Recent Advancements in the Recovery and Reuse of Organic Solvents Using Novel Nanomaterial-Based Membranes for Renewable Energy Applications
Abstract
1. Introduction
2. Membrane Materials for Organic Solvent Recovery
2.1. Graphene Oxide Quantum Dots
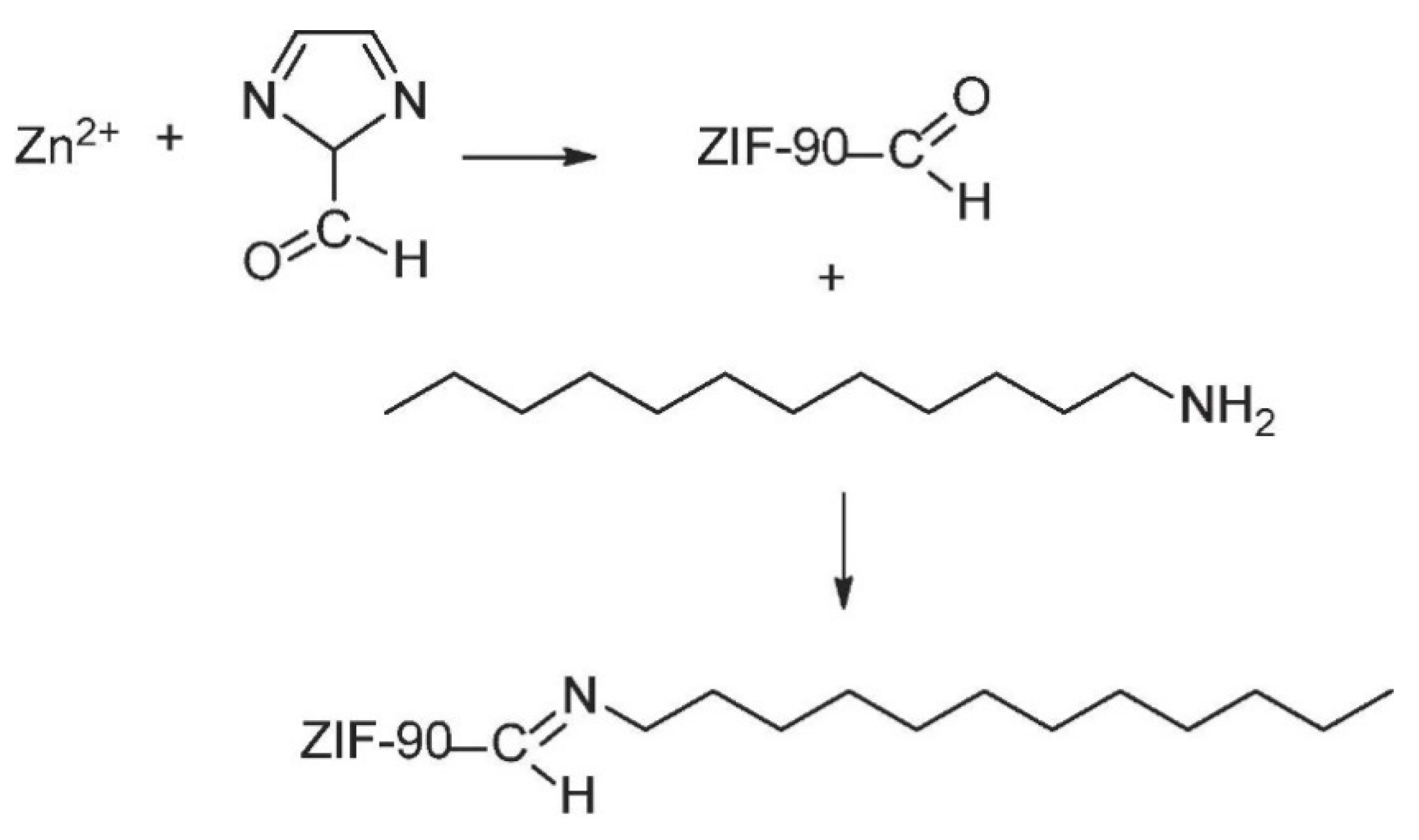
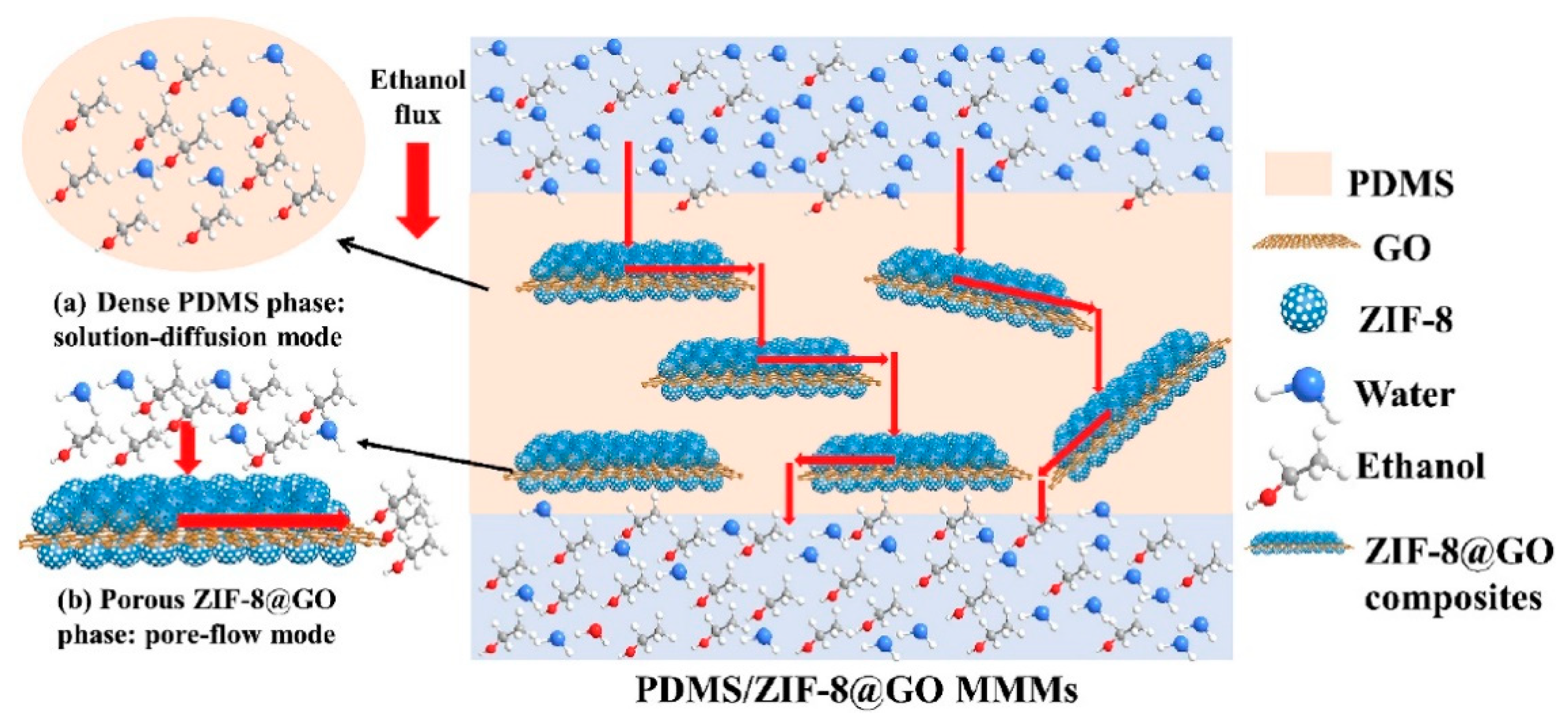
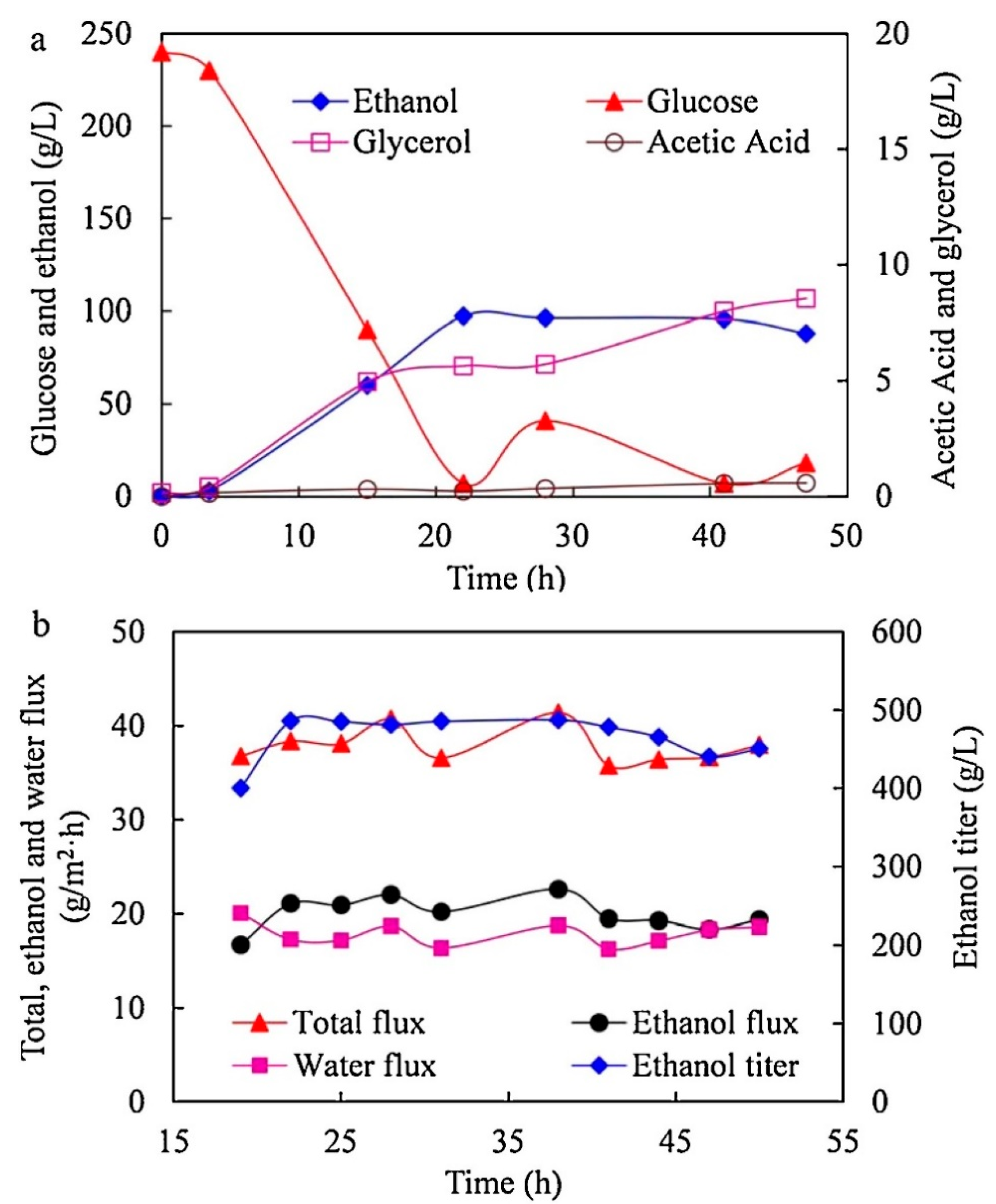
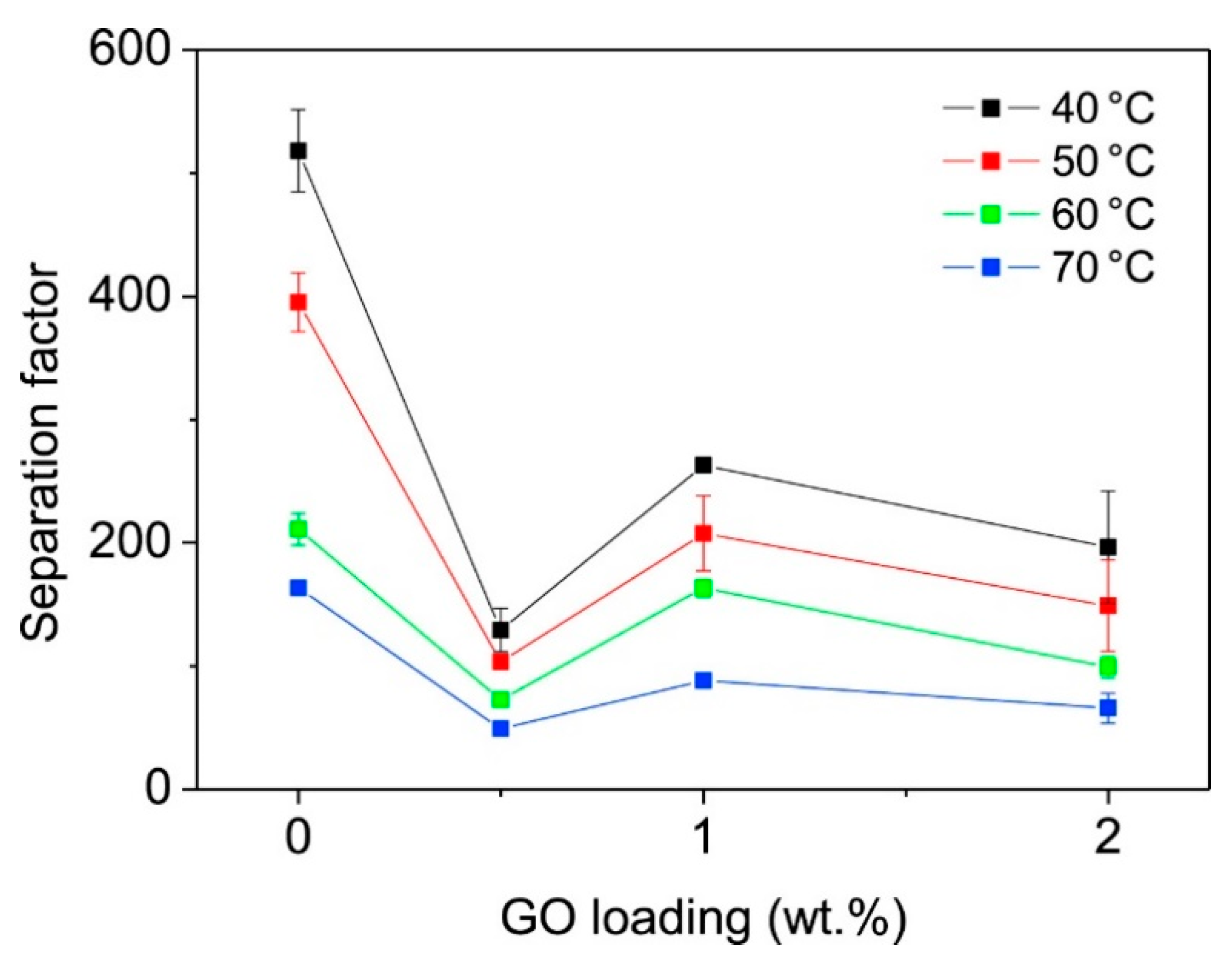
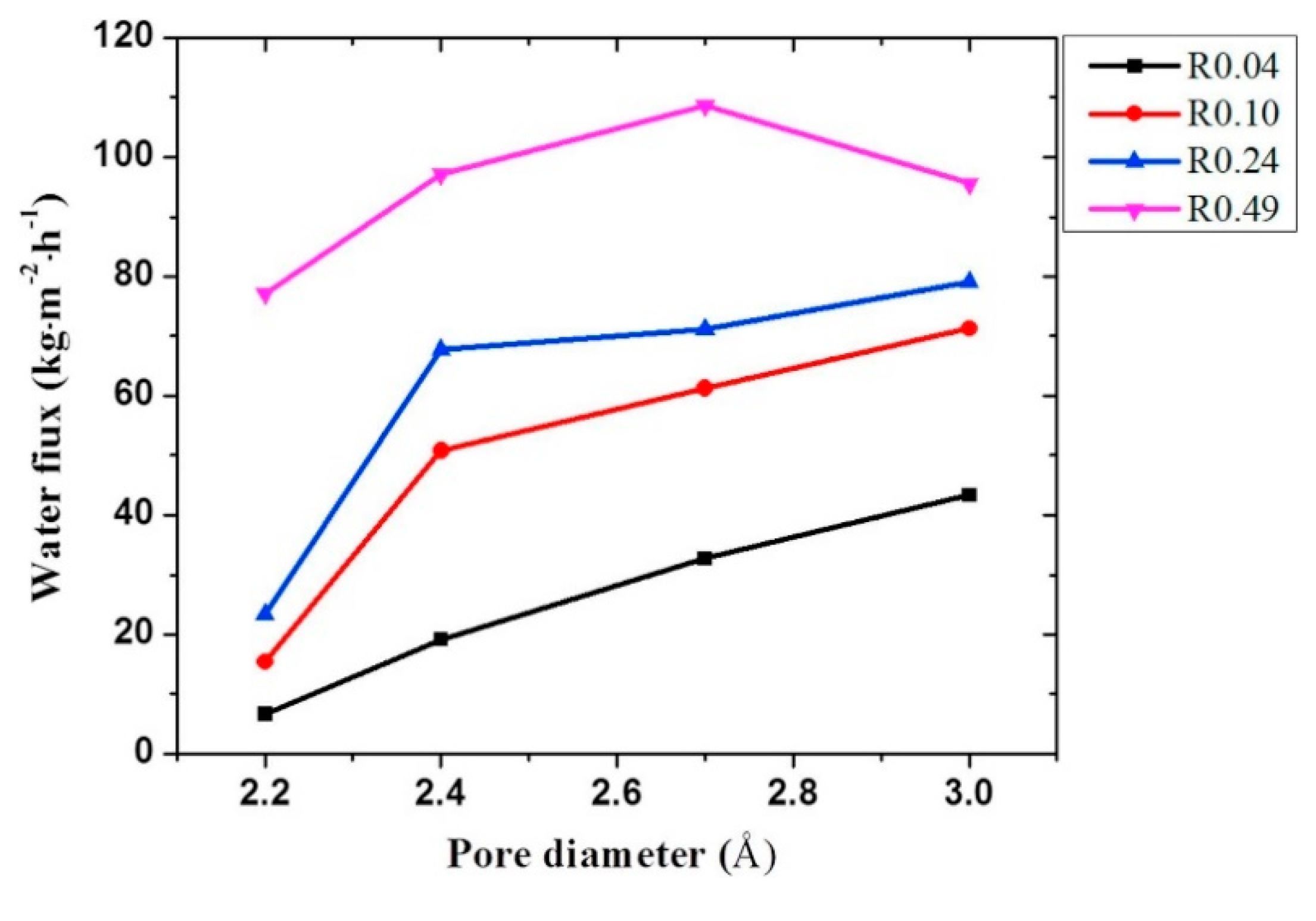
2.2. Zeolitic Imidazolate Frameworks (ZIFs)
2.3. Carbon Nanotubes
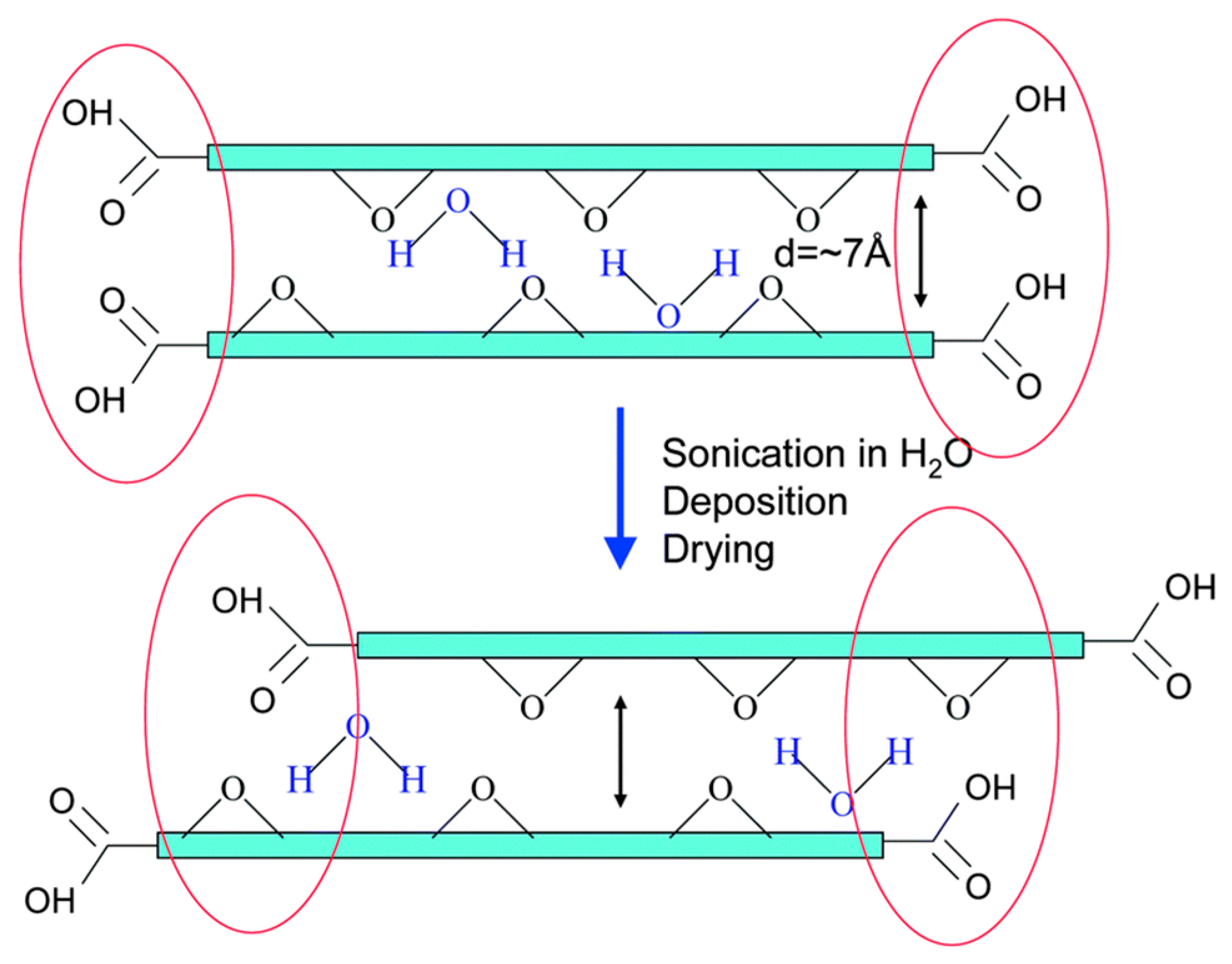
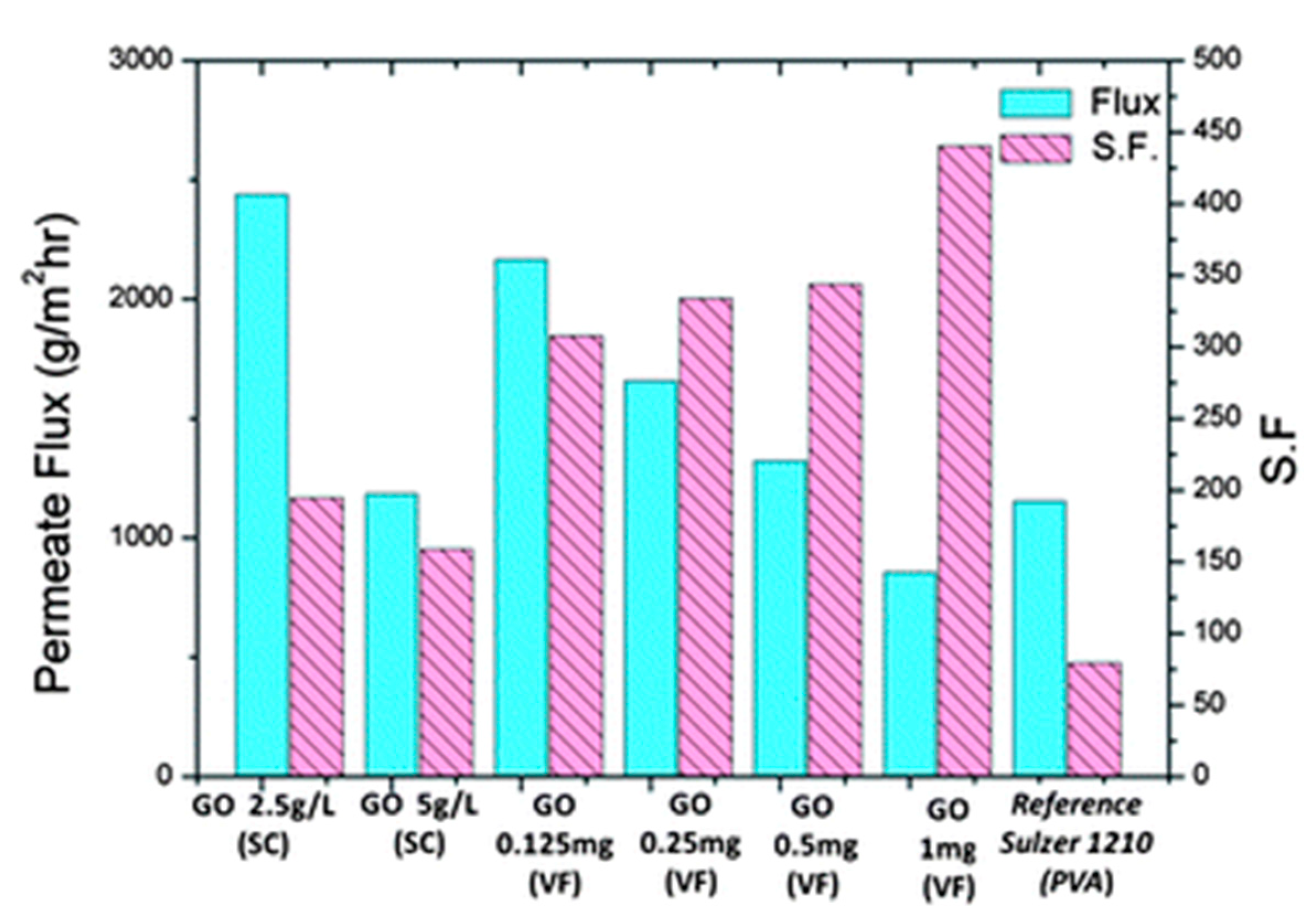
2.4. Graphene Oxide Membranes
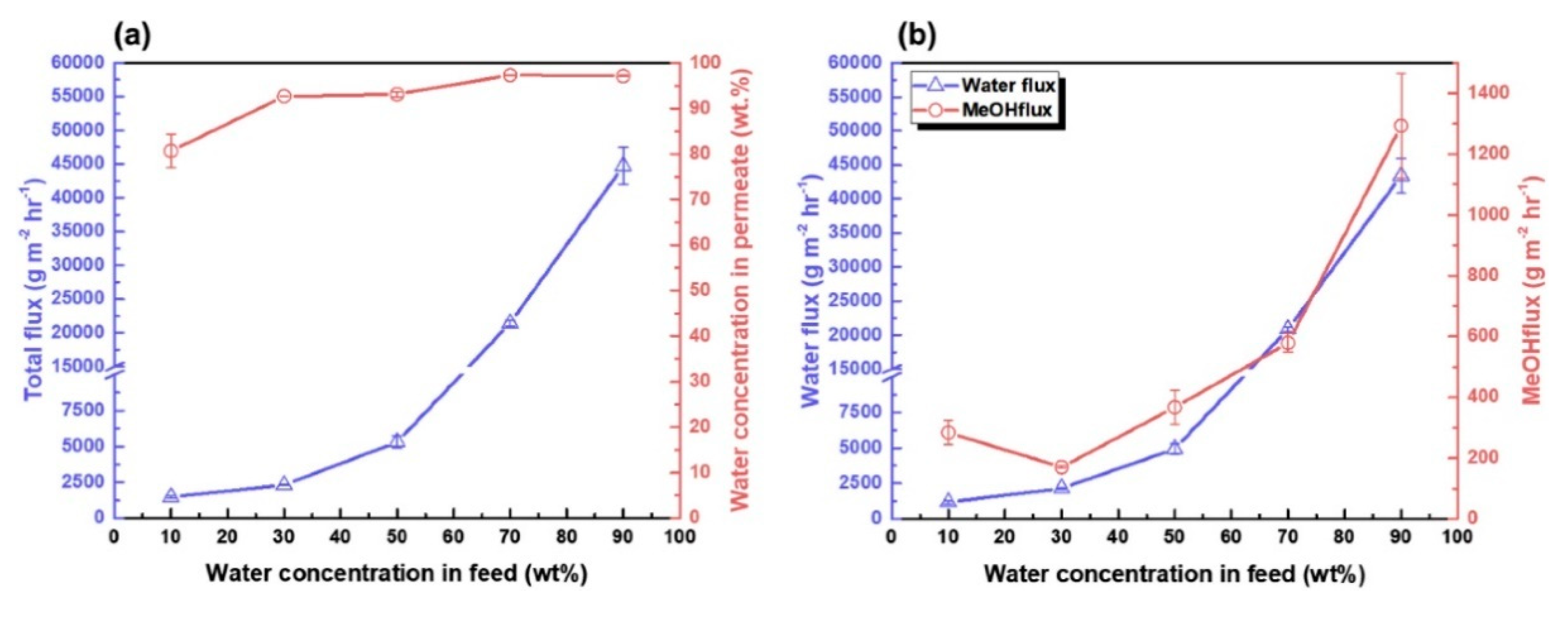
2.5. Cellulose Nanocrystals
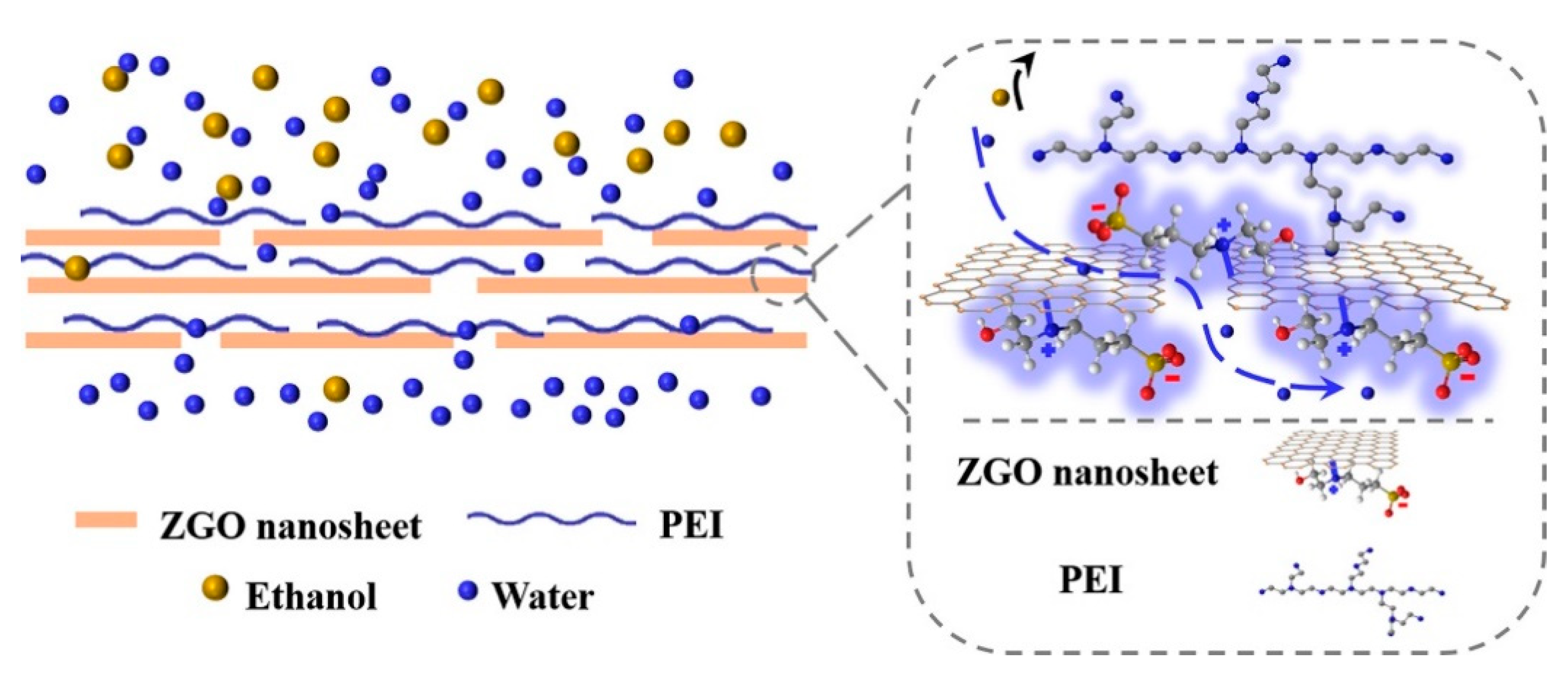
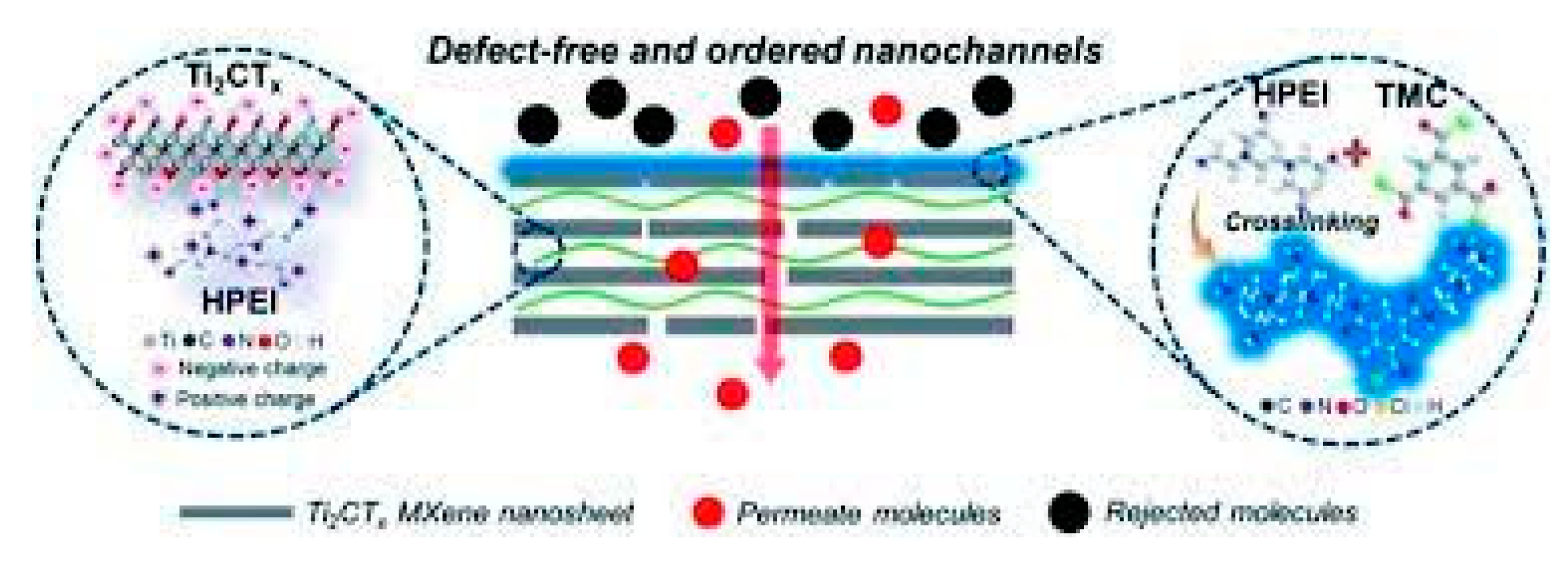
2.6. MXenes
2.7. Covalent Organic Frameworks (COFs)
2.8. Transition Metal Dichalcogenides (TMD)
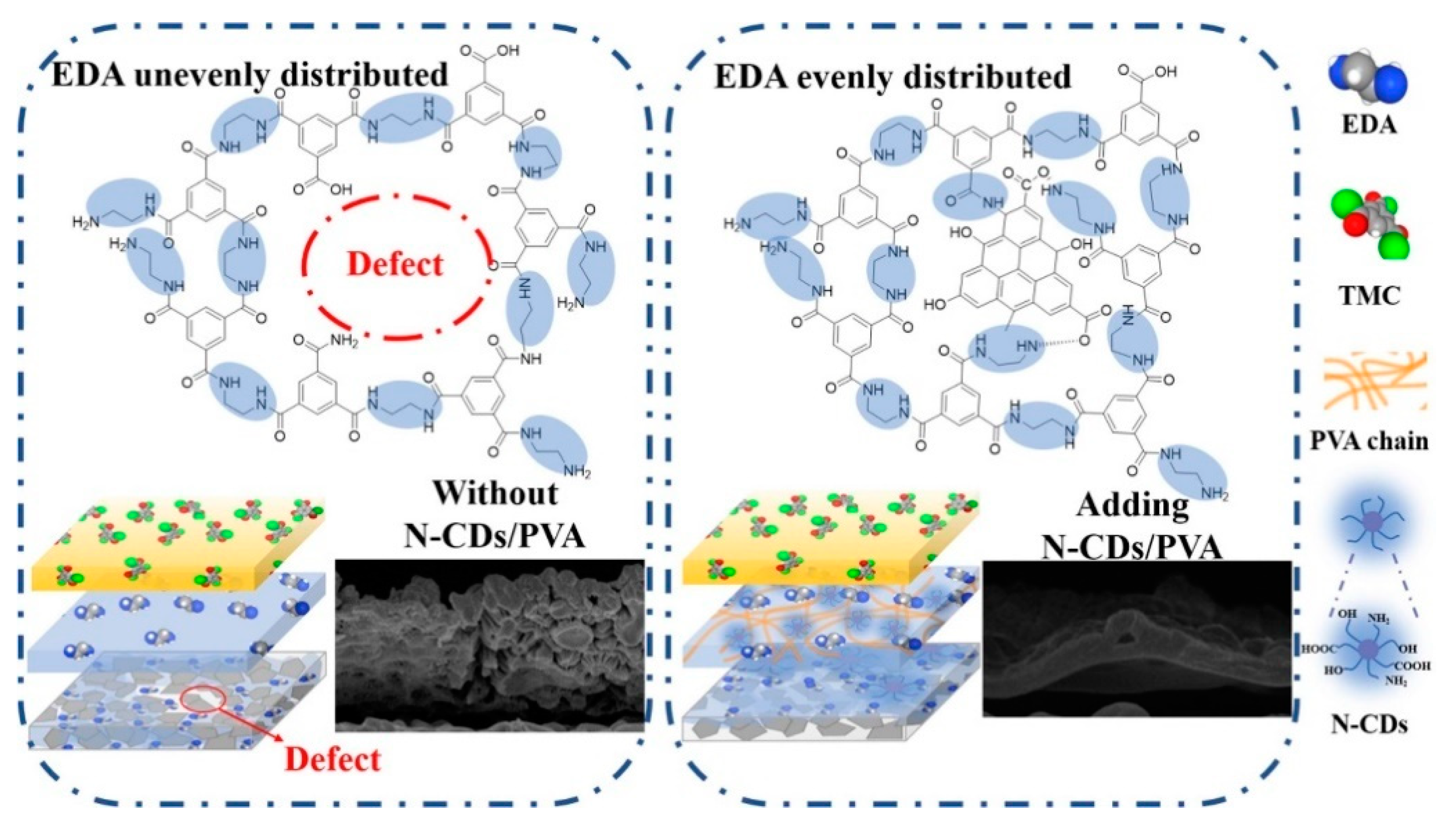
2.9. Metal Organic Framework (MOFs)
3. Limitations and Future Perspectives
4. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Le, N.L.; Wang, Y.; Chung, T.-S. Pebax/POSS mixed matrix membranes for ethanol recovery from aqueous solutions via pervaporation. J. Membr. Sci. 2011, 379, 174–183. [Google Scholar] [CrossRef]
- Vane, L.M. Separation technologies for the recovery and dehydration of alcohols from fermentation broths. Biofuels Bioprod. Biorefining 2008, 2, 553–588. [Google Scholar] [CrossRef]
- Marchetti, P.; Jimenez Solomon, M.F.; Szekely, G.; Livingston, A.G. Molecular separation with organic solvent nanofiltration: A critical review. Chem. Rev. 2014, 114, 10735–10806. [Google Scholar] [CrossRef] [PubMed]
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 16018. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef]
- Pandey, P.; Chauhan, R. Membranes for gas separation. Prog. Polym. Sci. 2001, 26, 853–893. [Google Scholar] [CrossRef]
- Robeson, L.M. Polymer membranes for gas separation. Curr. Opin. Solid State Mater. Sci. 1999, 4, 549–552. [Google Scholar] [CrossRef]
- Gupta, I.; Chakraborty, J.; Roy, S.; Farinas, E.T.; Mitra, S. Synergistic Effects of Microwave Radiation and Nanocarbon Immobilized Membranes in the Generation of Bacteria-Free Water via Membrane Distillation. Ind. Eng. Chem. Res. 2021, 61, 1453–1463. [Google Scholar] [CrossRef]
- Gupta, I.; Chakraborty, J.; Roy, S.; Farinas, E.T.; Mitra, S. Nanocarbon immobilized membranes for generating bacteria and endotoxin free water via membrane distillation. Sep. Purif. Technol. 2021, 259, 118133. [Google Scholar] [CrossRef]
- Geise, G.M.; Lee, H.S.; Miller, D.J.; Freeman, B.D.; McGrath, J.E.; Paul, D.R. Water purification by membranes: The role of polymer science. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 1685–1718. [Google Scholar] [CrossRef]
- Fane, A.G.; Wang, R.; Hu, M.X. Synthetic membranes for water purification: Status and future. Angew. Chem. Int. Ed. 2015, 54, 3368–3386. [Google Scholar] [CrossRef]
- Liang, B.; He, X.; Hou, J.; Li, L.; Tang, Z. Membrane separation in organic liquid: Technologies, achievements, and opportunities. Adv. Mater. 2019, 31, 1806090. [Google Scholar] [CrossRef]
- Blume, I.; Wijmans, J.; Baker, R. The separation of dissolved organics from water by pervaporation. J. Membr. Sci. 1990, 49, 253–286. [Google Scholar] [CrossRef]
- Lively, R.P.; Sholl, D.S. From water to organics in membrane separations. Nat. Mater. 2017, 16, 276–279. [Google Scholar] [CrossRef]
- Vandezande, P.; Gevers, L.E.; Vankelecom, I.F. Solvent resistant nanofiltration: Separating on a molecular level. Chem. Soc. Rev. 2008, 37, 365–405. [Google Scholar] [CrossRef]
- Moriarty, P.; Honnery, D. What is the global potential for renewable energy? Renew. Sustain. Energy Rev. 2012, 16, 244–252. [Google Scholar] [CrossRef]
- Milestone, N.B.; Bibby, D.M. Concentration of alcohols by adsorption on silicalite. J. Chem. Technol. Biotechnol. 1981, 31, 732–736. [Google Scholar] [CrossRef]
