Membrane-Based Technologies for Post-Combustion CO2 Capture from Flue Gases: Recent Progress in Commonly Employed Membrane Materials
Abstract
:1. Introduction
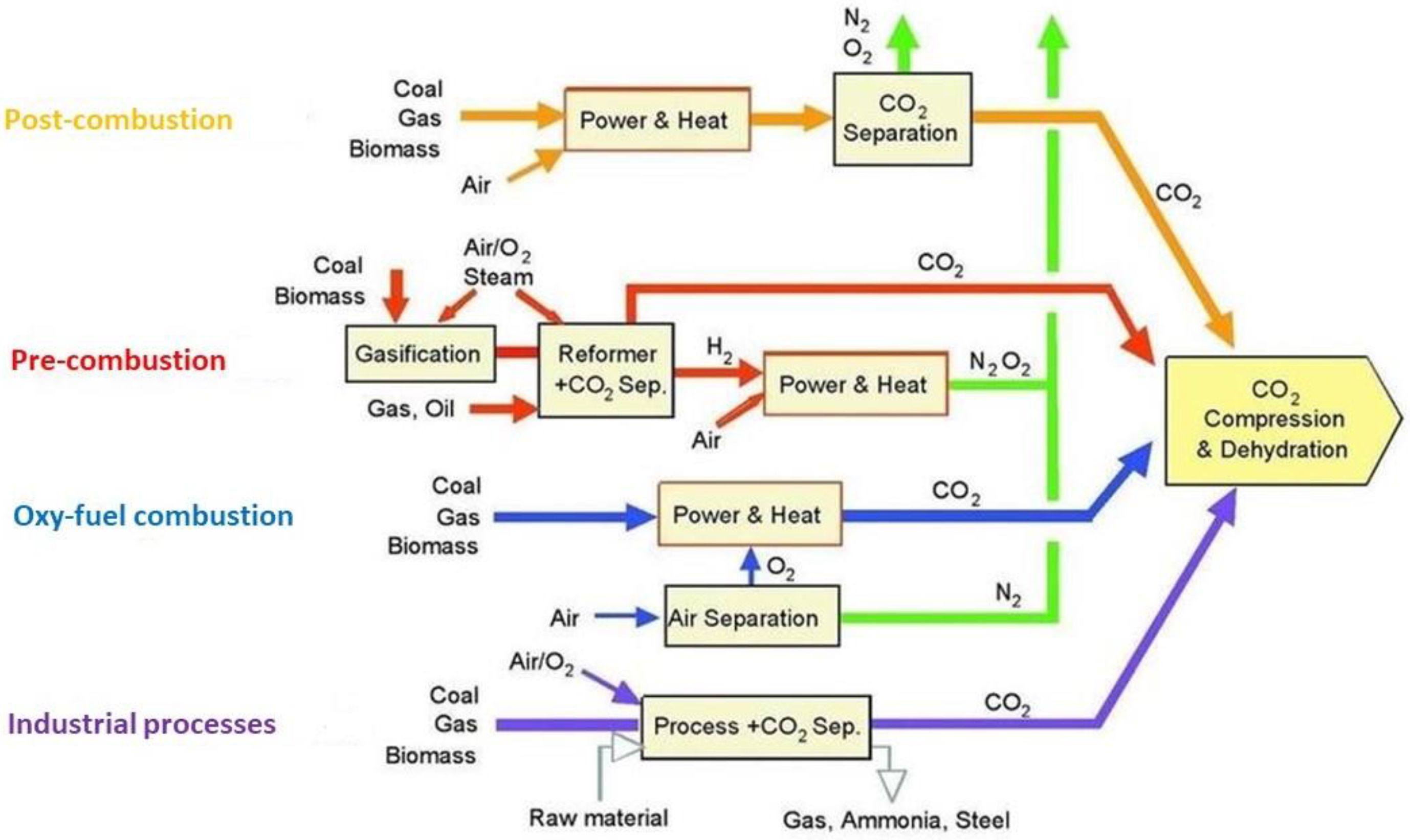
2. Main Processes for CO2 Capture
2.1. Post-Combustion Capture
2.2. Pre-Combustion Capture
2.3. Oxy-Fuel Combustion Capture
2.4. Capture from Industrial Process Streams
3. Post-Combustion CO2 Capture Technologies
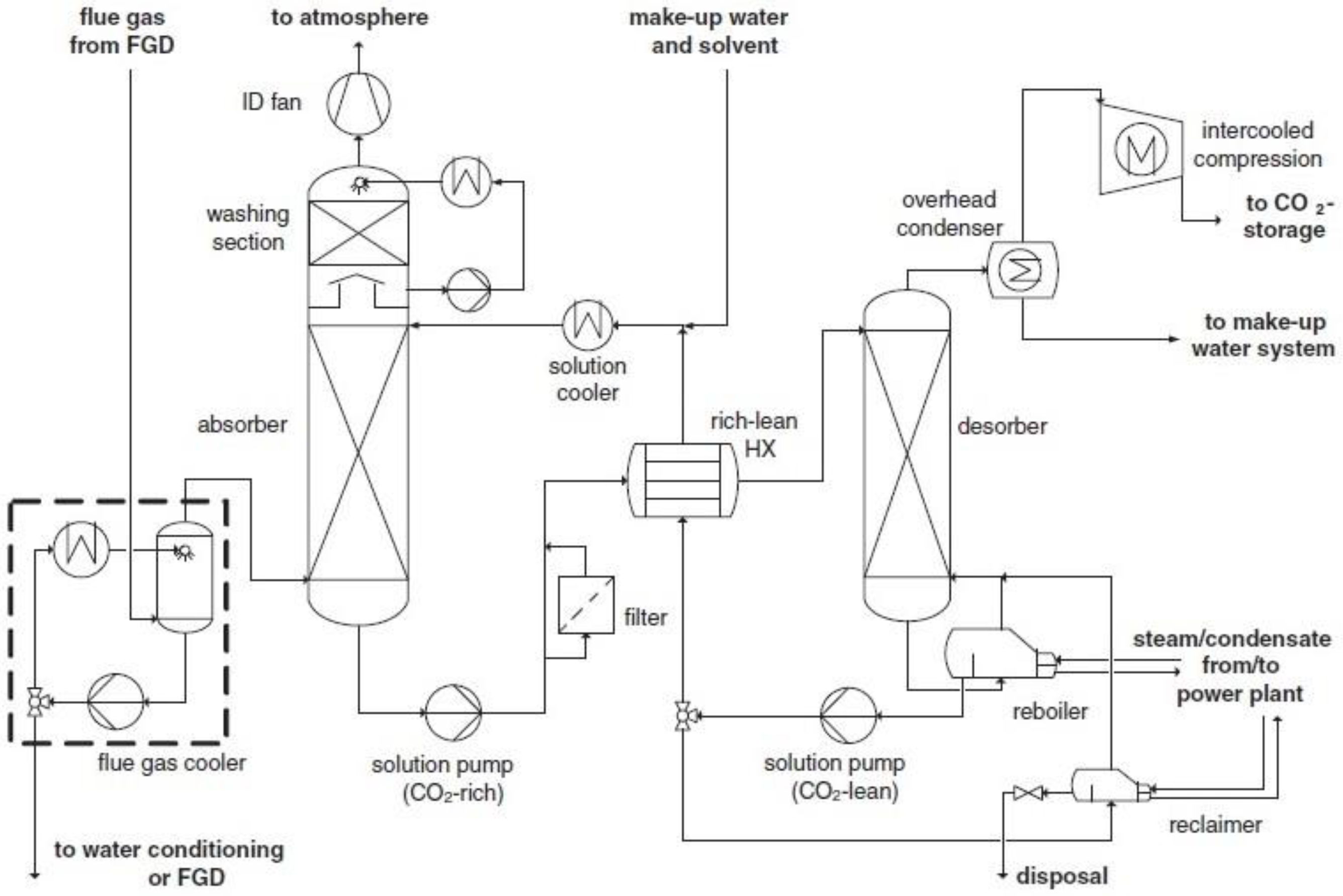
3.1. Chemical Absorption
3.2. Adsorption
