Platinum Nanoparticles Immobilized on Electrospun Membranes for Catalytic Oxidation of Volatile Organic Compounds
Abstract
1. Introduction
2. Materials and Methods
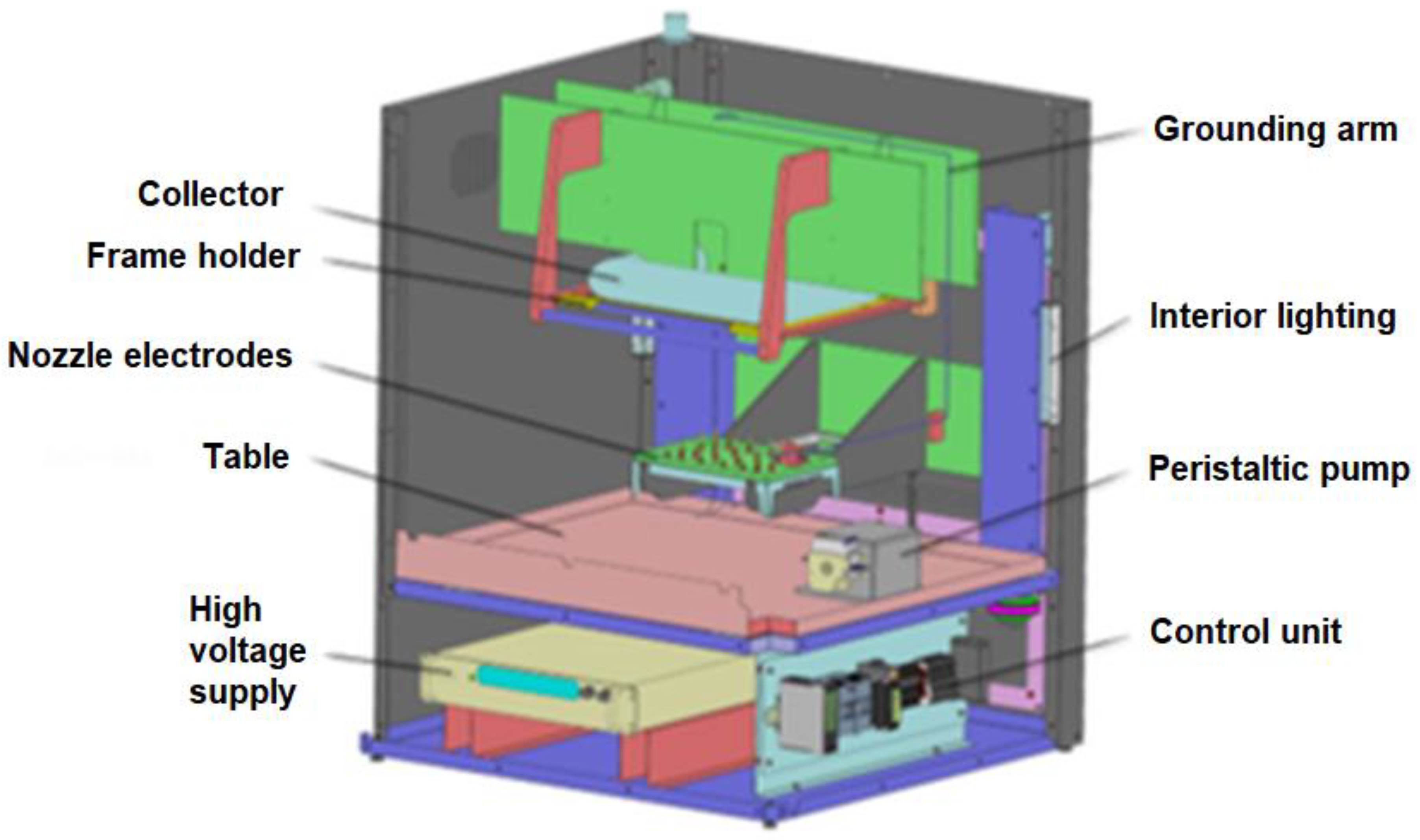
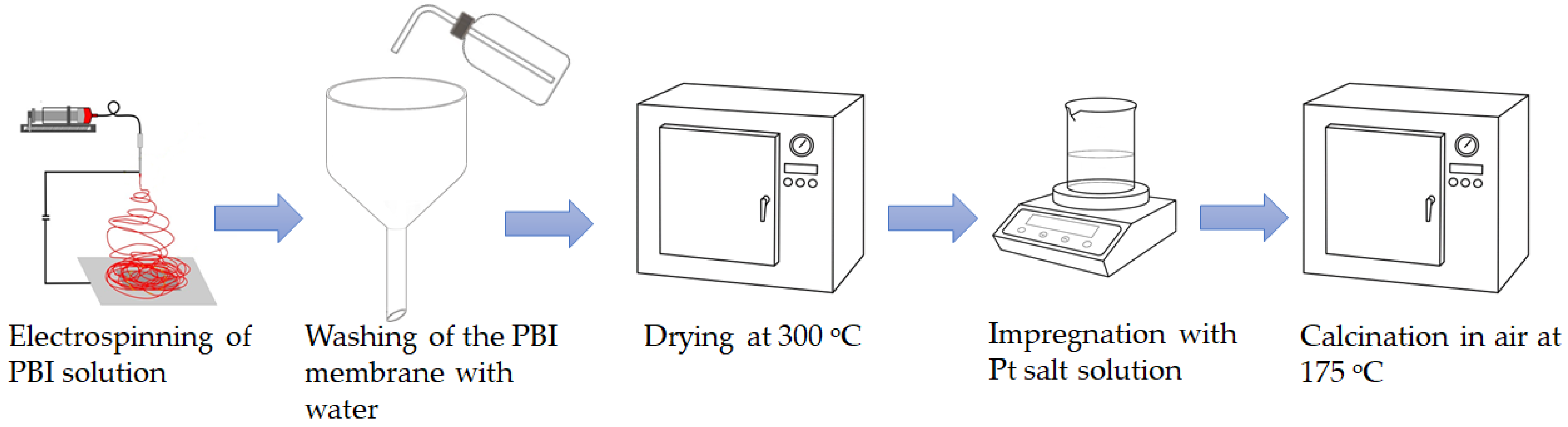
2.1. Electrospun Membrane Preparation
2.2. Electrospun Catalyst Preparation
2.3. Electrospun Membranes and Catalyst Characterization
2.4. Catalytic Experiments
3. Results and Discussion
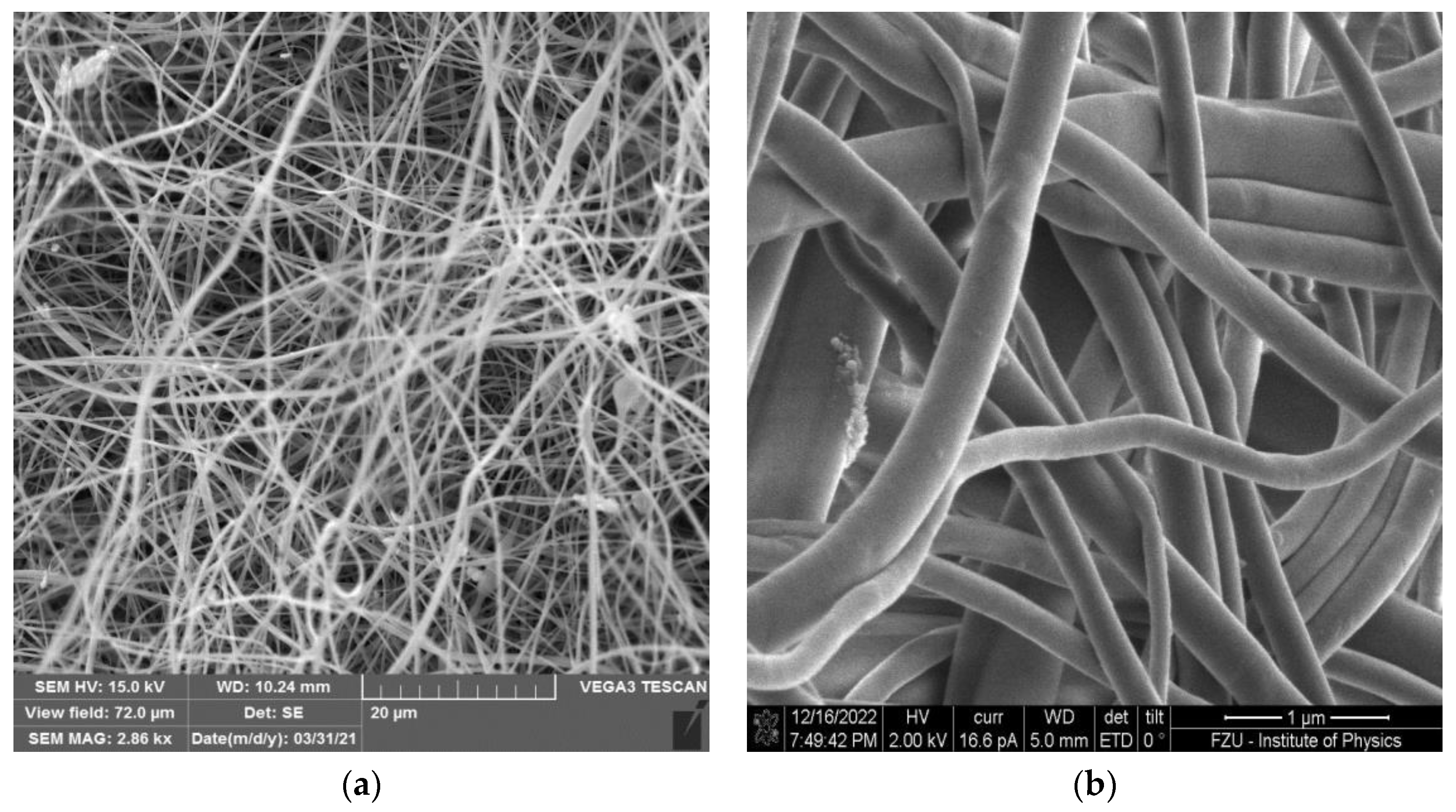
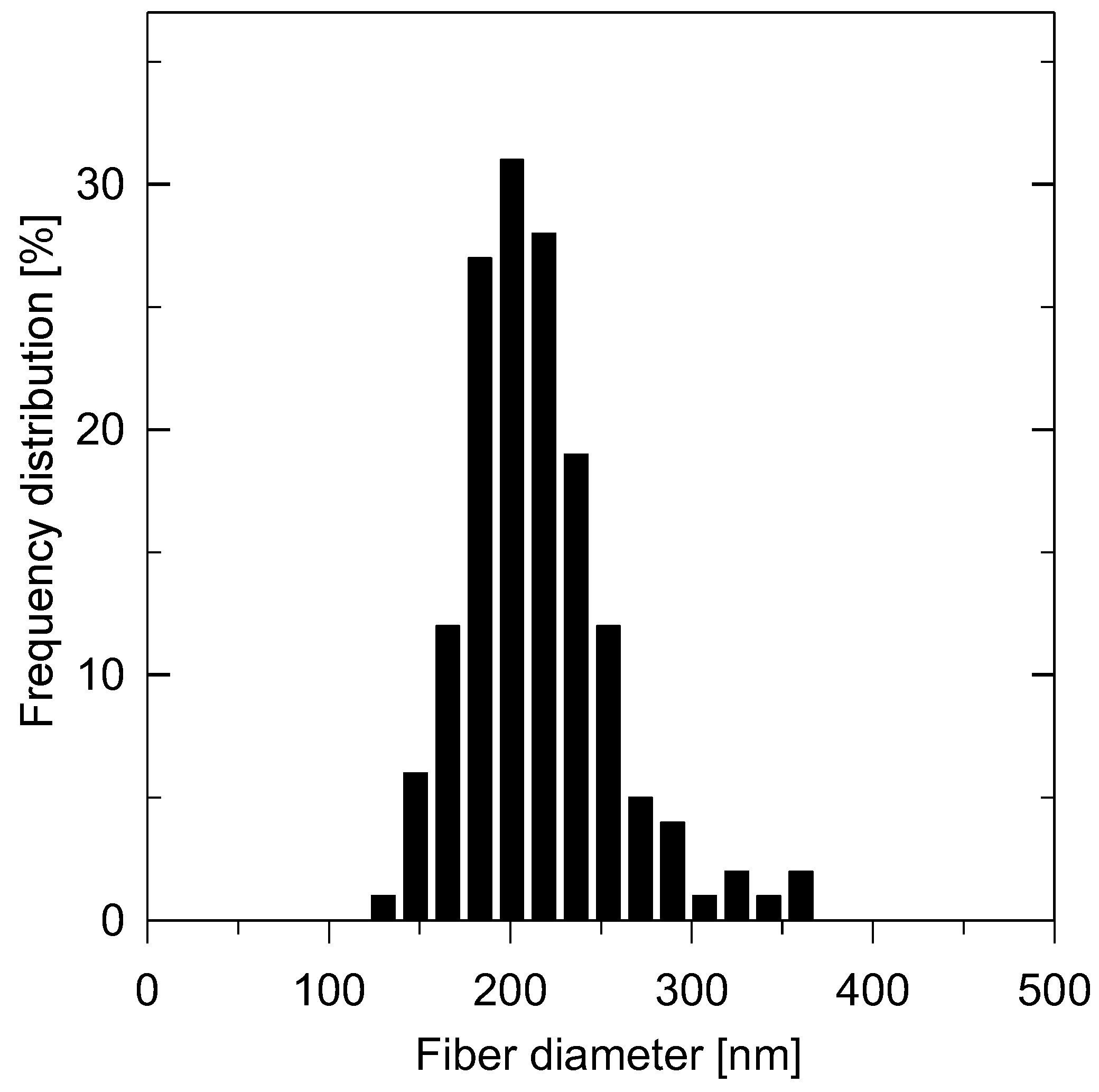
3.1. Morphology
3.2. Textural Properties
3.3. Physicochemical Properties of the Catalysts
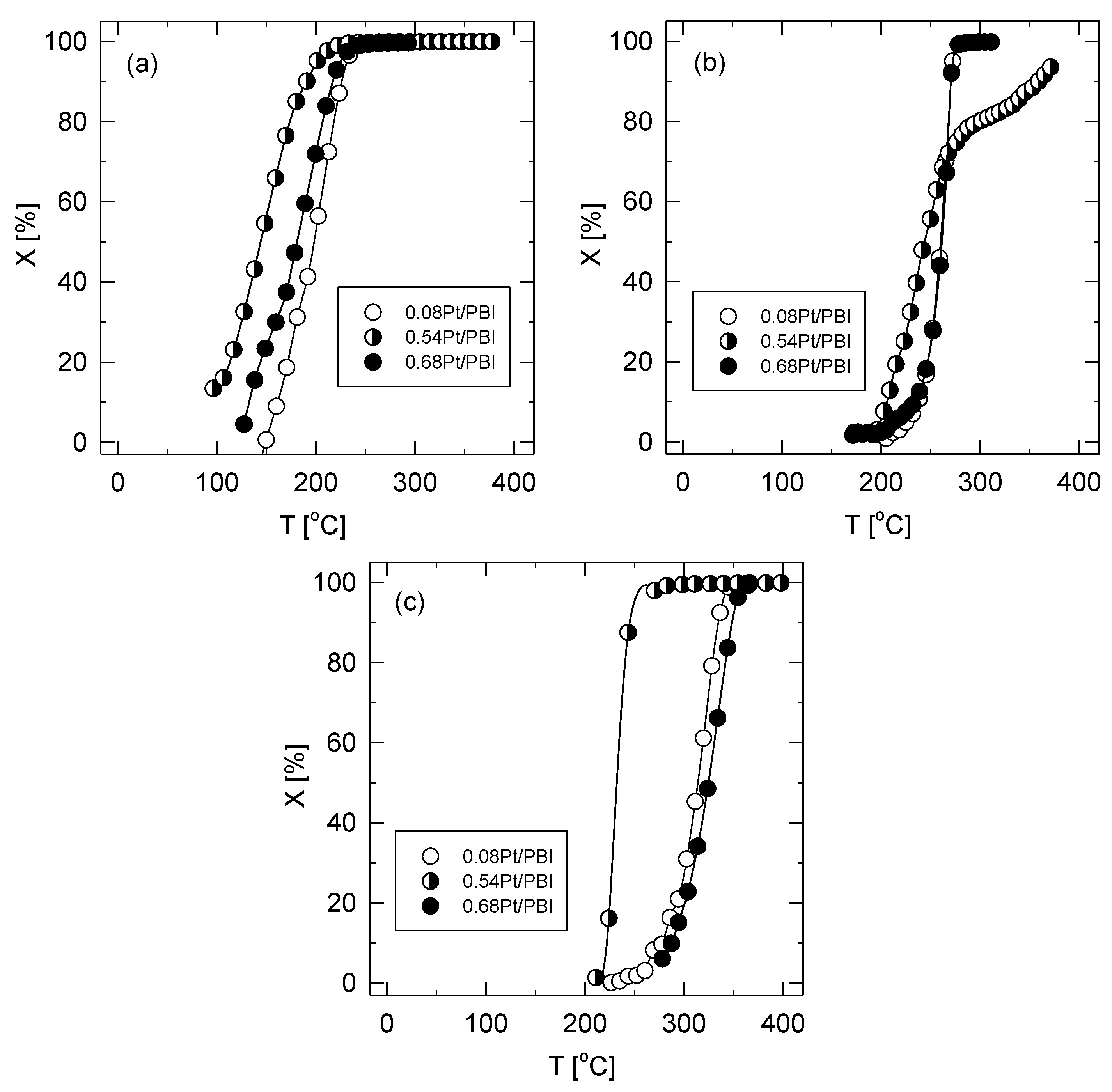
3.4. Catalytic Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| a | Equilibrium adsorbed amount |
| BET | Brunauer–Emmett–Teller approach |
