Membranes Coated with Graphene-Based Materials: A Review
Abstract
1. Introduction
2. Graphene Derivatives
3. Summary of Graphene’s Physicochemical Properties and Synthesis Approaches
3.1. Physicochemical Properties
3.2. Synthesis Processes
3.3. Challenges at Synthesis of Graphene-Based Membranes
3.4. Challenges and Specified Requirements to Achieve Graphene-Based Membranes at a Large Scale
4. Green Methods of the Preparation of Graphene-Based Materials
5. Graphene-Based Membrane Categories Based on Microstructure
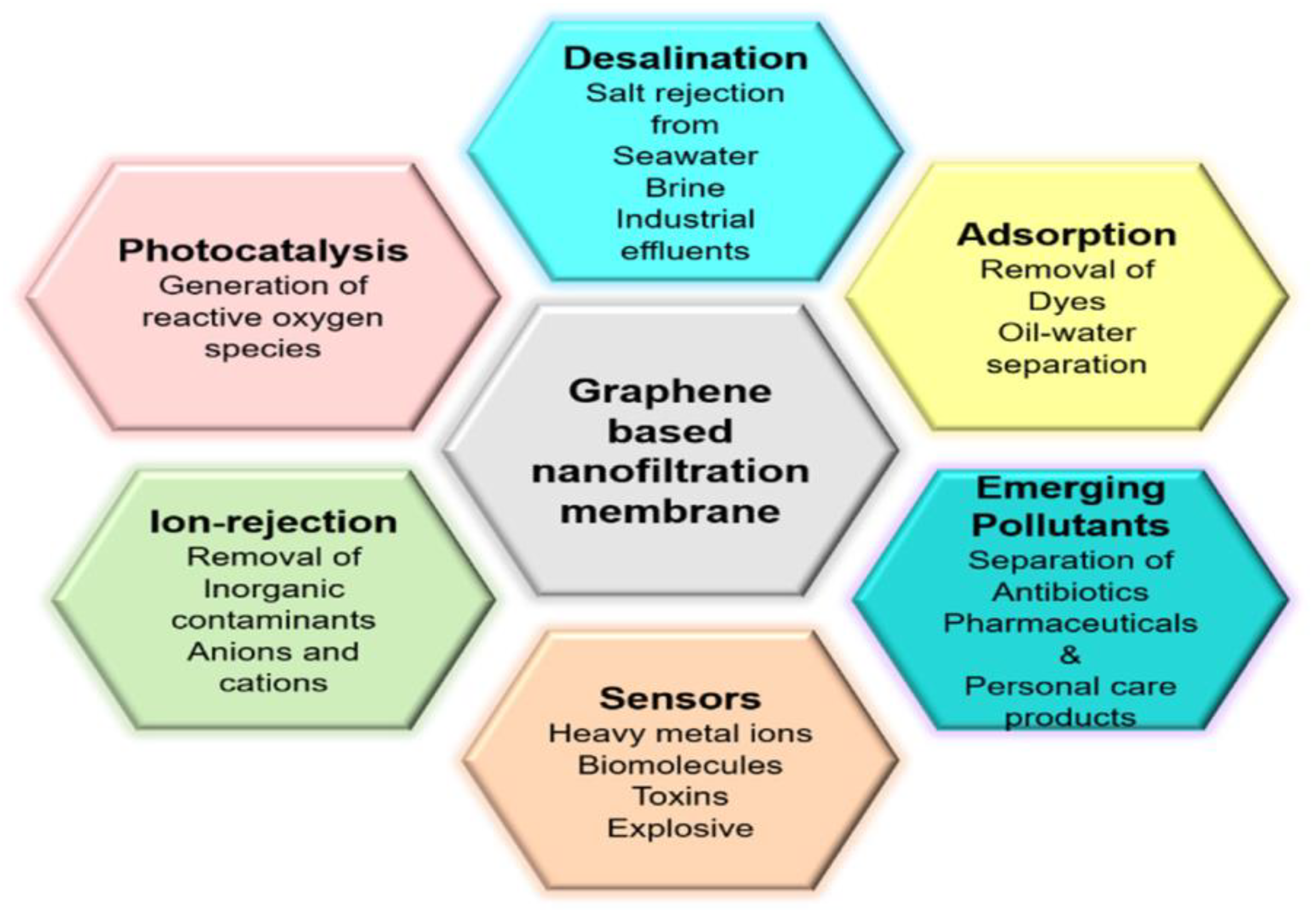
6. Graphene-Based Membranes in Water Purification
6.1. Graphene-Based Membranes in Water Purification
6.2. Desalination
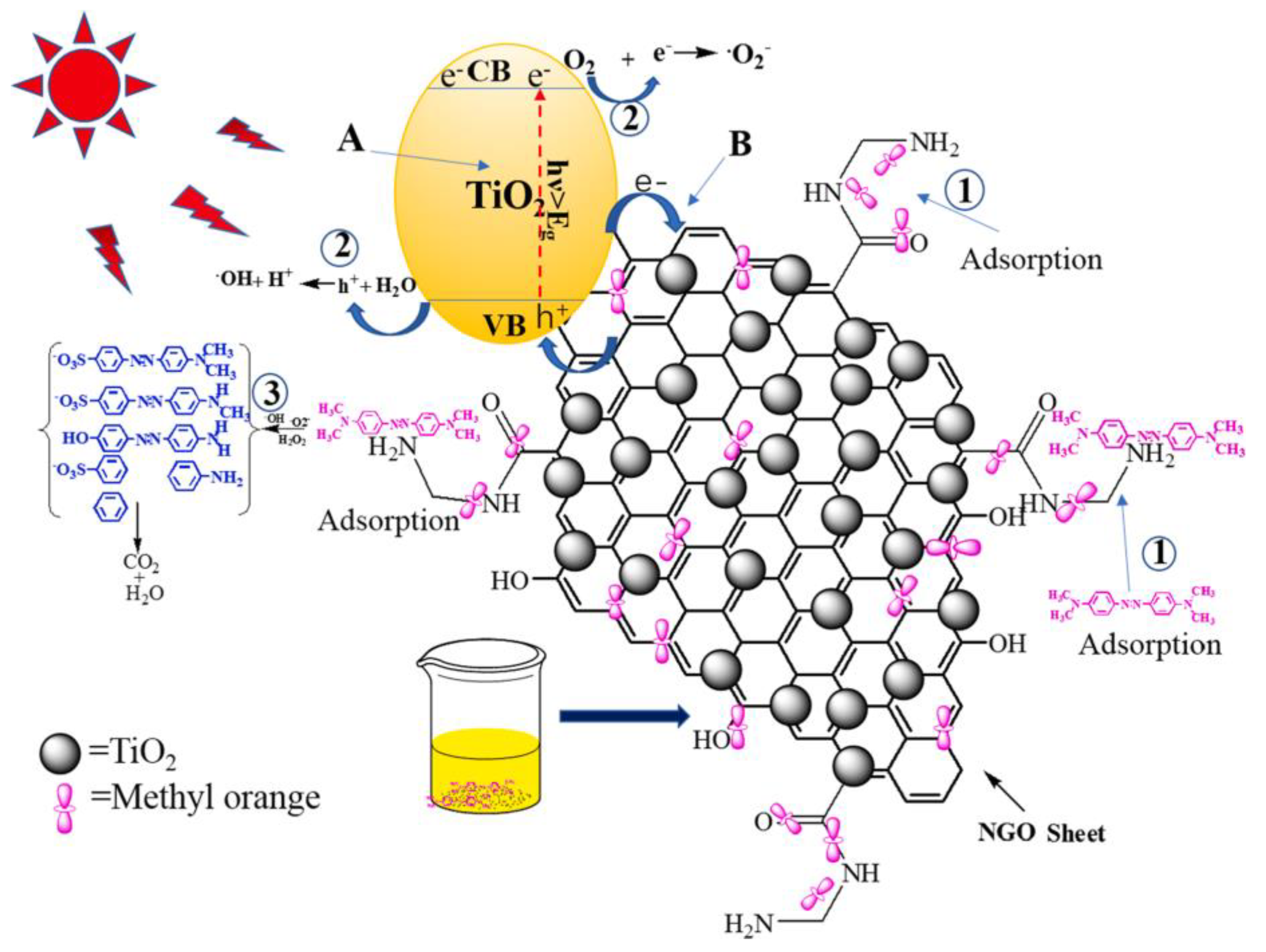
6.3. Photocatalysis
6.4. Adsorption
6.5. Ion Rejection
6.6. Sensors
6.7. Emerging Pollutants
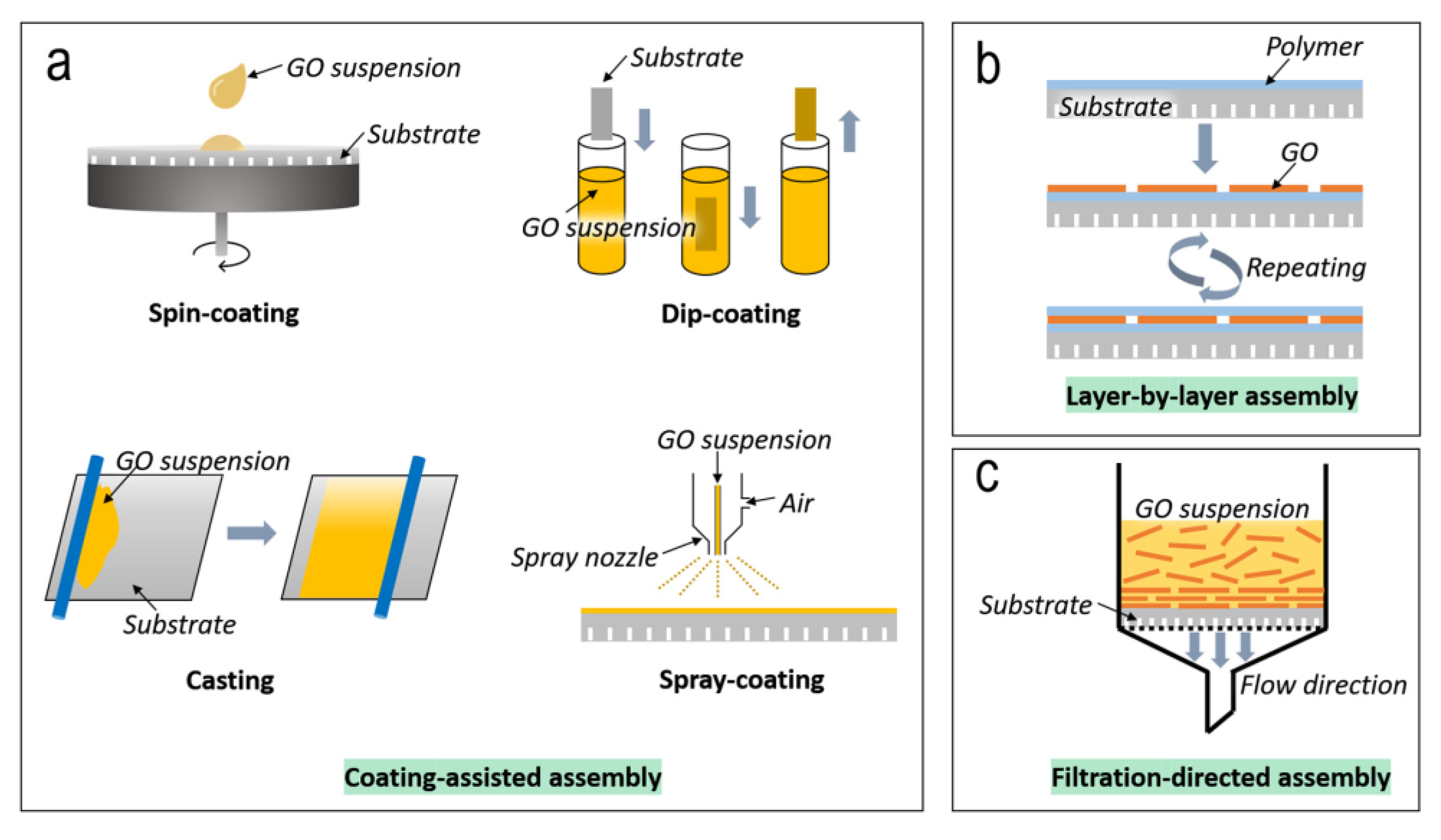
7. Fabrication and Performance of Graphene-Based Membranes
| Materials | Fabrication Method | Application | Membrane Performance | Reference | ||
|---|---|---|---|---|---|---|
| Water Permeance (LMH/bar) | Rejection (%) | |||||
| Hybrid 2D WS2/GO nanosheets | Vacuum filtration | Water filtration | 156.3 | 96.30% | Methylene Blue | [127] |
| Semi-permeable graphene/GO membranes | Vacuum filtration | Water treatment | 3.22 | 74.4% | Methylene Blue | [128] |
| 98.2% | rhodamine B | |||||
| High flux nanofiltration (NF) membranes prepared from GO quantum dots and sheets | Vacuum filtration | Nanofiltration (yellow, bovine serum albumin, humic acid, and Au NPs (>99%)) | 45.89 | 28.4 | NaCl | [129] |
| 74.9 | Na2SO4 | |||||
| 98.6 | Methyl orange | |||||
| GO/MB composite membrane | Vacuum filtration | Dye separation | 7.67 | 82.60% | rhodamine B | [130] |
| Niobate nanosheet-GO composite | Vacuum filtration | Nanofiltration/advanced molecular separation | 20 | 15.0% | NaCl | [131] |
| 60.0% | Na2SO4 | |||||
| 100% | Evans blue | |||||
| Photocatalytic self-cleaning titanium dioxide nanorods inserted GO based NF membrane | Vacuum filtration | water treatment | 68.1 | 33.0% | NaCl | [132] |
| 57.1% | Na2SO4 | |||||
| 99.3% | Methylene Blue | |||||
| 99.3% | Methyl Orange | |||||
| GO and Graphene | Vacuum-assisted filtration | 7.2 | 88.3% | NaCl | [117] | |
| Laser-induced graphene/poly (vinyl alcohol) | Cross Linking | Water treatment | 225 | - | - | [135] |
| High performance hierarchically nanostructured graphene oxide/covalent organic framework (GO/COF) hybrid membranes | Intercalating imine-based COF nanoparticles. | Organic solvent nanofiltration | 51−60 | 99%, | Methylene Blue | [136] |
| 99.82% | Congo Red | |||||
| oxygenated GO NF membrane | Slot-die coating | Nanofiltration | ∼30 | 89.8%, | Methylene Red | [137] |
| 99.4% | Methylene Blue | |||||
| 96.8% | Briliant Blue | |||||
| 72.6% | Evans Blue | |||||
| 63.9% | rhodamine B | |||||
| Graphene oxide membrane | Mild annealing process | Nanofiltration | 7.37 | 57.73% | Na2SO4 | [138] |
| GO/molybdenum disulphide (MoS2)-PVA composite membranes | Pressure filtration | Water and landfill leachate treatment | 0.592−1.416 | 89% | NaCl | [35] |
| Reduced graphene oxide membranes | Layer-by-layer | Desalination/water purification | - | 27.38% | Na+ | [134] |
| 47.44% | MG+ | |||||
8. Factors Influencing the Separation
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, G.; Jin, W.; Xu, N. Graphene-Based Membranes. Chem. Soc. Rev. 2015, 44, 5016–5030. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.; Choi, Y.; Choi, E.; Kim, M.; Woo, Y.C.; Kim, D.W. Fabrication Techniques for Graphene Oxide-Based Molecular Separation Membranes: Towards Industrial Application. Nanomaterials 2021, 11, 757. [Google Scholar] [CrossRef] [PubMed]
- Bhol, P.; Yadav, S.; Altaee, A.; Saxena, M.; Misra, P.K.; Samal, A.K. Graphene-Based Membranes for Water and Wastewater Treatment: A Review. ACS Appl. Nano Mater. 2021, 4, 3274–3293. [Google Scholar] [CrossRef]
- Miculescu, M.; Thakur, V.K.; Miculescu, F.; Voicu, S.I. Graphene-Based Polymer Nanocomposite Membranes: A Review: Polymer Nanocomposite Membranes. Polym. Adv. Technol. 2016, 27, 844–859. [Google Scholar] [CrossRef]
- Nidamanuri, N.; Li, Y.; Li, Q.; Dong, M.; Boni, B.O.O.; Lamboni, L.; Bakadia, B.M.; Hussein, S.A.; Yang, G. Graphene and Graphene Oxide-Based Membranes for Gas Separation. Eng. Sci. 2020, 9, 3–16. [Google Scholar] [CrossRef]
- Remanan, S.; Padmavathy, N.; Ghosh, S.; Mondal, S.; Bose, S.; Das, N.C. Porous Graphene-Based Membranes: Preparation and Properties of a Unique Two-Dimensional Nanomaterial Membrane for Water Purification. Sep. Purif. Rev. 2021, 50, 262–282. [Google Scholar] [CrossRef]
- Ma, J.; Ping, D.; Dong, X. Recent Developments of Graphene Oxide-Based Membranes: A Review. Membranes 2017, 7, 52. [Google Scholar] [CrossRef]
- Mohan, V.B.; Lau, K.; Hui, D.; Bhattacharyya, D. Graphene-Based Materials and Their Composites: A Review on Production, Applications and Product Limitations. Compos. Part B Eng. 2018, 142, 200–220. [Google Scholar] [CrossRef]