- Nielsen, L.; Larsson, M.; Holst, O.; Mattiasson, B. Adsorbents for extractive bioconversion applied to the acetone-butanol fermentation. Appl. Microbiol. Biotechnol. 1988, 28, 335–339. [Google Scholar] [CrossRef]
- Ezeji, T.; Qureshi, N.; Blaschek, H. Production of acetone, butanol and ethanol by Clostridium beijerinckii BA101 and in situ recovery by gas stripping. World J. Microbiol. Biotechnol. 2003, 19, 595–603. [Google Scholar] [CrossRef]
- Maddox, I.; Qureshi, N.; Roberts-Thomson, K. Production of acetone-butanol-ethanol from concentrated substrate using clostridium acetobutylicum in an integrated fermentation-product removal process. Process Biochem. 1995, 30, 209–215. [Google Scholar]
- Ezeji, T.C.; Karcher, P.M.; Qureshi, N.; Blaschek, H.P. Improving performance of a gas stripping-based recovery system to remove butanol from Clostridium beijerinckii fermentation. Bioprocess Biosyst. Eng. 2005, 27, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Rawa-Adkonis, M.; Wolska, L.; Przyjazny, A.; Namieśnik, J. Sources of errors associated with the determination of PAH and PCB analytes in water samples. Anal. Lett. 2006, 39, 2317–2331. [Google Scholar] [CrossRef]
- Cichy, W.; Schlosser, Š.; Szymanowski, J. Extraction and pertraction of phenol through bulk liquid membranes. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2005, 80, 189–197. [Google Scholar] [CrossRef]
- Groot, W.; Soedjak, H.; Donck, P.; Van der Lans, R.; Luyben, K.; Timmer, J. Butanol recovery from fermentations by liquid-liquid extraction and membrane solvent extraction. Bioprocess Eng. 1990, 5, 203–216. [Google Scholar] [CrossRef]
- Lei, Z.; Li, C.; Chen, B. Extractive distillation: A review. Sep. Purif. Rev. 2003, 32, 121–213. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, P.; Ma, Y.; Zhang, Z. Extractive distillation and pressure-swing distillation for THF/ethanol separation. J. Chem. Technol. Biotechnol. 2015, 90, 1463–1472. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, W.; Ziemann, E.; Be’er, A.; Lu, X.; Elimelech, M.; Bernstein, R. Functionalization of ultrafiltration membrane with polyampholyte hydrogel and graphene oxide to achieve dual antifouling and antibacterial properties. J. Membr. Sci. 2018, 565, 293–302. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, H.; Bernstein, R. Zwitterionic hydrogel modified reduced graphene oxide/ZnO nanocomposite blended membrane with high antifouling and antibiofouling performances. J. Colloid Interface Sci. 2022, 613, 426–434. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, Y.; Ziemann, E.; Be’Er, A.; Bashouti, M.Y.; Elimelech, M.; Bernstein, R. One-step sonochemical synthesis of a reduced graphene oxide–ZnO nanocomposite with antibacterial and antibiofouling properties. Environ. Sci. Nano 2019, 6, 3080–3090. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, Z.; Kaufman, Y.; Bernstein, R. Surface and anti-fouling properties of a polyampholyte hydrogel grafted onto a polyethersulfone membrane. J. Colloid Interface Sci. 2018, 517, 155–165. [Google Scholar] [CrossRef]
- Nagase, Y.; Takamura, Y.; Matsui, K. Chemical modification of poly (substituted-acetylene). V. Alkylsilylation of poly (1-trimethylsilyl-1-propyne) and improved liquid separating property at pervaporation. J. Appl. Polym. Sci. 1991, 42, 185–190. [Google Scholar] [CrossRef]
- Te Hennepe, H.; Bargeman, D.; Mulder, M.; Smolders, C. Zeolite-filled silicone rubber membranes: Part 1. Membrane preparation and pervaporation results. J. Membr. Sci. 1987, 35, 39–55. [Google Scholar] [CrossRef]
- Sukitpaneenit, P.; Chung, T.-S.; Jiang, L.Y. Modified pore-flow model for pervaporation mass transport in PVDF hollow fiber membranes for ethanol-water separation. J. Membr. Sci. 2010, 362, 393–406. [Google Scholar] [CrossRef]
