Design Strategies for Forward Osmosis Membrane Substrates with Low Structural Parameters—A Review
Abstract
1. Introduction
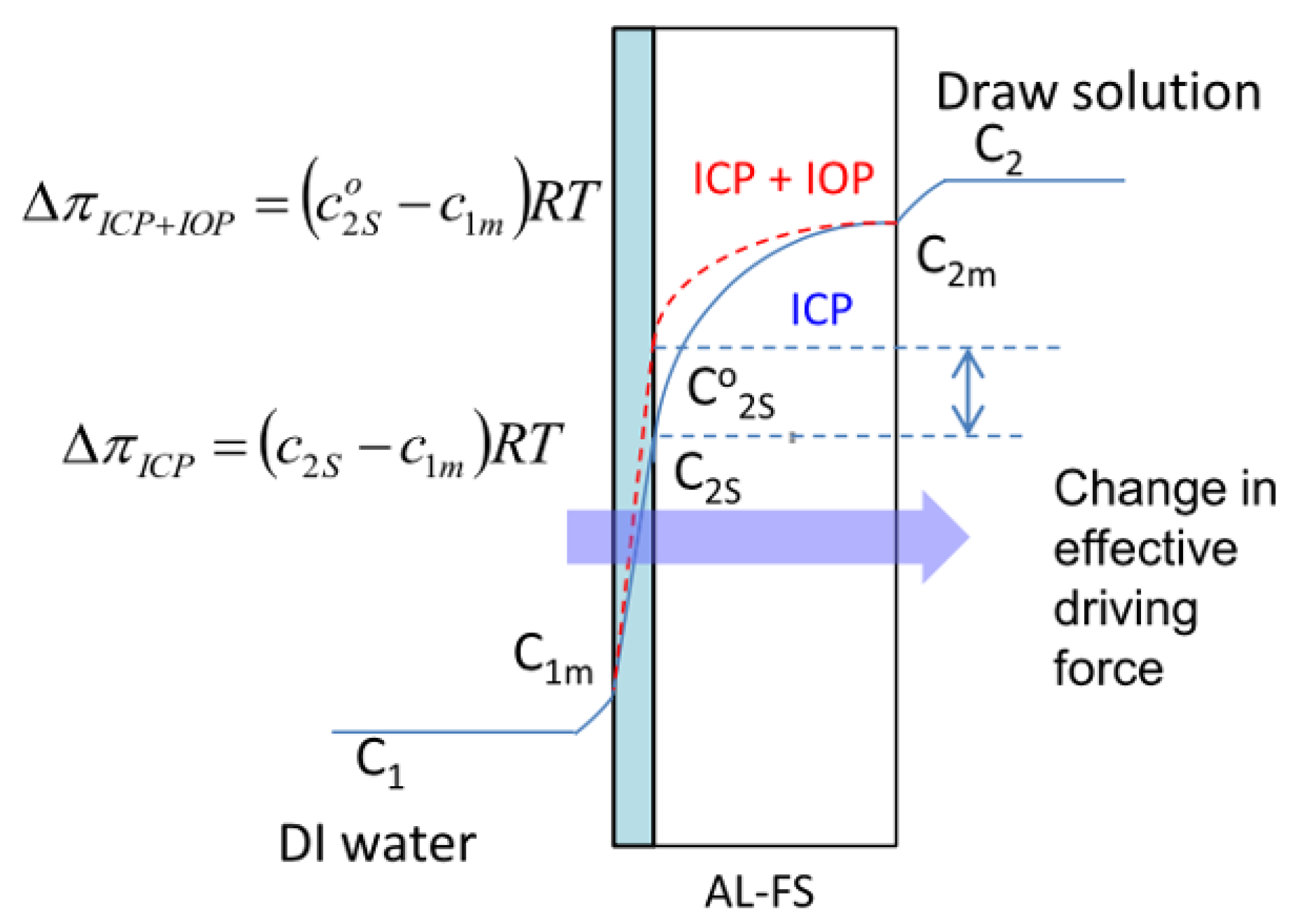
1.1. Structural Parameter (S) and Its Role in Reducing ICP Effects in FO Membranes
2. Methods for Measuring the Transport Parameters of FO Membrane
2.1. Transport-Based “Indirect” Techniques
2.1.1. RO-FO-Based Method
- (a)
- Pressurized RO-FO method
- (b)
- Ultra-low-pressure RO/FO-based method
2.1.2. FO-Based Methods for Determining Transport Properties of FO Membranes
2.2. Analytical Approach-Based “Direct” Techniques
2.2.1. Using Tortuosity Calculation Models
2.2.2. Using Mercury Intrusion Porosimetry and X-ray Microscopy
3. Modification Strategies of FO Membrane Substrates with S-Values
3.1. Electrospinning
3.1.1. Electrospinning without the Incorporation of Nanomaterials
| Support Layer Materials | FO Draw Solution | (LMH/bar) b | S-Value (μm) | References | ||
|---|---|---|---|---|---|---|
| PVDF/PVA | 0.5 M NaCl | 24.8 | 1.94 | #3 | 154 | [87] |
| PAN | 1.0 M NaCl | 50.7 | 3.23 | #2 | 86 | [86] |
| PAN | 2.0 M NaCl | 41.0 | 1.47 | #3 | 168 | [90] |
| PSf/PAN | 1.0 M NaCl | 38.3 c | 3.68 | #1 | 340 | [91] |
| PVDF | 1.0 M NaCl | 22.0 | 1.28 | #2 | 193 | [70] |
| PET/PSf | 1.0 M NaCl | 13.0 | 1.13 | #1 | 651 | [92] |
| Nylon 6, 6 | 1.0 M NaCl | 21.0 | 1.66 | #1 | 190 | [89] |
| PVDF | 1.0 M NaCl | 28.0 | 3.15 | #1 | 325 | [85] |
| PAN/CA | 1.5 M NaCl | 25.0 | 1.79 | #1 | 311 | [88] |
| CA/PVDF | 0.5 M NaCl | 31.3 | 2.79 | #3 | 190 | [93] |
3.1.2. Electrospinning with the Incorporation of Nanomaterials
3.2. Solvent Casting
3.2.1. Solvent Casting without the Incorporation of Nanomaterials
| Support Layer Materials | FO Draw Solution | (LMH/bar) b | Method to Calculate A | S-Value (μm) | References | |
|---|---|---|---|---|---|---|
| PSf/PSf-g-PDMA | 1.0 M NaCl | 16.4 d | 2.05 | #1 | 546 | [107] |
| SPSU/PVC | 1.0 M NaCl | 27.9 d | 2.80 c | #1 | 286 | [106] |
| PVDF/PFSA | 1.0 M NaCl | 27.0 d | 2.97 | #2 | 335 | [108] |
| PES/SPSU | 2.0 M NaCl | 26.0 | 0.77 | #1 | 238 | [101] |
| PSf/BPSH100-BPS0 f | 2.0 M NaCl | 40.9 | 1.57 c | #1 | 186 | [98] |
| PSf/SPPO | 2.0 M NaCl | 39.0 | 3.55 | #1 | 293 | [104] |
| PSf/SPEK | 2.0 M NaCl | 35.0 | 0.75 c | #1 | 107 | [105] |
| sPPSU | 2.0 M NaCl e | 17.5 | 3.70 c | #2 | 256 | [109] |
| PES/PESU-co-sPPSU | 2.0 M NaCl | 21.0 | 0.73 c | #1 | 324 | [110] |
| PES/SPES | 2.0 M NaCl | 35.1 | 2.90 c | #1 | 245 | [99] |
| PES/NaHCO3/PEG | 1.0 M NaCl | 26.6 | 2.13 | #1 | 257 | [111] |
3.2.2. Solvent Casting with the Incorporation of Nanomaterials
| Support Layer Materials | FO Draw Solution | (LMH/bar) b | S-Value (μm) | References | ||
|---|---|---|---|---|---|---|
| GO/PSf | 0.5 M NaCl | 19.8 | 1.76 | #1 | 191 | [97] |
| Al2O3/PSf | 1.0 M NaCl | 27.6 | 8.43 | #1 | 1028 | [117] |
| Zn2GeO4/PES | 1.0 M NaCl | 15.0 d | 2.47 c | #1 | 540 | [96] |
| INTs/PSf f | 1.0 M NaCl | 7.5 | 3.03 c | #1 | 2090 | [118] |
| TiO2/PSf | 2.0 M NaCl | 33.0 | 2.63 c | #1 | 390 | [113] |
| HNT/PSf g | 2.0 M NaCl e | 27.7 | 2.00 c | #1 | 370 | [114] |
| NaY(zeolite)/PSf | 2.0 M NaCl | 40.0 d | 3.30 | #1 | 340 | [112] |
| PSf/UiO-66 h | 1.0 M NaCl | 24.5 d | 3.31 c | #2 | 351 | [115] |
| GP/PSf i | 1.0 M NaCl | 15.6 | 3.12 | #1 | 711 | [119] |
| MWCNT/PES j | 2.0 M glucose e | 12.0 d,k | 2.31 c | #1 | 2042 | [95] |
3.3. Hollow Fiber FO Membrane Support

| Support Layer Materials | FO Draw Solution | (LMH/bar) c | S-Value (μm) | References | ||
|---|---|---|---|---|---|---|
| PAN | 1.0 M NaCl | 24.7 | 2.15 | #2 | 305 | [131] |
| CAB/PDA | 1.0 M NaCl | 37.0 b | 1.70 d | #2 | 250 | [129] |
| sPPSU | 0.5 M NaCl | 22.5 | 1.99 d | #2 | 163 | [127] |
| PES | 2.0 M NaCl | 32.1 | 1.18 d | #2 | 219 | [128] |
| PES | 1.0 M NaCl | 30.2 e | 2.26 | #3 | 190 | [132] |
| Polyketone | 0.5 M NaCl | 40.0 b,e | 1.20 | #3 | 250 | [133] |
| PPSU | 3.0 M NaCl | 13.5 g | 2.25 | #2 | 467 | [134] |
| GO/PES | 1.0 M NaCl | 43.7 b,e | 1.27 | #1 | 522 | [135] |
| P84 copolyimide f | 1.0 M NaCl | 22.0 e | 1.22 d | #2 | 232 | [136] |
| PEI | 1.0 M NaCl | 38.5 | 3.66 d | #2 | 172 | [137] |
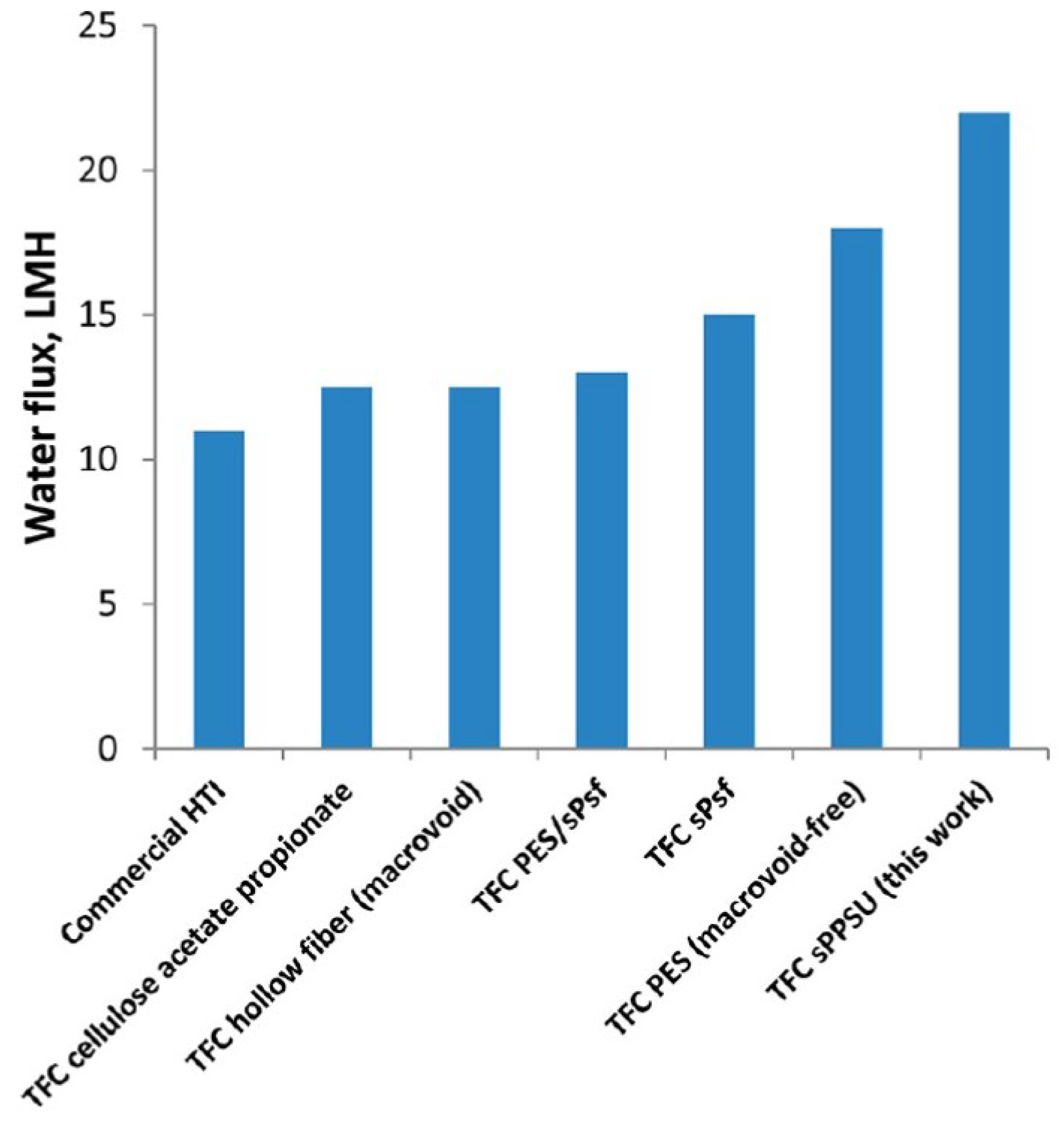
4. Commercial FO Membrane Substrates
5. Perspective and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| FO mode/AL-FS | Active layer faces feed solution |
| Al2O3 | Aluminum oxide nanoparticles |
| CNT | Carbon nanotubes |
| CA | Cellulose acetate |
| CAB | Cellulose acetate butyrate |
| P84 copolyimide | Copolyimide of 3,3′, 4,4′-benzophenone tetra-carboxylic dianhydride with 80% methylphenylene diamine and 20% methylene diamine |
| BPSH100-BPS0 | Disulfonated poly(arylene ether sulfone) multiblock copolymer |
| ECP | External concentration polarization |
| FO | Forward osmosis |
| f-CNT | Functionalized multi-walled carbon nanotube |
| GO | Graphene oxide |
| GP | Graphene oxide-graft-poly(2-hydroxy ethyl methacrylate) nanoparticles |
| HNT | Halloysite nanotubes |
| HTI | Hydration Technology Innovations |
| ICP | Internal concentration polarization |
| INTs | Imogolite nanotubes |
| MIP | Mercury intrusion porosimetry |
| Micro-XRM | Micro X-ray microscopy |
| MMcf | Million cubic feet |
| MPD | m-phenylene diamine |
| MWCNT | Multi-walled carbon nanotubes |
| NF | Nanofiltration |