- Sun, P.; Wang, K.; Zhu, H. Recent Developments in Graphene-Based Membranes: Structure, Mass-Transport Mechanism and Potential Applications. Adv. Mater. 2016, 28, 2287–2310. [Google Scholar] [CrossRef]
- Kumari, P.; Tripathi, K.M.; Jangir, L.K.; Gupta, R.; Awasthi, K. Recent Advances in Application of the Graphene-Based Membrane for Water Purification. Mater. Today Chem. 2021, 22, 100597. [Google Scholar] [CrossRef]
- Mahalingam, D.K.; Falca, G.; Upadhya, L.; Abou-Hamad, E.; Batra, N.; Wang, S.; Musteata, V.; da Costa, P.M.; Nunes, S.P. Spray-Coated Graphene Oxide Hollow Fibers for Nanofiltration. J. Membr. Sci. 2020, 606, 118006. [Google Scholar] [CrossRef]
- Seo, D.H.; Pineda, S.; Woo, Y.C.; Xie, M.; Murdock, A.T.; Ang, E.Y.M.; Jiao, Y.; Park, M.J.; Lim, S.I.; Lawn, M.; et al. Anti-Fouling Graphene-Based Membranes for Effective Water Desalination. Nat. Commun. 2018, 9, 683. [Google Scholar] [CrossRef] [PubMed]
- O’Hern, S.C.; Jang, D.; Bose, S.; Idrobo, J.-C.; Song, Y.; Laoui, T.; Kong, J.; Karnik, R. Nanofiltration across Defect-Sealed Nanoporous Monolayer Graphene. Nano Lett. 2015, 15, 3254–3260. [Google Scholar] [CrossRef] [PubMed]
- Banszerus, L.; Schmitz, M.; Engels, S.; Dauber, J.; Oellers, M.; Haupt, F.; Watanabe, K.; Taniguchi, T.; Beschoten, B.; Stampfer, C. Ultrahigh-Mobility Graphene Devices from Chemical Vapor Deposition on Reusable Copper. Sci. Adv. 2015, 1, e1500222. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A. Phononics of Graphene and Related Materials. ACS Nano 2020, 14, 5170–5178. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Homem, N.C.; de Camargo Lima Beluci, N.; Amorim, S.; Reis, R.; Vieira, A.M.S.; Vieira, M.F.; Bergamasco, R.; Amorim, M.T.P. Surface Modification of a Polyethersulfone Microfiltration Membrane with Graphene Oxide for Reactive Dyes Removal. Appl. Surf. Sci. 2019, 486, 499–507. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, X.; Yang, J.; Lv, Y.; Zhang, C.; Xu, Z.-K. Ultra-Thin Graphene Oxide Films via Contra-Diffusion Method: Fast Fabrication for Ion Rejection. J. Membr. Sci. 2020, 595, 117586. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, W.; Weyland, M.; Yuan, S.; Xia, Y.; Liu, H.; Jian, M.; Yang, J.; Easton, C.D.; Selomulya, C.; et al. Thermally Reduced Nanoporous Graphene Oxide Membrane for Desalination. Environ. Sci. Technol. 2019, 53, 8314–8323. [Google Scholar] [CrossRef]
- Mao, Y.; Huang, Q.; Meng, B.; Zhou, K.; Liu, G.; Gugliuzza, A.; Drioli, E.; Jin, W. Roughness-Enhanced Hydrophobic Graphene Oxide Membrane for Water Desalination via Membrane Distillation. J. Membr. Sci. 2020, 611, 118364. [Google Scholar] [CrossRef]
- Xu, Y.; Peng, G.; Liao, J.; Shen, J.; Gao, C. Preparation of Molecular Selective GO/DTiO2-PDA-PEI Composite Nanofiltration Membrane for Highly Pure Dye Separation. J. Membr. Sci. 2020, 601, 117727. [Google Scholar] [CrossRef]
- Lin, H.; Li, Y.; Zhu, J. Cross-Linked GO Membranes Assembled with GO Nanosheets of Differently Sized Lateral Dimensions for Organic Dye and Chromium Separation. J. Membr. Sci. 2020, 598, 117789. [Google Scholar] [CrossRef]
- Shao, L.; Yu, Z.; Li, X.; Zeng, H.; Liu, Y. One-Step Preparation of Sepiolite/Graphene Oxide Membrane for Multifunctional Oil-in-Water Emulsions Separation. Appl. Clay Sci. 2019, 181, 105208. [Google Scholar] [CrossRef]
- Alammar, A.; Park, S.-H.; Williams, C.J.; Derby, B.; Szekely, G. Oil-in-Water Separation with Graphene-Based Nanocomposite Membranes for Produced Water Treatment. J. Membr. Sci. 2020, 603, 118007. [Google Scholar] [CrossRef]
- Shen, W.; Zhao, G.; Zhang, X.; Bu, F.; Yun, J.; Tang, J. Using Dual Microresonant Cavity and Plasmonic Effects to Enhance the Photovoltaic Efficiency of Flexible Polymer Solar Cells. Nanomaterials 2020, 10, 944. [Google Scholar] [CrossRef]
- Chuah, C.; Lee, J.; Bae, T.-H. Graphene-Based Membranes for H2 Separation: Recent Progress and Future Perspective. Membranes 2020, 10, 336. [Google Scholar] [CrossRef]
- Han, Z.; Huang, L.; Qu, H.; Wang, Y.; Zhang, Z.; Rong, Q.; Sang, Z.; Wang, Y.; Kipper, M.J.; Tang, J. A Review of Performance Improvement Strategies for Graphene Oxide-Based and Graphene-Based Membranes in Water Treatment. J. Mater. Sci. 2021, 56, 9545–9574. [Google Scholar] [CrossRef]
- Junaidi, N.F.D.; Othman, N.H.; Fuzil, N.S.; Mat Shayuti, M.S.; Alias, N.H.; Shahruddin, M.Z.; Marpani, F.; Lau, W.J.; Ismail, A.F.; Aba, N.D. Recent Development of Graphene Oxide-Based Membranes for Oil–Water Separation: A Review. Sep. Purif. Technol. 2021, 258, 118000. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Elbahri, M. Graphene Membranes for Water Desalination. NPG Asia Mater. 2017, 9, e427. [Google Scholar] [CrossRef]
- Bianco, A.; Cheng, H.-M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M.D.; et al. All in the Graphene Family—A Recommended Nomenclature for Two-Dimensional Carbon Materials. Carbon 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Khan, M.; Tahir, M.N.; Adil, S.F.; Khan, H.U.; Siddiqui, M.R.H.; Al-warthan, A.A.; Tremel, W. Graphene Based Metal and Metal Oxide Nanocomposites: Synthesis, Properties and Their Applications. J. Mater. Chem. A 2015, 3, 18753–18808. [Google Scholar] [CrossRef]
