Investigation on Human Serum Protein Depositions Inside Polyvinylidene Fluoride-Based Dialysis Membrane Layers Using Synchrotron Radiation Micro-Computed Tomography (SR-μCT)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
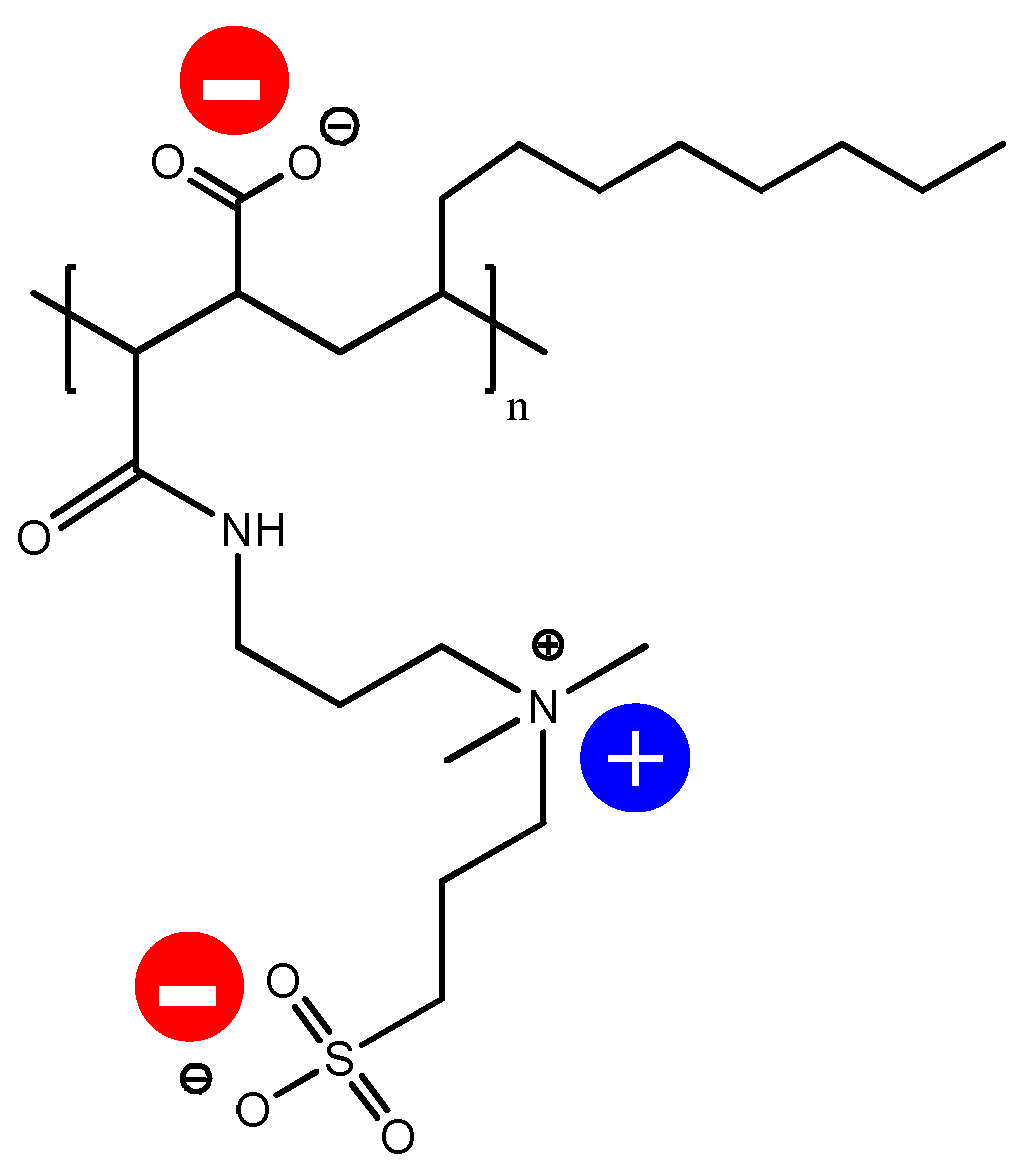
2.2. Membrane Surface Modification
2.3. Human Serum Protein Solution Preparation
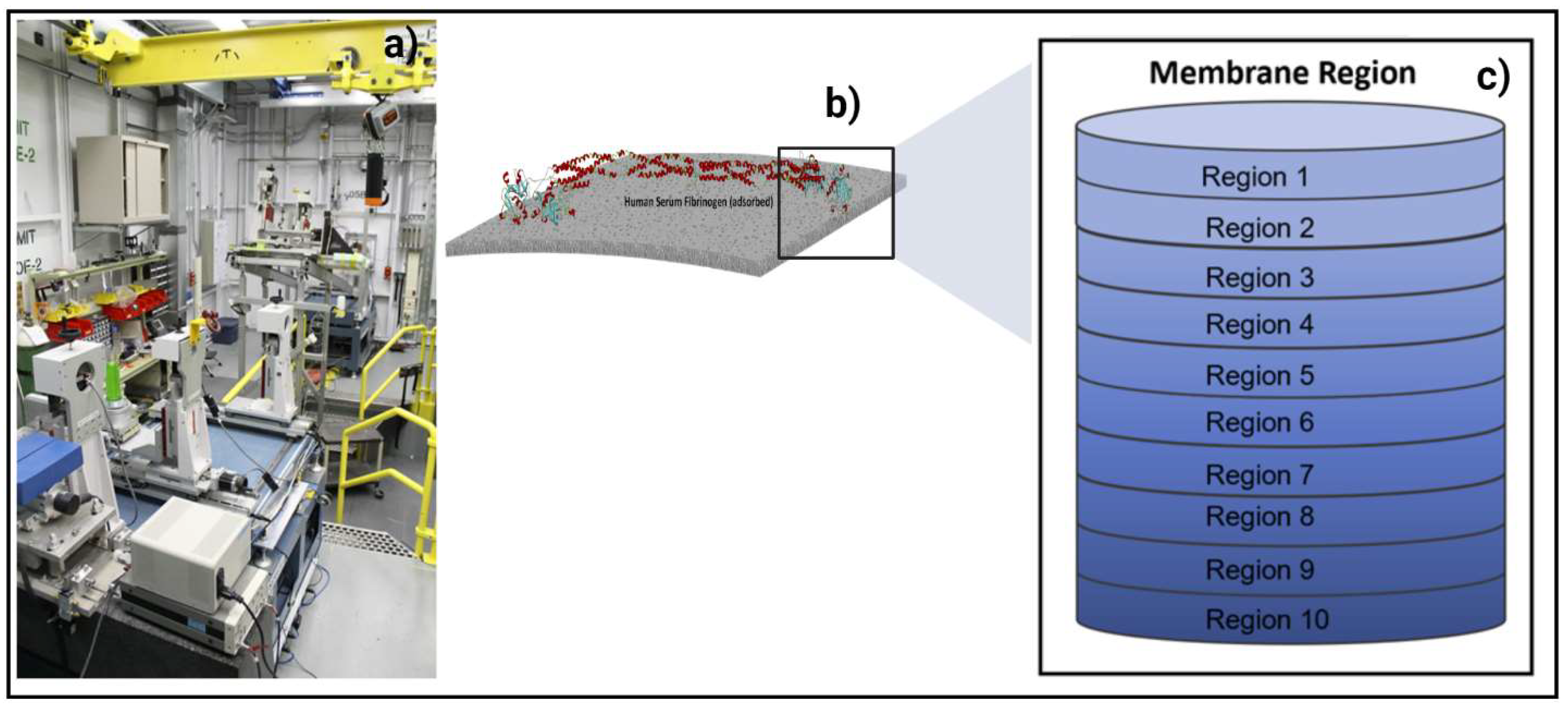
2.4. In Situ Synchrotron Advanced Imaging Techniques at BioMedical Imaging and Therapy (BMIT) Beamline
2.5. Analytical Techniques Used for Membrane Characterization
2.5.1. Fourier Transform Infrared Spectroscopy ATR-FTIR Analyses
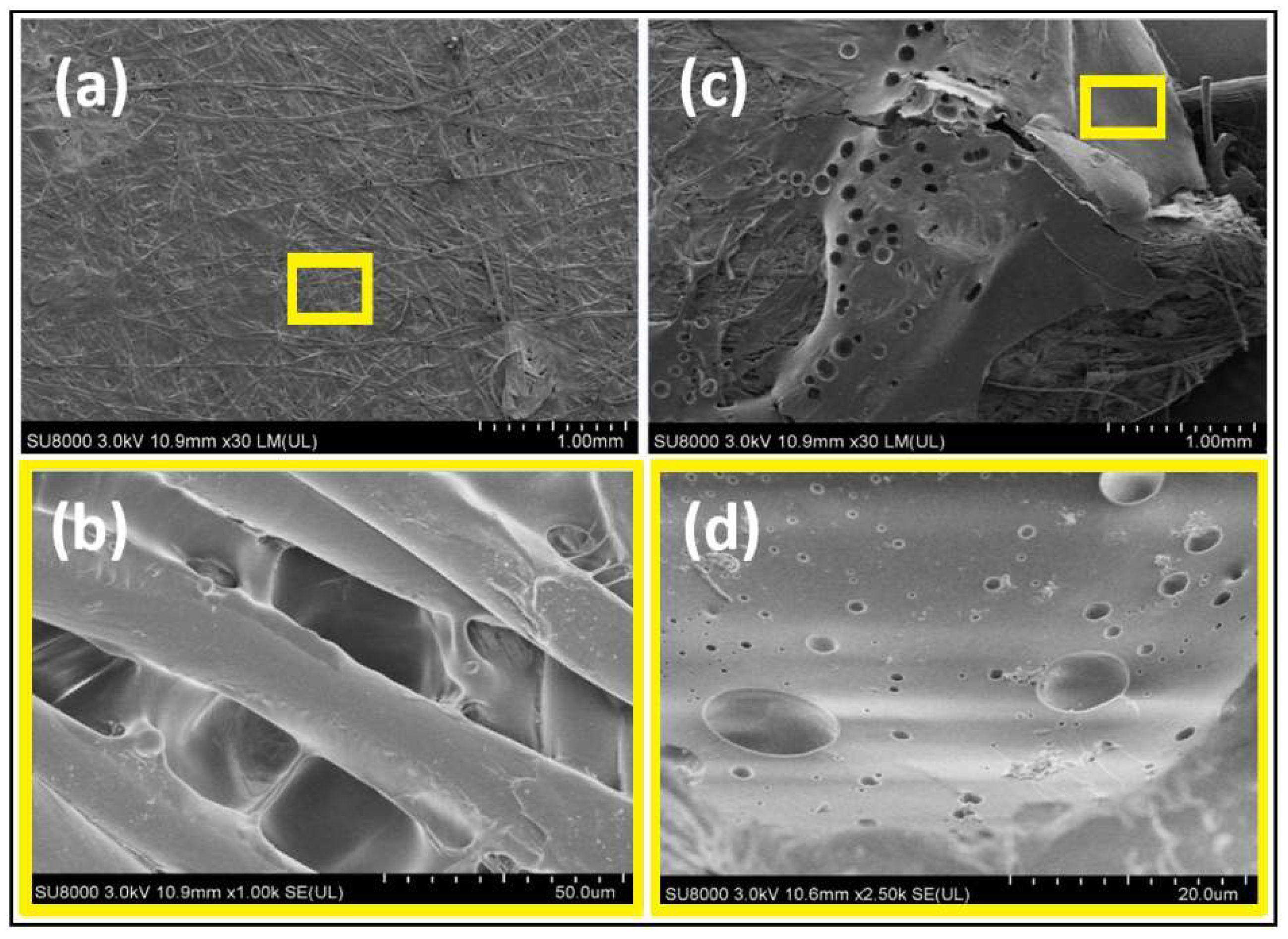
2.5.2. Scanning Electron Microscope (SEM)
2.5.3. Zeta Potential Analyzer
3. Results and Discussion
3.1. Zwitterionic-Polymer-Modified and Unmodified PVDF Membrane Characteristics
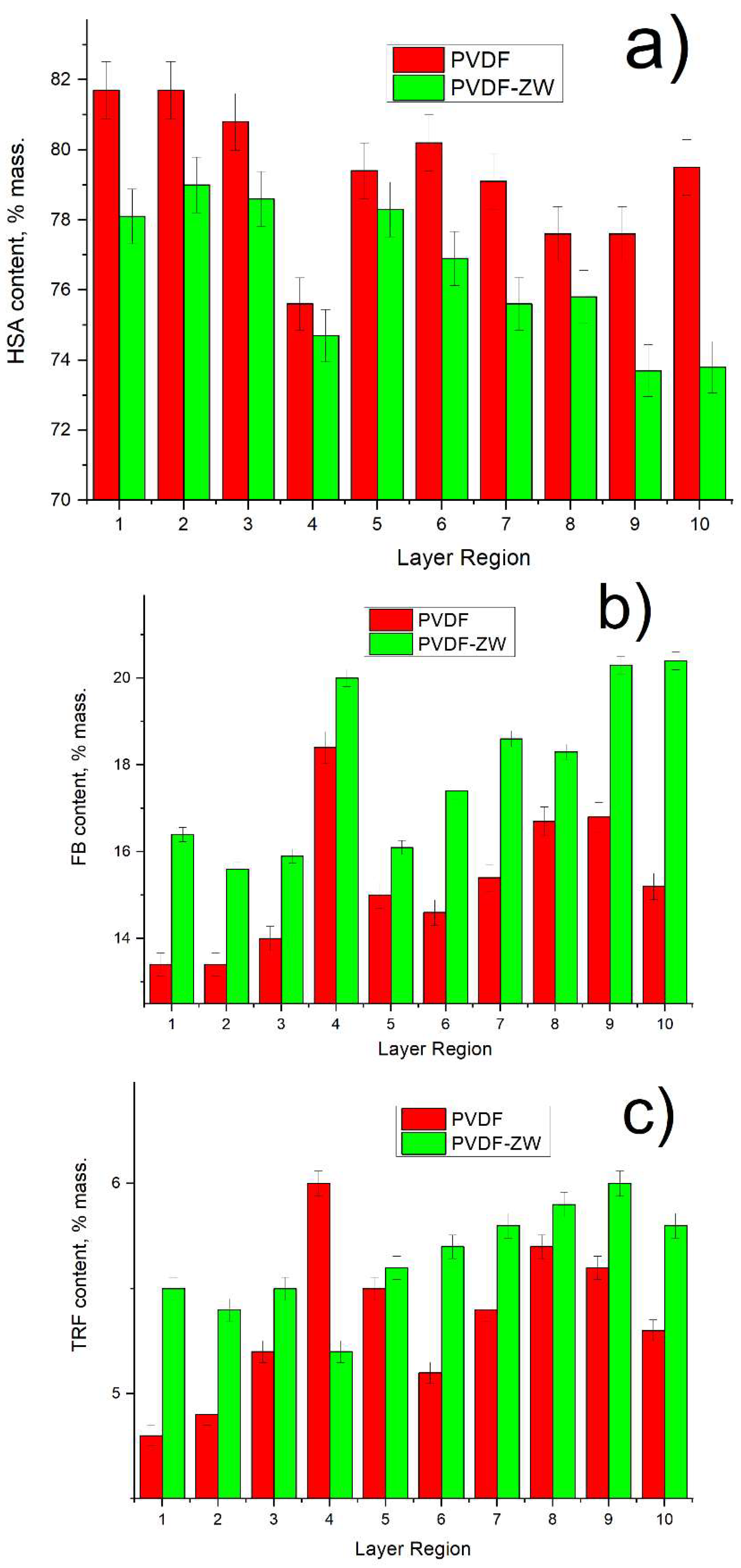
3.2. Human Serum Protein Adsorption from Protein Mixture Solution
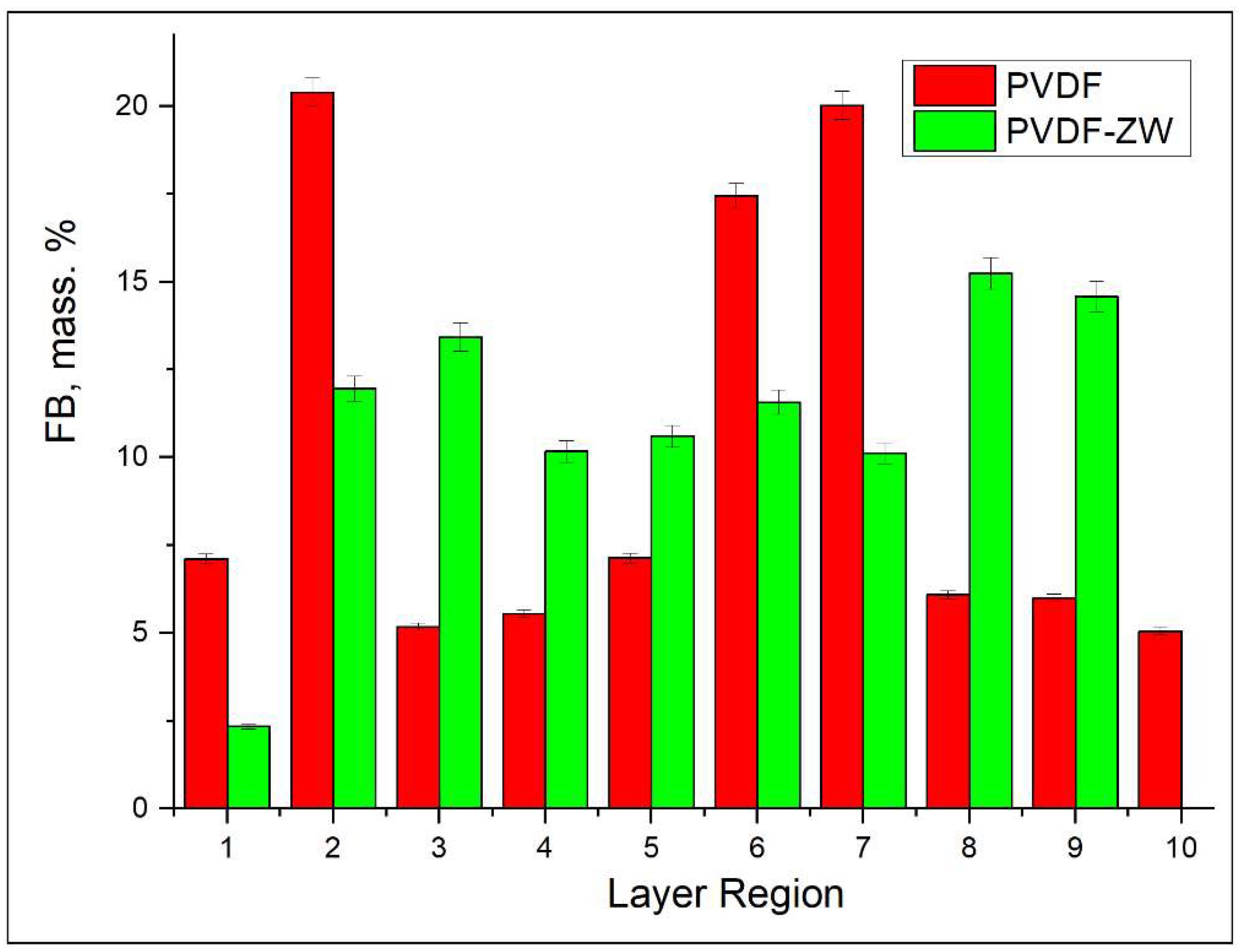
3.3. FB Adsorption from Its Single Solution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kolff, W.J.; Berk, H.T.J.; Welle, N.M.; Van Der Ley, A.J.W.; Van Dijk, E.C.; Van Noordwijk, J. The artificial kidney: A dialyzer with a great area. Acta Med. Scand. 1944, 117, 121–134. [Google Scholar] [CrossRef]
- Takeyama, T.; Sakai, Y. Polymethylmethacrylate: One biomaterial for a series of membranes. Contrib. Nephrol. 1998, 125, 9–24. [Google Scholar]
- Seifert, B.; Mihanetzis, G.; Groth, T.; Albrecht, W.; Richau, K.; Missirlis, Y.; Paul, D.; Von Sengbusch, G. Polyetherimide: A new membrane-forming polymer for biomedical applications. Artif. Organs 2002, 26, 189–199. [Google Scholar] [CrossRef]
- Kerr, P.G.; Huang, L. Membranes for haemodialysis. Nephrology 2010, 15, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Carpi, A.; Donadio, C.; Tramonti, G. (Eds.) Progress in Hemodialysis from Emergent Biotechnology to Clinical Practice; Books on Demand: Stoughton, WI, USA, 2011. [Google Scholar]