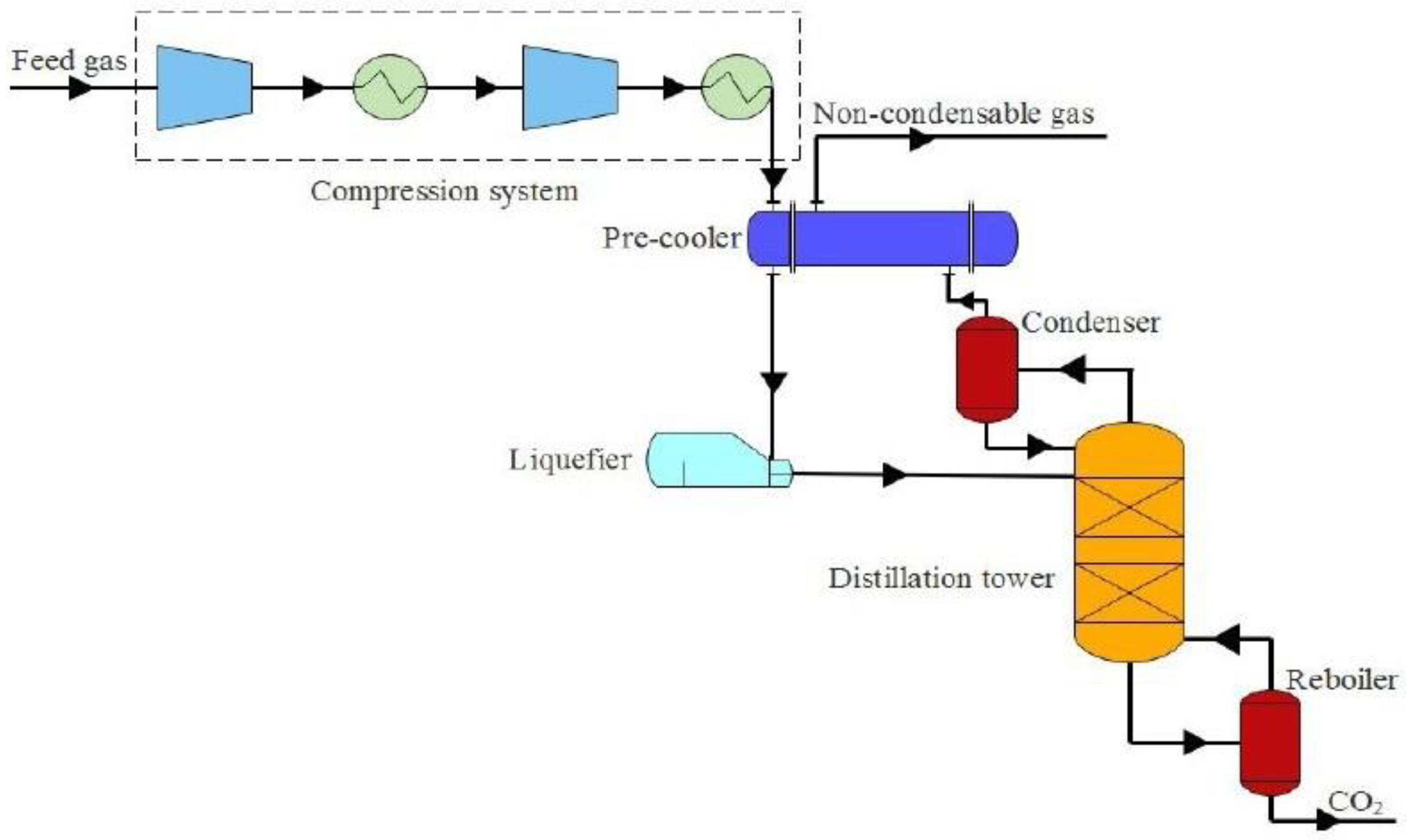
3.3. Cryogenic Separation
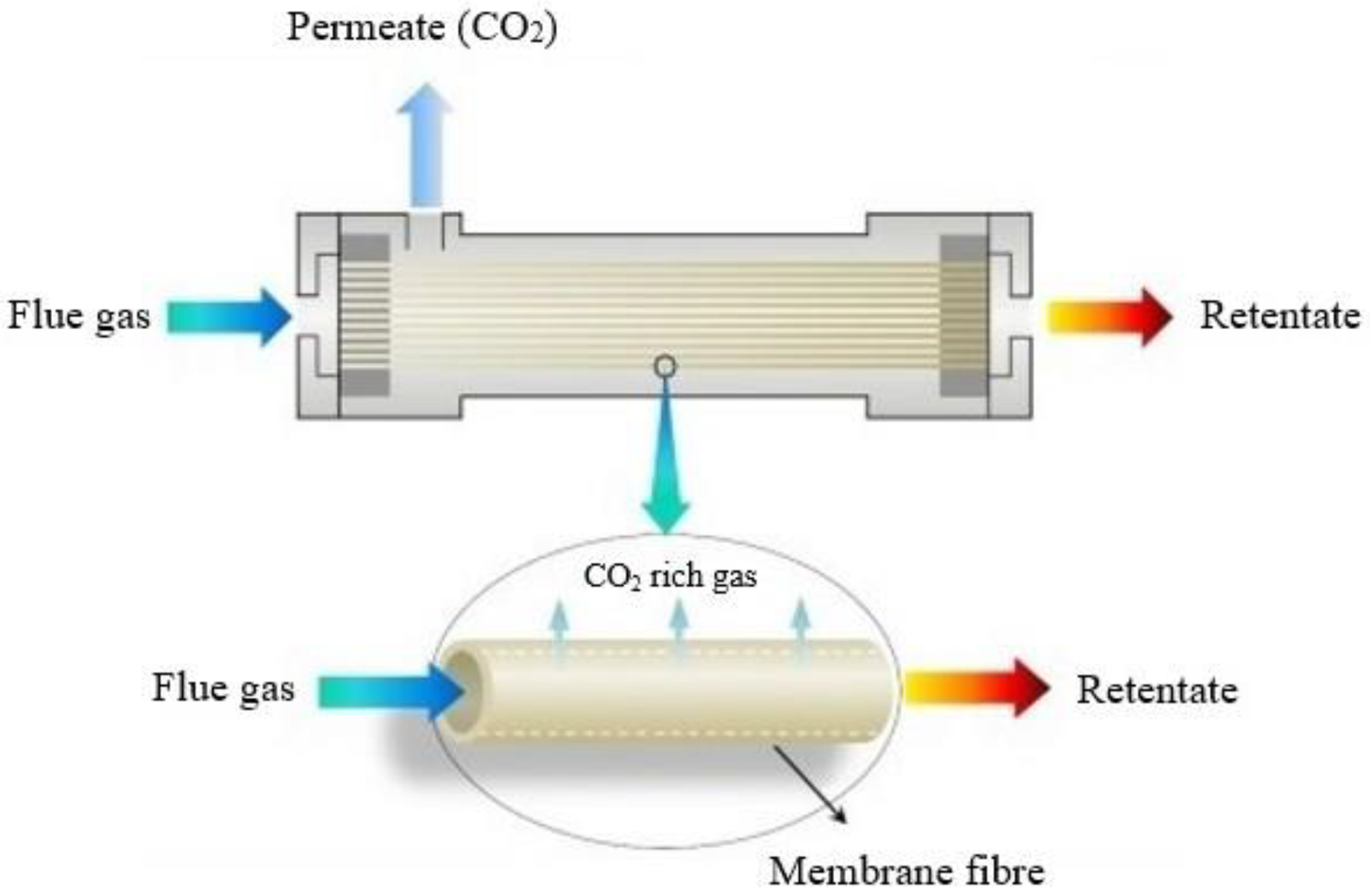
3.4. Membrane Separation
4. CO2 capture with Membrane Technologies
4.1. Basic Principles and Mechanism of Membrane Gas Separation
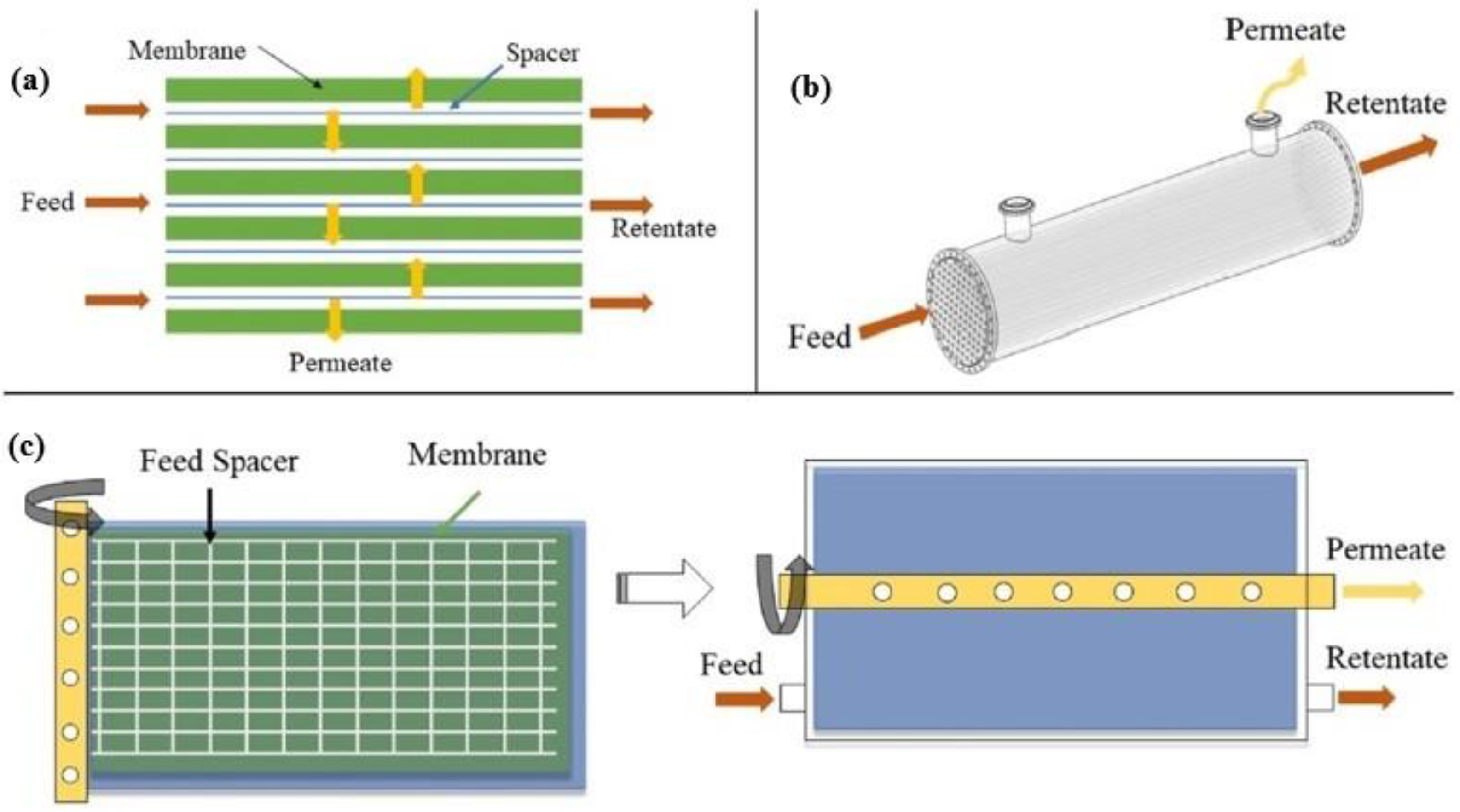
4.2. Membrane Configurations and Process Engineering
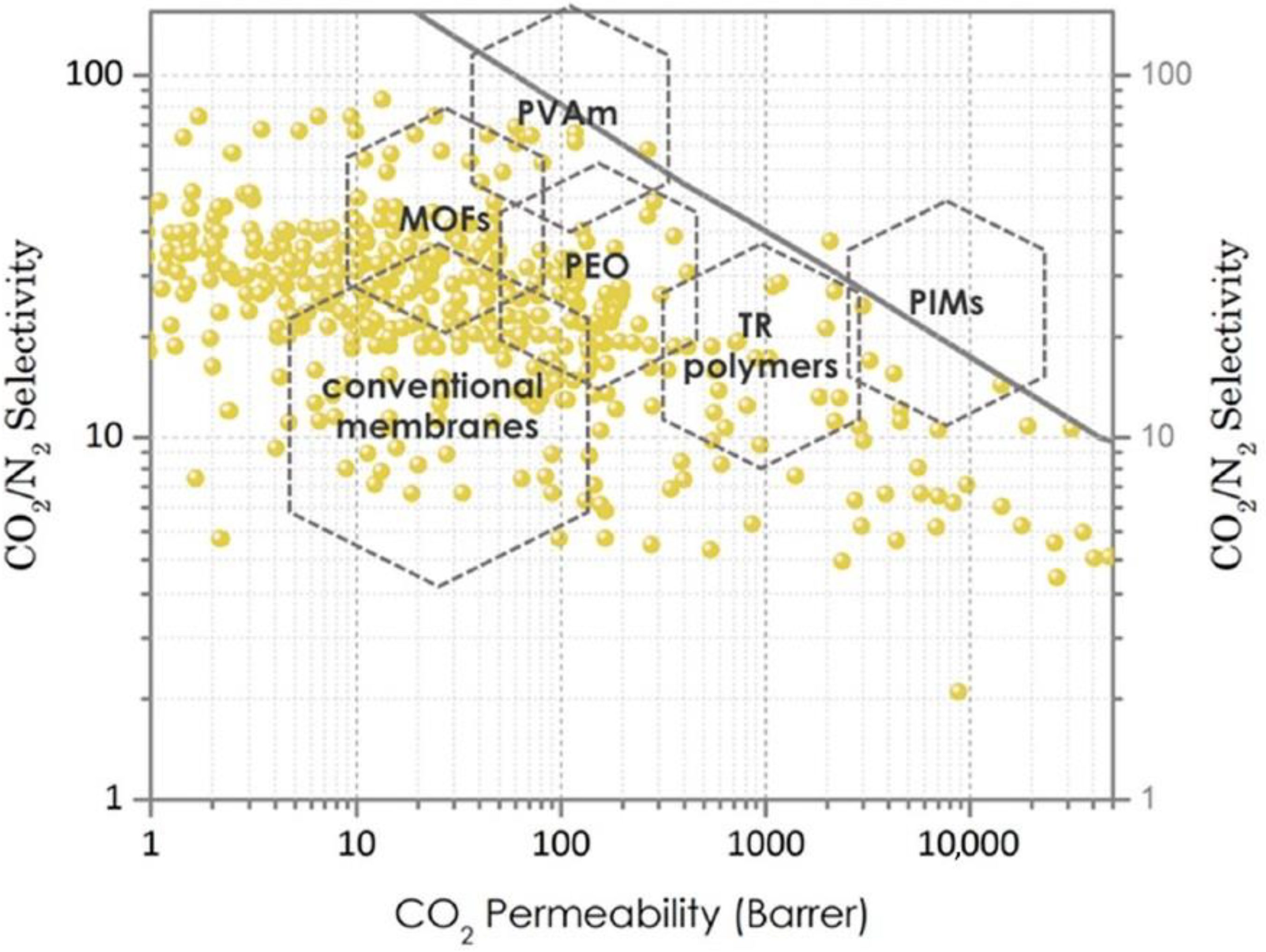
4.3. Membrane Materials
5. State of the Art in CO2 Capture with the Use of Membrane Technologies
- (i)
- Inorganic materials, e.g., zeolites.