- Su, N.C.; Sun, D.T.; Beavers, C.M.; Britt, D.K.; Queen, W.L.; Urban, J.J. Enhanced permeation arising from dual transport pathways in hybrid polymer—MOF membranes. Energy Environ. Sci. 2016, 9, 922–931. [Google Scholar] [CrossRef]
- Ma, D.; Peh, S.B.; Han, G.; Chen, S.B. Thin-film nanocomposite (TFN) membranes incorporated with super-hydrophilic metal–organic framework (MOF) UiO-66: Toward enhancement of water flux and salt rejection. ACS Appl. Mater. Interfaces 2017, 9, 7523–7534. [Google Scholar] [CrossRef]
- Deng, Y.H.; Chen, J.T.; Chang, C.H.; Liao, K.S.; Tung, K.L.; Price, W.E.; Yamauchi, Y.; Wu, K.C.W. A drying-free, water-based process for fabricating mixed-matrix membranes with outstanding pervaporation performance. Angew. Chem. 2016, 128, 12985–12988. [Google Scholar] [CrossRef]
- Wu, K.C.-W.; Kang, C.-H.; Lin, Y.-F.; Tung, K.-L.; Deng, Y.-H.; Ahamad, T.; Alshehri, S.M.; Suzuki, N.; Yamauchi, Y. Towards acid-tolerated ethanol dehydration: Chitosan-based mixed matrix membranes containing cyano-bridged coordination polymer nanoparticles. J. Nanosci. Nanotechnol. 2016, 16, 4141–4146. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, H.; Wang, J.; Ding, R.; Du, Z.; Liu, J.; Cao, S. Mineralization-inspired preparation of composite membranes with polyethyleneimine-nanoparticle hybrid active layer for solvent resistant nanofiltration. J. Membr. Sci. 2014, 470, 70–79. [Google Scholar] [CrossRef]
- Li, Y.; Mao, H.; Zhang, H.; Yang, G.; Ding, R.; Wang, J. Tuning the microstructure and permeation property of thin film nanocomposite membrane by functionalized inorganic nanospheres for solvent resistant nanofiltration. Sep. Purif. Technol. 2016, 165, 60–70. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots: Properties, syntheses, and biological applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Tang, S.; Qiao, C.; Wang, L.; Wang, H.; Liu, X.; Li, B.; Li, Y.; Yu, W. Surface chemistry routes to modulate the photoluminescence of graphene quantum dots: From fluorescence mechanism to up-conversion bioimaging applications. Adv. Funct. Mater. 2012, 22, 4732–4740. [Google Scholar] [CrossRef]
- Wang, F.; Gu, Z.; Lei, W.; Wang, W.; Xia, X.; Hao, Q. Graphene quantum dots as a fluorescent sensing platform for highly efficient detection of copper (II) ions. Sens. Actuators B Chem. 2014, 190, 516–522. [Google Scholar] [CrossRef]
- Geng, X.; Niu, L.; Xing, Z.; Song, R.; Liu, G.; Sun, M.; Cheng, G.; Zhong, H.; Liu, Z.; Zhang, Z. Aqueous-processable noncovalent chemically converted graphene-quantum dot composites for flexible and transparent optoelectronic films. Adv. Mater. 2010, 22, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wei, K.; Zhang, W.; Bai, Y.; Sun, Y.; Gu, J. Graphene oxide quantum dots incorporated into a thin film nanocomposite membrane with high flux and antifouling properties for low-pressure nanofiltration. ACS Appl. Mater. Interfaces 2017, 9, 11082–11094. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.X.; Zhao, D.; Zhao, Q.; Wang, P.; Lu, X. Na (+)-functionalized carbon quantum dots: A new draw solute in forward osmosis for seawater desalination. Chem. Commun. 2014, 50, 7318–7321. [Google Scholar] [CrossRef]
- Wang, M.; Pan, F.; Yang, L.; Song, Y.; Wu, H.; Cheng, X.; Liu, G.; Yang, H.; Wang, H.; Jiang, Z. Graphene oxide quantum dots incorporated nanocomposite membranes with high water flux for pervaporative dehydration. J. Membr. Sci. 2018, 563, 903–913. [Google Scholar] [CrossRef]
- Lecaros, R.L.G.; Deseo, K.M.; Hung, W.-S.; Tayo, L.L.; Hu, C.-C.; An, Q.-F.; Tsai, H.-A.; Lee, K.-R.; Lai, J.-Y. Influence of integrating graphene oxide quantum dots on the fine structure characterization and alcohol dehydration performance of pervaporation composite membrane. J. Membr. Sci. 2019, 576, 36–47. [Google Scholar] [CrossRef]