- Thomas, M.; Moriyama, K.; Ledebo, I. AN69: Evolution of the world’s first high permeability membrane. Contrib. Nephrol. 2011, 173, 119–129. [Google Scholar]
- Houis, S.; Engelhardt, E.M.; Wurm, F.; Gries, T. Application of Polywnylidene Fluoride (pvdf) asa Biomaterial in Medical Textiles; Woodhead Publishing Limited: Cambridge, UK, 2010; pp. 342–352. [Google Scholar]
- Venault, A.; Chang, Y.; Yang, H.-S.; Lin, P.-Y.; Shih, Y.-J.; Higuchi, A. Surface self-assembled zwitterionization of poly (vinylidene fluoride) microfiltration membranes via hydrophobic-driven coating for improved blood compatibility. J. Membr. Sci. 2014, 454, 253–263. [Google Scholar] [CrossRef]
- Ruan, L.; Yao, X.; Chang, Y.; Zhou, L.; Qin, G.; Zhang, X. Properties and Applications of the β Phase Poly(vinylidene fluoride). Polymers 2018, 10, 228. [Google Scholar] [CrossRef]
- Liang, H.-Q.; Li, H.-N.; Yu, H.-H.; Zhou, Y.-T.; Xu, Z.-K. Polysulfone membranes via thermally induced phase separation. Chin. J. Polym. Sci. 2017, 35, 846–856. [Google Scholar] [CrossRef]
- Irfan, M.; Idris, A. Overview of PES biocompatible/hemodialysis membranes: PES–blood interactions and modification techniques. Mater. Sci. Eng. 2015, 56, 574–592. [Google Scholar] [CrossRef]
- Röckel, A.; Hertel, J.; Fiegel, P.; Abdelhamid, S.; Panitz, N.; Walb, D. Permeability and secondary membrane formation of a high flux polysulfone hemofilter. Kidney Int. 1986, 30, 429–432. [Google Scholar] [CrossRef]
- Chang, T.; DeFine, L.; Alexander, T.; Kyu, T. In vitro investigation of antioxidant, anti-Inflammatory, and antiplatelet adhesion properties of genistein–modified poly (ethersulfone)/poly (vinylpyrrolidone) hemodialysis membranes. J. Biomed. Mater. Res. Appl. Biomater. 2015, 103, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Cvelbar, U.; Junkar, I.; Modic, M. Hemocompatible poly(ethylene terephthalate) polymer modified via reactive plasma treatment. Jpn. J. Appl. Phys. 2011, 50, 08JF02. [Google Scholar] [CrossRef]