- (ii)
- Carbon-based materials, e.g., carbon nanotubes and carbon molecular sieves (CMSs).
- (iii)
- Organic-based materials, such as (a) porous organic frameworks (POFs), which include covalent organic frameworks (COFs), porous aromatic frameworks (PAFs), covalent organic polymers (COPs) and porous organic polymers (POPs), and (b) microporous polymers, which include polymers of intrinsic microporosity (PIM) and thermally rearranged (TR) polymers.
- (iv)
- Hybrid materials, which are also known as metal–organic frameworks (MOFs).
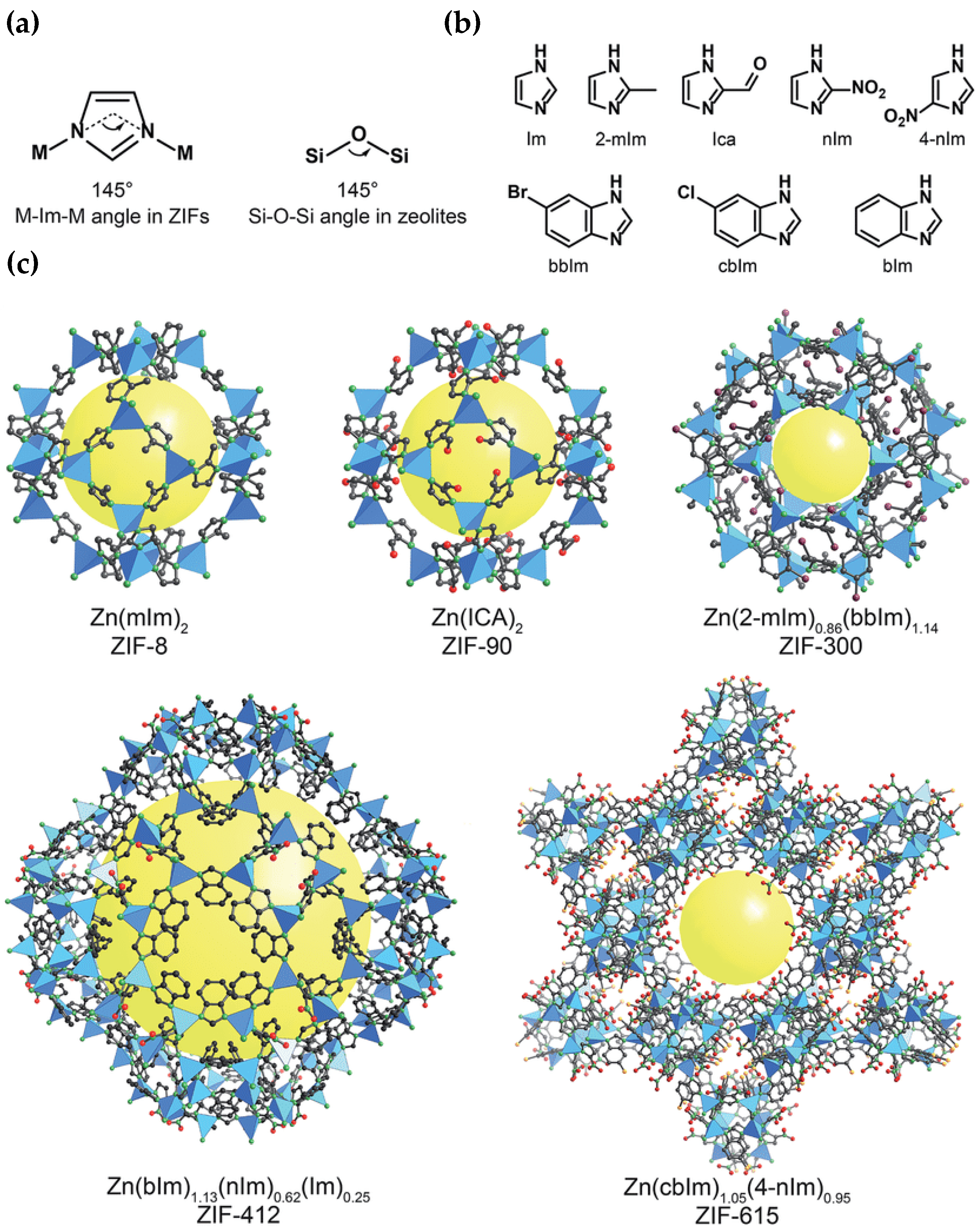
5.1. Metal–Organic Framework (MOF) Membraness
5.2. Carbon Molecular Sieve (CMS) Membranes
5.3. Nanocomposite Membranes
5.4. Ionic Liquid (IL)-Based Membranes
5.5. Facilitated Transport Membranes (FTMs)
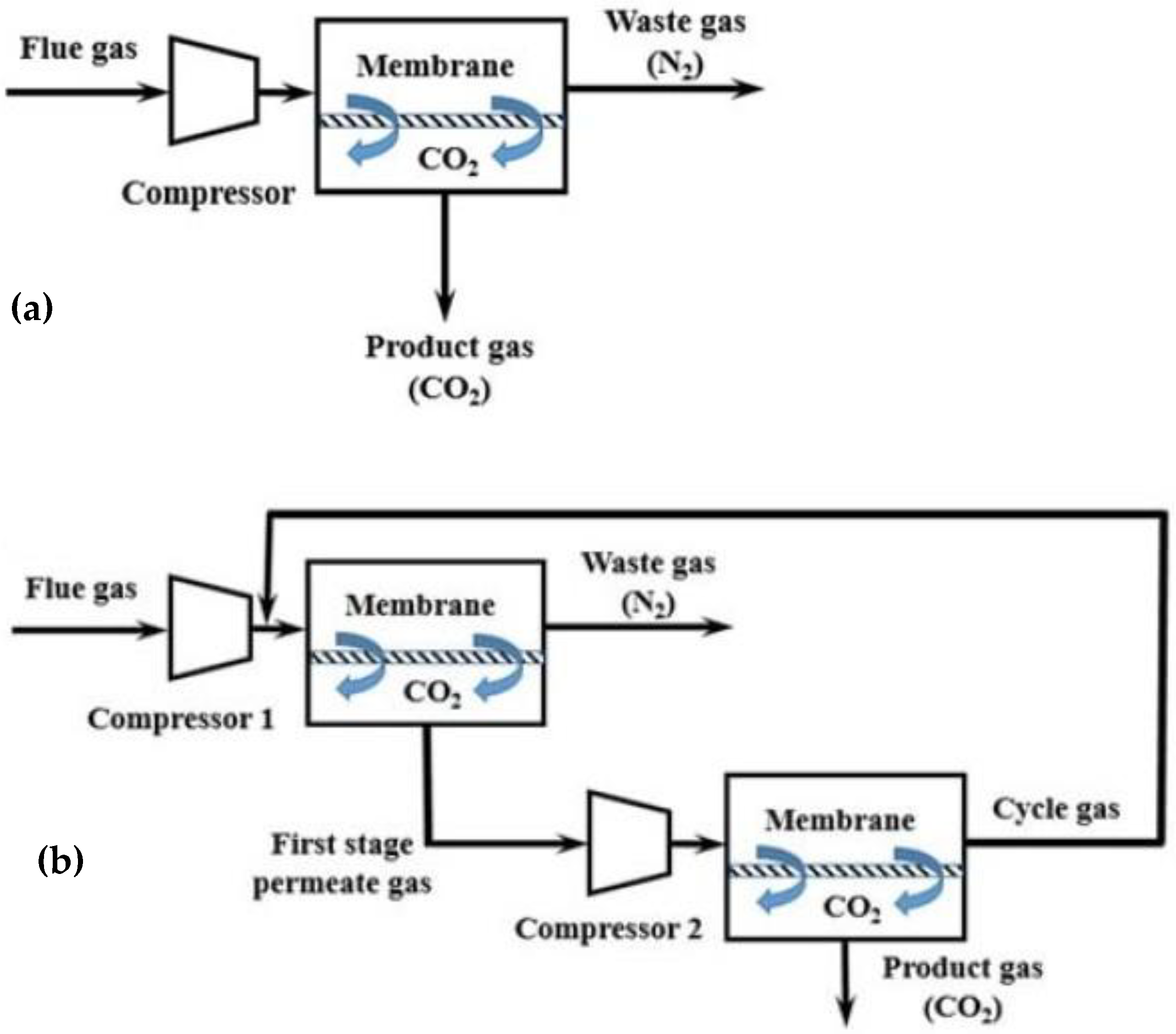
6. Commercially Applied Membrane Modules for Industrial CO2 Capture
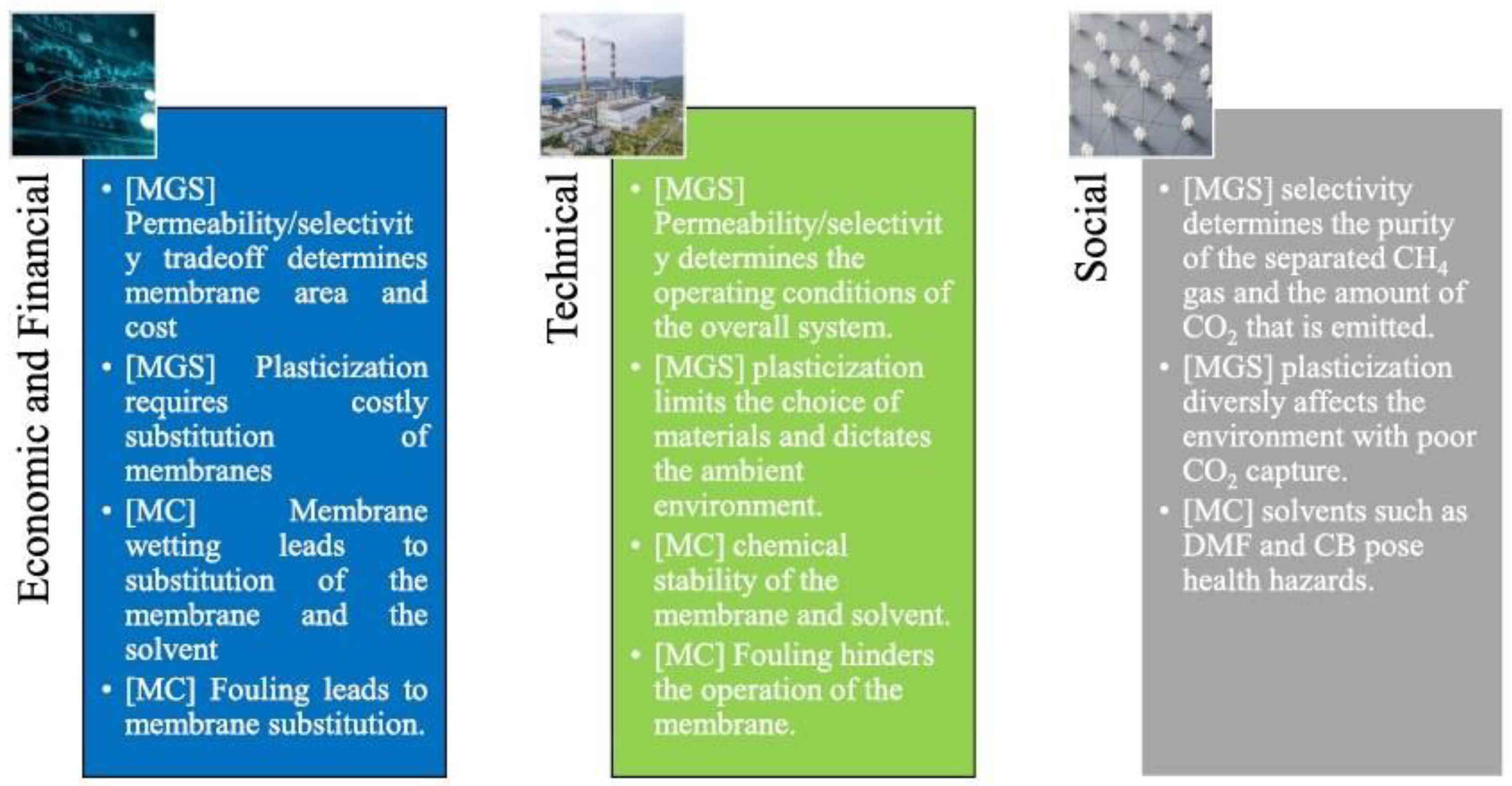
7. Main Challenges and Future Perspectives
8. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chakrabortty, S.; Kumar, R.; Nayak, J.; Jeon, B.-H.; Dargar, S.K.; Tripathy, S.K.; Pal, P.; Ha, G.-S.; Kim, K.K.; Jasiński, M. Green synthesis of MeOH derivatives through in situ catalytic transformations of captured CO2 in a membrane integrated photo-microreactor system: A state-of-art review for carbon capture and utilization. Renew. Sust. Energ. Rev. 2023, 182, 113417. [Google Scholar] [CrossRef]
- Wu, H.; Li, Q.; Sheng, M.; Wang, Z.; Zhao, S.; Wang, J.; Mao, S.; Wang, D.; Guo, B.; Ye, N.; et al. Membrane technology for CO2 capture: From pilot-scale investigation of two-stage plant to actual system design. J. Membr. Sci. 2021, 624, 119137. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, Y.; Ma, H.; He, G.; Jiang, Z. Advanced organic molecular sieve membranes for carbon capture: Current status, challenges and prospects. Adv. Membr. 2022, 2, 100028. [Google Scholar] [CrossRef]
- Tao, Y.R.; Xu, H.J. A critical review on potential applications of Metal-Organic frameworks (MOFs) in adsorptive carbon capture technologies. Appl. Therm. Eng. 2024, 236, 121504. [Google Scholar] [CrossRef]
- Langie, K.M.G.; Tak, K.; Kim, C.; Lee, H.W.; Park, K.; Kim, D.; Jung, W.; Lee, C.W.; Oh, H.-S.; Lee, D.K.; et al. Toward economical application of carbon capture and utilization technology with near-zero carbon emission. Nat. Commun. 2022, 13, 7482. [Google Scholar] [CrossRef]
- Odunlami, O.A.; Vershima, D.A.; Oladimeji, T.E.; Nkongho, S.; Ogunlade, S.K.; Fakinle, B.S. Advanced techniques for the capturing and separation of CO2—A review. Results Eng. 2022, 15, 100512. [Google Scholar] [CrossRef]
- Katare, A.; Kumar, S.; Kundu, S.; Sharma, S.; Kundu, L.M.; Mandal, B. Mixed Matrix Membranes for Carbon Capture and Sequestration: Challenges and Scope. ACS Omega 2023, 8, 17511–17522. [Google Scholar] [CrossRef]
- Zanco, S.E.; Pérez-Calvo, J.-F.; Gasós, A.; Cordiano, B.; Becattini, B.; Mazzotti, M. Post-combustion CO2 Capture: A Comparative Techno-Economic Assessment of Three Technologies Using a Solvent, an Adsorbent, and a Membrane. ACS Engin. Au 2021, 1, 50–72. [Google Scholar] [CrossRef]
- Ngu, L.H. Carbon Capture Technologies. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Kim, S.; Scholes, C.A.; Heath, D.E.; Kentish, S.E. Gas-liquid membrane contactors for carbon dioxide separation: A review. Chem. Eng. J. 2021, 41, 128468. [Google Scholar] [CrossRef]
- Sun, S.; Billings, A.; Zhang, K.; Huang, K. Direct, efficient and selective capture of low concentration of CO2 from natural gas flue gas using a high temperature tubular carbon capture membrane. J. Membr. Sci. 2022, 661, 120929. [Google Scholar] [CrossRef]
- Gkotsis, P.; Kougias, P.; Mitrakas, M.; Zouboulis, A. Biogas upgrading technologies—Recent advances in membrane-based processes. Int. J. Hydrogen Energy 2023, 48, 3965–3993. [Google Scholar] [CrossRef]
- Kárászová, M.; Zach, B.; Petrusová, Z.; Červenka, V.; Bobák, M.; Šyc, M.; Izák, P. Post-combustion carbon capture by membrane separation, Review. Sep. Purif. Technol. 2020, 238, 116448. [Google Scholar] [CrossRef]
- Da Conceicao, M.; Nemetz, L.; Rivero, J.; Hornbostel, K.; Lipscomb, G. Gas Separation Membrane Module Modeling: A Comprehensive Review. Membranes 2023, 13, 639. [Google Scholar] [CrossRef]
- Singh, P.; Shah, S.; Rai, S.K. Post-combustion carbon capture by polymeric membrane: A review. Mater. Today Proc. 2022, 62, 318–324. [Google Scholar] [CrossRef]
- Favre, E. Membrane Separation Processes and Post-Combustion Carbon Capture: State of the Art and Prospects. Membranes 2022, 12, 884. [Google Scholar] [CrossRef]
- Dowson, G.; Styring, P. Chapter 9—Conversion of Carbon Dioxide to Oxygenated Organics. In Carbon Dioxide Utilisation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 141–159. [Google Scholar]
- Ericsson, K. Biogenic carbon dioxide as feedstock for production of chemicals and fuels: A techno-economic assessment with a European perspective. In IMES/EESS Report No 103; Lund University Publications: Lund, Sweden, 2017. [Google Scholar]
- IPCC. IPCC Special Report on Carbon Dioxide Capture and Storage (Chapter 3); Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Kanniche, M.; Gros-Bonnivard, R.; Jaud, P.; Valle-Marcos, J.; Amann, J.-M.; Bouallou, C. Pre-combustion, post-combustion and oxy-combustion in thermal power plant for CO2 capture. Appl. Therm. Eng. 2010, 30, 53–62. [Google Scholar] [CrossRef]
- Kheirinik, M.; Ahmed, S.; Rahmanian, N. Comparative Techno-Economic Analysis of Carbon Capture Processes: Pre-Combustion, Post-Combustion, and Oxy-Fuel Combustion Operations. Sustainability 2021, 13, 13567. [Google Scholar] [CrossRef]
- De Mello, L.F.; Gobbo, R.; Moure, G.T.; Miracca, I. Oxy-combustion technology development for fluid catalytic crackers (FCC)—Large pilot scale demonstration. Energy Procedia 2023, 37, 7815–7824. [Google Scholar] [CrossRef]
- Khan, U.; Ogbaba, C.C.; Abiodun, O.-A.O.; Adeleke, A.A.; Ikubanni, P.P.; Okoye, P.U.; Okolie, J.A. Assessing absorption-based CO2 capture: Research progress and techno-economic assessment overview. Carbon Capture Sci. Technol. 2023, 8, 100125. [Google Scholar] [CrossRef]