- Wick, P.; Louw-Gaume, A.E.; Kucki, M.; Krug, H.F.; Kostarelos, K.; Fadeel, B.; Dawson, K.A.; Salvati, A.; Vázquez, E.; Ballerini, L.; et al. Classification Framework for Graphene-Based Materials. Angew. Chem. Int. Ed. 2014, 53, 7714–7718. [Google Scholar] [CrossRef] [PubMed]
- Koulivand, H.; Shahbazi, A.; Vatanpour, V.; Rahmandoust, M. Development of Carbon Dot-Modified Polyethersulfone Membranes for Enhancement of Nanofiltration, Permeation and Antifouling Performance. Sep. Purif. Technol. 2020, 230, 115895. [Google Scholar] [CrossRef]
- Anand, A.; Unnikrishnan, B.; Mao, J.-Y.; Lin, H.-J.; Huang, C.-C. Graphene-Based Nanofiltration Membranes for Improving Salt Rejection, Water Flux and Antifouling–A Review. Desalination 2018, 429, 119–133. [Google Scholar] [CrossRef]
- Yadav, S.; Ibrar, I.; Altaee, A.; Samal, A.K.; Ghobadi, R.; Zhou, J. Feasibility of Brackish Water and Landfill Leachate Treatment by GO/MoS2-PVA Composite Membranes. Sci. Total Environ. 2020, 745, 141088. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Parekh, S.H. Linking Graphene-Based Material Physicochemical Properties with Molecular Adsorption, Structure and Cell Fate. Commun. Chem. 2020, 3, 8. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Cheng, Z.; Jia, L.; Mo, S.; Liu, Z. Ultrahigh Specific Surface Area of Graphene for Eliminating Subcooling of Water. Appl. Energy 2014, 130, 824–829. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, K.; Dixit, A.R. A Review of the Mechanical and Thermal Properties of Graphene and Its Hybrid Polymer Nanocomposites for Structural Applications. J. Mater. Sci. 2019, 54, 5992–6026. [Google Scholar] [CrossRef]
- Song, N.; Gao, X.; Ma, Z.; Wang, X.; Wei, Y.; Gao, C. A Review of Graphene-Based Separation Membrane: Materials, Characteristics, Preparation and Applications. Desalination 2018, 437, 59–72. [Google Scholar] [CrossRef]
- Prekodravac, J.R.; Kepić, D.P.; Colmenares, J.C.; Giannakoudakis, D.A.; Jovanović, S.P. A Comprehensive Review on Selected Graphene Synthesis Methods: From Electrochemical Exfoliation through Rapid Thermal Annealing towards Biomass Pyrolysis. J. Mater. Chem. C 2021, 9, 6722–6748. [Google Scholar] [CrossRef]
- Moon, J.-Y.; Kim, M.; Kim, S.-I.; Xu, S.; Choi, J.-H.; Whang, D.; Watanabe, K.; Taniguchi, T.; Park, D.S.; Seo, J.; et al. Layer-Engineered Large-Area Exfoliation of Graphene. Sci. Adv. 2020, 6, eabc6601. [Google Scholar] [CrossRef]
- Avouris, P.; Dimitrakopoulos, C. Graphene: Synthesis and Applications. Mater. Today 2012, 15, 86–97. [Google Scholar] [CrossRef]
- Folorunso, O.; Kumar, N.; Hamam, Y.; Sadiku, R.; Ray, S.S. Recent Progress on 2D Metal Carbide/Nitride (MXene) Nanocomposites for Lithium-Based Batteries. FlatChem 2021, 29, 100281. [Google Scholar] [CrossRef]
- Katsnelson, M.I.; Novoselov, K.S.; Geim, A.K. Chiral Tunnelling and the Klein Paradox in Graphene. Nat. Phys. 2006, 2, 620–625. [Google Scholar] [CrossRef]
- Folorunso, O.; Hamam, Y.; Sadiku, R.; Ray, S.S.; Adekoya, G.J. Synthesis Methods of Borophene, Graphene-Loaded Polypyrrole Nanocomposites and Their Benefits for Energy Storage Applications: A Brief Overview. FlatChem 2021, 26, 100211. [Google Scholar] [CrossRef]
- Garaj, S.; Hubbard, W.; Golovchenko, J.A. Graphene Synthesis by Ion Implantation. Appl. Phys. Lett. 2010, 97, 183103. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Boeckl, J.; Motta, N.; Iacopi, F. Graphene Growth on Silicon Carbide: A Review. Phys. Status Solidi A 2016, 213, 2277–2289. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Z.; Dong, J.; Yi, D.; Niu, J.; Wu, M.; Lin, L.; Yin, R.; Li, M.; Zhou, J.; et al. Ultrafast Epitaxial Growth of Metre-Sized Single-Crystal Graphene on Industrial Cu Foil. Sci. Bull. 2017, 62, 1074–1080. [Google Scholar] [CrossRef]
- Ma, Y.; Zhi, L. Graphene-Based Transparent Conductive Films: Material Systems, Preparation and Applications. Small Methods 2019, 3, 1800199. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical Properties of Graphene and Graphene-Based Nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Liu, L.; Cheng, Y.; Zhang, X.; Shan, Y.; Zhang, X.; Wang, W.; Li, D. Graphene-Based Transparent Conductive Films with Enhanced Transmittance and Conductivity by Introducing Antireflection Nanostructure. Surf. Coat. Technol. 2017, 325, 611–616. [Google Scholar] [CrossRef]
- Compton, O.C.; Nguyen, S.T. Graphene Oxide, Highly Reduced Graphene Oxide, and Graphene: Versatile Building Blocks for Carbon-Based Materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.; Saleem, M.; Ullah, S.; Saeed, N.; Afridi, A.; Khan, M.; Arif, M. Modified and Improved Hummer’s Synthesis of Graphene Oxide for Capacitors Applications. Mod. Electron. Mater. 2017, 3, 110–116. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, B.; Bulin, C.; Li, R.; Xing, R. High-Efficient Synthesis of Graphene Oxide Based on Improved Hummers Method. Sci. Rep. 2016, 6, 36143. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Kovtyukhova, N.I.; Ollivier, P.J.; Martin, B.R.; Mallouk, T.E.; Chizhik, S.A.; Buzaneva, E.V.; Gorchinskiy, A.D. Layer-by-Layer Assembly of Ultrathin Composite Films from Micron-Sized Graphite Oxide Sheets and Polycations. Chem. Mater. 1999, 11, 771–778. [Google Scholar] [CrossRef]