- Lecaros, R.L.G.; Bismonte, M.E.; Doma, B.T., Jr.; Hung, W.-S.; Hu, C.-C.; Tsai, H.-A.; Huang, S.-H.; Lee, K.-R.; Lai, J.-Y. Alcohol dehydration performance of pervaporation composite membranes with reduced graphene oxide and graphene quantum dots homostructured filler. Carbon 2020, 162, 318–327. [Google Scholar] [CrossRef]
- Wu, Y.-Z.; Shareef, U.; Xu, J.-P.; Xu, Z.-L.; Li, P.-P.; Li, Y.-X.; Li, P.; Gao, P.; Zhang, X.; Xu, S.-J. Carbon quantum dots doped thin-film nanocomposite (TFN) membrane on macroporous ceramic hollow fiber support via one-step interfacial polymerization. Sep. Purif. Technol. 2021, 266, 118572. [Google Scholar] [CrossRef]
- Lecaros, R.L.G.; Valbuena, R.E.; Tayo, L.L.; Hung, W.-S.; Hu, C.-C.; Tsai, H.-A.; Huang, S.-H.; Lee, K.-R.; Lai, J.-Y. Tannin-based thin-film composite membranes integrated with nitrogen-doped graphene quantum dots for butanol dehydration through pervaporation. J. Membr. Sci. 2021, 623, 119077. [Google Scholar] [CrossRef]
- Fan, H.; Shi, Q.; Yan, H.; Ji, S.; Dong, J.; Zhang, G. Simultaneous Spray Self-Assembly of Highly Loaded ZIF-8–PDMS Nanohybrid Membranes Exhibiting Exceptionally High Biobutanol-Permselective Pervaporation. Angew. Chem. 2014, 126, 5684–5688. [Google Scholar] [CrossRef]
- Si, Z.; Cai, D.; Li, S.; Zhang, C.; Qin, P.; Tan, T. Carbonized ZIF-8 incorporated mixed matrix membrane for stable ABE recovery from fermentation broth. J. Membr. Sci. 2019, 579, 309–317. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, H.; Yu, F.; Zhao, X.; Wang, Y. Enhanced ethanol recovery of PDMS mixed matrix membranes with hydrophobically modified ZIF-90. Sep. Purif. Technol. 2018, 206, 80–89. [Google Scholar] [CrossRef]
- Pan, Y.; Zhu, T.; Xia, Q.; Yu, X.; Wang, Y. Constructing superhydrophobic ZIF-8 layer with bud-like surface morphology on PDMS composite membrane for highly efficient ethanol/water separation. J. Environ. Chem. Eng. 2021, 9, 104977. [Google Scholar] [CrossRef]
- Zhu, T.; Xu, S.; Yu, F.; Yu, X.; Wang, Y. ZIF-8@ GO composites incorporated polydimethylsiloxane membrane with prominent separation performance for ethanol recovery. J. Membr. Sci. 2020, 598, 117681. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Wang, N.; Li, X.; Zhang, Y.; Ye, Q.; Ji, S.; An, Q.-F. Recovery of bio-butanol from aqueous solution with ZIF-8 modified graphene oxide composite membrane. J. Membr. Sci. 2020, 598, 117671. [Google Scholar] [CrossRef]
- Ismail, A.; Goh, P.; Sanip, S.; Aziz, M. Transport and separation properties of carbon nanotube-mixed matrix membrane. Sep. Purif. Technol. 2009, 70, 12–26. [Google Scholar] [CrossRef]
- Georgakilas, V.; Bourlinos, A.; Gournis, D.; Tsoufis, T.; Trapalis, C.; Mateo-Alonso, A.; Prato, M. Multipurpose organically modified carbon nanotubes: From functionalization to nanotube composites. J. Am. Chem. Soc. 2008, 130, 8733–8740. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Rana, S.; Cho, J.W.; Li, L.; Chan, S.H. Polymer nanocomposites based on functionalized carbon nanotubes. Prog. Polym. Sci. 2010, 35, 837–867. [Google Scholar]
- Choi, J.H.; Jegal, J.; Kim, W.N. Modification of performances of various membranes using MWNTs as a modifier. In Macromolecular Symposia; Wiley-VCH: Weinheim, Germany, 2007; pp. 610–617. [Google Scholar]
- Yan, K.-K.; Jiao, L.; Lin, S.; Ji, X.; Lu, Y.; Zhang, L. Superhydrophobic electrospun nanofiber membrane coated by carbon nanotubes network for membrane distillation. Desalination 2018, 437, 26–33. [Google Scholar] [CrossRef]
- Yang, D.; Tian, D.; Xue, C.; Gao, F.; Liu, Y.; Li, H.; Bao, Y.; Liang, J.; Zhao, Z.; Qiu, J. Tuned fabrication of the aligned and opened CNT membrane with exceptionally high permeability and selectivity for bioalcohol recovery. Nano Lett. 2018, 18, 6150–6156. [Google Scholar] [CrossRef] [PubMed]