- Mukherjee, A.; Okolie, J.A.; Abdelrasoul, A.; Niu, C.; Dalai, A.K. Review of post-combustion carbon dioxide capture technologies using activated carbon. J. Environ. Sci. 2019, 83, 46–63. [Google Scholar] [CrossRef]
- Wang, X.; Song, C. Carbon Capture from Flue Gas and the Atmosphere: A Perspective. Front. Energy Res. 2020, 8, 560849. [Google Scholar] [CrossRef]
- Spigarelli, B.P.; Kawatra, S.K. Opportunities and challenges in carbon dioxide capture. J. CO2 Util. 2013, 1, 69–87. [Google Scholar] [CrossRef]
- Khalifa, O.; Alkhatib, I.I.I.; Bahamon, D.; Alhajaj, A.; Abu-Zahra, M.R.M.; Vega, L.F. Modifying absorption process configurations to improve their performance for Post-Combustion CO2 capture—What have we learned and what is still Missing? Chem. Eng. J. 2022, 430, 1330996. [Google Scholar] [CrossRef]
- Oexmann, J.; Kather, A.; Linnenberg, S.; Liebenthal, U. Post-combustion CO2 capture: Chemical absorption processes in coal-fired steam power plants. Greenh. Gases Sci. Technol. 2012, 2, 80–98. [Google Scholar] [CrossRef]
- Ghiat, I.; Al-Ansari, T. A review of carbon capture and utilization as a CO2 abatement opportunity within the EWF nexus. J. CO2 Util. 2021, 45, 101432. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Otto, A.; Robinius, M.; Stolten, D. A Review of Post-combustion CO2 Capture Technologies from Coal-fired Power Plants. Energy Procedia 2017, 114, 650–665. [Google Scholar] [CrossRef]
- Song, C.; Liu, Q.; Deng, S.; Li, H.; Kitamura, Y. Cryogenic-based CO2 capture technologies: State-of-the-art developments and current challenges. Renew. Sust. Energ. Rev. 2019, 101, 265–278. [Google Scholar] [CrossRef]
- Hou, R.; Fong, C.; Freeman, B.D.; Hill, M.R.; Xie, Z. Current status and advances in membrane technology for carbon capture. Sep. Purif. Technol. 2022, 300, 121863. [Google Scholar] [CrossRef]
- Hongjun, Y.; Shuanshi, F.; Xuemei, L.; Yanhong, W.; Jianghua, N. Economic Comparison of Three Gas Separation Technologies for CO2 Capture from Power Plant Flue Gas. Chin. J. Chem. Eng. 2011, 19, 615–620. [Google Scholar]
- Lin, Q.; Zhang, X.; Wang, T.; Zheng, C.; Gao, X. Technical Perspective of Carbon Capture, Utilization, and Storage. Engineering 2022, 14, 27–32. [Google Scholar] [CrossRef]
- Arshad, N.; Alhajaj, A. Process synthesis for amine-based CO2 capture from combined cycle gas turbine power plant. Energy 2023, 274, 127391. [Google Scholar] [CrossRef]
- Artanto, Y.; Jansen, J.; Pearson, P.; Do, T.; Cottrell, A.; Meuleman, E.; Feron, P. Performance of MEA and amine-blends in the CSIRO PCC pilot plant at Loy Yang Power in Australia. Fuel 2012, 101, 264–275. [Google Scholar] [CrossRef]
- Wang, M.; Lawal, A.; Stephenson, P.; Sidders, J.; Ramshaw, C. Post-combustion CO2 capture with chemical absorption: A state-of-the-art review. Chem. Eng. Res. Des. 2011, 89, 1609–1624. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, F.; Cui, Z.; Liu, C.; Yue, H.; Tang, S. Enhancing the energetic efficiency of MDEA/PZ-based CO2 capture technology for a 650 MW power plant: Process improvement. Appl. Energy 2017, 185, 362–375. [Google Scholar] [CrossRef]
- Li, K.; Leigh, W.; Feron, P.; Yu, H.; Tade, M. Systematic study of aqueous monoethanolamine (MEA)-based CO2 capture process: Techno-economic assessment of the MEA process and its improvements. Appl. Energy 2016, 165, 648–659. [Google Scholar] [CrossRef]
- Raynal, L.; Bouillon, P.A.; Gomez, A.; Broutin, P. From MEA to demixing solvents and future steps, a roadmap for lowering the cost of post-combustion carbon capture. Chem. Eng. J. 2011, 171, 742–752. [Google Scholar] [CrossRef]
- Rao, A.B.; Rubin, E.S. A technical, economic, and environmental assessment of amine-based CO2 capture technology for power plant greenhouse gas control. Environ. Sci. Technol. 2002, 36, 4467–4475. [Google Scholar] [CrossRef] [PubMed]
- Braakhuis, L.; Knuutila, H.N. Predicting solvent degradation in absorption-based CO2 capture from industrial flue gases. Chem. Eng. Sci. 2023, 279, 118940. [Google Scholar] [CrossRef]
- Raganati, F.; Chirone, R.; Ammendola, P. CO2 Capture by Temperature Swing Adsorption: Working Capacity as Affected by Temperature and CO2 Partial Pressure. Ind. Eng. Chem. Res. 2020, 59, 3593–3605. [Google Scholar] [CrossRef]
- Zeng, H.; Qu, X.; Xu, D.; Luo, Y. Porous Adsorption Materials for Carbon Dioxide Capture in Industrial Flue Gas. Front. Chem. 2022, 10, 939701. [Google Scholar] [CrossRef]
- Guo, Z.; Deng, S.; Li, S.; Lian, Y.; Zhao, L.; Yuan, X. Entropy Analysis of Temperature Swing Adsorption for CO2 Capture Using the Computational Fluid Dynamics (CFD) Method. Entropy 2019, 21, 285. [Google Scholar] [CrossRef] [PubMed]
- Akdag, A.S.; Durán, I.; Gullu, G.; Pevida, C. Performance of TSA and VSA post-combustion CO2 capture processes with a biomass waste-based adsorbent. J. Environ. Chem. Eng. 2022, 10, 108759. [Google Scholar] [CrossRef]
- Golmakani, A.; Fatemi, S.; Tamnanloo, J. Investigating PSA, VSA, and TSA methods in SMR unit of refineries for hydrogen production with fuel cell specification. Separ. Purif. Technol. 2017, 176, 73–91. [Google Scholar] [CrossRef]
- Dhoke, C.; Cloete, S.; Krishnamurthy, S.; Seo, H.; Luz, I.; Soukri, M.; Park, Y.-k.; Blom, R.; Amini, S.; Zaabout, A. Sorbents screening for post-combustion CO2 capture via combined temperature and pressure swing adsorption. Chem. Eng. J. 2020, 380, 122201. [Google Scholar] [CrossRef]
- Gunawardene, O.H.P.; Gunathilake, C.A.; Vikrant, K.; Amaraweera, S.M. Carbon Dioxide Capture through Physical and Chemical Adsorption Using Porous Carbon Materials: A Review. Atmosphere 2022, 13, 397. [Google Scholar] [CrossRef]
- Rashidi, N.A.; Yusup, S. An overview of activated carbons utilization for the post-combustion carbon dioxide capture. J. CO2 Util. 2016, 13, 1–16. [Google Scholar] [CrossRef]
- Karimi, M.; Shirzad, M.; Silva, J.A.C.; Rodrigues, A.E. Carbon dioxide separation and capture by adsorption: A review. Environ. Chem. Lett. 2023, 21, 2041–2084. [Google Scholar] [CrossRef]
- Lai, J.Y.; Ngu, L.H.; Hashim, S.S. A review of CO2 adsorbents performance for different carbon capture technology processes conditions. Greenh. Gases Sci. Technol. 2021, 11, 1076–1117. [Google Scholar] [CrossRef]
- Raganati, F.; Miccio, F.; Ammendola, P. Adsorption of Carbon Dioxide for Post-combustion Capture: A Review. Energy Fuels 2021, 35, 12845–12868. [Google Scholar] [CrossRef]