| BJH | Barrett–Joyner–Halenda |
| CCD | Charge-coupled device |
| CPS | Counts per second |
| DC | Direct current |
| EDX | Energy-dispersive X-ray spectrometer |
| FEG | Field emission electron gun |
| fcc | Face centered cubic |
| FTIR | Fourier transform infrared |
| GHSV | Gas hourly space velocity |
| HAADF | High angle annular dark field detector |
| HRTEM | High-resolution transmission spectroscopy |
| IUPAC | International Union of Pure and Applied Chemistry |
| liq. | Corresponding to liquid nitrogen |
| MP-AES | Microwave plasma atomic emission spectroscopy |
| P | Pressure |
| P0 | Vapor pressure |
| PBI | Polybenzimidazole |
| PSD | Pore-size distribution |
| r | Pore radius |
| SBET | The specific surface area |
| Smeso | The specific area of mesopores |
| SEM | Scanning electron microscopy |
| STEM | Scanning transmission electron microscopy |
| STP | Standard temperature and pressure (273 K, 101.325 kPa) |
| T | Temperature |
| TEM | Transmission electron microscopy |
| TOF | Turn over frequency |
| T50 | Temperature corresponding to 50% conversion |
| T90 | Temperature corresponding to 90% conversion |
| V | Volume |
| VOC | Volatile organic compound |
| vol. | Volume |
| Vmicro | The specific volume of micropores |
| Vtot | The specific total pore volume |
| wt. | Weight |
| X | Conversion |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
References
- Official Journal of the European Union Home Page. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2004:143:0087:0096:EN:PDF (accessed on 15 November 2022).