| NMP | N-Methyl-2-pyrrolidone |
| DMF | N,N- dimethylformamide |
| OSRO | Osmotically assisted reverse osmosis |
| PAN | Polyacrylonitrile |
| PFSA | Perfluorosulfonic acid |
| PA-TFC | Polyamide thin-film composite |
| PEG | Polyethylene glycol |
| PET | Polyethylene terephthalate |
| PEI | Polyetherimide |
| PES | Polyethersulfone |
| PESU-co-sPPSU | Sulfonated copolymer of polyethersulfone and polyphenylsulfone |
| PDA | Polydopamine |
| PPSU | Poly(phenyl sulfone) |
| PSf | Polysulfone |
| PSf-g-PDMA | Polysulfone-graft-poly(2-dimethylaminoethyl methacrylate) |
| PVA | Polyvinyl alcohol |
| PVC | Polyvinyl chloride |
| PVDF | Polyvinylidene fluoride |
| PRO mode/AL-DS | Pressure retarded osmosis/Active layer faces draw solution |
| RO | Reverse osmosis |
| Salt permeability | |
| SEM | Scanning electron microscopy |
| SPEK | Sulfonated poly(ether ketone) |
| SPES | Sulfonated polyethersulfone |
| SPPO | Sulfonated poly(phenylene oxide) |
| sPPSU | Sulfonated polyphenylenesulfone |
| SPSU | Sulfonated polysulfone |
| -value | Structural parameter |
| TiO2 | Titanium dioxide |
| TDS | Total dissolved solids |
| TFC | Thin-film composite |
| TMC | Trimesoyl chloride |
| Water permeability | |
| NaY | Zeolite nanoparticles |
| Zn2GeO4 | Zinc germanate |
References
- Chang, H.; Li, T.; Liu, B.; Vidic, R.D.; Elimelech, M.; Crittenden, J.C. Potential and implemented membrane-based technologies for the treatment and reuse of flowback and produced water from shale gas and oil plays: A review. Desalination 2019, 455, 34–57. [Google Scholar] [CrossRef]
- Shaffer, D.L.; Chavez, L.H.A.; Ben-Sasson, M.; Castrillón, S.R.-V.; Yip, N.Y.; Elimelech, M. Desalination and Reuse of High-Salinity Shale Gas Produced Water: Drivers, Technologies, and Future Directions. Environ. Sci. Technol. 2013, 47, 9569–9583. [Google Scholar] [CrossRef] [PubMed]
- Asatekin, A.; Mayes, A.M. Oil Industry Wastewater Treatment with Fouling Resistant Membranes Containing Amphiphilic Comb Copolymers. Environ. Sci. Technol. 2009, 43, 4487–4492. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, B.R.; Reedy, R.C.; Xu, P.; Engle, M.; Nicot, J.P.; Yoxtheimer, D.; Yang, Q.; Ikonnikova, S. Can we beneficially reuse produced water from oil and gas extraction in the U.S.? Sci. Total Environ. 2020, 717, 137085. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.Z.; Wang, H.; Hu, L.; Zhang, Y.; Xu, P. Treatment of Produced Water in the Permian Basin for Hydraulic Fracturing: Comparison of Different Coagulation Processes and Innovative Filter Media. Water 2020, 12, 770. [Google Scholar] [CrossRef]
- Hanson, A.J.; Luek, J.L.; Tummings, S.S.; McLaughlin, M.; Blotevogel, J.; Mouser, P.J. High total dissolved solids in shale gas wastewater inhibit biodegradation of alkyl and nonylphenol ethoxylate surfactants. Sci. Total Environ. 2019, 668, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Horner, R.M.; Harto, C.B.; Jackson, R.B.; Lowry, E.R.; Brandt, A.R.; Yeskoo, T.W.; Murphy, D.J.; Clark, C.E. Water Use and Management in the Bakken Shale Oil Play in North Dakota. Environ. Sci. Technol. 2016, 50, 3275–3282. [Google Scholar] [CrossRef]
- Jiang, W.; Xu, X.; Hall, R.; Zhang, Y.; Carroll, K.C.; Ramos, F.; Engle, M.A.; Lin, L.; Wang, H.; Sayer, M.; et al. Characterization of produced water and surrounding surface water in the Permian Basin, the United States. J. Hazard. Mater. 2022, 430, 128409. [Google Scholar] [CrossRef]
- Rautenbach, R.; Linn, T.; Eilers, L. Treatment of severely contaminated waste water by a combination of RO, high-pressure RO and NF—Potential and limits of the process. J. Membr. Sci. 2000, 174, 231–241. [Google Scholar] [CrossRef]
- Qin, D.; Liu, Z.; Sun, D.D.; Song, X.; Bai, H. A new nanocomposite forward osmosis membrane custom-designed for treating shale gas wastewater. Sci. Rep. 2015, 5, 14530. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Al-Kaabi, M.A.; Ashfaq, M.Y.; Da’Na, D.A. Produced water characteristics, treatment and reuse: A review. J. Water Process Eng. 2019, 28, 222–239. [Google Scholar] [CrossRef]
- Zolghadr, E.; Firouzjaei, M.D.; Amouzandeh, G.; LeClair, P.; Elliott, M. The Role of Membrane-Based Technologies in Environmental Treatment and Reuse of Produced Water. Front. Environ. Sci. 2021, 9, 629767. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F.; Ng, B.C.; Abdullah, M.S. Recent Progresses of Forward Osmosis Membranes Formulation and Design for Wastewater Treatment. Water 2019, 11, 2043. [Google Scholar] [CrossRef]
- Bell, E.A.; Poynor, T.E.; Newhart, K.B.; Regnery, J.; Coday, B.D.; Cath, T.Y. Produced water treatment using forward osmosis membranes: Evaluation of extended-time performance and fouling. J. Membr. Sci. 2017, 525, 77–88. [Google Scholar] [CrossRef]
- Shaffer, D.L.; Werber, J.R.; Jaramillo, H.; Lin, S.; Elimelech, M. Forward osmosis: Where are we now? Desalination 2015, 356, 271–284. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; Elimelech, M. Influence of concentrative and dilutive internal concentration polarization on flux behavior in forward osmosis. J. Membr. Sci. 2006, 284, 237–247. [Google Scholar] [CrossRef]
- Chekli, L.; Phuntsho, S.; Shon, H.K.; Vigneswaran, S.; Kandasamy, J.; Chanan, A. A review of draw solutes in forward osmosis process and their use in modern applications. Desalin. Water Treat. 2012, 43, 167–184. [Google Scholar] [CrossRef]