- Aneesh, A.M.; Sam, A.A. A mini-review on cryogenic carbon capture technology by desublimation: Theoretical and modeling aspects. Front. Energy Res. 2023, 11, 1167099. [Google Scholar] [CrossRef]
- Knapik, E.; Kosowski, P.; Stopa, J. Cryogenic liquefaction and separation of CO2 using nitrogen removal unit cold energy. Chem. Eng. Res. Des. 2018, 131, 66–79. [Google Scholar] [CrossRef]
- Mokhatab, S.; Poe, W.A.; Mak, J.Y. Chapter 7—Natural Gas Treating. In Handbook of Natural Gas Transmission and Processing—Principles and Practices, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Liu, B.; Yang, X.; Chiang, P.-C.; Wang, T. Energy Consumption Analysis of Cryogenic-membrane Hybrid Process for CO2 Capture from CO2-EOR Extraction Gas. Aerosol Air Qual. Res. 2020, 20, 820–832. [Google Scholar] [CrossRef]
- Asghar, U.; Rafiq, S.; Anwar, A.; Iqbal, T.; Ahmed, A.; Jamil, F.; Khurram, M.S.; Akbar, M.M.; Farooq, A.; Shah, N.S.; et al. Review on the progress in emission control strategies for the abatement of CO2, SOx and NOx from fuel combustion. J. Environ. Chem. Eng. 2021, 9, 106064. [Google Scholar] [CrossRef]
- Seong, M.S.; Kong, C.I.; Park, B.R.; Lee, Y.; Na, B.K.; Kim, J.H. Optimization of pilot-scale 3-stage membrane process using asymmetric polysulfone hollow fiber membranes for production of high-purity CH4 and CO2 from crude biogas. Chem. Eng. J. 2020, 384, 123342. [Google Scholar] [CrossRef]
- Gkotsis, P.K.; Zouboulis, A.I. The use of bio-carriers and zeolite in a lab-scale MBR for membrane fouling mitigation. Glob. Nest J. 2018, 21, 58–63. [Google Scholar]
- Ji, G.; Zhao, M. Membrane Separation Technology in Carbon Capture. In Recent Advances in Carbon Capture and Storage; Yun, Y., Ed.; InTech: Rijeka, Croatia, 2017. [Google Scholar]
- Rafiq, S.; Deng, L.; Hägg, M.B. Role of Facilitated Transport Membranes and Composite Membranes for Efficient CO2 Capture—A Review. ChemBioEng Rev. 2016, 3, 68–85. [Google Scholar] [CrossRef]
- Tong, Z.; Sekizkardes, A.K. Recent Developments in High-Performance Membranes for CO2 Separation. Membranes 2021, 11, 156. [Google Scholar] [CrossRef]
- Castro-Dominguez, B.; Leelachaikul, P.; Messaoud, S.B.; Takagaki, A.; Sugawara, T.; Kikuchi, R.; Oyama, S.T. The optimal point within the Robeson upper boundary. Chem. Eng. Res. Des. 2015, 97, 109–119. [Google Scholar] [CrossRef]
- Rivero, J.R.; Nemetz, L.R.; Da Conceicao, M.M.; Lipscomb, G.; Hornbostel, K. Modeling gas separation in flat sheet membrane modules: Impact of flow channel size variation. Carbon Capture Sci. Technol. 2023, 6, 100093. [Google Scholar] [CrossRef]
- Wu, H.; Li, Q.; Guo, B.; Sheng, M.; Wang, D.; Mao, S.; Ye, N.; Qiao, Z.; Kang, G.; Cao, Y.; et al. Industrial-scale spiral-wound facilitated transport membrane modules for post-combustion CO2 capture: Development, investigation and optimization. J. Membr. Sci. 2023, 670, 121368. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Tarannum, K.; Chowdhury, A.T.; Rafa, N.; Nuzhat, S.; Kumar, P.S.; Vo, D.-V.N.; Lichtfouse, E.; Mahlia, T.M.I. Biogas upgrading, economy and utilization: A review. Environ. Chem. Lett. 2021, 19, 4137–4164. [Google Scholar] [CrossRef]
- Hidalgo, D.; Sanz-Bedate, S.; Martín-Marroquín, J.M.; Castro, J.; Antolín, G. Selective separation of CH4 and CO2 using membrane contactors. Renew. Energy 2020, 150, 935–942. [Google Scholar] [CrossRef]
- Xu, J.; Wu, H.; Wang, Z.; Qiao, Z.; Zhao, S.; Wang, J. Recent advances on the membrane processes for CO2 separation. Chin. J. Chem. Eng. 2018, 26, 2280–2291. [Google Scholar] [CrossRef]
- He, X. A review of material development in the field of carbon capture and the application of membrane-based processes in power plants and energy-intensive industries. Energy Sustain. Soc. 2018, 8, 34. [Google Scholar] [CrossRef]
- Zhu, B.; He, S.; Yang, Y.; Li, S.; Lau, C.H.; Liu, S.; Shao, L. Boosting membrane carbon capture via multifaceted polyphenol-mediated soldering. Nat. Commun. 2023, 14, 1697. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Chen, C.; Guo, Y.; Chu, Z.; Pan, Y.; Liu, G.; Liu, G.; Han, Y.; Jin, W.; Xu, N. Solid-solvent processing of ultrathin, highly loaded mixed-matrix membrane for gas separation. Science 2023, 381, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hu, G.; Shen, T.; Zhang, H.; Sun, J.Z.; Tang, B.Z. Applications of Polyacetylene Derivatives in Gas and Liquid Separation. Molecules 2023, 28, 2748. [Google Scholar] [CrossRef] [PubMed]
- Vostiňáková, M.; Lupínková, S.; Ryšánek, P.; Stejskal, J.; Kolská, Z. Polyaniline/aramid composite for carbon dioxide capture. Polym. Int. 2023, 72, 552–557. [Google Scholar] [CrossRef]
- Quian, H.; Zheng, J.; Zhang, S. Preparation of microporous polyamide networks for carbon dioxide capture and nanofiltration. Polymer 2013, 54, 557–564. [Google Scholar] [CrossRef]
- Jankowski, A.; Grabiec, E.; Nocoń-Szmajda, K.; Marcinkowski, A.; Janeczek, H.; Wolińska-Grabczyk, A. Polyimide-Based Membrane Materials for CO2 Separation: A Comparison of Segmented and Aromatic (Co)polyimides. Membranes 2021, 11, 274. [Google Scholar] [CrossRef]
- Naim, R.; Ismail, A.F.; Matsuura, T.; Rudaini, I.A.; Abdullah, S. Polyetherimide hollow fiber membranes for CO2 absorption and stripping in membrane contactor application. RSC Adv. 2018, 8, 3556. [Google Scholar] [CrossRef] [PubMed]
- Hauenstein, O.; Rahman, M.M.; Elsayed, M.; Krause-Rehberg, R.; Agarwal, S.; Abetz, V.; Greiner, A. Biobased Polycarbonate as a Gas Separation Membrane and “Breathing Glass” for Energy Saving Applications. Adv. Mater. Technol. 2017, 2, 1700026. [Google Scholar] [CrossRef]
- Sridhar, S.; Smitha, B.; Ramakrishna, M.; Aminabhavi, T.M. Modified poly(phenylene oxide) membranes for the separation of carbon dioxide from methane. J. Membr. Sci. 2006, 280, 202–209. [Google Scholar] [CrossRef]
- Zhu, B.; Jiang, X.; He, S.; Yang, X.; Long, J.; Zhang, Y.; Shao, L. Rational design of poly(ethylene oxide) based membranes for sustainable CO2 capture. J. Mater. Chem. A. 2020, 8, 24233–24252. [Google Scholar] [CrossRef]