- Official Journal of the European Union Home Page. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:334:0017:0119:en:PDF (accessed on 15 November 2022).
- Kaštánek, F.; Topka, P.; Soukup, K.; Maléterová, Y.; Demnerová, K.; Kaštánek, P.; Šolcová, O. Remediation of Contaminated Soils by Thermal Desorption; Effect of Benzoyl Peroxide Addition. J. Clean Prod. 2016, 125, 309–313. [Google Scholar] [CrossRef]
- Gaálová, J.; Topka, P. Gold and Ceria as Catalysts for VOC Abatement: A Review. Catalysts 2021, 11, 789. [Google Scholar] [CrossRef]
- Topka, P.; Dvořáková, M.; Kšírová, P.; Perekrestov, R.; Čada, M.; Balabánová, J.; Koštejn, M.; Jirátová, K.; Kovanda, F. Structured Cobalt Oxide Catalysts for VOC Abatement: The effect of preparation method. Environ. Sci. Pollut. Res. 2020, 27, 7608–7617. [Google Scholar] [CrossRef] [PubMed]
- Topka, P.; Jirátová, K.; Dvořáková, M.; Balabánová, J.; Koštejn, M.; Kovanda, F. Hydrothermal Deposition as a Novel Method for the Preparation of Co-Mn Mixed Oxide Catalysts Supported on Stainless Steel Meshes: Application to VOC Oxidation. Environ. Sci. Pollut. Res. 2022, 29, 5172–5183. [Google Scholar] [CrossRef]
- Jirátová, K.; Perekrestov, R.; Dvořáková, M.; Balabánová, J.; Koštejn, M.; Veselý, M.; Čada, M.; Topka, P.; Pokorná, D.; Hubička, Z.; et al. Modification of Cobalt Oxide Electrochemically Deposited on Stainless Steel Meshes with Co-Mn Thin Films Prepared by Magnetron Sputtering: Effect of Preparation Method and Application to Ethanol Oxidation. Catalysts 2021, 11, 1453. [Google Scholar] [CrossRef]
- Gaálová, J.; Bourassi, M.; Soukup, K.; Trávníčková, T.; Bouša, D.; Sundararajan, S.; Losada, O.; Kasher, R.; Friess, K.; Sofer, Z. Modified Single-Walled Carbon Nanotube Membranes for the Elimination of Antibiotics from Water. Membranes 2021, 11, 720. [Google Scholar] [CrossRef]
- Bourassi, M.; Pasichnyk, M.; Oesch, O.; Sundararajan, S.; Trávničková, T.; Soukup, K.; Kasher, R.; Gaálová, J. Glycidyl and Methyl Methacrylate UV-Grafted PDMS Membrane Modification toward Tramadol Membrane Selectivity. Membranes 2021, 11, 752. [Google Scholar] [CrossRef]
- Matei, E.; Covaliu-Mierla, C.I.; Ţurcanu, A.A.; Râpă, M.; Predescu, A.M.; Predescu, C. Multifunctional Membranes—A Versatile Approach for Emerging Pollutants Removal. Membranes 2022, 12, 67. [Google Scholar] [CrossRef]
- Nasir, A.M.; Awang, N.; Jaafar, J.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Aziz, F.; Yajid, M.A.M. Recent Progress on Fabrication and Application of Electrospun Nanofibrous Photocatalytic Membranes for Wastewater Treatment: A Review. J. Water Process Eng. 2021, 40, 101878. [Google Scholar] [CrossRef]
- Zhu, J.; Shao, C.; Li, X.; Han, C.; Yang, S.; Ma, J.; Li, X.; Liu, Y. Immobilization of ZnO/Polyaniline Heterojunction on Electrospun Polyacrylonitrile Nanofibers and Enhanced Photocatalytic Activity. Mater. Chem. Phys. 2018, 214, 507–515. [Google Scholar] [CrossRef]
- Soukup, K.; Hejtmánek, V.; Šolcová, O. Determination of Microstructural Characteristics of Advanced Biocompatible Nanofibrous Membranes. Micropor. Mesopor. Mat. 2020, 304, 1–7. [Google Scholar] [CrossRef]
- Cao, A.; Lu, R.; Veser, G. Stabilizing Metal Nanoparticles for Heterogeneous Catalysis. Phys. Chem. Chem. Phys. 2010, 12, 13499–13510. [Google Scholar] [CrossRef] [PubMed]
- Balcar, H.; Topka, P.; Žilková, N.; Perez-Pariente, J.; Čejka, J. Metathesis of Linear Alpha-Olefins with MoO3 Supported on MCM-41 Catalyst. In Nanoporous Materials IV. Studies in Surface Science and Catalysis; Sayari, A., Jaroniec, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 155, pp. 795–802. ISBN 0-444-51748-0. [Google Scholar]
- Yonezawa, T.; Imamura, K.; Kimizuka, N. Direct Preparation and Size Control of Palladium Nanoparticle Hydrosols by Water-Soluble Isocyanide Ligands. Langmuir 2001, 17, 4701–4703. [Google Scholar] [CrossRef]
- Itoh, H.; Naka, K.; Chujo, Y. Synthesis of Palladium Clusters with Surface Initiator for Polymerization of 2-Methyl-2-Oxazoline. Polym. Bull. 2001, 46, 357–362. [Google Scholar] [CrossRef]
- Hirai, H.; Yakaru, N.I.; Seta, Y.; Hodosima, S. Characterization of Palladium Nanoparticles Protected with Polymer as Hydrogenation Catalyst. React. Funct. Polym. 1998, 37, 121–131. [Google Scholar] [CrossRef]
- Soukup, K.; Petráš, D.; Topka, P.; Slobodian, P.; Šolcová, O. Preparation and Characterization of Electrospun Poly(p-Phenylene Oxide) Membranes. Catal. Today 2012, 193, 165–171. [Google Scholar] [CrossRef]
- Demir, M.M.; Gulgun, M.A.; Menceloglu, Y.Z.; Erman, B.; Abramchuk, S.S.; Makhaeva, V.G.; Khorkhlov, A.R.; Matveeva, V.G.; Sulman, M.G. Palladium Nanoparticles by Electrospinning from Poly(Acronitrile-Co-Acrylic Acid)–PdCl2 Solutions. Relations between Preparation Conditions, Particle Size, and Catalytic Activity. Macromolecules 2004, 37, 1787–1792. [Google Scholar] [CrossRef]
- Ebert, K.; Bengtson, G.; Just, R.; Oehring, M.; Fritsch, D. Catalytically Active Poly (amideimide) Nanofibre Mats with High Activity Tested in the Hydrogenation of Methyl-cis-9-Octadecenoate. Appl. Catal. A-Gen. 2008, 346, 72–78. [Google Scholar] [CrossRef]
- Wendorff, J.H.; Agarwal, S.; Greiner, A. Electrospinning. Materials, Processing, and Applications; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Ramakrishna, S.; Fujihara, K.; Teo, W.-E.; Lim, T.-C.; Ma, Z. An Introduction to Electrospinning and Nanofibers; World Scientific Publishing: Singapore, 2005. [Google Scholar]
- Elmarco Company. Available online: http://www.elmarco.cz (accessed on 7 November 2022).