- Johnson, D.J.; Suwaileh, W.A.; Mohammed, A.W.; Hilal, N. Osmotic’s potential: An overview of draw solutes for forward osmosis. Desalination 2018, 434, 100–120. [Google Scholar] [CrossRef]
- Long, Q.; Jia, Y.; Li, J.; Yang, J.; Liu, F.; Zheng, J.; Yu, B. Recent Advance on Draw Solutes Development in Forward Osmosis. Processes 2018, 6, 165. [Google Scholar] [CrossRef]
- Chaoui, I.; Abderafi, S.; Vaudreuil, S.; Bounahmidi, T. Water desalination by forward osmosis: Draw solutes and recovery methods—Review. Environ. Technol. Rev. 2019, 8, 25–46. [Google Scholar] [CrossRef]
- Dutta, S.; Nath, K. Prospect of ionic liquids and deep eutectic solvents as new generation draw solution in forward osmosis process. J. Water Process Eng. 2018, 21, 163–176. [Google Scholar] [CrossRef]
- Han, G.; Zhang, S.; Li, X.; Chung, T.-S. Progress in pressure retarded osmosis (PRO) membranes for osmotic power generation. Prog. Polym. Sci. 2015, 51, 1–27. [Google Scholar] [CrossRef]
- Haupt, A.; Marx, C.; Lerch, A. Modelling Forward Osmosis Treatment of Automobile Wastewaters. Membranes 2019, 9, 106. [Google Scholar] [CrossRef]
- Ibrar, I.; Naji, O.; Sharif, A.; Malekizadeh, A.; Alhawari, A.; Alanezi, A.A.; Altaee, A. A Review of Fouling Mechanisms, Control Strategies and Real-Time Fouling Monitoring Techniques in Forward Osmosis. Water 2019, 11, 695. [Google Scholar] [CrossRef]
- Suzaimi, N.D.; Goh, P.S.; Ismail, A.F.; Mamah, S.C.; Malek, N.A.N.N.; Lim, J.W.; Wong, K.C.; Hilal, N. Strategies in Forward Osmosis Membrane Substrate Fabrication and Modification: A Review. Membranes 2020, 10, 332. [Google Scholar] [CrossRef]
- Chun, Y.; Mulcahy, D.; Zou, L.; Kim, I.S. A Short Review of Membrane Fouling in Forward Osmosis Processes. Membranes 2017, 7, 30. [Google Scholar] [CrossRef]
- Zhao, Y.; Duan, L.; Liu, X.; Song, Y. Forward Osmosis Technology and Its Application on Microbial Fuel Cells: A Review. Membranes 2022, 12, 1254. [Google Scholar] [CrossRef]
- Han, G.; Cheng, Z.L.; Chung, T.-S. Thin-film composite (TFC) hollow fiber membrane with double-polyamide active layers for internal concentration polarization and fouling mitigation in osmotic processes. J. Membr. Sci. 2017, 523, 497–504. [Google Scholar] [CrossRef]
- Lu, X.; Chavez, L.H.A.; Castrillón, S.R.-V.; Ma, J.; Elimelech, M. Influence of Active Layer and Support Layer Surface Structures on Organic Fouling Propensity of Thin-Film Composite Forward Osmosis Membranes. Environ. Sci. Technol. 2015, 49, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; McCutcheon, J.R. Impact of support layer pore size on performance of thin film composite membranes for forward osmosis. J. Membr. Sci. 2015, 483, 25–33. [Google Scholar] [CrossRef]
- Alshwairekh, A.M.; Alghafis, A.A.; Usta, M.; Alwatban, A.M.; Krysko, R.; Oztekin, A. The Effect of Porous Support Layer in Forward Osmosis Membranes: A Computational Fluid Dynamics Simulation. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Proceedings (IMECE), Pittsburgh, PA, USA, 9–15 November 2018; Volume 7. [Google Scholar]
- Song, X.; Liu, Z.; Sun, D.D. Nano Gives the Answer: Breaking the Bottleneck of Internal Concentration Polarization with a Nanofiber Composite Forward Osmosis Membrane for a High Water Production Rate. Adv. Mater. 2011, 23, 3256–3260. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Lee, J.J.; Lee, S.; Kim, J.H. Determination of a constant membrane structure parameter in forward osmosis processes. J. Membr. Sci. 2011, 375, 241–248. [Google Scholar] [CrossRef]
- Tiraferri, A.; Yip, N.Y.; Phillip, W.A.; Schiffman, J.D.; Elimelech, M. Relating performance of thin-film composite forward osmosis membranes to support layer formation and structure. J. Membr. Sci. 2011, 367, 340–352. [Google Scholar] [CrossRef]
- Ren, J.; Chowdhury, M.R.; Xia, L.; Ma, C.; Bollas, G.M.; McCutcheon, J.R. A computational fluid dynamics model to predict performance of hollow fiber membrane modules in forward osmosis. J. Membr. Sci. 2020, 603, 117973. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; Elimelech, M. Influence of membrane support layer hydrophobicity on water flux in osmotically driven membrane processes. J. Membr. Sci. 2008, 318, 458–466. [Google Scholar] [CrossRef]
- Suwaileh, W.; Pathak, N.; Shon, H.; Hilal, N. Forward osmosis membranes and processes: A comprehensive review of research trends and future outlook. Desalination 2020, 485, 114455. [Google Scholar] [CrossRef]
- Jafarinejad, S. Forward osmosis membrane technology for nutrient removal/recovery from wastewater: Recent advances, proposed designs, and future directions. Chemosphere 2021, 263, 128116. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X. Forward osmosis technology for water treatment: Recent advances and future perspectives. J. Clean. Prod. 2021, 280, 124354. [Google Scholar] [CrossRef]
- Mohammadifakhr, M.; de Grooth, J.; Roesink, H.D.W.; Kemperman, A.J.B. Forward Osmosis: A Critical Review. Processes 2020, 8, 404. [Google Scholar] [CrossRef]
- Lee, D.-J.; Hsieh, M.-H. Forward osmosis membrane processes for wastewater bioremediation: Research needs. Bioresour. Technol. 2019, 290, 121795. [Google Scholar] [CrossRef]