- Gupta, O.; Roy, S.; Mitra, S. Microwave induced membrane distillation for enhanced ethanol–water separation on a carbon nanotube immobilized membrane. Ind. Eng. Chem. Res. 2019, 58, 18313–18319. [Google Scholar] [CrossRef]
- Gupta, O.; Roy, S.; Mitra, S. Low temperature recovery of acetone–butanol–ethanol (ABE) fermentation products via microwave induced membrane distillation on carbon nanotube immobilized membranes. Sustain. Energy Fuels 2020, 4, 3487–3499. [Google Scholar] [CrossRef]
- Xue, C.; Du, G.-Q.; Chen, L.-J.; Ren, J.-G.; Sun, J.-X.; Bai, F.-W.; Yang, S.-T. A carbon nanotube filled polydimethylsiloxane hybrid membrane for enhanced butanol recovery. Sci. Rep. 2014, 4, 5925. [Google Scholar] [CrossRef]
- Xue, C.; Wang, Z.-X.; Du, G.-Q.; Fan, L.-H.; Mu, Y.; Ren, J.-G.; Bai, F.-W. Integration of ethanol removal using carbon nanotube (CNT)-mixed membrane and ethanol fermentation by self-flocculating yeast for antifouling ethanol recovery. Process Biochem. 2016, 51, 1140–1146. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.-S.; Bozoklu, G.; Cai, W.; Nguyen, S.T.; Ruoff, R.S. Graphene oxide papers modified by divalent ions—Enhancing mechanical properties via chemical cross-linking. ACS Nano 2008, 2, 572–578. [Google Scholar] [CrossRef]
- Shin, Y.; Liu, W.; Schwenzer, B.; Manandhar, S.; Chase-Woods, D.; Engelhard, M.H.; Devanathan, R.; Fifield, L.S.; Bennett, W.D.; Ginovska, B. Graphene oxide membranes with high permeability and selectivity for dehumidification of air. Carbon 2016, 106, 164–170. [Google Scholar] [CrossRef]
- Nair, R.; Wu, H.; Jayaram, P.N.; Grigorieva, I.V.; Geim, A. Unimpeded permeation of water through helium-leak–tight graphene-based membranes. Science 2012, 335, 442–444. [Google Scholar] [CrossRef]
- Gupta, O.; Roy, S.; Rao, L.; Mitra, S. Graphene Oxide-Carbon Nanotube (GO-CNT) Hybrid Mixed Matrix Membrane for Pervaporative Dehydration of Ethanol. Membranes 2022, 12, 1227. [Google Scholar] [CrossRef]
- Shin, Y.; Taufique, M.F.N.; Devanathan, R.; Cutsforth, E.C.; Lee, J.; Liu, W.; Fifield, L.S.; Gotthold, D.W. Highly selective supported graphene oxide membranes for water-ethanol separation. Sci. Rep. 2019, 9, 2251. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Buera-González, J.; de la Iglesia, O.; Galiano, F.; Fíla, V.; Malankowska, M.; Rubio, C.; Figoli, A.; Téllez, C.; Coronas, J. Towards the dehydration of ethanol using pervaporation cross-linked poly (vinyl alcohol)/graphene oxide membranes. J. Membr. Sci. 2019, 582, 423–434. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, Y.; Wang, X.; Liu, G.; Zhu, Y.; Tu, Y.; Lu, X.; Jin, W. Molecular dynamics simulation of water-ethanol separation through monolayer graphene oxide membranes: Significant role of O/C ratio and pore size. Sep. Purif. Technol. 2019, 224, 219–226. [Google Scholar] [CrossRef]
- Talyzin, A.V.; Hausmaninger, T.; You, S.; Szabó, T. The structure of graphene oxide membranes in liquid water, ethanol and water–ethanol mixtures. Nanoscale 2014, 6, 272–281. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, M.; Mao, Y.; Liu, G. Theoretical study on Janus graphene oxide membrane for water transport. Front. Chem. Sci. Eng. 2021, 15, 913–921. [Google Scholar] [CrossRef]
- Liang, F.; Zheng, J.; He, M.; Mao, Y.; Liu, G.; Zhao, J.; Jin, W. Exclusive and fast water channels in zwitterionic graphene oxide membrane for efficient water–ethanol separation. AIChE J. 2021, 67, e17215. [Google Scholar] [CrossRef]
- Yeh, T.-M.; Wang, Z.; Mahajan, D.; Hsiao, B.S.; Chu, B. High flux ethanol dehydration using nanofibrous membranes containing graphene oxide barrier layers. J. Mater. Chem. A 2013, 1, 12998–13003. [Google Scholar] [CrossRef]
- Hao, W.; Tong, Z.; Liu, X.; Zhang, B. Optimizing nanostrands-inserted graphene oxide membrane with polyelectrolyte protective layer for enhanced ethanol pervaporation dehydration. Sep. Purif. Technol. 2020, 251, 117322. [Google Scholar] [CrossRef]