- Soukup, K.; Topka, P.; Hejtmánek, V.; Petráš, D.; Valeš, V.; Šolcová, O. Noble Metal Catalysts Supported on Nanofibrous Polymeric Membranes for Environmental Applications. Catal. Today 2014, 236, 3–11. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- de Boer, J.H.; Lippens, B.C.; Linsen, B.G.; Broekhoff, J.C.P.; van den Heuvel, A.; Osinga, T.J. The t-Curve of Multimolecular N2-Adsorption. J. Colloid Interface Sci. 1966, 21, 405–414. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Roberts, B.F. A Procedure for Estimating Pore Volume and Area Distributions from Sorption Isotherms. J. Colloid Interface Sci. 1967, 23, 266–273. [Google Scholar] [CrossRef]
- Jura, G.; Harkins, W.D. A New Adsorption Isotherm Which is Valid Over a Very Wide Range of Pressure. J. Chem. Phys. 1943, 11, 430–431. [Google Scholar] [CrossRef]
- Soukup, K.; Petráš, D.; Klusoň, P.; Šolcová, O. Nanofiber Membranes—Evaluation of Gas Transport. Catal. Today 2010, 156, 316–321. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAS Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Topka, P.; Hejtmánek, V.; Cruz, G.J.F.; Šolcová, O.; Soukup, K. Activated Carbon from Renewable Material as an Efficient Support for Palladium Oxidation Catalysts. Chem. Eng. Technol. 2019, 42, 851–858. [Google Scholar] [CrossRef]
- Liotta, L.F. Catalytic Oxidation of Volatile Organic Compounds on Supported Noble Metals. Appl. Catal. B 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Lauterbach, J.; Rotermund, H.H. Gas-Phase Coupling in the Co Oxidation Reaction on Polycrystalline Platinum. Catal. Lett. 1994, 27, 27–32. [Google Scholar] [CrossRef]

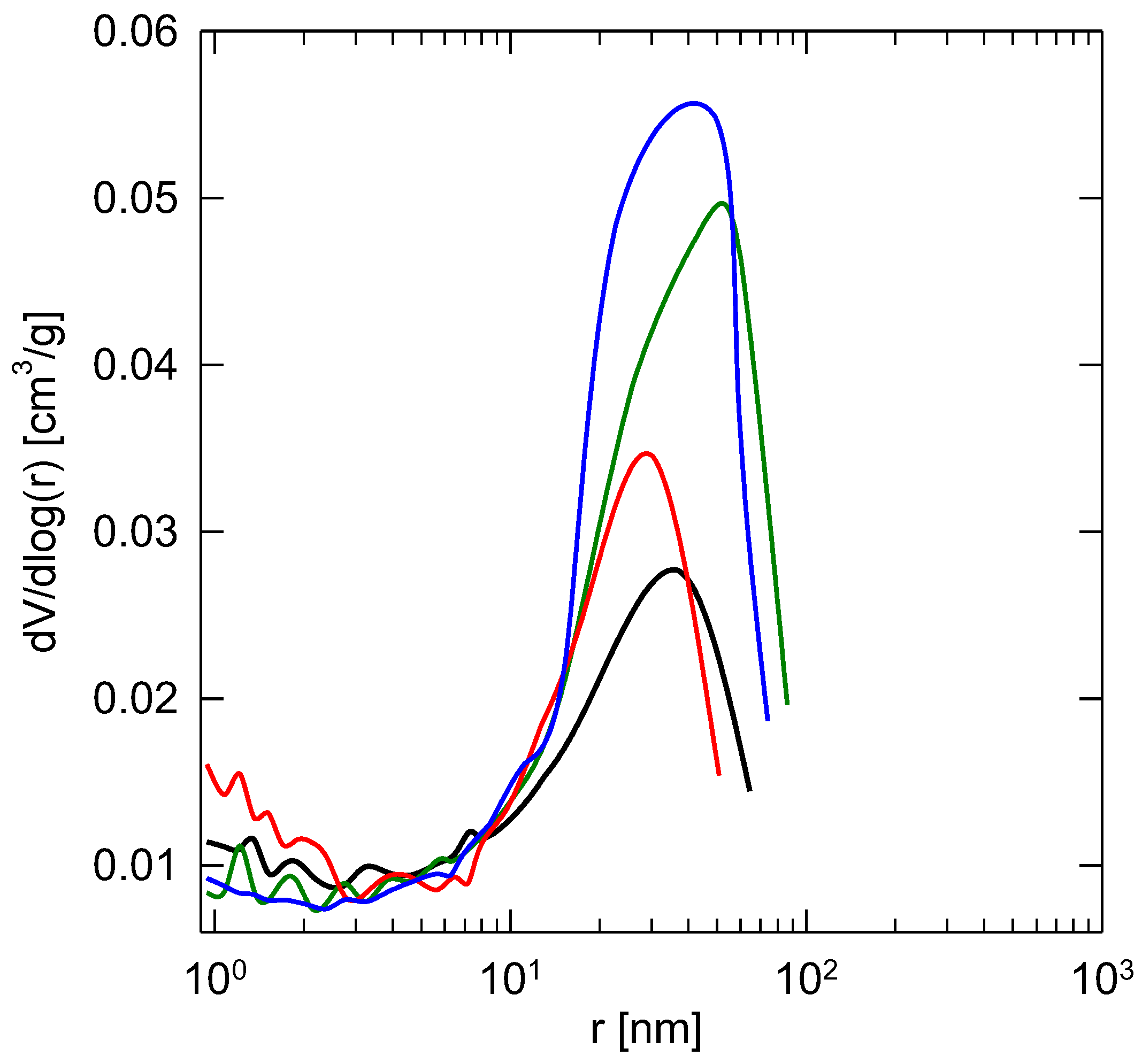
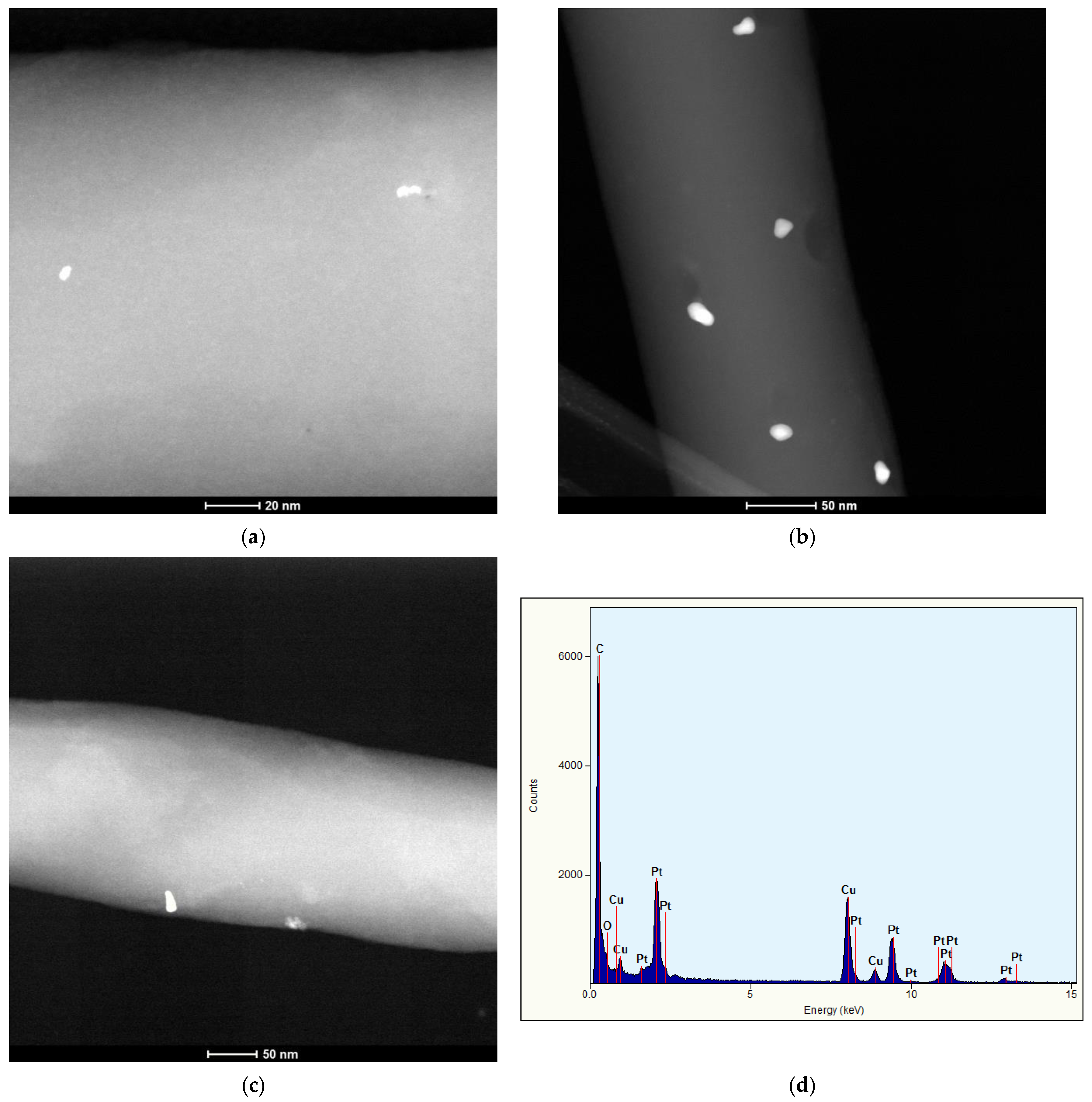
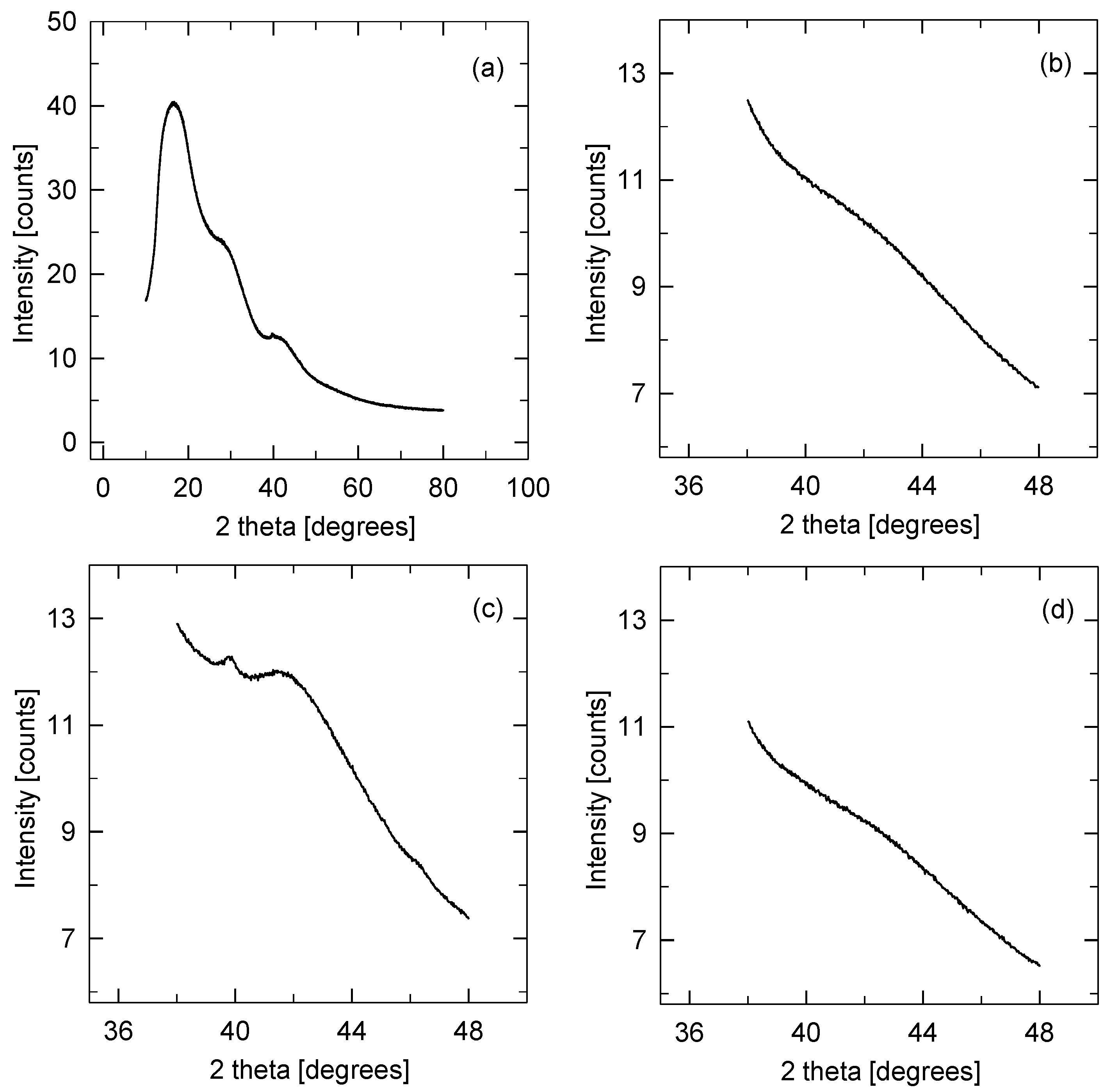