- Rosca, I.D.; Watari, F.; Uo, M.; Akasaka, T. Oxidation of Multiwalled Carbon Nanotubes by Nitric Acid. Carbon 2005, 43, 3124–3131. [Google Scholar] [CrossRef]
- Istrate, O.M.; Paton, K.R.; Khan, U.; O’Neill, A.; Bell, A.P.; Coleman, J.N. Reinforcement in Melt-Processed Polymer–Graphene Composites at Extremely Low Graphene Loading Level. Carbon 2014, 78, 243–249. [Google Scholar] [CrossRef]
- Mohan, V.B.; Brown, R.; Jayaraman, K.; Bhattacharyya, D. Characterisation of Reduced Graphene Oxide: Effects of Reduction Variables on Electrical Conductivity. Mater. Sci. Eng. B 2015, 193, 49–60. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Chemical Reduction of Graphene Oxide: A Synthetic Chemistry Viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Shen, G.; Cheng, P.; Zhang, J.; Guo, S. Reduction of Graphene Oxide via L -Ascorbic Acid. Chem. Commun. 2010, 46, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Hu, Y.; Shi, M.; Lu, X.; Qin, C.; Li, C.; Ye, M. Fast and Facile Preparation of Graphene Oxide and Reduced Graphene Oxide Nanoplatelets. Chem. Mater. 2009, 21, 3514–3520. [Google Scholar] [CrossRef]
- Tang, H.; Ehlert, G.J.; Lin, Y.; Sodano, H.A. Highly Efficient Synthesis of Graphene Nanocomposites. Nano Lett. 2012, 12, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Jang, J.; Nagase, S. Hydrazine and Thermal Reduction of Graphene Oxide: Reaction Mechanisms, Product Structures, and Reaction Design. J. Phys. Chem. C 2010, 114, 832–842. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, D.; Kim, S.; Kim, S.-Y.; Hu, Y.; Yakes, M.K.; Laracuente, A.R.; Dai, Z.; Marder, S.R.; Berger, C.; et al. Nanoscale Tunable Reduction of Graphene Oxide for Graphene Electronics. Science 2010, 328, 1373–1376. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Liu, S.; Li, H.; Wang, L.; Sun, X. Production of Reduced Graphene Oxide by UV Irradiation. J. Nanosci. Nanotechnol. 2011, 11, 10078–10081. [Google Scholar] [CrossRef]
- Ghorbani, M.; Abdizadeh, H.; Golobostanfard, M.R. Reduction of Graphene Oxide via Modified Hydrothermal Method. Procedia Mater. Sci. 2015, 11, 326–330. [Google Scholar] [CrossRef]
- Lee, H.C.; Liu, W.-W.; Chai, S.-P.; Mohamed, A.R.; Aziz, A.; Khe, C.-S.; Hidayah, N.M.S.; Hashim, U. Review of the Synthesis, Transfer, Characterization and Growth Mechanisms of Single and Multilayer Graphene. RSC Adv. 2017, 7, 15644–15693. [Google Scholar] [CrossRef]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.; Lotya, M.; Istrate, O.M.; King, P.; et al. Scalable Production of Large Quantities of Defect-Free Few-Layer Graphene by Shear Exfoliation in Liquids. Nat. Mater. 2014, 13, 624–630. [Google Scholar] [CrossRef]
- Edwards, R.S.; Coleman, K.S. Graphene Synthesis: Relationship to Applications. Nanoscale 2013, 5, 38–51. [Google Scholar] [CrossRef]
- Ayán-Varela, M.; Paredes, J.I.; Villar-Rodil, S.; Rozada, R.; Martínez-Alonso, A.; Tascón, J.M.D. A Quantitative Analysis of the Dispersion Behavior of Reduced Graphene Oxide in Solvents. Carbon 2014, 75, 390–400. [Google Scholar] [CrossRef]
- Johnson, D.W.; Dobson, B.P.; Coleman, K.S. A Manufacturing Perspective on Graphene Dispersions. Curr. Opin. Colloid Interface Sci. 2015, 20, 367–382. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, G.; Prakash, J.; Chen, Z.; Gauthier, M.; Sun, S. Chemical Vapour Deposition of Graphene: Layer Control, the Transfer Process, Characterisation, and Related Applications. Int. Rev. Phys. Chem. 2019, 38, 149–199. [Google Scholar] [CrossRef]
- Karfa, P.; Chandra Majhi, K.; Madhuri, R. Synthesis of Two-Dimensional Nanomaterials. In Two-Dimensional Nanostructures for Biomedical Technology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 35–71. ISBN 978-0-12-817650-4. [Google Scholar]
- Moosa, A.A.; Abed, M.S. Graphene Preparation and Graphite Exfoliation. Turk. J. Chem. 2021, 45, 493–519. [Google Scholar] [CrossRef]
- Abbas, Q.; Shinde, P.A.; Abdelkareem, M.A.; Alami, A.H.; Mirzaeian, M.; Yadav, A.; Olabi, A.G. Graphene Synthesis Techniques and Environmental Applications. Materials 2022, 15, 7804. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, M.; Alam, S.; Uddin, M.; Islam, M.; Bipasha, F.A.; Hossain, S.S. Synthesis of Graphene. Int. Nano Lett. 2016, 6, 65–83. [Google Scholar] [CrossRef]
- Saha, J.K.; Dutta, A. A Review of Graphene: Material Synthesis from Biomass Sources. Waste Biomass Valorization 2022, 13, 1385–1429. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The Chemistry of Graphene Oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Yu, W.; Sisi, L.; Haiyan, Y.; Jie, L. Progress in the Functional Modification of Graphene/Graphene Oxide: A Review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar] [CrossRef]
- Costa, M.C.F.; Marangoni, V.S.; Ng, P.R.; Nguyen, H.T.L.; Carvalho, A.; Castro Neto, A.H. Accelerated Synthesis of Graphene Oxide from Graphene. Nanomaterials 2021, 11, 551. [Google Scholar] [CrossRef]