- Junkar, I.; Cvelbar, U.; Lehocký, M. Plasma treatment of biomedical materials. Mater. Technol. 2011, 45, 221–226. [Google Scholar]
- Modic, M.; Junkar, I.; Vesel, A.; Mozetic, M. Aging of plasma treated surfaces and their effects on platelet adhesion and activation. Surf Coat Technol. 2012, 213, 98–104. [Google Scholar] [CrossRef]
- Recek, N. Biocompatibility of plasma-treated polymeric implan. Materials 2019, 12, 240. [Google Scholar] [CrossRef] [PubMed]
- Grunkemeier, J.M.; Tsai, W.B.; Horbett, T.A. Hemocompatibility of treated polystyrene substrates: Contact activation, platelet adhesion, and procoagulant activity of adherent platelets. J. Biomed. Mater. Res. 1998, 41, 657–670. [Google Scholar] [CrossRef]
- Sin, M.C.; Chen, S.H.; Chang, Y. Hemocompatibility of zwitterionic interfaces and membranes. Polym. J. 2014, 46, 436. [Google Scholar] [CrossRef]
- Yoshikawa, C.; Goto, A.; Tsujii, Y.; Fukuda, T.; Kimura, T.; Yamamoto, K.; Kishida, A. Protein repellency of well-defined, concentrated poly (2-hydroxyethyl methacrylate) brushes by the size-exclusion effect. Macromolecules 2006, 39, 2284–2290. [Google Scholar] [CrossRef]
- Mrabet, B.; Nguyen, M.N.; Majbri, A.; Mahouche, S.; Turmine, M.; Bakhrouf, A.; Chehimi, M.M. Anti-fouling poly (2-hydoxyethyl methacrylate) surface coatings with specific bacteria recognition capabilities. Surf. Sci. 2009, 603, 2422–2429. [Google Scholar] [CrossRef]
- Rodrigues, S.N.; Gonçalves, I.C.; Martins MC, L.; Barbosa, M.A.; Ratner, B.D. Fibrinogen adsorption, platelet adhesion and activation on mixed hydroxyl-/methyl-terminated self-assembled monolayers. Biomaterials 2006, 27, 5357–5367. [Google Scholar] [CrossRef]
- Liu, P.-S.; Chen, Q.; Wu, S.-S.; Shen, J.; Lin, S.-C. Surface modification of cellulose membranes with zwitterionic polymers for resistance to protein adsorption and platelet adhesion. J. Membr. Sci. 2010, 350, 387–394. [Google Scholar] [CrossRef]