- Manickam, S.S.; McCutcheon, J.R. Understanding mass transfer through asymmetric membranes during forward osmosis: A historical perspective and critical review on measuring structural parameter with semi-empirical models and characterization approaches. Desalination 2017, 421, 110–126. [Google Scholar] [CrossRef]
- Ang, W.L.; Mohammad, A.W.; Johnson, D.; Hilal, N. Unlocking the application potential of forward osmosis through integrated/hybrid process. Sci. Total Environ. 2020, 706, 136047. [Google Scholar] [CrossRef]
- Li, L.; Shi, W.; Yu, S. Research on Forward Osmosis Membrane Technology Still Needs Improvement in Water Recovery and Wastewater Treatment. Water 2019, 12, 107. [Google Scholar] [CrossRef]
- Sreedhar, I.; Khaitan, S.; Gupta, R.; Reddy, B.M.; Venugopal, A. An odyssey of process and engineering trends in forward osmosis. Environ. Sci. Water Res. Technol. 2018, 4, 129–168. [Google Scholar] [CrossRef]
- Ndiaye, I.; Vaudreuil, S.; Bounahmidi, T. Forward Osmosis Process: State-Of-The-Art of Membranes. Sep. Purif. Rev. 2021, 50, 53–73. [Google Scholar] [CrossRef]
- Francis, L.; Ogunbiyi, O.; Saththasivam, J.; Lawler, J.; Liu, Z. A comprehensive review of forward osmosis and niche applications. Environ. Sci. Water Res. Technol. 2020, 6, 1986–2015. [Google Scholar] [CrossRef]
- Zhan, M.; Kim, Y.; Hong, S. Recent developments in forward osmosis and its implication in expanding applications. In Osmosis Engineering; Elsevier: Amsterdam, The Netherlands, 2021; pp. 149–186. [Google Scholar] [CrossRef]
- Blandin, G.; Ferrari, F.; Lesage, G.; Le-Clech, P.; Héran, M.; Martinez-Lladó, X. Forward Osmosis as Concentration Process: Review of Opportunities and Challenges. Membranes 2020, 10, 284. [Google Scholar] [CrossRef]
- Yazdanabad, S.K.; Samimi, A.; Shokrollahzadeh, S.; Kalhori, D.M.; Moazami, N.; González, M.J.I.; Sobczuk, T.M.; Grima, E.M. Microalgae biomass dewatering by forward osmosis: Review and critical challenges. Algal Res. 2021, 56, 102323. [Google Scholar] [CrossRef]
- Wibisono, Y.; Nugroho, W.A.; Devianto, L.A.; Sulianto, A.A.; Bilad, M.R. Microalgae in Food-Energy-Water Nexus: A Review on Progress of Forward Osmosis Applications. Membranes 2019, 9, 166. [Google Scholar] [CrossRef]
- Das, P.; Singh, K.K.K.; Dutta, S. Insight into emerging applications of forward osmosis systems. J. Ind. Eng. Chem. 2019, 72, 1–17. [Google Scholar] [CrossRef]
- Haupt, A.; Lerch, A. Forward Osmosis Application in Manufacturing Industries: A Short Review. Membranes 2018, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Ding, C.; Zhu, T.; Wang, Y. A review on the forward osmosis applications and fouling control strategies for wastewater treatment. Front. Chem. Sci. Eng. 2022, 16, 661–680. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.-P.; Li, H.-Q. A review of forward osmosis membrane fouling: Types, research methods and future prospects. Environ. Technol. Rev. 2017, 6, 26–46. [Google Scholar] [CrossRef]
- Yadav, S.; Ibrar, I.; Bakly, S.; Khanafer, D.; Altaee, A.; Padmanaban, V.C.; Samal, A.K.; Hawari, A.H. Organic Fouling in Forward Osmosis: A Comprehensive Review. Water 2020, 12, 1505. [Google Scholar] [CrossRef]
- She, Q.; Wang, R.; Fane, A.G.; Tang, C.Y. Membrane fouling in osmotically driven membrane processes: A review. J. Membr. Sci. 2016, 499, 201–233. [Google Scholar] [CrossRef]
- Lee, W.J.; Ng, Z.C.; Hubadillah, S.K.; Goh, P.S.; Lau, W.J.; Othman, M.H.D.; Ismail, A.F.; Hilal, N. Fouling mitigation in forward osmosis and membrane distillation for desalination. Desalination 2020, 480, 114338. [Google Scholar] [CrossRef]
- Field, R.W.; She, Q.; Siddiqui, F.A.; Fane, A.G. Reverse osmosis and forward osmosis fouling: A comparison. Discov. Chem. Eng. 2021, 1, 6. [Google Scholar] [CrossRef]
- Singh, S.K.; Sharma, C.; Maiti, A. A comprehensive review of standalone and hybrid forward osmosis for water treatment: Membranes and recovery strategies of draw solutions. J. Environ. Chem. Eng. 2021, 9, 105473. [Google Scholar] [CrossRef]
- Cai, Y.; Hu, X.M. A critical review on draw solutes development for forward osmosis. Desalination 2016, 391, 16–29. [Google Scholar] [CrossRef]
- Aende, A.; Gardy, J.; Hassanpour, A. Seawater Desalination: A Review of Forward Osmosis Technique, Its Challenges, and Future Prospects. Processes 2020, 8, 901. [Google Scholar] [CrossRef]
- Zou, S.; Yuan, H.; Childress, A.; He, Z. Energy Consumption by Recirculation: A Missing Parameter When Evaluating Forward Osmosis. Environ. Sci. Technol. 2016, 50, 6827–6829. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.-S.; Li, X.; Ong, R.C.; Ge, Q.; Wang, H.; Han, G. Emerging forward osmosis (FO) technologies and challenges ahead for clean water and clean energy applications. Curr. Opin. Chem. Eng. 2012, 1, 246–257. [Google Scholar] [CrossRef]
- Nasr, P.; Sewilam, H. Forward osmosis: An alternative sustainable technology and potential applications in water industry. Clean Technol. Environ. Policy 2015, 17, 2079–2090. [Google Scholar] [CrossRef]
- Lugito, G.; Ariono, D.; Putra, M.R.T.; Zafra, Z.N. Progress, Challenges, and Prospects of Forward Osmosis (FO) and Pressure Retarded Osmosis (PRO) as An Alternative solution for Water and Energy Crisis. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1143, 012060. [Google Scholar] [CrossRef]