- Garg, R.; Dutta, N.; Choudhury, N. Work Function Engineering of Graphene. Nanomaterials 2014, 4, 267–300. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cao, H.; Xue, Y.; Li, B.; Cai, W. Liquid-Phase Exfoliation of Graphene: An Overview on Exfoliation Media, Techniques, and Challenges. Nanomaterials 2018, 8, 942. [Google Scholar] [CrossRef] [PubMed]
- Santhiran, A.; Iyngaran, P.; Abiman, P.; Kuganathan, N. Graphene Synthesis and Its Recent Advances in Applications—A Review. C 2021, 7, 76. [Google Scholar] [CrossRef]
- Muscatello, J.; Jaeger, F.; Matar, O.K.; Müller, E.A. Optimizing Water Transport through Graphene-Based Membranes: Insights from Nonequilibrium Molecular Dynamics. ACS Appl. Mater. Interfaces 2016, 8, 12330–12336. [Google Scholar] [CrossRef]
- Khan, M.; Al-Marri, A.H.; Khan, M.; Shaik, M.R.; Mohri, N.; Adil, S.F.; Kuniyil, M.; Alkhathlan, H.Z.; Al-Warthan, A.; Tremel, W.; et al. Green Approach for the Effective Reduction of Graphene Oxide Using Salvadora Persica L. Root (Miswak) Extract. Nanoscale Res. Lett. 2015, 10, 281. [Google Scholar] [CrossRef]
- Al-Marri, A.; Khan, M.; Khan, M.; Adil, S.; Al-Warthan, A.; Alkhathlan, H.; Tremel, W.; Labis, J.; Siddiqui, M.; Tahir, M. Pulicaria Glutinosa Extract: A Toolbox to Synthesize Highly Reduced Graphene Oxide-Silver Nanocomposites. Int. J. Mol. Sci. 2015, 16, 1131–1142. [Google Scholar] [CrossRef]
- Guan, K.; Liu, G.; Matsuyama, H.; Jin, W. Graphene-Based Membranes for Pervaporation Processes. Chin. J. Chem. Eng. 2020, 28, 1755–1766. [Google Scholar] [CrossRef]
- Kim, H.W.; Yoon, H.W.; Yoon, S.-M.; Yoo, B.M.; Ahn, B.K.; Cho, Y.H.; Shin, H.J.; Yang, H.; Paik, U.; Kwon, S.; et al. Selective Gas Transport Through Few-Layered Graphene and Graphene Oxide Membranes. Science 2013, 342, 91–95. [Google Scholar] [CrossRef]
- Chi, C.; Wang, X.; Peng, Y.; Qian, Y.; Hu, Z.; Dong, J.; Zhao, D. Facile Preparation of Graphene Oxide Membranes for Gas Separation. Chem. Mater. 2016, 28, 2921–2927. [Google Scholar] [CrossRef]
- Shen, J.; Liu, G.; Huang, K.; Chu, Z.; Jin, W.; Xu, N. Subnanometer Two-Dimensional Graphene Oxide Channels for Ultrafast Gas Sieving. ACS Nano 2016, 10, 3398–3409. [Google Scholar] [CrossRef]
- Akbari, A.; Sheath, P.; Martin, S.T.; Shinde, D.B.; Shaibani, M.; Banerjee, P.C.; Tkacz, R.; Bhattacharyya, D.; Majumder, M. Large-Area Graphene-Based Nanofiltration Membranes by Shear Alignment of Discotic Nematic Liquid Crystals of Graphene Oxide. Nat. Commun. 2016, 7, 10891. [Google Scholar] [CrossRef] [PubMed]
- Tsou, C.-H.; An, Q.-F.; Lo, S.-C.; De Guzman, M.; Hung, W.-S.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Effect of Microstructure of Graphene Oxide Fabricated through Different Self-Assembly Techniques on 1-Butanol Dehydration. J. Membr. Sci. 2015, 477, 93–100. [Google Scholar] [CrossRef]
- Chae, H.-R.; Lee, J.; Lee, C.-H.; Kim, I.-C.; Park, P.-K. Graphene Oxide-Embedded Thin-Film Composite Reverse Osmosis Membrane with High Flux, Anti-Biofouling, and Chlorine Resistance. J. Membr. Sci. 2015, 483, 128–135. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Q.; Hou, J.; Sutrisna, P.D.; Chen, V. Shear-Aligned Graphene Oxide Laminate/Pebax Ultrathin Composite Hollow Fiber Membranes Using a Facile Dip-Coating Approach. J. Mater. Chem. A 2017, 5, 7732–7737. [Google Scholar] [CrossRef]
- Qin, D.; Liu, Z.; Bai, H.; Sun, D.D. Three-Dimensional Architecture Constructed from a Graphene Oxide Nanosheet–Polymer Composite for High-Flux Forward Osmosis Membranes. J. Mater. Chem. A 2017, 5, 12183–12192. [Google Scholar] [CrossRef]
- Fan, Y.; Quan, X.; Zhao, H.; Chen, S.; Yu, H.; Zhang, Y.; Zhang, Q. Poly(Vinylidene Fluoride) Hollow-Fiber Membranes Containing Silver/Graphene Oxide Dope with Excellent Filtration Performance. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, X.; Xue, Q.; He, D.; Zhu, L.; Guo, Q. Antifouling Hydrolyzed Polyacrylonitrile/Graphene Oxide Membrane with Spindle-Knotted Structure for Highly Effective Separation of Oil-Water Emulsion. J. Membr. Sci. 2017, 532, 38–46. [Google Scholar] [CrossRef]
- Lee, J.; Chae, H.-R.; Won, Y.J.; Lee, K.; Lee, C.-H.; Lee, H.H.; Kim, I.-C.; Lee, J. Graphene Oxide Nanoplatelets Composite Membrane with Hydrophilic and Antifouling Properties for Wastewater Treatment. J. Membr. Sci. 2013, 448, 223–230. [Google Scholar] [CrossRef]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H. Preparation of a Novel Antifouling Mixed Matrix PES Membrane by Embedding Graphene Oxide Nanoplates. J. Membr. Sci. 2014, 453, 292–301. [Google Scholar] [CrossRef]
- Ding, R.; Zhang, H.; Li, Y.; Wang, J.; Shi, B.; Mao, H.; Dang, J.; Liu, J. Graphene Oxide-Embedded Nanocomposite Membrane for Solvent Resistant Nanofiltration with Enhanced Rejection Ability. Chem. Eng. Sci. 2015, 138, 227–238. [Google Scholar] [CrossRef]
- Lai, G.S.; Lau, W.J.; Goh, P.S.; Ismail, A.F.; Yusof, N.; Tan, Y.H. Graphene Oxide Incorporated Thin Film Nanocomposite Nanofiltration Membrane for Enhanced Salt Removal Performance. Desalination 2016, 387, 14–24. [Google Scholar] [CrossRef]