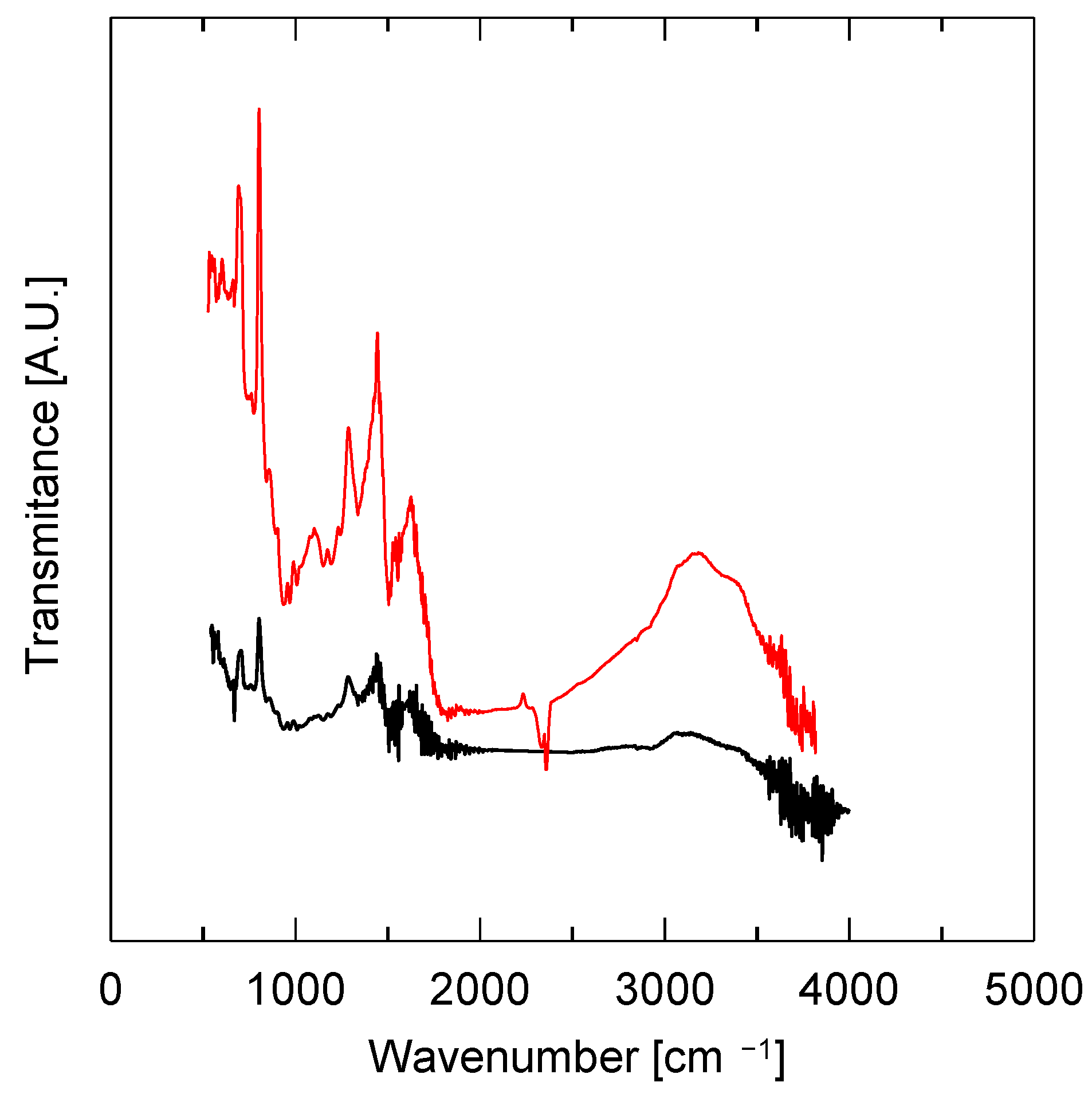

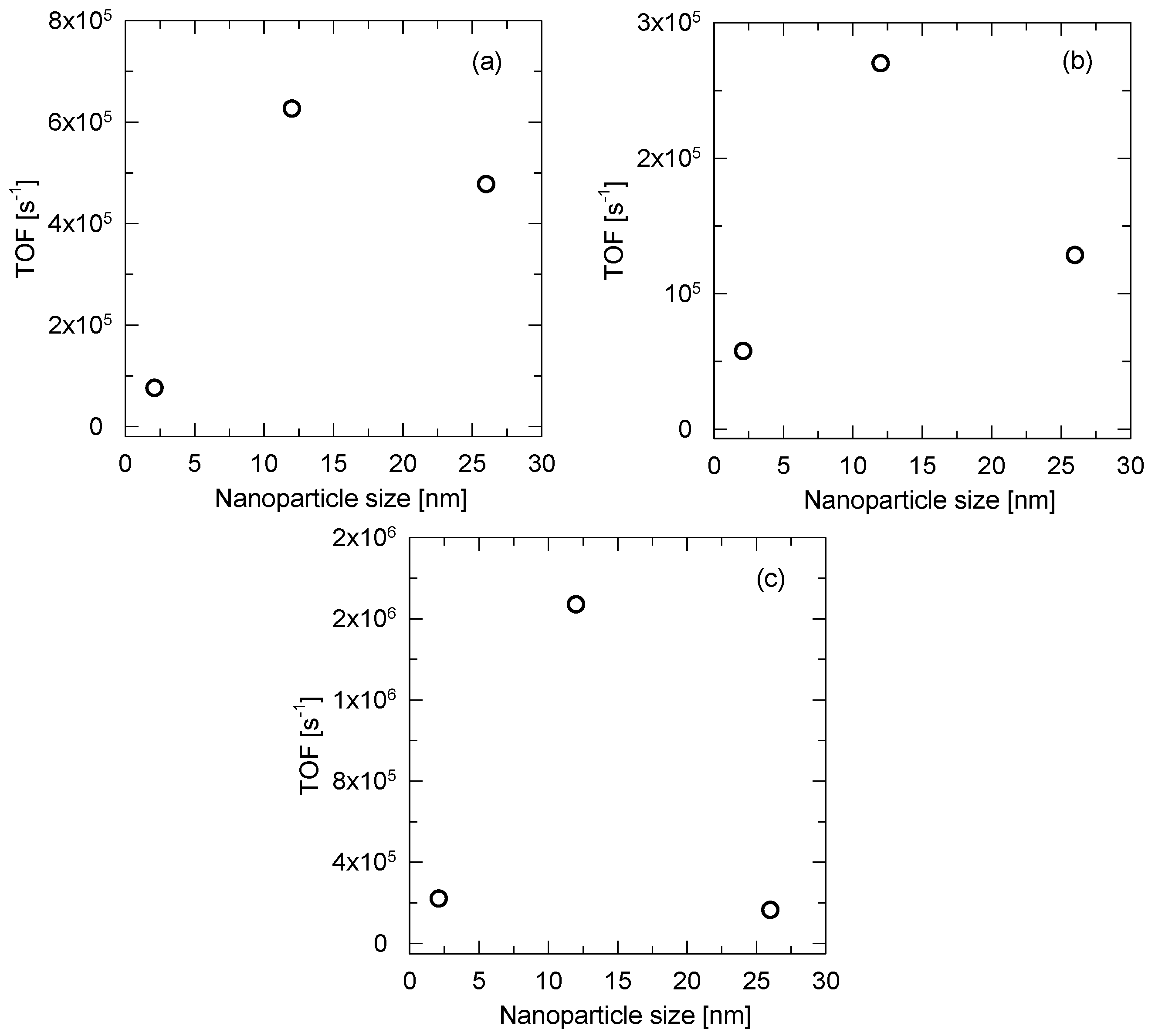
| Sample | SBET | Smeso | Vtot | Vmicro |
|---|---|---|---|---|
| [m2/g] | [m2/g] | (mm3 liq./g) | (mm3 liq./g) | |
| PBI | 16 | 14 | 26 | <1 |
| 0.08 Pt/PBI | 14 | 13 | 28 | <1 |
| 0.54 Pt/PBI | 16 | 16 | 25 | <1 |
| 0.68 Pt/PBI | 14 | 13 | 30 | <1 |
| Catalyst | Ethanol | Acetone | Toluene | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T50 | T90 | TOF*105 (s−1) | T50 | T90 | TOF*105 (s−1) | T50 | T90 | TOF*105 (s−1) | |
| 0.08 Pt/PBI | 203 | 229 | 0.5 | 260 | 271 | 0.4 | 314 | 334 | 1.6 |
| 0.54 Pt/PBI | 144 | 190 | 6.3 | 244 | 359 | 2.7 | 234 | 251 | 16.7 |
| 0.68 Pt/PBI | 181 | 217 | 4.8 | 261 | 270 | 1.3 | 325 | 349 | 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soukup, K.; Topka, P.; Kupčík, J.; Solcova, O. Platinum Nanoparticles Immobilized on Electrospun Membranes for Catalytic Oxidation of Volatile Organic Compounds. Membranes 2023, 13, 110. https://doi.org/10.3390/membranes13010110
Soukup K, Topka P, Kupčík J, Solcova O. Platinum Nanoparticles Immobilized on Electrospun Membranes for Catalytic Oxidation of Volatile Organic Compounds. Membranes. 2023; 13(1):110. https://doi.org/10.3390/membranes13010110
Chicago/Turabian StyleSoukup, Karel, Pavel Topka, Jaroslav Kupčík, and Olga Solcova. 2023. "Platinum Nanoparticles Immobilized on Electrospun Membranes for Catalytic Oxidation of Volatile Organic Compounds" Membranes 13, no. 1: 110. https://doi.org/10.3390/membranes13010110
APA StyleSoukup, K., Topka, P., Kupčík, J., & Solcova, O. (2023). Platinum Nanoparticles Immobilized on Electrospun Membranes for Catalytic Oxidation of Volatile Organic Compounds. Membranes, 13(1), 110. https://doi.org/10.3390/membranes13010110