- Suhail, F.; Batool, M.; Anjum, T.; Shah, A.T.; Tabassum, S.; Khan, A.L.; AlMohamadi, H.; Najam, M.; Gilani, M.A. Enhanced CO2 separation performance of polysulfone membranes via incorporation of pyrazole modified MCM-41 mesoporous silica as a nano-filler. Fuel 2023, 350, 128840. [Google Scholar] [CrossRef]
- Akbarzadeh, E.; Shockravi, A.; Vatanpour, V. High performance compatible thiazole-based polymeric blend cellulose acetate membrane as selective CO2 absorbent and molecular sieve. Carbohydr. Polym. 2021, 252, 117215. [Google Scholar] [CrossRef]
- Chen, G.; Wang, T.; Zhang, G.; Li, G.; Jin, W. Membrane materials targeting carbon capture and utilization. Adv. Membr. 2022, 2, 100025. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Jawad, Z.A.; Low, S.C.; Zein, S.H.S. A cellulose acetate/multi-walled carbon nanotube mixed matrix membrane for CO2/N2 separation. J. Membr. Sci. 2014, 451, 55–66. [Google Scholar] [CrossRef]
- Al-Masr, M.; Fritsch, D.; Kricheldorf, H.R. New polyimides for gas separation. 2. Polyimides derived from substituted catechol bis(etherphthalic anhydride)s. Macromolecules 2000, 33, 7127–7135. [Google Scholar] [CrossRef]
- De Abajo, J.; De la Campa, J.G.; Lozano, A.E. Designing aromatic polyamides and polyimides for gas separation membranes. Macromol. Symp. 2003, 199, 293–306. [Google Scholar] [CrossRef]
- Dai, Y.; Guiver, M.D.; Robertson, G.P.; Kang, Y.S.; Lee, K.J. Enhancement in the gas permeabilities of novel polysulfones with pendant 4-trimethylsilyl-α-hydroxylbenzyl substituents. Macromolecules 2003, 36, 6807–6816. [Google Scholar] [CrossRef]
- Dai, Y.; Guiver, M.D.; Robertson, G.P.; Kang, Y.S.; Lee, K.J.; Jho, J.Y. Preparation and characterization of polysulfones containing both hexafluoroisopropylidene and trimethylsilyl groups as gas separation membrane materials. Macromolecules 2004, 37, 1403–1410. [Google Scholar] [CrossRef]
- Dai, Y.; Guiver, M.D.; Robertson, G.P.; Bilodeau, F.; Kang, Y.S.; Lee, K.J.; Jho, J.Y.; Won, J. Modified polysulfones 5: Synthesis and characterization of tetramethyl polysulfones containing trimethylsilyl groups and their gas transport properties. Polymer 2002, 43, 5369–5378. [Google Scholar] [CrossRef]
- Hellums, M.W.; Koros, W.J.; Husk, G.R.; Paul, D.R. Fluorinated polycarbonates for gas separation applications. J. Membr. Sci. 1989, 46, 93–112. [Google Scholar] [CrossRef]
- Aguilar-Vega, M.; Paul, D.R. Gas transport properties of polycarbonates and polysulfones with aromatic substitutions on the bisphenol connector group. J. Polym. Sci. B Polym. Phys. 1993, 31, 1599–1610. [Google Scholar] [CrossRef]
- Koros, W.J.; Zhang, C. Materials for next-generation molecularly selective synthetic membranes. Nat. Mater. 2017, 16, 289–297. [Google Scholar] [CrossRef]
- Golmakani, A.; Nabavi, S.A.; Wadi, B.; Manovic, V. Advances, challenges, and perspectives of biogas cleaning, upgrading, and utilisation. Fuel 2022, 317, 123085. [Google Scholar] [CrossRef]
- Luo, W.; Li, F.; Li, H.; Zhang, Z.; Zhang, X.; Liang, Y.; Huang, G. From 0D to 3D nanomaterial-based composite membranes for CO2 capture: Recent advances and perspectives. J. Environ. Chem. Eng. 2023, 11, 110657. [Google Scholar] [CrossRef]
- Chen, D.; Wang, K.; Yuan, Z.; Lin, Z.; Zhang, M.; Li, Y.; Tang, J.; Liang, Z.; Li, Y.; Chen, L.; et al. Boosting membranes for CO2 capture toward industrial decarbonization. Carbon Capture Sci. Technol. 2023, 7, 100117. [Google Scholar] [CrossRef]
- Basile, A.; Gugliuzza, A.; Iulianelli, A.; Morrone, P. Membrane technology for carbon dioxide (CO2) capture in power plants. In Advanced Membrane Science and Technology for Sustainable Energy and Environmental Applications; Woodhead Publishing Limited; Elsevier: Sawston, UK, 2021; pp. 113–159. [Google Scholar]
- Shah, Y.T. CO2 Capture, Utilization and Sequestration Strategies; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Wu, H.; Li, X.; Li, Y.; Wang, S.; Guo, R.; Jiang, Z.; Wu, C.; Xin, Q.; Lu, X. Facilitated transport mixed matrix membranes incorporated with amine functionalized MCM-41 for enhanced gas separation properties. J. Membr. Sci. 2014, 465, 78–90. [Google Scholar] [CrossRef]
- Prasetya, N.; Himma, N.F.; Sutrisna, P.D.; Wenten, I.G.; Ladewig, B.P. A review on emerging organic-containing microporous material membranes for carbon capture and separation. Chem. Eng. J. 2020, 391, 123575. [Google Scholar] [CrossRef]
- Shan, M.; Liu, X.; Wang, X.; Yarulina, I.; Seoane, B.; Karteijin, F.; Gascon, J. Facile manufacture of porous organic framework membranes for precombustion CO2 capture. Sci. Adv. 2018, 4, 1698. [Google Scholar] [CrossRef]
- Zhao, M.; Guo, J.; Xin, Q.; Zhang, Y.; Li, X.; Ding, X.; Zhang, L.; Zhao, L.; Ye, H.; Li, H.; et al. Novel aminated F-Ce nanosheet mixed matrix membranes with controllable channels for CO2 capture. Sep. Purif. Technol. 2023, 324, 124512. [Google Scholar] [CrossRef]
- Liu, M.; Xie, K.; Nothling, M.D.; Gurr, P.A.; Tan, S.S.L.; Fu, Q.; Webley, P.A.; Qiao, G. Ultrathin Metal-Organic Framework Nanosheets as A Gutter Layer for Flexible Composite Gas Separation Membranes. ACS Nano 2018, 12, 11591–11599. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Nothling, M.D.; Webley, P.A.; Jin, J.; Fu, Q.; Qiao, G.G. High-throughput CO2 capture using PIM-1@MOF based thin film composite membranes. Chem. Eng. J. 2020, 396, 125328. [Google Scholar] [CrossRef]
- Yao, B.; Wang, Y.; Fang, Z.; Hu, Y.; Ye, Z.; Peng, X. Electrodepositing MOFs into laminated graphene oxide membrane for CO2 capture. Microporous Mesoporous Mater. 2023, 361, 112758. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Li, X.; Zhang, J. Constructing double-faced CO2-capture domains by sandwich-like fillers in membranes for efficient CO2 separation. Chem. Eng. Sci. 2024, 283, 119374. [Google Scholar] [CrossRef]
- Demir, H.; Aksu, G.O.; Gulbalkan, H.C.; Keskin, S. MOF Membranes for CO2 Capture: Past, Present and Future. Carbon Capture Sci. Technol. 2022, 2, 100026. [Google Scholar] [CrossRef]
- Rungtaweevoranit, B.; Diercks, C.S.; Kalmutzki, M.J.; Yaghi, O.M. Spiers Memorial Lecture: Progress and prospects of reticular chemistry. Faraday Discuss. 2017, 201, 9–45. [Google Scholar] [CrossRef]
- Gutierrez-Ortega, A.; Montes-Morán, M.A.; Parra, J.P.; Sempere, J.; Nomen, R.; Gonzalez-Olmos, R. Comparative study of binderless zeolites and carbon molecular sieves as adsorbents for CO2 capture processes. J. CO2 Util. 2022, 61, 102012. [Google Scholar] [CrossRef]