- Cai, N.; Li, Q.; Zhang, J.; Xu, T.; Zhao, W.; Yang, J.; Zhang, L. Antifouling zwitterionic hydrogel coating improves hemocompatibility of activated carbon hemoadsorbent. J. Colloids Interface Sci. 2017, 503, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Erfani, A.; Khosharay, S.; Flynn, N.H.; Ramsey, J.D.; Aichele, C.P. Effect of zwitterionic betaine surfactant on interfacial behavior of bovine serum albumin (BSA). J. Mol. Liq. 2020, 318, 114067. [Google Scholar] [CrossRef]
- Abdelrasoul, A.; Shoker, A. Influence of Hydration Shell of Hemodialysis Clinical Membranes on Surrogate Biomarkers Activation in Uremic Serum of Dialysis Patients. Biomed. Eng. Adv. 2022, 4, 100049. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, C.; Xu, S.; Pang, X.; Cai, G.; Wang, J. Preparation of Zwitterionic Polymer-Functionalized Cotton Fabrics and the Performance of Anti-Biofouling and Long-Term Biofilm Resistance. Colloid Interface Sci. Commun. 2018, 24, 98–104. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Z.; Liu, Y.; Huo, P.; Gu, J.; Zhao, F. Synthesis and characterization of novel zwitterionic poly(aryl ether oxadiazole) ultrafiltration membrane with good antifouling and antibacterial properties. J. Membr. Sci. 2020, 611, 118337. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chao, C.-M.; Wang, D.K.; Liu, K.-M.; Tseng, H.-H. Enhancing the antifouling properties of a PVDF membrane for protein separation by grafting branch-like zwitterions via a novel amphiphilic SMA-HEA linker. J. Membr. Sci. 2021, 624, 119126. [Google Scholar] [CrossRef]
- Venault, A.; Wei, T.-C.; Shih, H.-L.; Yeh, C.-C.; Chinnathambi, A.; Alharbi, S.A.; Carretier, S.; Aimar, P.; Lai, J.-Y.; Chang, Y. Antifouling pseudo-zwitterionic poly(vinylidene fluoride) membranes with efficient mixed-charge surface grafting via glow dielectric barrier discharge plasma-induced copolymerization. J. Membr. Sci. 2016, 516, 13–25. [Google Scholar] [CrossRef]