- Perreault, F.; Tousley, M.E.; Elimelech, M. Thin-Film Composite Polyamide Membranes Functionalized with Biocidal Graphene Oxide Nanosheets. Environ. Sci. Technol. Lett. 2014, 1, 71–76. [Google Scholar] [CrossRef]
- Zhang, J.; Xue, Q.; Pan, X.; Jin, Y.; Lu, W.; Ding, D.; Guo, Q. Graphene Oxide/Polyacrylonitrile Fiber Hierarchical-Structured Membrane for Ultra-Fast Microfiltration of Oil-Water Emulsion. Chem. Eng. J. 2017, 307, 643–649. [Google Scholar] [CrossRef]
- Cohen-Tanugi, D.; Grossman, J.C. Nanoporous Graphene as a Reverse Osmosis Membrane: Recent Insights from Theory and Simulation. Desalination 2015, 366, 59–70. [Google Scholar] [CrossRef]
- Xu, Z.; Yan, X.; Du, Z.; Li, J.; Cheng, F. Effect of Oxygenic Groups on Desalination Performance Improvement of Graphene Oxide-Based Membrane in Membrane Distillation. Sep. Purif. Technol. 2020, 251, 117304. [Google Scholar] [CrossRef]
- Romaniak, G.; Dybowski, K.; Jeziorna, A.; Kula, P.; Kaźmierczak, T. Synthesis and Characterization of Semi-Permeable Graphene/Graphene Oxide Membranes for Water Desalination. J. Mater. Sci. 2020, 55, 9775–9786. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Sun, H.; Zhang, D.; Zhao, Y.; Chen, L. Self-Assembling TiO2 on Aminated Graphene Based on Adsorption and Catalysis to Treat Organic Dyes. Appl. Surf. Sci. 2021, 539, 147889. [Google Scholar] [CrossRef]
- Yu, Z.; Zeng, H.; Min, X.; Zhu, X. High-performance Composite Photocatalytic Membrane Based on Titanium Dioxide Nanowire/Graphene Oxide for Water Treatment. J. Appl. Polym. Sci. 2020, 137, 48488. [Google Scholar] [CrossRef]
- Alyarnezhad, S.; Marino, T.; Parsa, J.B.; Galiano, F.; Ursino, C.; Garcìa, H.; Puche, M.; Figoli, A. Polyvinylidene Fluoride-Graphene Oxide Membranes for Dye Removal under Visible Light Irradiation. Polymers 2020, 12, 1509. [Google Scholar] [CrossRef]
- Gao, P.; Zhou, W.; Zhang, J.; Yang, Z.; Yuan, X.; Wang, Z. The Effect of Acidified Graphite Carbon Nitride on the Removal of Pollutants by Coupling Filtration and Photocatalysis. Appl. Surf. Sci. 2021, 542, 148675. [Google Scholar] [CrossRef]
- Zhan, B.; Liu, Y.; Zhou, W.-T.; Li, S.-Y.; Chen, Z.-B.; Stegmaier, T.; Aliabadi, M.; Han, Z.-W.; Ren, L.-Q. Multifunctional 3D GO/g-C3N4/TiO2 Foam for Oil-Water Separation and Dye Adsorption. Appl. Surf. Sci. 2021, 541, 148638. [Google Scholar] [CrossRef]
- Dou, W.; Liu, J.; Li, M. Competitive Adsorption of Cu2+ in Cu2+, Co2+ and Ni2+ Mixed Multi–Metal Solution onto Graphene Oxide (GO)–Based Hybrid Membranes. J. Mol. Liq. 2021, 322, 114516. [Google Scholar] [CrossRef]
- Croitoru, A.-M.; Ficai, A.; Ficai, D.; Trusca, R.; Dolete, G.; Andronescu, E.; Turculet, S.C. Chitosan/Graphene Oxide Nanocomposite Membranes as Adsorbents with Applications in Water Purification. Materials 2020, 13, 1687. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, Z.; Sun, R.; Chen, Z.; Liu, M.; Zhou, X.; Yao, M.; Wang, G. Self-Supported Reduced Graphene Oxide Membrane and Its Cu2+ Adsorption Capability. Materials 2020, 14, 146. [Google Scholar] [CrossRef] [PubMed]
- Medhat Bojnourd, F.; Pakizeh, M. Preparation and Characterization of a PVA/PSf Thin Film Composite Membrane after Incorporation of PSSMA into a Selective Layer and Its Application for Pharmaceutical Removal. Sep. Purif. Technol. 2018, 192, 5–14. [Google Scholar] [CrossRef]
- Hung, W.-S.; Lin, T.-J.; Chiao, Y.-H.; Sengupta, A.; Hsiao, Y.-C.; Wickramasinghe, S.R.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Graphene-Induced Tuning of the d -Spacing of Graphene Oxide Composite Nanofiltration Membranes for Frictionless Capillary Action-Induced Enhancement of Water Permeability. J. Mater. Chem. A 2018, 6, 19445–19454. [Google Scholar] [CrossRef]
- Yuan, S.; Li, Y.; Xia, Y.; Kang, Y.; Yang, J.; Uddin, M.H.; Liu, H.; Selomulya, C.; Zhang, X. Minimizing Non-Selective Nanowrinkles of Reduced Graphene Oxide Laminar Membranes for Enhanced NaCl Rejection. Environ. Sci. Technol. Lett. 2020, 7, 273–279. [Google Scholar] [CrossRef]
- Hong, S.; Constans, C.; Surmani Martins, M.V.; Seow, Y.C.; Guevara Carrió, J.A.; Garaj, S. Scalable Graphene-Based Membranes for Ionic Sieving with Ultrahigh Charge Selectivity. Nano Lett. 2017, 17, 728–732. [Google Scholar] [CrossRef]
- Kar, P.; Jain, P.; Gupta, R.K.; Tripathi, K.M. Emerging Carbon-Based Nanocomposites for Remediation of Heavy Metals and Organic Pollutants from Wastewater. In Emerging Carbon-Based Nanocomposites for Environmental Applications; Mishra, A.K., Hussain, C.M., Mishra, S.B., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 1–29. ISBN 978-1-119-55485-1. [Google Scholar]
- Santhosh, C.; Daneshvar, E.; Tripathi, K.M.; Baltrėnas, P.; Kim, T.; Baltrėnaitė, E.; Bhatnagar, A. Synthesis and Characterization of Magnetic Biochar Adsorbents for the Removal of Cr(VI) and Acid Orange 7 Dye from Aqueous Solution. Environ. Sci. Pollut. Res. 2020, 27, 32874–32887. [Google Scholar] [CrossRef]
- Wang, S.; Liang, S.; Chen, L.; Mu, L.; Xu, G.; Fang, H. Effects of Cationic Concentration on Controlling the Interlayer Spacings for Highly Effective Ion Rejection via Graphene Oxide Membranes. Chem. Commun. 2020, 56, 2743–2746. [Google Scholar] [CrossRef]