- Chekli, L.; Phuntsho, S.; Kim, J.E.; Kim, J.; Choi, J.Y.; Choi, J.-S.; Kim, S.; Kim, J.H.; Hong, S.; Sohn, J.; et al. A comprehensive review of hybrid forward osmosis systems: Performance, applications and future prospects. J. Membr. Sci. 2016, 497, 430–449. [Google Scholar] [CrossRef]
- Kahrizi, M.; Gonzales, R.R.; Kong, L.; Matsuyama, H.; Lu, P.; Lin, J.; Zhao, S. Significant roles of substrate properties in forward osmosis membrane performance: A review. Desalination 2022, 528, 115615. [Google Scholar] [CrossRef]
- Kim, B.; Gwak, G.; Hong, S. Review on methodology for determining forward osmosis (FO) membrane characteristics: Water permeability (A), solute permeability (B), and structural parameter (S). Desalination 2017, 422, 5–16. [Google Scholar] [CrossRef]
- Huang, L.; Arena, J.T.; McCutcheon, J.R. Surface modified PVDF nanofiber supported thin film composite membranes for forward osmosis. J. Membr. Sci. 2016, 499, 352–360. [Google Scholar] [CrossRef]
- Cath, T.Y.; Elimelech, M.; McCutcheon, J.R.; McGinnis, R.L.; Achilli, A.; Anastasio, D.; Brady, A.R.; Childress, A.E.; Farr, I.V.; Hancock, N.T.; et al. Standard Methodology for Evaluating Membrane Performance in Osmotically Driven Membrane Processes. Desalination 2013, 312, 31–38. [Google Scholar] [CrossRef]
- Tiraferri, A.; Yip, N.Y.; Straub, A.P.; Castrillon, S.R.-V.; Elimelech, M. A method for the simultaneous determination of transport and structural parameters of forward osmosis membranes. J. Membr. Sci. 2013, 444, 523–538. [Google Scholar] [CrossRef]
- Kim, W.-J.; Heldman, D.R. A mathematical estimation of the structural parameter for prediction of Forward Osmosis (FO) performance. J. Water Process Eng. 2021, 39, 101719. [Google Scholar] [CrossRef]
- Manickam, S.S.; Gelb, J.; McCutcheon, J.R. Pore structure characterization of asymmetric membranes: Non-destructive characterization of porosity and tortuosity. J. Membr. Sci. 2014, 454, 549–554. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology, 2nd ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1997. [Google Scholar]
- Baker, R.W. Membrane Technology and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Loeb, S.; Titelman, L.; Korngold, E.; Freiman, J. Effect of porous support fabric on osmosis through a Loeb-Sourirajan type asymmetric membrane. J. Membr. Sci. 1997, 129, 243–249. [Google Scholar] [CrossRef]
- Siefert, N.S.; Arena, J.; Mauter, M. Dewatering of High Salinity Brines by Osmotically Assisted Reverse Osmosis; ASME 2017 Power Conference; No. NETL-PUB-21273; National Energy Technology Laboratory (NETL): Pittsburgh, PA, USA; Morgantown, WV, USA, 2017. [Google Scholar]
- Arena, J.T.; Manickam, S.S.; Reimund, K.K.; Brodskiy, P.; McCutcheon, J.R. Characterization and Performance Relationships for a Commercial Thin Film Composite Membrane in Forward Osmosis Desalination and Pressure Retarded Osmosis. Ind. Eng. Chem. Res. 2015, 54, 11393–11403. [Google Scholar] [CrossRef]
- Kalla, S.; Piash, K.P.S.; Sanyal, O. Anti-fouling and anti-wetting membranes for membrane distillation. J. Water Process Eng. 2022, 46, 102634. [Google Scholar] [CrossRef]
- Reneker, D.H.; Chun, I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996, 7, 216–223. [Google Scholar] [CrossRef]
- Dzenis, Y. Spinning Continuous Fibers for Nanotechnology. Science 2004, 304, 1917–1919. [Google Scholar] [CrossRef]
- Huang, L.; Manickam, S.S.; McCutcheon, J.R. Increasing strength of electrospun nanofiber membranes for water filtration using solvent vapor. J. Membr. Sci. 2013, 436, 213–220. [Google Scholar] [CrossRef]
- Jayaraman, S.; Aravindan, V.; Kumar, P.S.; Ling, W.C.; Ramakrishna, S.; Madhavi, S. Synthesis of porous LiMn2O4 hollow nanofibers by electrospinning with extraordinary lithium storage properties. Chem. Commun. 2013, 49, 6677–6679. [Google Scholar] [CrossRef]
- Tian, M.; Qiu, C.; Liao, Y.; Chou, S.; Wang, R. Preparation of polyamide thin film composite forward osmosis membranes using electrospun polyvinylidene fluoride (PVDF) nanofibers as substrates. Sep. Purif. Technol. 2013, 118, 727–736. [Google Scholar] [CrossRef]
- Han, C.; Zhang, X.; Ding, C.; Xiong, S.; Yu, X.; Wang, Y. Improved performance of thin-film composite membrane supported by aligned nanofibers substrate with slit-shape pores for forward osmosis. J. Membr. Sci. 2020, 612, 118447. [Google Scholar] [CrossRef]
- Park, M.J.; Gonzales, R.R.; Abdel-Wahab, A.; Phuntsho, S.; Shon, H.K. Hydrophilic polyvinyl alcohol coating on hydrophobic electrospun nanofiber membrane for high performance thin film composite forward osmosis membrane. Desalination 2018, 426, 50–59. [Google Scholar] [CrossRef]
- Bui, N.-N.; McCutcheon, J.R. Hydrophilic Nanofibers as New Supports for Thin Film Composite Membranes for Engineered Osmosis. Environ. Sci. Technol. 2013, 47, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; McCutcheon, J.R. Hydrophilic nylon 6,6 nanofibers supported thin film composite membranes for engineered osmosis. J. Membr. Sci. 2014, 457, 162–169. [Google Scholar] [CrossRef]
- Pan, S.-F.; Dong, Y.; Zheng, Y.-M.; Zhong, L.-B.; Yuan, Z.-H. Self-sustained hydrophilic nanofiber thin film composite forward osmosis membranes: Preparation, characterization and application for simulated antibiotic wastewater treatment. J. Membr. Sci. 2017, 523, 205–215. [Google Scholar] [CrossRef]