- Liang, F.; Wang, H.; Liu, G.; Zhao, J.; Jin, W. Designing highly selective and stable water transport channel through graphene oxide membranes functionalized with polyhedral oligomeric silsesquioxane for ethanol dehydration. J. Membr. Sci. 2021, 638, 119675. [Google Scholar] [CrossRef]
- Tang, W.; Lou, H.; Li, Y.; Kong, X.; Wu, Y.; Gu, X. Ionic liquid modified graphene oxide-PEBA mixed matrix membrane for pervaporation of butanol aqueous solutions. J. Membr. Sci. 2019, 581, 93–104. [Google Scholar] [CrossRef]
- Mariano, M.; El Kissi, N.; Dufresne, A. Cellulose nanocrystals and related nanocomposites: Review of some properties and challenges. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 791–806. [Google Scholar] [CrossRef]
- Dagnon, K.L.; Shanmuganathan, K.; Weder, C.; Rowan, S.J. Water-triggered modulus changes of cellulose nanofiber nanocomposites with hydrophobic polymer matrices. Macromolecules 2012, 45, 4707–4715. [Google Scholar] [CrossRef]
- Kamtsikakis, A.; Baales, J.; Zeisler-Diehl, V.V.; Vanhecke, D.; Zoppe, J.O.; Schreiber, L.; Weder, C. Asymmetric water transport in dense leaf cuticles and cuticle-inspired compositionally graded membranes. Nat. Commun. 2021, 12, 1267. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Qu, P.; Li, S.; Gao, Y.; Zhang, L.P. Poly (vinyl alcohol)/cellulose nanocomposite pervaporation membranes for ethanol dehydration. In Materials Science Forum; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2011; pp. 383–386. [Google Scholar]
- Kamtsikakis, A.; Delepierre, G.; Weder, C. Cellulose nanocrystals as a tunable nanomaterial for pervaporation membranes with asymmetric transport properties. J. Membr. Sci. 2021, 635, 119473. [Google Scholar] [CrossRef]
- Liu, G.; Shen, J.; Liu, Q.; Liu, G.; Xiong, J.; Yang, J.; Jin, W. Ultrathin two-dimensional MXene membrane for pervaporation desalination. J. Membr. Sci. 2018, 548, 548–558. [Google Scholar] [CrossRef]
- Ren, C.E.; Hatzell, K.B.; Alhabeb, M.; Ling, Z.; Mahmoud, K.A.; Gogotsi, Y. Charge-and size-selective ion sieving through Ti3C2Tx MXene membranes. J. Phys. Chem. Lett. 2015, 6, 4026–4031. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Ding, B.; Chang, Z.; Zhang, X.; Yamauchi, Y.; Wu, K.C.W. Confined self-assembly in two-dimensional interlayer space: Monolayered mesoporous carbon nanosheets with in-plane orderly arranged mesopores and a highly graphitized framework. Angew. Chem. Int. Ed. 2018, 57, 2894–2898. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, G.; Ye, H.; Jin, W.; Cui, Z. Two-dimensional MXene incorporated chitosan mixed-matrix membranes for efficient solvent dehydration. J. Membr. Sci. 2018, 563, 625–632. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, L.; Lu, Z.; Deng, J.; Wei, Y. Two-dimensional MXene membrane for ethanol dehydration. J. Membr. Sci. 2019, 590, 117300. [Google Scholar] [CrossRef]
- Liu, G.; Shen, J.; Ji, Y.; Liu, Q.; Liu, G.; Yang, J.; Jin, W. Two-dimensional Ti2CTx MXene membranes with integrated and ordered nanochannels for efficient solvent dehydration. J. Mater. Chem. A 2019, 7, 12095–12104. [Google Scholar] [CrossRef]
- Cai, W.; Cheng, X.; Chen, X.; Li, J.; Pei, J. Poly (vinyl alcohol)-Modified Membranes by Ti3C2Tx for Ethanol Dehydration via Pervaporation. ACS Omega 2020, 5, 6277–6287. [Google Scholar] [CrossRef]
- Li, S.; Dai, J.; Geng, X.; Li, J.; Li, P.; Lei, J.; Wang, L.; He, J. Highly selective sodium alginate mixed-matrix membrane incorporating multi-layered MXene for ethanol dehydration. Sep. Purif. Technol. 2020, 235, 116206. [Google Scholar] [CrossRef]
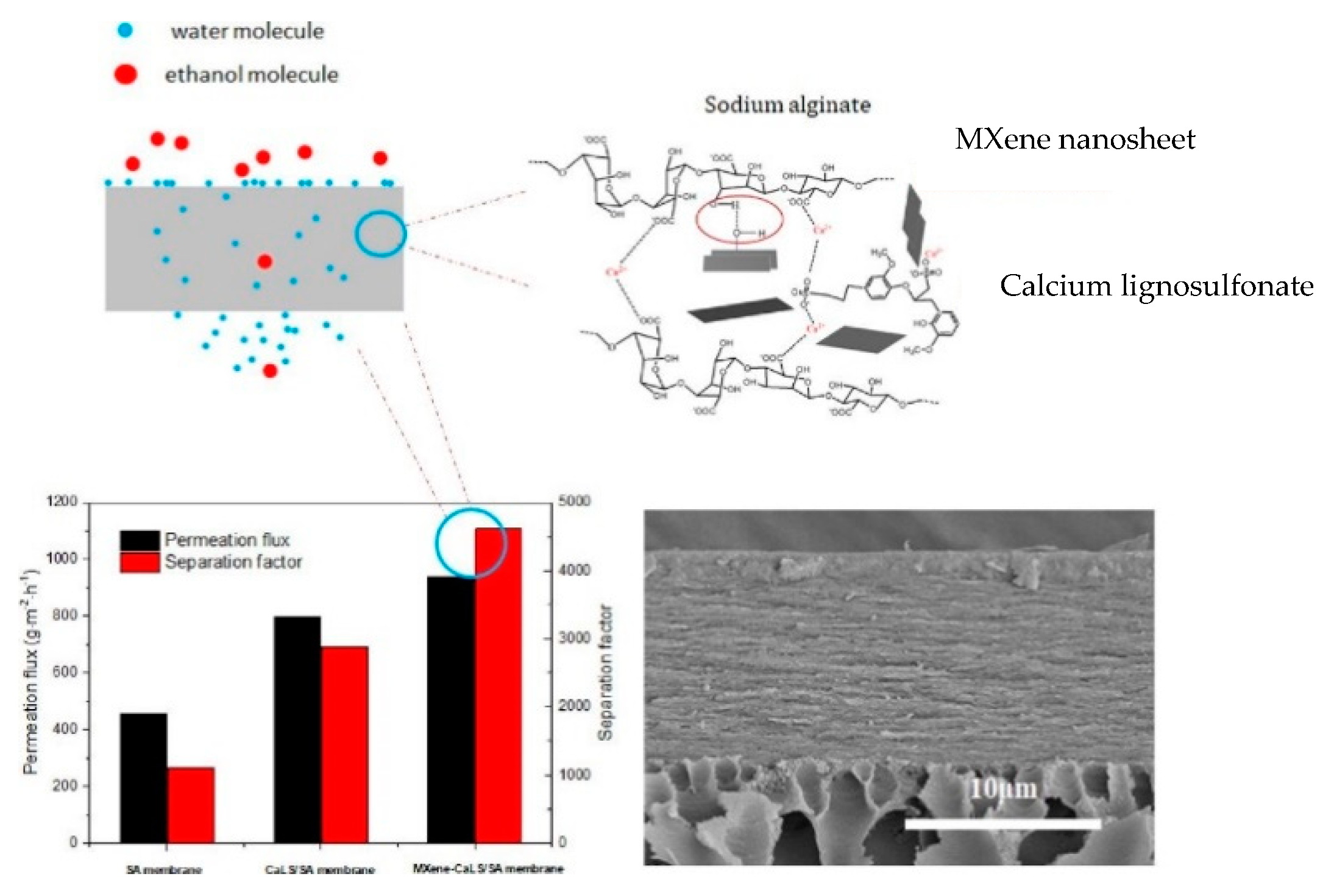
- Li, S.; Geng, X.; Ma, C.; Zhan, X.; Li, J.; Ma, M.; He, J.; Wang, L. Improved performance of three-component structure mixed membrane for pervaporation modified by lignosulfonates@ 2D-MXene. Sep. Purif. Technol. 2021, 276, 119294. [Google Scholar] [CrossRef]