- Sanyal, O.; Hays, S.S.; León, N.E.; Guta, Y.A.; Itta, A.K.; Lively, R.P.; Koros, W.J. A Self-Consistent Model for Sorption and Transport in Polyimide-Derived Carbon Molecular Sieve Gas Separation Membranes. Angew. Chem. 2020, 132, 20525–20891. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Zhang, X.; Li, J. Co@NC@ZIF-8-hybridized carbon molecular sieve membranes for highly efficient gas separation. J. Membr. Sci. 2023, 682, 121781. [Google Scholar] [CrossRef]
- Cheng, L.-H.; Fu, Y.-J.; Liao, K.-S.; Chen, J.-T.; Hu, C.-C.; Hung, W.-S.; Lee, K.-R.; Lai, J.-Y. A high-permeance supported carbon molecular sieve membrane fabricated by plasma-enhanced chemical vapor deposition followed by carbonization for CO2 capture. J. Membr. Sci. 2014, 460, 1–8. [Google Scholar] [CrossRef]
- Dasgupta, S.; Rajasekaran, M.; Roy, P.K.; Thakkar, F.M.; Pathak, A.D.; Ayappa, K.G.; Maiti, P.K. Influence of chain length on structural properties of carbon molecular sieving membranes and their effects on CO2, CH4 and N2 adsorption: A molecular simulation study. J. Membr. Sci. 2022, 664, 121044. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, M.; Li, L.; Xu, R.; Pan, Y.; Wang, T. Carbon molecular sieve/ZSM-5 mixed matrix membranes with enhanced gas separation performance and the performance recovery of the aging membranes. J. Membr. Sci. 2023, 660, 120869. [Google Scholar] [CrossRef]
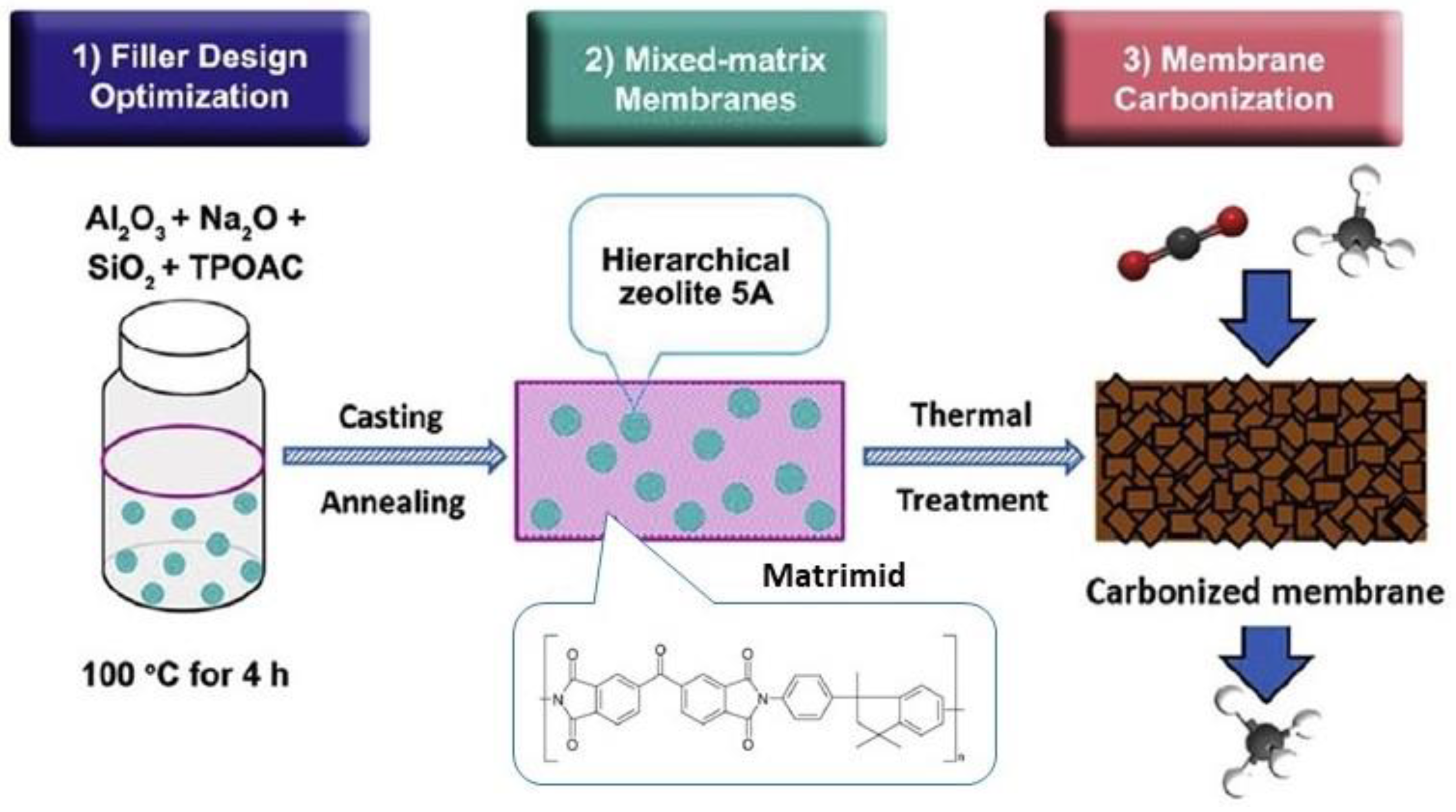
- Li, W.; Goh, K.; Chuah, C.Y.; Bae, T.-H. Mixed-matrix carbon molecular sieve membranes using hierarchical zeolite: A simple approach towards high CO2 permeability enhancements. J. Membr. Sci. 2019, 588, 117220. [Google Scholar] [CrossRef]
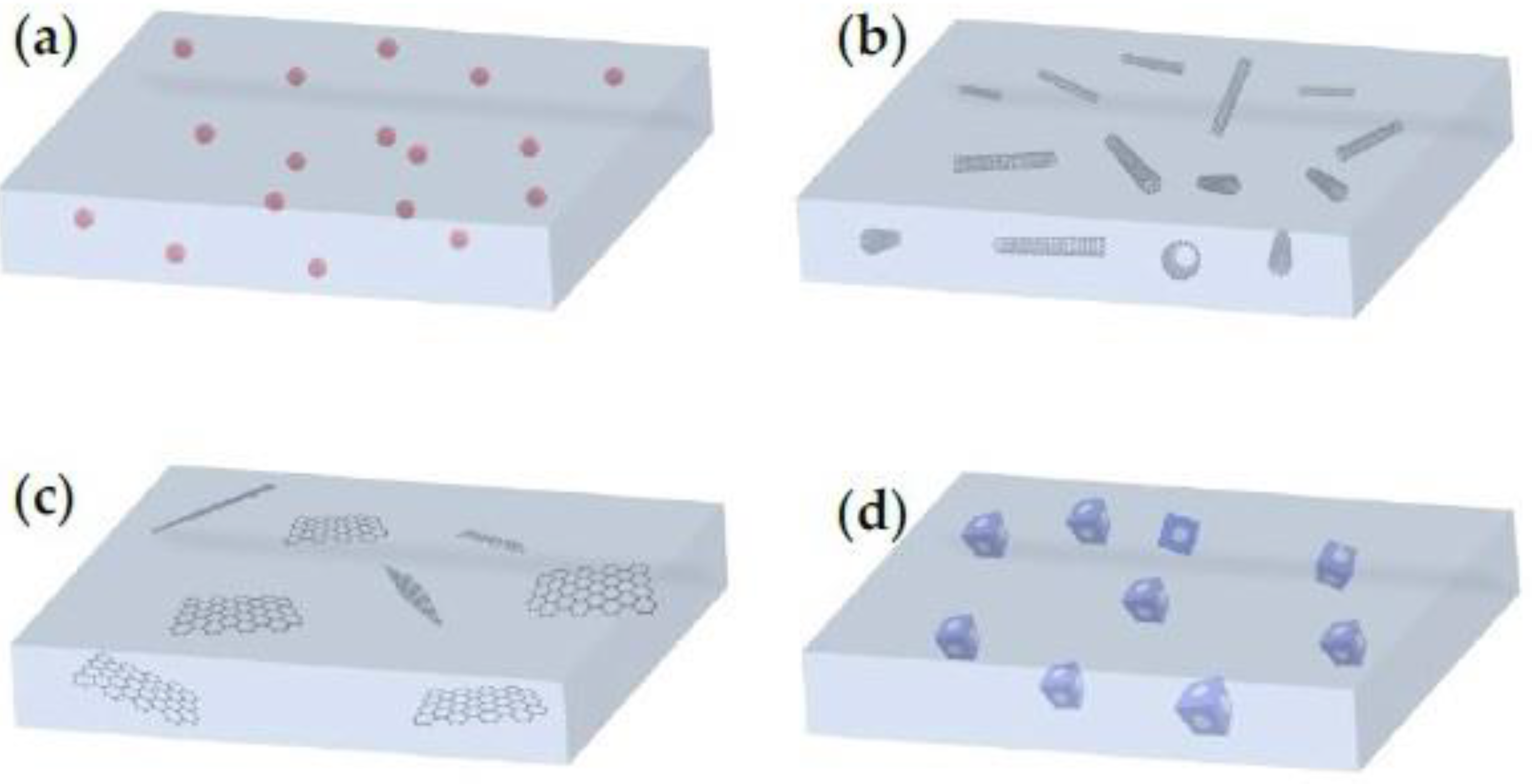
- Yang, G.; Xie, Z.; Cran, M.; Wu, C.; Gray, S. Dimensional Nanofillers in Mixed Matrix Membranes for Pervaporation Separations: A Review. Membranes 2020, 10, 193. [Google Scholar] [CrossRef]
- Dai, Y.; Niu, Z.; Fang, T.; Wang, Y.; Zhong, S.; Mu, P.; Li, J. 1D-2D intercalated network CMC@g-C3N4/IL membrane with high permeability and selectivity for the CO2 capture. J. Membr. Sci. 2023, 686, 122019. [Google Scholar] [CrossRef]
- Maleh, M.S.; Kiani, S.; Raisi, K. Study on the advantageous effect of nano-clay and polyurethane on structure and CO2 separation performance of polyethersulfone based ternary mixed matrix membranes. Chem. Eng. Res. Des. 2022, 179, 27–40. [Google Scholar] [CrossRef]
- Mahboubi, R.; Joudaki, E.; Behbahani, R.M.; Azizi, N. Enhancing CO2/(light gases) separation performance of Pebax-based mixed-matrix membranes by [BMIM][AC] ionic liquid. Mater. Today Commun. 2023, 36, 106542. [Google Scholar] [CrossRef]
- Mulk, W.U.; Ali, S.A.; Shah, S.N.; Shah, M.U.H.; Zhang, Q.-J.; Younas, M.; Fatehizadeh, A.; Sheikh, M.; Rezakazemi, M. Breaking boundaries in CO2 capture: Ionic liquid-based membrane separation for post-combustion applications. J. CO2 Util. 2023, 75, 102555. [Google Scholar] [CrossRef]
- Sanni, S.E.; Vershima, D.A.; Okoro, E.E.; Oni, B.A. Technological advancements in the use of ionic liquid-membrane systems for CO2 capture from biogas/flue gas—A review. Heliyon 2022, 8, 12233. [Google Scholar] [CrossRef]
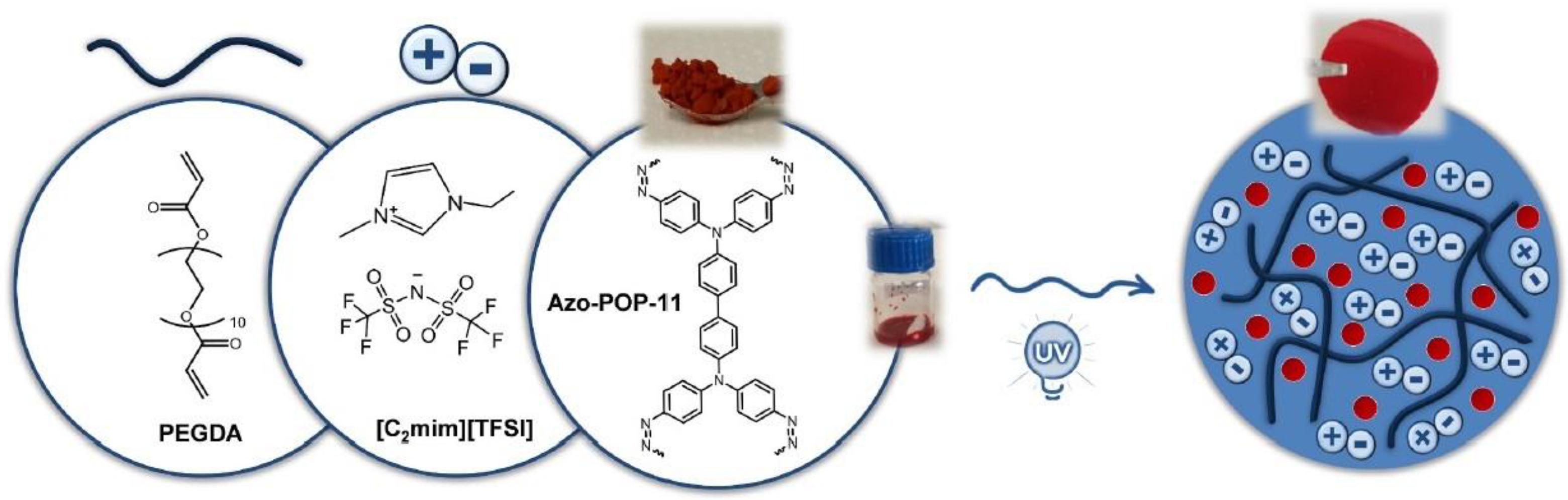
- Nabais, A.R.; Ahmed, S.; Younis, M.; Zhou, J.-X.; Pereira, J.R.; Freitas, F.; Mecerreyes, D.; Crespo, J.G.; Huang, M.H.; Neves, L.A.; et al. Mixed matrix membranes based on ionic liquids and porous organic polymers for selective CO2 separation. J. Membr. Sci. 2022, 660, 120841. [Google Scholar] [CrossRef]
- Ahmad, N.N.R.; Leo, C.P.; Mohammad, A.W.; Ahmad, A.L. Interfacial sealing and functionalization of polysulfone/SAPO-34 mixed matrix membrane using acetate-based ionic liquid in post-impregnation for CO2 capture. Sep. Purif. Technol. 2018, 197, 439–448. [Google Scholar] [CrossRef]
- Ahmad, N.N.R.; Leo, C.P.; Mohammad, A.W. Enhancement on the CO2 separation performance of mixed matrix membrane using ionic liquid. Mater. Lett. 2021, 304, 130736. [Google Scholar] [CrossRef]
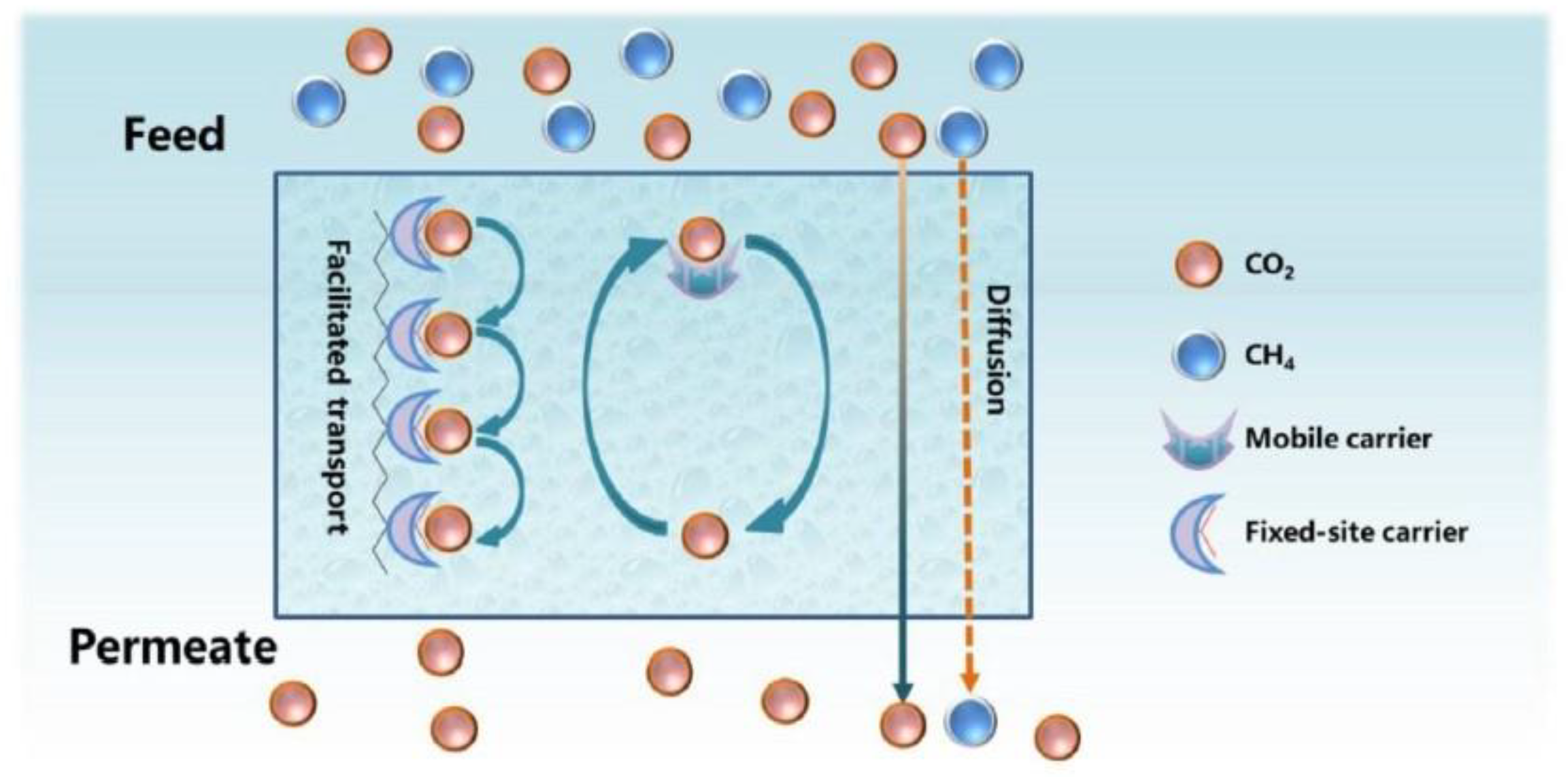
- Guo, H.; Wei, J.; Ma, Y.; Deng, J.; Yi, S.; Wang, B.; Deng, L.; Jiang, X.; Dai, Z. Facilitated transport membranes for CO2/CH4 separation—State of the art. Adv. Membr. 2022, 2, 100040. [Google Scholar] [CrossRef]
- Zheng, W.; Tian, Z.; Wang, Z.; Peng, D.; Zhang, Y.; Wang, J.; Zhang, Y. Dual-function biomimetic carrier based facilitated transport mixed matrix membranes with high stability for efficient CO2/N2 separation. Sep. Purif. Technol. 2022, 285, 120371. [Google Scholar] [CrossRef]
- Kilner, J.A.; Skinner, S.J.; Irvine, S.J.C.; Edwards, P.P. Chapter 7.3.2—Facilitated transport membranes. In Functional Materials for Sustainable Energy Applications; Woodhead Publishing Limited; Elsevier: Sawston, UK, 2012. [Google Scholar]
- He, X. Polyvinylamine-Based Facilitated Transport Membranes for Post-Combustion CO2 Capture: Challenges and Perspectives from Materials to Processes. Engineering 2021, 7, 124–131. [Google Scholar] [CrossRef]
- Janakiram, S.; Yu, X.; Ansaloni, L.; Dai, Z.; Deng, L. Manipulation of fibril surfaces in nanocellulose-based facilitated transport membranes for enhanced CO2 capture. ACS Appl. Mater. Interfaces 2019, 11, 33302–33313. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, S.; Santinelli, F.; Costi, R.; Lindbråthen, A.; Nardelli, G.M.; Milkowski, K.; Ansaloni, L.; Deng, L. Field trial of hollow fiber modules of hybrid facilitated transport membranes for flue gas CO2 capture in cement industry. Chem. Eng. J. 2021, 413, 127405. [Google Scholar] [CrossRef]
- Janakiram, S.; Lindbråthen, A.; Ansaloni, L.; Peters, T.; Deng, L. Two-stage membrane cascades for post-combustion CO2 capture using facilitated transport membranes: Importance on sequence of membrane types. Int. J. Greenh. Gas Control. 2022, 119, 103698. [Google Scholar] [CrossRef]
- He, X.; Chen, D.; Liang, Z.; Yang, F. Insight and Comparison of Energy-efficient Membrane Processes for CO2 Capture from Flue Gases in Power Plant and Energy-intensive Industry. Carbon Capture Sci. Technol. 2022, 2, 100020. [Google Scholar] [CrossRef]