- Zhai, W.; Wang, M.; Song, J.; Zhang, L.; Li, X.-M.; He, T. Fouling resistance of 3-[[3-(trimethoxysilane)-propyl] amino] propane-1-sulfonic acid zwitterion modified poly (vinylidene fluoride) membranes. Sep. Purif. Technol. 2020, 239, 116589. [Google Scholar] [CrossRef]
- Mollahosseini, A.; Abdelrasoul, A. Novel insights in hemodialysis: Most Recent theories on the membrane hemocompatibility improvement. Biomed. Eng. Adv. 2022, 3, 100034. [Google Scholar] [CrossRef]
- Mollahosseini, A.; Abdelrasoul, A.; Shoker, A. Latest advances in zwitterionic structures modified dialysis membranes. Mater. Today Chem. 2020, 15, 100227. [Google Scholar] [CrossRef]
- Nazari, S.; Abdelrasoul, A. Surface zwitterionization of hemodialysismembranesfor hemocompatibility enhancement and protein-mediated anti-adhesion: A critical review. Biomed. Eng. Adv. 2022, 3, 100026. [Google Scholar] [CrossRef]
- Saadati, S.; Westphalen, H.; Eduok, U.; Abdelrasoul, A.; Shoker, A.; Choi, P.; Doan, H.; Ein-Mozaffari, F.; Zhu, N. Biocompatibility enhancement of hemodialysis membranes using a novel zwitterionic copolymer: Experimental, in situ synchrotron imaging, molecular docking, and clinical inflammatory biomarkers investigations. Mater. Sci. Eng. Mater. Biol. Appl. 2020, 117, 111301. [Google Scholar] [CrossRef]
- Maghami, M.; Abdelrasoul, A. A comprehensive computational study and simulation of innovative zwitterionic materials for enhanced poly (vinylidene fluoride) membrane hydrophilicity. J. Mol. Graph. Model. 2020, 100, 107656. [Google Scholar] [CrossRef] [PubMed]
- Bui, V.T.; Abdelrasoul, A.; McMartin, D.W. Investigation on the stability and antifouling properties of polyvinylidene fluoride (PVDF)-zwitterion mixed matrix membranes (MMMs) using molecular dynamics simulation (MDS). Comput. Mater. Sci. 2021, 187, 110079. [Google Scholar] [CrossRef]