- Shokrollahzadeh, S.; Tajik, S. Fabrication of thin film composite forward osmosis membrane using electrospun polysulfone/polyacrylonitrile blend nanofibers as porous substrate. Desalination 2018, 425, 68–76. [Google Scholar] [CrossRef]
- Hoover, L.A.; Schiffman, J.D.; Elimelech, M. Nanofibers in thin-film composite membrane support layers: Enabling expanded application of forward and pressure retarded osmosis. Desalination 2013, 308, 73–81. [Google Scholar] [CrossRef]
- Shibuya, M.; Park, M.J.; Lim, S.; Phuntsho, S.; Matsuyama, H.; Shon, H.K. Novel CA/PVDF nanofiber supports strategically designed via coaxial electrospinning for high performance thin-film composite forward osmosis membranes for desalination. Desalination 2018, 445, 63–74. [Google Scholar] [CrossRef]
- Tian, M.; Wang, Y.-N.; Wang, R. Synthesis and characterization of novel high-performance thin film nanocomposite (TFN) FO membranes with nanofibrous substrate reinforced by functionalized carbon nanotubes. Desalination 2015, 370, 79–86. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, R.; Ge, Q.; Wang, H.; Xu, T. Preparation of polyethersulfone/carbon nanotube substrate for high-performance forward osmosis membrane. Desalination 2013, 330, 70–78. [Google Scholar] [CrossRef]
- Low, Z.-X.; Liu, Q.; Shamsaei, E.; Zhang, X.; Wang, H. Preparation and Characterization of Thin-Film Composite Membrane with Nanowire-Modified Support for Forward Osmosis Process. Membranes 2015, 5, 136–149. [Google Scholar] [CrossRef]
- Park, M.J.; Phuntsho, S.; He, T.; Nisola, G.M.; Tijing, L.D.; Li, X.-M.; Chen, G.; Chung, W.-J.; Shon, H.K. Graphene oxide incorporated polysulfone substrate for the fabrication of flat-sheet thin-film composite forward osmosis membranes. J. Membr. Sci. 2015, 493, 496–507. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, J.; Ren, Z.; Shi, W.; Zhang, Z.; Xu, Y.; Gao, S.; Cui, F. High performance thin-film composite (TFC) forward osmosis (FO) membrane fabricated on novel hydrophilic disulfonated poly(arylene ether sulfone) multiblock copolymer/polysulfone substrate. J. Membr. Sci. 2016, 520, 529–539. [Google Scholar] [CrossRef]
- Sahebi, S.; Phuntsho, S.; Woo, Y.C.; Park, M.J.; Tijing, L.D.; Hong, S.; Shon, H.K. Effect of sulphonated polyethersulfone substrate for thin film composite forward osmosis membrane. Desalination 2016, 389, 129–136. [Google Scholar] [CrossRef]
- Yip, N.Y.; Tiraferri, A.; Phillip, W.A.; Schiffman, J.D.; Elimelech, M. High Performance Thin-Film Composite Forward Osmosis Membrane. Environ. Sci. Technol. 2010, 44, 3812–3818. [Google Scholar] [CrossRef]
- Wang, K.Y.; Chung, T.-S.; Amy, G. Developing thin-film-composite forward osmosis membranes on the PES/SPSf substrate through interfacial polymerization. AIChE J. 2012, 58, 770–781. [Google Scholar] [CrossRef]
- Han, G.; Zhang, S.; Li, X.; Widjojo, N.; Chung, T.-S. Thin film composite forward osmosis membranes based on polydopamine modified polysulfone substrates with enhancements in both water flux and salt rejection. Chem. Eng. Sci. 2012, 80, 219–231. [Google Scholar] [CrossRef]
- Zahid, M.; Rashid, A.; Akram, S.; Rehan, Z.A.; Razzaq, W. A Comprehensive Review on Polymeric Nano-Composite Membranes for Water Treatment. J. Membr. Sci. Technol. 2018, 8, 1–20. [Google Scholar] [CrossRef]
- Zhou, Z.; Lee, J.Y.; Chung, T.-S. Thin film composite forward-osmosis membranes with enhanced internal osmotic pressure for internal concentration polarization reduction. Chem. Eng. J. 2014, 249, 236–245. [Google Scholar] [CrossRef]
- Han, G.; Chung, T.-S.; Toriida, M.; Tamai, S. –Thin-film composite forward osmosis membranes with novel hydrophilic supports for desalination. J. Membr. Sci. 2012, 423–424, 543–555. [Google Scholar] [CrossRef]
- Zheng, K.; Zhou, S.; Zhou, X. A low-cost and high-performance thin-film composite forward osmosis membrane based on an SPSU/PVC substrate. Sci. Rep. 2018, 8, 10022. [Google Scholar] [CrossRef] [PubMed]
- Salehi, H.; Shakeri, A.; Mahdavi, H.; Lammertink, R.G.H. Improved performance of thin-film composite forward osmosis membrane with click modified polysulfone substrate. Desalination 2020, 496, 114731. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, L.; Lang, W.-Z.; Wang, Y. Improved performance of thin-film composite membrane with PVDF/PFSA substrate for forward osmosis process. J. Membr. Sci. 2017, 535, 188–199. [Google Scholar] [CrossRef]
- Han, G.; Zhao, B.; Fu, F.; Chung, T.-S.; Weber, M.; Staudt, C.; Maletzko, C. High performance thin-film composite membranes with mesh-reinforced hydrophilic sulfonated polyphenylenesulfone (sPPSU) substrates for osmotically driven processes. J. Membr. Sci. 2016, 502, 84–93. [Google Scholar] [CrossRef]
- Widjojo, N.; Chung, T.-S.; Weber, M.; Maletzko, C.; Warzelhan, V. The role of sulphonated polymer and macrovoid-free structure in the support layer for thin-film composite (TFC) forward osmosis (FO) membranes. J. Membr. Sci. 2011, 383, 214–223. [Google Scholar] [CrossRef]
- Shakeri, A.; Babaheydari, S.M.M.; Salehi, H.; Razavi, S.R. Reduction of the Structure Parameter of Forward Osmosis Membranes by Using Sodium Bicarbonate as Pore-Forming Agent. Langmuir 2021, 37, 7591–7599. [Google Scholar] [CrossRef]