- Bandehali, S.; Moghadassi, A.; Parvizian, F.; Zhang, Y.; Hosseini, S.M.; Shen, J. New Mixed Matrix PEI Nanofiltration Membrane Decorated by Glycidyl-POSS Functionalized Graphene Oxide Nanoplates with Enhanced Separation and Antifouling Behaviour: Heavy Metal Ions Removal. Sep. Purif. Technol. 2020, 242, 116745. [Google Scholar] [CrossRef]
- Khalil, A.M.E.; Memon, F.A.; Tabish, T.A.; Salmon, D.; Zhang, S.; Butler, D. Nanostructured Porous Graphene for Efficient Removal of Emerging Contaminants (Pharmaceuticals) from Water. Chem. Eng. J. 2020, 398, 125440. [Google Scholar] [CrossRef]
- Sheng, J.; Yin, H.; Qian, F.; Huang, H.; Gao, S.; Wang, J. Reduced Graphene Oxide-Based Composite Membranes for in-Situ Catalytic Oxidation of Sulfamethoxazole Operated in Membrane Filtration. Sep. Purif. Technol. 2020, 236, 116275. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, G.; Liu, B.; Kong, H.; Xiong, Z.; Guo, L.; Wei, G. Biomineralization of ZrO2 Nanoparticles on Graphene Oxide-Supported Peptide/Cellulose Binary Nanofibrous Membranes for High-Performance Removal of Fluoride Ions. Chem. Eng. J. 2022, 430, 132721. [Google Scholar] [CrossRef]
- Cheng, P.; Chen, Y.; Gu, Y.-H.; Yan, X.; Lang, W.-Z. Hybrid 2D WS2/GO Nanofiltration Membranes for Finely Molecular Sieving. J. Membr. Sci. 2019, 591, 117308. [Google Scholar] [CrossRef]
- Sun, J.; Hu, C.; Liu, Z.; Liu, H.; Qu, J. Surface Charge and Hydrophilicity Improvement of Graphene Membranes via Modification of Pore Surface Oxygen-Containing Groups to Enhance Permeability and Selectivity. Carbon 2019, 145, 140–148. [Google Scholar] [CrossRef]
- Zhao, G.; Hu, R.; Zhao, X.; He, Y.; Zhu, H. High Flux Nanofiltration Membranes Prepared with a Graphene Oxide Homo-Structure. J. Membr. Sci. 2019, 585, 29–37. [Google Scholar] [CrossRef]
- Hou, J.; Chen, Y.; Shi, W.; Bao, C.; Hu, X. Graphene Oxide/Methylene Blue Composite Membrane for Dyes Separation: Formation Mechanism and Separation Performance. Appl. Surf. Sci. 2020, 505, 144145. [Google Scholar] [CrossRef]
- Kunimatsu, M.; Nakagawa, K.; Yoshioka, T.; Shintani, T.; Yasui, T.; Kamio, E.; Tsang, S.C.E.; Li, J.; Matsuyama, H. Design of Niobate Nanosheet-Graphene Oxide Composite Nanofiltration Membranes with Improved Permeability. J. Membr. Sci. 2020, 595, 117598. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Z.; Peng, Y.; Shao, L.; Li, X.; Zeng, H. A Novel Photocatalytic Self-Cleaning TiO2 Nanorods Inserted Graphene Oxide-Based Nanofiltration Membrane. Chem. Phys. Lett. 2020, 749, 137424. [Google Scholar] [CrossRef]
- Jia, T.; Shen, S.; Xiao, L.; Jin, J.; Zhao, J.; Che, Q. Constructing Multilayered Membranes with Layer-by-Layer Self-Assembly Technique Based on Graphene Oxide for Anhydrous Proton Exchange Membranes. Eur. Polym. J. 2020, 122, 109362. [Google Scholar] [CrossRef]
- Arenas, B.; Gutiérrez, N.; Cabanzo, R.; Mejía, E. A Methodology for Synthesis of Reduced Graphene Oxide Membranes for Desalination of Produced Water. J. Phys. Conf. Ser. 2019, 1159, 012005. [Google Scholar] [CrossRef]
- Thakur, A.K.; Singh, S.P.; Kleinberg, M.N.; Gupta, A.; Arnusch, C.J. Laser-Induced Graphene–PVA Composites as Robust Electrically Conductive Water Treatment Membranes. ACS Appl. Mater. Interfaces 2019, 11, 10914–10921. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, W.; Fang, Q.; Zuo, K.; Hou, G.; Ai, Q.; Li, Q.; Ci, L.; Lou, J. High Performance Hierarchically Nanostructured Graphene Oxide/Covalent Organic Framework Hybrid Membranes for Stable Organic Solvent Nanofiltration. Appl. Mater. Today 2020, 20, 100791. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, Y.; Kang, J.; Choi, E.; Choi, S.E.; Kwon, O.; Kim, D.W. Scalable Fabrication of Deoxygenated Graphene Oxide Nanofiltration Membrane by Continuous Slot-Die Coating. J. Membr. Sci. 2020, 612, 118454. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, S.; Xia, Y.; Zhao, W.; Easton, C.D.; Selomulya, C.; Zhang, X. Mild Annealing Reduced Graphene Oxide Membrane for Nanofiltration. J. Membr. Sci. 2020, 601, 117900. [Google Scholar] [CrossRef]
- Kulkarni, H.B.; Tambe, P.; M. Joshi, G. Influence of Covalent and Non-Covalent Modification of Graphene on the Mechanical, Thermal and Electrical Properties of Epoxy/Graphene Nanocomposites: A Review. Compos. Interfaces 2018, 25, 381–414. [Google Scholar] [CrossRef]
- Alyobi, M.; Barnett, C.; Cobley, R. Effects of Thermal Annealing on the Properties of Mechanically Exfoliated Suspended and On-Substrate Few-Layer Graphene. Crystals 2017, 7, 349. [Google Scholar] [CrossRef]
- Kaplas, T.; Jakstas, V.; Biciunas, A.; Luksa, A.; Setkus, A.; Niaura, G.; Kasalynas, I. Effect of High-Temperature Annealing on Graphene with Nickel Contacts. Condens. Matter 2019, 4, 21. [Google Scholar] [CrossRef]
- Liu, J.; Yu, L.; Yue, G.; Wang, N.; Cui, Z.; Hou, L.; Li, J.; Li, Q.; Karton, A.; Cheng, Q.; et al. Thermoresponsive Graphene Membranes with Reversible Gating Regularity for Smart Fluid Control. Adv. Funct. Mater. 2019, 29, 1808501. [Google Scholar] [CrossRef]