- Saadati, S.; Eduok, U.; Camara, H.; Abdelrasoul, A.; Shoker, A.; Choi, P.; Doan, H.; Ein-Mozaffari, F.; Zhu, N. In situ Synchrotron Imaging of Human Serum Proteins Interactions, Molecular Docking and Inflammatory Biomarkers of Hemocompatible Synthesized Zwitterionic Polymer Coated- Polyvinylidene Fluoride (PVDF) Dialysis Membranes. Surf. Interfaces 2021, 27, 101505. [Google Scholar] [CrossRef]
- Abdelrasoul, A.; Doan, H.; Lohi, A.; Zhu, N. Synchrotron based Micro Tomography (SR-μCT), Experimental, and Computational Studies to Investigate the Influences of Cross Flow Air Injection in Ultrafiltration Process. J. Environ. Chem. Eng. 2021, 9, 104611. [Google Scholar] [CrossRef]
- Ehsani, M.; Zhu, N.; Doan, H.; Lohi, A.; Abdelrasoul, A. In-situ synchrotron X-ray imaging of ultrasound (US)-generated bubbles: Influence of US frequency on microbubble cavitation for membrane fouling remediation. Ultrason. Sonochem. 2021, 77, 105697. [Google Scholar] [CrossRef]
- Mollahosseini, A.; Min Lee, K.; Abdelrasoul, A.; Doan, H.; Zhu, N. Innovative in situ investigations using synchrotron-based micro tomography and molecular dynamics simulation for fouling assessment in ceramic membranes for dairy and food industry. Int. J. Appl. Ceram. Technol. 2021, 18, 2143–2157. [Google Scholar] [CrossRef]
- Saadati, S.; Eduok, U.; Westphalen, H.; Abdelrasoul, A.; Shoker, A.; Choi, P.; Doan, H.; Ein-Mozaffari, F.; Zhu, N. Assessment of polyethersulfone and polyacrylonitrile hemodialysis clinical membranes: In situ synchrotron-based imaging of human serum proteins adsorption, interaction analyses, molecular docking and clinical inflammatory biomarkers investigations. Mater. Today Commun. 2021, 29, 102928. [Google Scholar] [CrossRef]
- Westphalen, H.; Saadati, S.; Eduok, U.; Abdelrasoul, A.; Shoker, A.; Choi, P.; Doan, H.; Ein-Mozaffari, F. Case studies of clinical hemodialysis membranes: Influences of membrane morphology and biocompatibility on uremic blood-membrane interactions and inflammatory biomarkers. Sci. Rep. 2020, 10, 14808. [Google Scholar] [CrossRef]