- Kniep, J.; Richard, B.; Casillas, C.; Chan, K.; Huang, I.; Merkel, T.; Nguyen, V.; Sun, Z.; Wang, X. Small Pilot Test Results from a Polaris Membrane 1 MWe CO2 Capture System. In Proceedings of the 14th Greenhouse Gas Control Technologies Conference, Melbourne, Australia, 21–26 October 2018. (GHGT-14). [Google Scholar]
- Baker, R.; Freeman, B.; Casillas, C.; Chan, K.; Hofmann, T.; Huang, I.; Kniep, J.; Merkerl, T.; Nguyen, V.; Sun, Z.; et al. CO2 Capture from Power Plant Flue Gas with the MTR PolarCap™ Process: Update on Field Demonstration Tests; Membrane Technology and Research (MTR), Report: Houston, TX, USA, 2019. [Google Scholar]
- Merkerl, T.; Kniep, J. Scale-up and Testing of Advanced Polaris Membrane CO2 Capture Technology (DE-FE0031591). Membrane Technology and Research (MTR), Report: Newark, CA, USA, 2018. [Google Scholar]
- Cui, Q.; Wang, B.; Zhao, X.; Zhang, G.; He, Z.; Long, Y.; Sun, Y.; Ku, A.Y. Post-combustion slipstream CO2-capture test facility at Jiangyou Power Plant, Sichuan, China: Performance of a membrane separation module under dynamic power-plant operations. Clean Ener. 2021, 5, 742–755. [Google Scholar] [CrossRef]
- Pohlmann, J.; Bram, M.; Wilkner, K.; Brinkmann, T. Pilot scale separation of CO2 from power plant flue gases by membrane technology. Int. J. Greenh. Gas Control. 2016, 53, 56–64. [Google Scholar] [CrossRef]
- Brinkmann, T.; Lillepärg, J.; Notzke, H.; Pohlmann, J.; Shishatskiy, S.; Wind, J.; Wolff, T. Development of CO2 selective poly(ethylene oxide)-based membranes: From laboratory to pilot plant scale. Engineering 2017, 3, 485–493. [Google Scholar] [CrossRef]
- Schuldt, K.; Pohlmann, J.; Shishatskiy, S.; Brinkmann, T. Applicability of polyactive™ thin film composite membranes for CO2 separation from C2H4 containing multicomponent gas mixtures at pressures up to 30 bar. Membranes 2018, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Scholes, C.A.; Qader, A.; Stevens, G.W.; Kentish, S.E. Membrane pilot plant trials of CO2 separation from flue gas. Greenh. Gases Sci. Technol. 2015, 5, 229–237. [Google Scholar] [CrossRef]
- Scholes, C.A. Challenges for CO2 capture by membranes. In Advances Carbon Capture; Methods, Technologies and Applications; Woodhead Publishing Limited; Elsevier: Sawston, UK, 2020; pp. 357–377. [Google Scholar]
- Chiwaye, N.; Majozi, T.; Daramola, M.O. On optimisation of N2 and CO2-selective hybrid membrane process systems for post-combustion CO2 capture from coal-fired power plants. J. Membr. Sci. 2021, 638, 119691. [Google Scholar] [CrossRef]
- Kamble, A.R.; Patel, C.M.; Murthy, Z.V.P. A review on the recent advances in mixed matrix membranes for gas separation processes. Renew. Sust. Energ. Rev. 2021, 145, 111062. [Google Scholar] [CrossRef]
- Olabi, A.G.; Alami, A.H.; Ayoub, M.; Aljaghoub, H.; Alasad, S.; Inayat, A.; Abdelkareem, M.A.; Chae, K.-J.; Sayed, E.T. Membrane-based carbon capture: Recent progress, challenges, and their role in achieving the sustainable development goals. Chemosphere 2023, 320, 137996. [Google Scholar] [CrossRef]

| Technology | Mechanism | Advantages | Drawbacks | Maturity |
|---|---|---|---|---|
| Absorption | Physical or chemical absorption of CO2 into a liquid carrier (solvent); regeneration via increase in temperature or reduction in pressure | High capture efficiency (>90%); aqueous amine scrubbing (MEA) is currently the benchmark carbon capture technology | Large energy penalty, estimated at 20–30% of the power plant output; solvent regeneration and CO2 recovery contributing ~50%; equipment corrosion and removal/disposal of solvent | TRL 9 |
| Adsorption | Physical or chemical adsorption of CO2 using a solid sorbent; regeneration via increase in temperature or reduction in pressure | Lower regeneration energies compared to solvents due to lower heat capacities | Heat transfer, stability and attrition challenges | TRL 7–9 |
| Cryogenic separation | Used for gas streams with high CO2 concentration (>90%) | Liquid CO2 produced is ready for transportation | Energy intensive | TRL 9 |
| Membrane separation | Selective transportation and separation of CO2 through a membrane under the driving force of pressure difference | No hazardous chemicals storage, handling, disposal, or emissions issues; simple operation; reduced plant footprint; diminished need for modifications to the existing power plant steam cycle | Relatively low partial pressure of CO2 in the flue gas; use of low-cost and durable membranes; efficient permeability and selectivity; thermal, physical and chemical stability must be improved | TRL 6 |
| Membrane Material | Permeance a (mol·s−1·m−2·Pa−1) or Permeability b (mol·s−1·m−1·Pa−1) | CO2/N2 Selectivity | Reference |
|---|---|---|---|
| Cellulose acetate | 2.48 × 10−7 a | 40.17 | [84] |
| Polyimides-TMeCat | 6.30 × 10−10 b | 25 | [85] |
| Polyimides-TMMPD | 1.89 × 10−9 b | 17.1 | [86] |
| Polyimides-IMDDM | 6.17 × 10−10 b | 18.1 | [86] |
| Polysulfone-HFPSF-o-HBTMS | 3.31 × 10−10 b | 18.6 | [87] |
| Polysulfone-HFPSF-TMS | 3.47 × 10−10 b | 18 | [88] |
| Polysulfone-TMPSF-HBTMS | 2.27 × 10−10 b | 21.4 | [89] |
| Polycarbonates-TMHFPC | 3.50 × 10−10 b | 15 | [90] |
| Polycarbonates-FBPC | 4.76 × 10−11 b | 25.5 | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkotsis, P.; Peleka, E.; Zouboulis, A. Membrane-Based Technologies for Post-Combustion CO2 Capture from Flue Gases: Recent Progress in Commonly Employed Membrane Materials. Membranes 2023, 13, 898. https://doi.org/10.3390/membranes13120898
Gkotsis P, Peleka E, Zouboulis A. Membrane-Based Technologies for Post-Combustion CO2 Capture from Flue Gases: Recent Progress in Commonly Employed Membrane Materials. Membranes. 2023; 13(12):898. https://doi.org/10.3390/membranes13120898
Chicago/Turabian StyleGkotsis, Petros, Efrosini Peleka, and Anastasios Zouboulis. 2023. "Membrane-Based Technologies for Post-Combustion CO2 Capture from Flue Gases: Recent Progress in Commonly Employed Membrane Materials" Membranes 13, no. 12: 898. https://doi.org/10.3390/membranes13120898
APA StyleGkotsis, P., Peleka, E., & Zouboulis, A. (2023). Membrane-Based Technologies for Post-Combustion CO2 Capture from Flue Gases: Recent Progress in Commonly Employed Membrane Materials. Membranes, 13(12), 898. https://doi.org/10.3390/membranes13120898