- Ma, N.; Wei, J.; Qi, S.; Zhao, Y.; Gao, Y.; Tang, C.Y. Nanocomposite substrates for controlling internal concentration polarization in forward osmosis membranes. J. Membr. Sci. 2013, 441, 54–62. [Google Scholar] [CrossRef]
- Emadzadeh, D.; Lau, W.J.; Matsuura, T.; Ismail, A.F.; Rahbari-Sisakht, M. Synthesis and characterization of thin film nanocomposite forward osmosis membrane with hydrophilic nanocomposite support to reduce internal concentration polarization. J. Membr. Sci. 2014, 449, 74–85. [Google Scholar] [CrossRef]
- Ghanbari, M.; Emadzadeh, D.; Lau, W.J.; Riazi, H.; Almasi, D.; Ismail, A.F. Minimizing structural parameter of thin film composite forward osmosis membranes using polysulfone/halloysite nanotubes as membrane substrates. Desalination 2016, 377, 152–162. [Google Scholar] [CrossRef]
- Ma, D.; Han, G.; Peh, S.B.; Chen, S.B. Water-Stable Metal–Organic Framework UiO-66 for Performance Enhancement of Forward Osmosis Membranes. Ind. Eng. Chem. Res. 2017, 56, 12773–12782. [Google Scholar] [CrossRef]
- Ma, D.; Peh, S.B.; Han, G.; Chen, S.B. Thin-Film Nanocomposite (TFN) Membranes Incorporated with Super-Hydrophilic Metal–Organic Framework (MOF) UiO-66: Toward Enhancement of Water Flux and Salt Rejection. ACS Appl. Mater. Interfaces 2017, 9, 7523–7534. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Li, Y.; Bao, M.; Zhang, J.; Zhang, C.; Lu, J. Highly permeable and stable forward osmosis (FO) membrane based on the incorporation of Al2O3 nanoparticles into both substrate and polyamide active layer. RSC Adv. 2017, 7, 40311–40320. [Google Scholar] [CrossRef]
- Pan, Y.-H.; Zhao, Q.-Y.; Gu, L.; Wu, Q.-Y. Thin film nanocomposite membranes based on imologite nanotubes blended substrates for forward osmosis desalination. Desalination 2017, 421, 160–168. [Google Scholar] [CrossRef]
- Razavi, S.R.; Shakeri, A.; Mahdavi, H. Polymer-Grafted Graphene Oxide as a High-Performance Nanofiller for Modification of Forward Osmosis Membrane Substrates. ACS Appl. Polym. Mater. 2022, 4, 8878–8891. [Google Scholar] [CrossRef]
- Peng, N.; Widjojo, N.; Sukitpaneenit, P.; Teoh, M.M.; Lipscomb, G.G.; Chung, T.-S.; Lai, J.-Y. Evolution of polymeric hollow fibers as sustainable technologies: Past, present, and future. Prog. Polym. Sci. 2012, 37, 1401–1424. [Google Scholar] [CrossRef]
- Wang, K.Y.; Chung, T.-S.; Qin, J.-J. Polybenzimidazole (PBI) nanofiltration hollow fiber membranes applied in forward osmosis process. J. Membr. Sci. 2007, 300, 6–12. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, K.Y.; Chung, N.T.-S. Dual-Layer Hollow Fibers with Enhanced Flux As Novel Forward Osmosis Membranes for Water Production. Environ. Sci. Technol. 2009, 43, 2800–2805. [Google Scholar] [CrossRef]
- Chou, S.; Shi, L.; Wang, R.; Tang, C.Y.; Qiu, C.; Fane, A.G. Characteristics and potential applications of a novel forward osmosis hollow fiber membrane. Desalination 2010, 261, 365–372. [Google Scholar] [CrossRef]
- Setiawan, L.; Wang, R.; Li, K.; Fane, A.G. Fabrication of novel poly(amide–imide) forward osmosis hollow fiber membranes with a positively charged nanofiltration-like selective layer. J. Membr. Sci. 2011, 369, 196–205. [Google Scholar] [CrossRef]
- Naim, R.; Sean, G.P.; Nasir, Z.; Mokhtar, N.M.; Muhammad, N.A.S. Recent Progress and Challenges in Hollow Fiber Membranes for Wastewater Treatment and Resource Recovery. Membranes 2021, 11, 839. [Google Scholar] [CrossRef]
- Kůdelová, T.; Bartuli, E.; Strunga, A.; Hvožďa, J.; Dohnal, M. Fully Polymeric Distillation Unit Based on Polypropylene Hollow Fibers. Polymers 2021, 13, 1031. [Google Scholar] [CrossRef] [PubMed]
- Zhong, P.; Fu, X.; Chung, N.T.-S.; Weber, M.; Maletzko, C. Development of Thin-Film Composite forward Osmosis Hollow Fiber Membranes Using Direct Sulfonated Polyphenylenesulfone (sPPSU) as Membrane Substrates. Environ. Sci. Technol. 2013, 47, 7430–7436. [Google Scholar] [CrossRef] [PubMed]
- Sukitpaneenit, P.; Chung, T.-S. High Performance Thin-Film Composite Forward Osmosis Hollow Fiber Membranes with Macrovoid-Free and Highly Porous Structure for Sustainable Water Production. Environ. Sci. Technol. 2012, 46, 7358–7365. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; de Wit, J.S.; Chung, T.-S. Water reclamation from emulsified oily wastewater via effective forward osmosis hollow fiber membranes under the PRO mode. Water Res. 2015, 81, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Qiu, W.; Miller, S.J.; Koros, W.J. Plasticization-resistant hollow fiber membranes for CO2/CH4 separation based on a thermally crosslinkable polyimide. J. Membr. Sci. 2011, 382, 212–221. [Google Scholar] [CrossRef]
- Ren, J.; McCutcheon, J.R. Polyacrylonitrile supported thin film composite hollow fiber membranes for forward osmosis. Desalination 2015, 372, 67–74. [Google Scholar] [CrossRef]
- Lim, S.; Tran, V.H.; Akther, N.; Phuntsho, S.; Shon, H.K. Defect-free outer-selective hollow fiber thin-film composite membranes for forward osmosis applications. J. Membr. Sci. 2019, 586, 281–291. [Google Scholar] [CrossRef]
- Shibuya, M.; Yasukawa, M.; Mishima, S.; Tanaka, Y.; Takahashi, T.; Matsuyama, H. A thin-film composite-hollow fiber forward osmosis membrane with a polyketone hollow fiber membrane as a support. Desalination 2017, 402, 33–41. [Google Scholar] [CrossRef]