- Zhanga, D.; Zhaodi, Y.; Mingcong HJianpeng, T.; Xiao, S.; Wang, K.; Wan, X.; Wang, Y.; Wu, Y.; Daib, F. Ultrathin coordination polymer nanosheets modified with carbon quantum dots for ultrasensitive ammonia sensors. J. Colloid Interface Sci. 2023, 630, 776–785. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, D.; Li, P.; Zhou, X.; Zong, X.; Dong, G. Facile fabrication of high-performance QCM humidity sensor based on layer-by-layer self-assembled polyaniline/graphene oxide nanocomposite film. Sens. Actuators Chem. 2018, 255, 1869–1877. [Google Scholar] [CrossRef]
- Maghami, M.; Abdelrasoul, A. Pair interaction energy decomposition analysis (PIEDA) and experimental approaches for investigating water interactions with hydrophilic and hydrophobic membranes. J. Mol. Graph. Model. 2020, 96, 107540. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, K.; Chao, C.; Wang, D.; Tung, K.; Tseng, H. Enhanced anti–protein fouling of PVDF membrane via hydrophobic–hydrophobic adsorption of styrene–terminated amphiphilic linker. Chem. Eng. Res. Des. 2020, 156, 273–280. [Google Scholar] [CrossRef]
- Chen, F.; Shi, X.; Chen, X.; Chen, W. Preparation and characterization of amphiphilic copolymer PVDF-g-PMABS and its application in improving hydrophilicity and protein fouling resistance of PVDF membrane. Appl. Surf. Sci. 2018, 427, 787–797. [Google Scholar] [CrossRef]
- Westphalen, H.; Kalugin, D.; Abdelrasoul, A. Structure, function, and adsorption of highly abundant blood proteins and its critical influence on hemodialysis patients: A critical review. Biomed. Eng. Adv. 2021, 2, 100021. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, J.; Gallardo-Moreno, A.M.; Bruque, J.M.; González-Martín, M.L. Adsorption of human fibrinogen and albumin onto hydrophobic and hydrophilic Ti6Al4V powder. Appl. Surf. Sci. 2016, 376, 269–275. [Google Scholar] [CrossRef]
- Gök, E.; Kiremitçi, M.; Ateş, I. Protein adsorption on functional hydrophilic polymer beads: Role of structural properties and medium conditions. React. Polym. 1994, 24, 41–48. [Google Scholar] [CrossRef]