- Zheng, S.; Tu, Q.; Urban, J.J.; Li, S.; Mi, B. Swelling of Graphene Oxide Membranes in Aqueous Solution: Characterization of Interlayer Spacing and Insight into Water Transport Mechanisms. ACS Nano 2017, 11, 6440–6450. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Vasu, K.S.; Williams, C.D.; Gopinadhan, K.; Su, Y.; Cherian, C.T.; Dix, J.; Prestat, E.; Haigh, S.J.; Grigorieva, I.V.; et al. Tunable Sieving of Ions Using Graphene Oxide Membranes. Nat. Nanotechnol. 2017, 12, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Mi, B.; Zheng, S.; Tu, Q. 2D Graphene Oxide Channel for Water Transport. Faraday Discuss. 2018, 209, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shi, G.; Shen, J.; Peng, B.; Zhang, B.; Wang, Y.; Bian, F.; Wang, J.; Li, D.; Qian, Z.; et al. Ion Sieving in Graphene Oxide Membranes via Cationic Control of Interlayer Spacing. Nature 2017, 550, 380–383. [Google Scholar] [CrossRef]
- Hung, W.-S.; Tsou, C.-H.; De Guzman, M.; An, Q.-F.; Liu, Y.-L.; Zhang, Y.-M.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Cross-Linking with Diamine Monomers To Prepare Composite Graphene Oxide-Framework Membranes with Varying d -Spacing. Chem. Mater. 2014, 26, 2983–2990. [Google Scholar] [CrossRef]
- Jia, Z.; Shi, W.; Wang, Y.; Wang, J. Dicarboxylic Acids Crosslinked Graphene Oxide Membranes for Salt Solution Permeation. Colloids Surf. Physicochem. Eng. Asp. 2016, 494, 101–107. [Google Scholar] [CrossRef]
- Zhang, P.; Gong, J.-L.; Zeng, G.-M.; Deng, C.-H.; Yang, H.-C.; Liu, H.-Y.; Huan, S.-Y. Cross-Linking to Prepare Composite Graphene Oxide-Framework Membranes with High-Flux for Dyes and Heavy Metal Ions Removal. Chem. Eng. J. 2017, 322, 657–666. [Google Scholar] [CrossRef]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.-S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Ri Kim, H.; Song, Y.I.; et al. Roll-to-Roll Production of 30-Inch Graphene Films for Transparent Electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef]
- Thebo, K.H.; Qian, X.; Zhang, Q.; Chen, L.; Cheng, H.-M.; Ren, W. Highly Stable Graphene-Oxide-Based Membranes with Superior Permeability. Nat. Commun. 2018, 9, 1486. [Google Scholar] [CrossRef]
- Huang, H.-H.; Joshi, R.K.; De Silva, K.K.H.; Badam, R.; Yoshimura, M. Fabrication of Reduced Graphene Oxide Membranes for Water Desalination. J. Membr. Sci. 2019, 572, 12–19. [Google Scholar] [CrossRef]
- Nam, Y.T.; Choi, J.; Kang, K.M.; Kim, D.W.; Jung, H.-T. Enhanced Stability of Laminated Graphene Oxide Membranes for Nanofiltration via Interstitial Amide Bonding. ACS Appl. Mater. Interfaces 2016, 8, 27376–27382. [Google Scholar] [CrossRef] [PubMed]
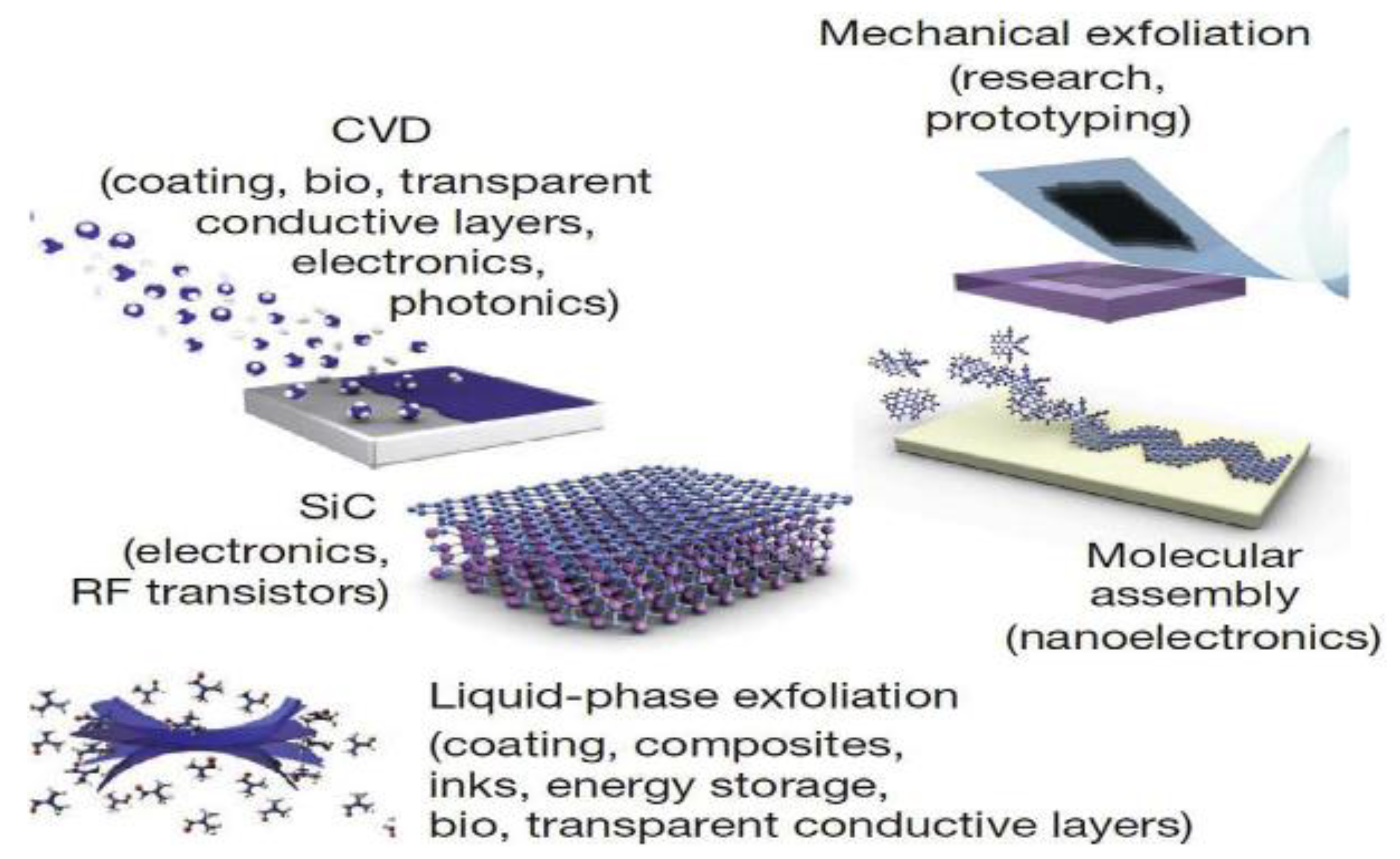
| A/A | Synthesis Process | Method | Strengths | Limitations | Economic Considerations |
|---|---|---|---|---|---|
| 1 | Chemical vapor deposition (CVD) | Bottom up | Allows for large-scale synthesis of single-crystal graphene [73] | Emits toxic gaseous by-products during reaction [74] | Requires highly expensive equipment [74] |
| 2 | Epitaxial growth | Bottom up | High-quality graphene with excellent properties, [75] | Energy intensive [76] Difficult to control at elevated temperatures [76] | Expensive process [77] High cost of the substrates [78] |
| 3 | Wet chemical synthesis (for ex the Hummers’ method) | Bottom up | Transparent conductive film, that can be used to synthesize graphene [79] | The Hummers’ method produces nitrogen dioxide, dinitrogen tetroxide causes heavy metal pollution. Additionally, the products contained sodium and nitrate anions, which were not easy to remove [80] | The process is expensive in terms of time, energy, and waste treatment [81] |
| 4 | Mechanical Exfoliation | Top down | Time saving method [82] | It is uncontrollable and not scalable [78] | Inexpensive method [82] |
| 5 | Liquid exfoliation | Top Down | Scalable method and inexpensive [83] | Yield that is not sufficient for industrial applications at macroscopic scale [84] Other disadvantages include, toxic and the reduction of the size of the nanosheets [84] | The required solvents are expensive [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkika, D.A.; Karmali, V.; Lambropoulou, D.A.; Mitropoulos, A.C.; Kyzas, G.Z. Membranes Coated with Graphene-Based Materials: A Review. Membranes 2023, 13, 127. https://doi.org/10.3390/membranes13020127
Gkika DA, Karmali V, Lambropoulou DA, Mitropoulos AC, Kyzas GZ. Membranes Coated with Graphene-Based Materials: A Review. Membranes. 2023; 13(2):127. https://doi.org/10.3390/membranes13020127
Chicago/Turabian StyleGkika, Despina A., Vasiliki Karmali, Dimitra A. Lambropoulou, Athanasios C. Mitropoulos, and George Z. Kyzas. 2023. "Membranes Coated with Graphene-Based Materials: A Review" Membranes 13, no. 2: 127. https://doi.org/10.3390/membranes13020127
APA StyleGkika, D. A., Karmali, V., Lambropoulou, D. A., Mitropoulos, A. C., & Kyzas, G. Z. (2023). Membranes Coated with Graphene-Based Materials: A Review. Membranes, 13(2), 127. https://doi.org/10.3390/membranes13020127