- Jaafer, M.J.; Al-Najar, J.A.; Alsalhy, Q.F. Poly(phenyl sulfone) hollow fiber forward osmosis membrane for saline water desalination. Chem. Eng. Process.—Process Intensif. 2020, 157, 108119. [Google Scholar] [CrossRef]
- Park, M.J.; Lim, S.; Gonzales, R.R.; Phuntsho, S.; Han, D.S.; Abdel-Wahab, A.; Adham, S.; Shon, H.K. Thin-film composite hollow fiber membranes incorporated with graphene oxide in polyethersulfone support layers for enhanced osmotic power density. Desalination 2019, 464, 63–75. [Google Scholar] [CrossRef]
- Li, X.; Ang, W.L.; Liu, Y.; Chung, T.-S. Engineering design of outer-selective tribore hollow fiber membranes for forward osmosis and oil-water separation. AIChE J. 2015, 61, 4491–4501. [Google Scholar] [CrossRef]
- Li, X.; Loh, C.H.; Wang, R.; Widjajanti, W.; Torres, J. Fabrication of a robust high-performance FO membrane by optimizing substrate structure and incorporating aquaporin into selective layer. J. Membr. Sci. 2017, 525, 257–268. [Google Scholar] [CrossRef]
- Ren, J.; McCutcheon, J.R. A new commercial thin film composite membrane for forward osmosis. Desalination 2014, 343, 187–193. [Google Scholar] [CrossRef]
- Xia, L.; Andersen, M.F.; Hélix-Nielsen, C.; McCutcheon, J.R. Novel Commercial Aquaporin Flat-Sheet Membrane for Forward Osmosis. Ind. Eng. Chem. Res. 2017, 56, 11919–11925. [Google Scholar] [CrossRef]
- Ren, J.; McCutcheon, J.R. A new commercial biomimetic hollow fiber membrane for forward osmosis. Desalination 2018, 442, 44–50. [Google Scholar] [CrossRef]
- Tian, M.; Wang, Y.-N.; Wang, R.; Fane, A.G. Synthesis and characterization of thin film nanocomposite forward osmosis membranes supported by silica nanoparticle incorporated nanofibrous substrate. Desalination 2017, 401, 142–150. [Google Scholar] [CrossRef]
- Phillip, W.A.; Yong, J.S.; Elimelech, M. Reverse Draw Solute Permeation in Forward Osmosis: Modeling and Experiments. Environ. Sci. Technol. 2010, 44, 5170–5176. [Google Scholar] [CrossRef]
- Wei, J.; Qiu, C.; Tang, C.Y.; Wang, R.; Fane, A.G. Synthesis and characterization of flat-sheet thin film composite forward osmosis membranes. J. Membr. Sci. 2011, 372, 292–302. [Google Scholar] [CrossRef]
- Coday, B.D.; Heil, D.M.; Xu, P.; Cath, T.Y. Effects of Transmembrane Hydraulic Pressure on Performance of Forward Osmosis Membranes. Environ. Sci. Technol. 2013, 47, 2386–2393. [Google Scholar] [CrossRef]
- Rodriguez, K.M.; Wu, W.-N.; Alebrahim, T.; Cao, Y.; Freeman, B.D.; Harrigan, D.; Jhalaria, M.; Kratochvil, A.; Kumar, S.; Lee, W.H.; et al. Multi-lab study on the pure-gas permeation of commercial polysulfone (PSf) membranes: Measurement standards and best practices. J. Membr. Sci. 2022, 659, 120746. [Google Scholar] [CrossRef]
- Chen, C.; Huang, X.; Prakash, P.; Chilekar, S.; Franks, R. Produced water desalination using high temperature membranes. Desalination 2021, 513, 115144. [Google Scholar] [CrossRef]





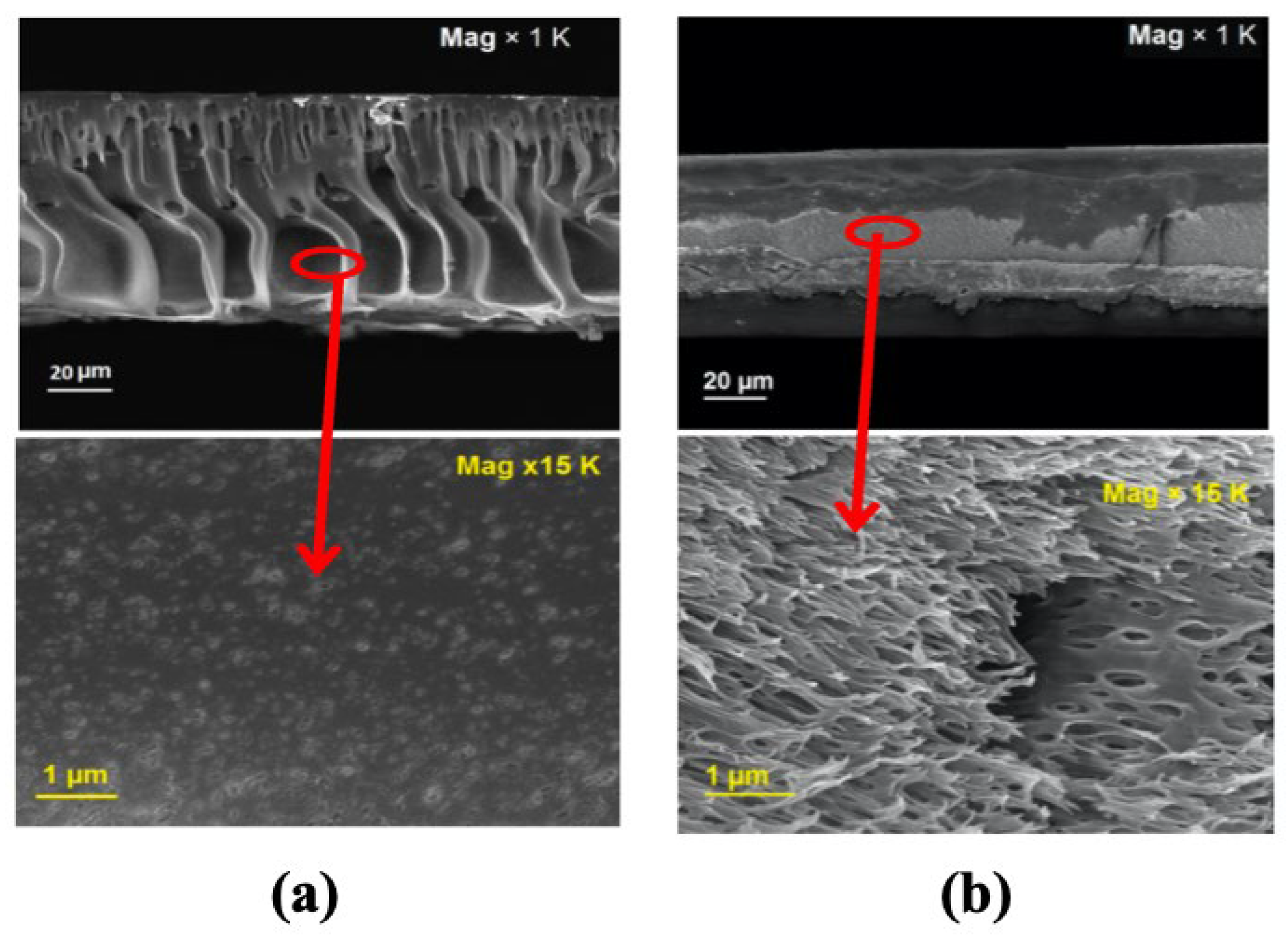



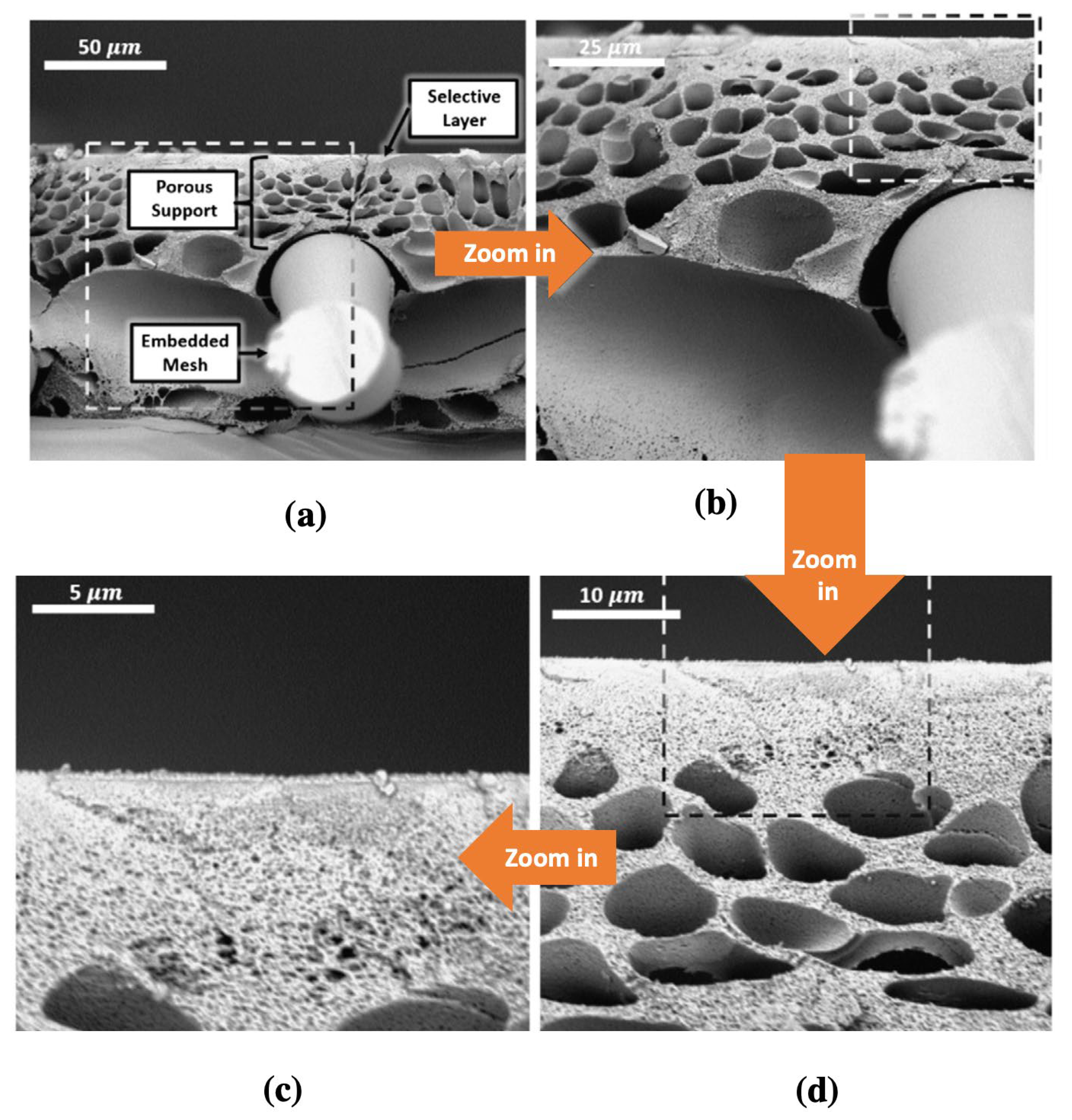
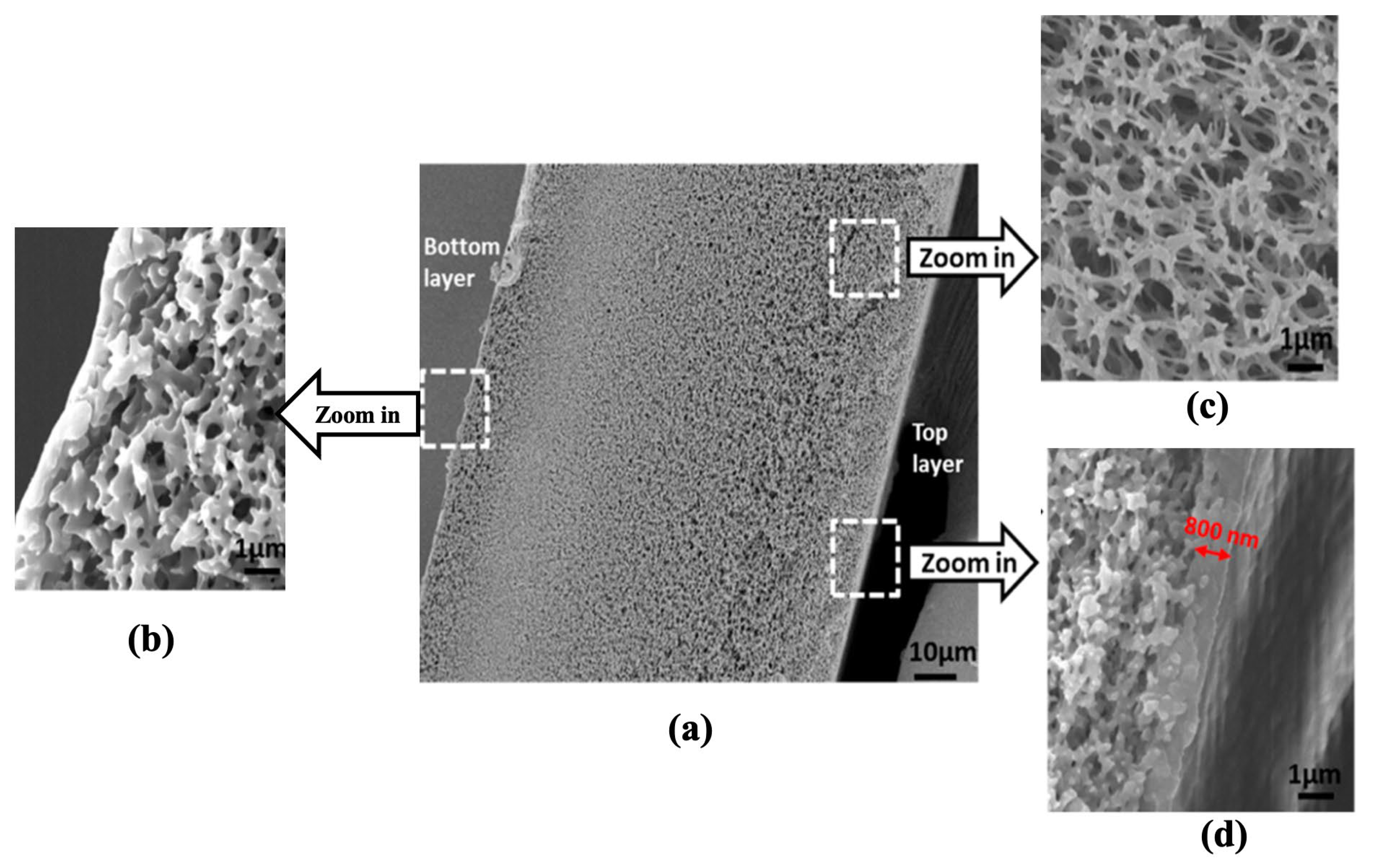
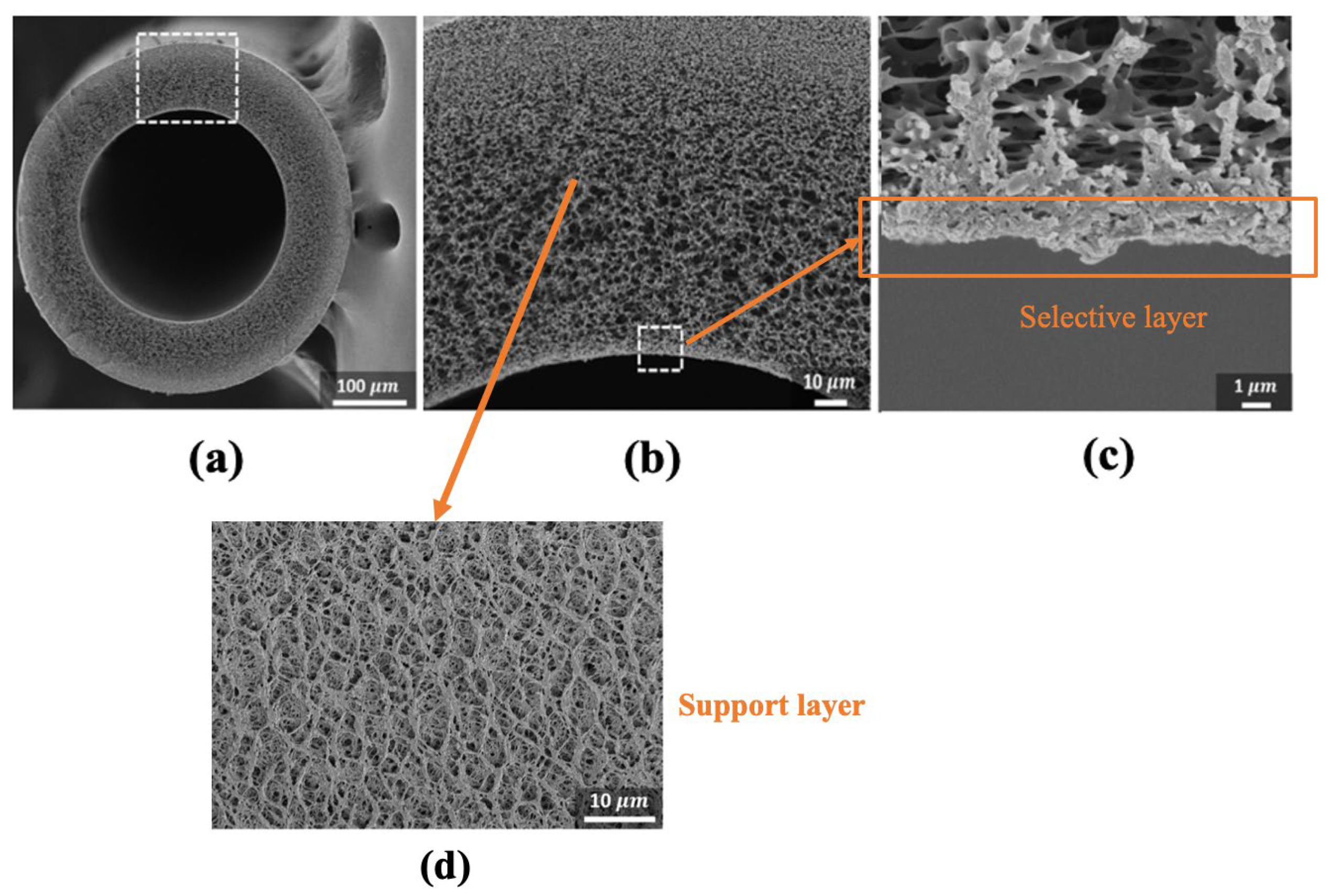
| (a) | ||
| Experimental Conditions | Value | Additional Notes |
| Feed and DS temperature | 20 °C | |
| DS concentration | 1.0 M NaCl | 58.44 g/L NaCl |
| Feed concentration | DI Water | |
| Feed and DS cross flow velocity | 0.25 m/s | Feed and draw solutions’ flow rate defined by multiplying flow velocity with a cross sectional area of the flow channel perpendicular to flow direction |
| (b) | ||
| Experimental Conditions | Value | Additional Notes |
| Feed temperature | 20 °C | |
| Feed pressure | 8.62 (125) bar (psi) |
|
| Feed concentration | DI Water 2000 mg/L NaCl |
|
| Cross flow velocity | 0.25 m/s |
|
| Membrane | (LMH/bar) | S-Value (μm) | References | |
|---|---|---|---|---|
| CTA-W a | 0.34 h | 950 | #1 | [141] |
| HTI-CTA a | 0.62 | 690 | #3 | [87] |
| HTI-CTA a | 0.44 | 481 | #1 | [142] |
| HTI-CTA a | 0.68 | 578 | #1 | [88] |
| HTI-CTA a | 0.36 | 595 | #1 | [100] |
| CTA-HW a | 1.19 h | 720 | #1 | [143] |
| HTI-CTA a | 0.55 | 463 | #1 | [144] |
| TFC-HTI b | 1.63 | 690 | #1 | [144] |
| TFC-Oasys d | 4.72 | 365 | #1 | [144] |
| HTI-M c | 0.64 h | 3074 i | #1 | [95] |
| TFC-Oasys d | 4.25 | 483 | #1 | [79] |
| Aquaporin FO e | 0.52 | 630 | #1 | [139] |
| Aquaporin f | 0.43 | 210 | #3 | [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piash, K.S.; Sanyal, O. Design Strategies for Forward Osmosis Membrane Substrates with Low Structural Parameters—A Review. Membranes 2023, 13, 73. https://doi.org/10.3390/membranes13010073
Piash KS, Sanyal O. Design Strategies for Forward Osmosis Membrane Substrates with Low Structural Parameters—A Review. Membranes. 2023; 13(1):73. https://doi.org/10.3390/membranes13010073
Chicago/Turabian StylePiash, KmProttoy Shariar, and Oishi Sanyal. 2023. "Design Strategies for Forward Osmosis Membrane Substrates with Low Structural Parameters—A Review" Membranes 13, no. 1: 73. https://doi.org/10.3390/membranes13010073
APA StylePiash, K. S., & Sanyal, O. (2023). Design Strategies for Forward Osmosis Membrane Substrates with Low Structural Parameters—A Review. Membranes, 13(1), 73. https://doi.org/10.3390/membranes13010073