- Bremmell, K.E.; Britcher, L.; Griesser, H.J. Griesser Steric and electrostatic surface forces on sulfonated PEG graft surfaces with selective albumin adsorption. Colloids Surf. 2013, 106, 102–108. [Google Scholar] [CrossRef]
- Gagliardi, M. In vitro haematic proteins adsorption and cytocompatibility study on acrylic copolymer to realise coatings for drug-eluting stents. Mater. Sci. Eng. 2012, 32, 2445–2451. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Wasilewska, M.; Nattich-Rak, M.; Barbasz, J. Mechanisms of Fibrinogen Adsorption on Mica. ACS Symp. Ser. 2012, 1120, 97–127. [Google Scholar]
- Tiscia, G.L.; Margaglione, M. Human Fibrinogen: Molecular and Genetic Aspects of Congenital Disorders. Int. J. Mol. Sci. 2018, 19, 1597. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelrasoul, A.; Zhu, N.; Shoker, A. Investigation on Human Serum Protein Depositions Inside Polyvinylidene Fluoride-Based Dialysis Membrane Layers Using Synchrotron Radiation Micro-Computed Tomography (SR-μCT). Membranes 2023, 13, 117. https://doi.org/10.3390/membranes13010117
Abdelrasoul A, Zhu N, Shoker A. Investigation on Human Serum Protein Depositions Inside Polyvinylidene Fluoride-Based Dialysis Membrane Layers Using Synchrotron Radiation Micro-Computed Tomography (SR-μCT). Membranes. 2023; 13(1):117. https://doi.org/10.3390/membranes13010117
Chicago/Turabian StyleAbdelrasoul, Amira, Ning Zhu, and Ahmed Shoker. 2023. "Investigation on Human Serum Protein Depositions Inside Polyvinylidene Fluoride-Based Dialysis Membrane Layers Using Synchrotron Radiation Micro-Computed Tomography (SR-μCT)" Membranes 13, no. 1: 117. https://doi.org/10.3390/membranes13010117
APA StyleAbdelrasoul, A., Zhu, N., & Shoker, A. (2023). Investigation on Human Serum Protein Depositions Inside Polyvinylidene Fluoride-Based Dialysis Membrane Layers Using Synchrotron Radiation Micro-Computed Tomography (SR-μCT). Membranes, 13(1), 117. https://doi.org/10.3390/membranes13010117