- Zou, C.; Li, Q.; Hua, Y.; Zhou, B.; Duan, J.; Jin, W. Mechanical synthesis of COF nanosheet cluster and its mixed matrix membrane for efficient CO2 removal. ACS Appl. Mater. Interfaces 2017, 9, 29093–29100. [Google Scholar] [CrossRef]
- Duan, K.; Wang, J.; Zhang, Y.; Liu, J. Covalent organic frameworks (COFs) functionalized mixed matrix membrane for effective CO2/N2 separation. J. Membr. Sci. 2019, 572, 588–595. [Google Scholar] [CrossRef]
- Liu, G.; Jiang, Z.; Yang, H.; Li, C.; Wang, H.; Wang, M.; Song, Y.; Wu, H.; Pan, F. High-efficiency water-selective membranes from the solution-diffusion synergy of calcium alginate layer and covalent organic framework (COF) layer. J. Membr. Sci. 2019, 572, 557–566. [Google Scholar] [CrossRef]
- Xu, L.; Xu, J.; Shan, B.; Wang, X.; Gao, C. TpPa-2-incorporated mixed matrix membranes for efficient water purification. J. Membr. Sci. 2017, 526, 355–366. [Google Scholar] [CrossRef]
- Duong, P.H.; Kuehl, V.A.; Mastorovich, B.; Hoberg, J.O.; Parkinson, B.A.; Li-Oakey, K.D. Carboxyl-functionalized covalent organic framework as a two-dimensional nanofiller for mixed-matrix ultrafiltration membranes. J. Membr. Sci. 2019, 574, 338–348. [Google Scholar] [CrossRef]
- Wu, G.; Li, Y.; Geng, Y.; Jia, Z. In situ preparation of COF-LZU1 in poly (ether-block-amide) membranes for efficient pervaporation of n-butanol/water mixture. J. Membr. Sci. 2019, 581, 1–8. [Google Scholar] [CrossRef]
- Wu, G.; Lu, X.; Li, Y.; Jia, Z.; Cao, X.; Wang, B.; Zhang, P. Two-dimensional covalent organic frameworks (COF-LZU1) based mixed matrix membranes for pervaporation. Sep. Purif. Technol. 2020, 241, 116406. [Google Scholar] [CrossRef]
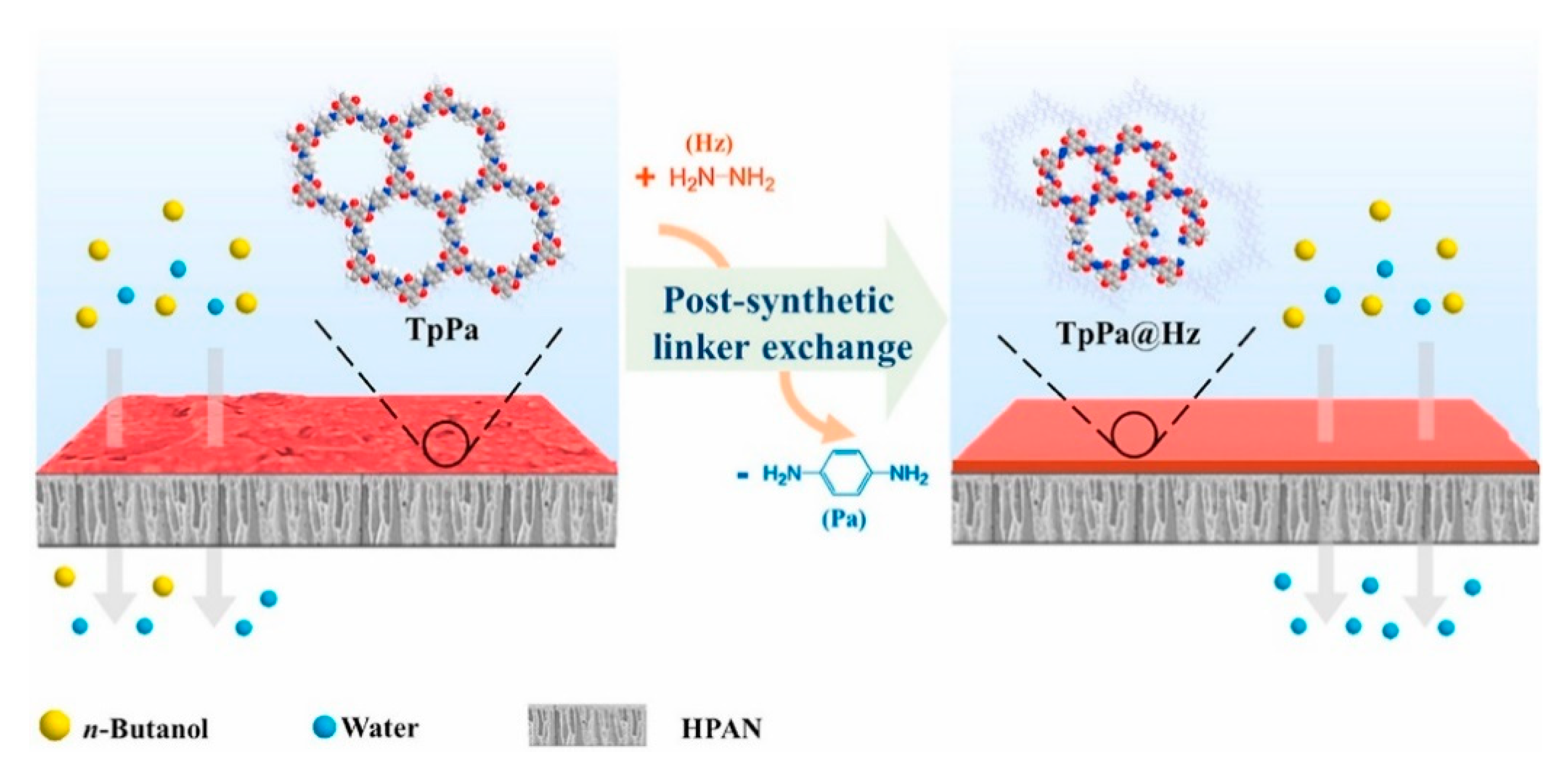
- Cao, C.; Wang, H.; Wang, M.; Liu, Y.; Zhang, Z.; Liang, S.; Yuhan, W.; Pan, F.; Jiang, Z. Conferring efficient alcohol dehydration to covalent organic framework membranes via post-synthetic linker exchange. J. Membr. Sci. 2021, 630, 119319. [Google Scholar] [CrossRef]
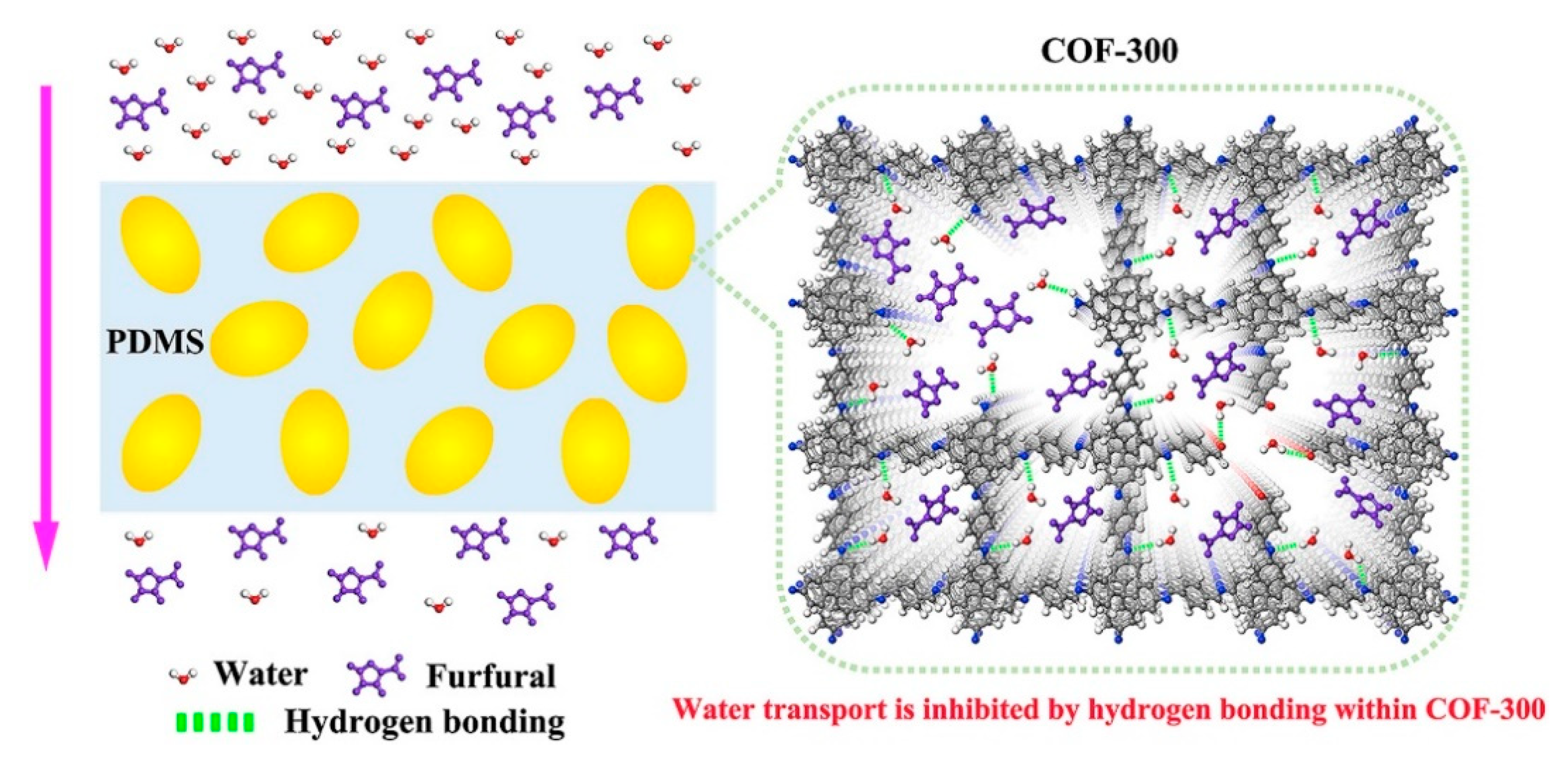
- Li, S.; Li, P.; Cai, D.; Shan, H.; Zhao, J.; Wang, Z.; Qin, P.; Tan, T. Boosting pervaporation performance by promoting organic permeability and simultaneously inhibiting water transport via blending PDMS with COF-300. J. Membr. Sci. 2019, 579, 141–150. [Google Scholar] [CrossRef]
- Yang, H.; Cheng, X.; Cheng, X.; Pan, F.; Wu, H.; Liu, G.; Song, Y.; Cao, X.; Jiang, Z. Highly water-selective membranes based on hollow covalent organic frameworks with fast transport pathways. J. Membr. Sci. 2018, 565, 331–341. [Google Scholar] [CrossRef]
- Yang, H.; Yang, L.; Wang, H.; Xu, Z.; Zhao, Y.; Luo, Y.; Nasir, N.; Song, Y.; Wu, H.; Pan, F. Covalent organic framework membranes through a mixed-dimensional assembly for molecular separations. Nat. Commun. 2019, 10, 2101. [Google Scholar] [CrossRef] [PubMed]
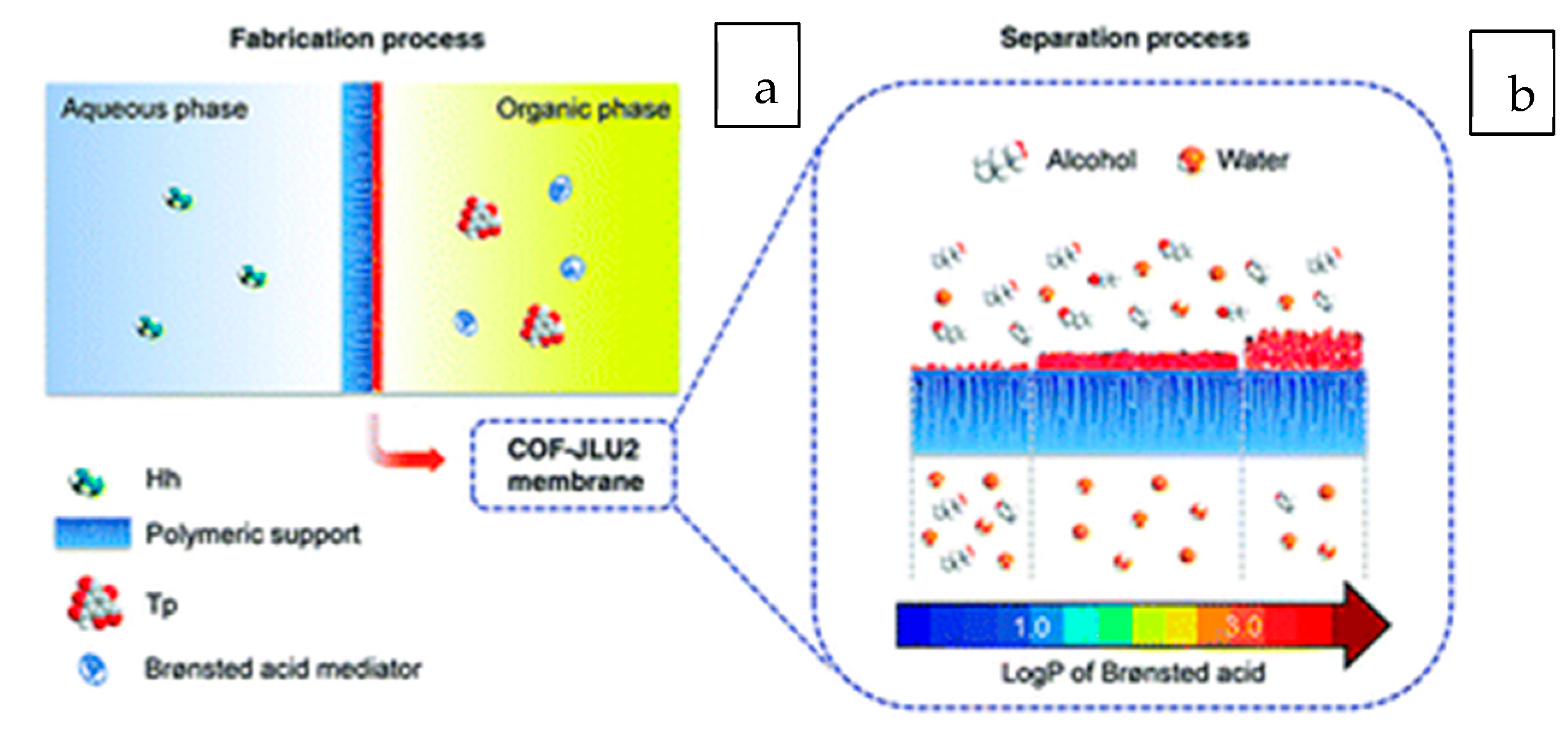
- Wang, H.; Chen, L.; Yang, H.; Wang, M.; Yang, L.; Du, H.; Cao, C.; Ren, Y.; Wu, Y.; Pan, F. Brønsted acid mediated covalent organic framework membranes for efficient molecular separation. J. Mater. Chem. A 2019, 7, 20317–20324. [Google Scholar] [CrossRef]
- Fan, H.; Xie, Y.; Li, J.; Zhang, L.; Zheng, Q.; Zhang, G. Ultra-high selectivity COF-based membranes for biobutanol production. J. Mater. Chem. A 2018, 6, 17602–17611. [Google Scholar] [CrossRef]
- Yang, H.; Wu, H.; Xu, Z.; Mu, B.; Lin, Z.; Cheng, X.; Liu, G.; Pan, F.; Cao, X.; Jiang, Z. Hierarchical pore architectures from 2D covalent organic nanosheets for efficient water/alcohol separation. J. Membr. Sci. 2018, 561, 79–88. [Google Scholar] [CrossRef]
- Castellanos-Gomez, A.; Poot, M.; Steele, G.A.; Van der Zant, H.S.; Agraït, N.; Rubio-Bollinger, G. Mechanical properties of freely suspended semiconducting graphene-like layers based on MoS2. Nanoscale Res. Lett. 2012, 7, 233. [Google Scholar] [CrossRef]
