−1) | Salt Concentration (g L−1) | Ref. |
|---|---|---|---|---|---|
| HNTs-G1 * | 90.25 | 96.88 | 5.65 | 3 | This work |
| ATP | 20 | 92 | 23 | 1 | [38] |
| GO | - | 96.56 | 15.63 | 1 | [39] |
| TiO2 @ GO | 6.2 | 98.8 | 5.60 | 1 | [40] |
| GO-COCl * | - | 97.1 | 3.76 | 1 | [41] |
| ZNGs | 41.1 | 97.8 | 10.63 | 1 | [42] |
| COFs (SNW-1) | - | 83.5 | 19.25 | 1 | [43] |
| SGO | - | 96.45 | 2.37 | 2.5 | [44] |
| PDA-Si * | 68 | 97 | 13.33 | 1 | [45] |
| NH2-SWCNT | 51.63 | 96.34 | 17.8 | 2 | [46] |
| Aluminosilicate SWCNT | - | 97 | <1.2 | 2 | [47] |
| PMMA- MWNT * | - | 99 | 7 | 2 | [48] |
| Poly(dopamine) MWCNT | 91.5 | 45.2 | 15.32 | 1 | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atashgar, A.; Emadzadeh, D.; Akbari, S.; Kruczek, B. Incorporation of Functionalized Halloysite Nanotubes (HNTs) into Thin-Film Nanocomposite (TFN) Nanofiltration Membranes for Water Softening. Membranes 2023, 13, 245. https://doi.org/10.3390/membranes13020245
Atashgar A, Emadzadeh D, Akbari S, Kruczek B. Incorporation of Functionalized Halloysite Nanotubes (HNTs) into Thin-Film Nanocomposite (TFN) Nanofiltration Membranes for Water Softening. Membranes. 2023; 13(2):245. https://doi.org/10.3390/membranes13020245
Chicago/Turabian StyleAtashgar, Amirsajad, Daryoush Emadzadeh, Somaye Akbari, and Boguslaw Kruczek. 2023. "Incorporation of Functionalized Halloysite Nanotubes (HNTs) into Thin-Film Nanocomposite (TFN) Nanofiltration Membranes for Water Softening" Membranes 13, no. 2: 245. https://doi.org/10.3390/membranes13020245
APA StyleAtashgar, A., Emadzadeh, D., Akbari, S., & Kruczek, B. (2023). Incorporation of Functionalized Halloysite Nanotubes (HNTs) into Thin-Film Nanocomposite (TFN) Nanofiltration Membranes for Water Softening. Membranes, 13(2), 245. https://doi.org/10.3390/membranes13020245