- Peng, Q.; De, S. Outstanding mechanical properties of monolayer MoS2 and its application in elastic energy storage. Phys. Chem. Chem. Phys. 2013, 15, 19427–19437. [Google Scholar] [CrossRef]
- Govind Rajan, A.; Sresht, V.; Pádua, A.A.; Strano, M.S.; Blankschtein, D. Dominance of dispersion interactions and entropy over electrostatics in determining the wettability and friction of two-dimensional MoS2 surfaces. ACS Nano 2016, 10, 9145–9155. [Google Scholar] [CrossRef]
- Wang, Z.; Mi, B. Environmental applications of 2D molybdenum disulfide (MoS2) nanosheets. Environ. Sci. Technol. 2017, 51, 8229–8244. [Google Scholar] [CrossRef]
- Deng, M.; Kwac, K.; Li, M.; Jung, Y.; Park, H.G. Stability, molecular sieving, and ion diffusion selectivity of a lamellar membrane from two-dimensional molybdenum disulfide. Nano Lett. 2017, 17, 2342–2348. [Google Scholar] [CrossRef]
- Zhang, H.; Taymazov, D.; Li, M.-P.; Huang, Z.-H.; Liu, W.-L.; Zhang, X.; Ma, X.-H.; Xu, Z.-L. Construction of MoS2 composite membranes on ceramic hollow fibers for efficient water desalination. J. Membr. Sci. 2019, 592, 117369. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, Z.; Gao, B.; Wang, H.; Wang, M.; He, Z.; Cao, X.; Pan, F. Embedding hydrophobic MoS2 nanosheets within hydrophilic sodium alginate membrane for enhanced ethanol dehydration. Chem. Eng. Sci. 2018, 185, 231–242. [Google Scholar] [CrossRef]
- Kan, L.; Li, G.; Liu, Y. Highly selective separation of C3H8 and C2H2 from CH4 within two water-stable Zn5 cluster-based metal–organic frameworks. ACS Appl. Mater. Interfaces 2020, 12, 18642–18649. [Google Scholar] [CrossRef]
- Peng, Y.L.; He, C.; Pham, T.; Wang, T.; Li, P.; Krishna, R.; Forrest, K.A.; Hogan, A.; Suepaul, S.; Space, B. Robust microporous metal–organic frameworks for highly efficient and simultaneous removal of propyne and propadiene from propylene. Angew. Chem. 2019, 131, 10315–10320. [Google Scholar] [CrossRef]
- Sarawade, P.; Tan, H.; Polshettiwar, V. Shape-and morphology-controlled Sustainable synthesis of Cu, Co, and in metal organic frameworks with high CO2 capture capacity. ACS Sustain. Chem. Eng. 2013, 1, 66–74. [Google Scholar] [CrossRef]
- Alezi, D.; Belmabkhout, Y.; Suyetin, M.; Bhatt, P.M.; Weseliński, Ł.J.; Solovyeva, V.; Adil, K.; Spanopoulos, I.; Trikalitis, P.N.; Emwas, A.-H. MOF crystal chemistry paving the way to gas storage needs: Aluminum-based soc-MOF for CH4, O2, and CO2 storage. J. Am. Chem. Soc. 2015, 137, 13308–13318. [Google Scholar] [CrossRef]
- Chen, Y.; Qiao, Z.; Lv, D.; Wu, H.; Shi, R.; Xia, Q.; Wang, H.; Zhou, J.; Li, Z. Selective adsorption of light alkanes on a highly robust indium based metal–organic framework. Ind. Eng. Chem. Res. 2017, 56, 4488–4495. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Q.; Zhao, J.; Hua, Y.; Sun, J.; Duan, J.; Jin, W. High efficient water/ethanol separation by a mixed matrix membrane incorporating MOF filler with high water adsorption capacity. J. Membr. Sci. 2017, 544, 68–78. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, C.; Peng, L.; Wang, X.; Kong, Q.; Gu, X. Enhanced stability of MFI zeolite membranes for separation of ethanol/water by eliminating surface Si–OH groups. ACS Appl. Mater. Interfaces 2018, 10, 3175–3180. [Google Scholar] [CrossRef]
- Wang, L.; Huang, H.; Chang, Y.; Zhong, C. Integrated High Water Affinity and Size Exclusion Effect on Robust Cu-Based Metal–Organic Framework for Efficient Ethanol–Water Separation. ACS Sustain. Chem. Eng. 2021, 9, 3195–3202. [Google Scholar] [CrossRef]
- Jiang, H.; Shi, W.; Liu, Q.; Wang, H.; Li, J.; Wu, C.; Li, Y.; Wei, Z. Intensification of water/ethanol separation by PVA hybrid membrane with different functional ligand UiO-66-X nanochannels in pervaporation process. Sep. Purif. Technol. 2021, 256, 117802. [Google Scholar] [CrossRef]
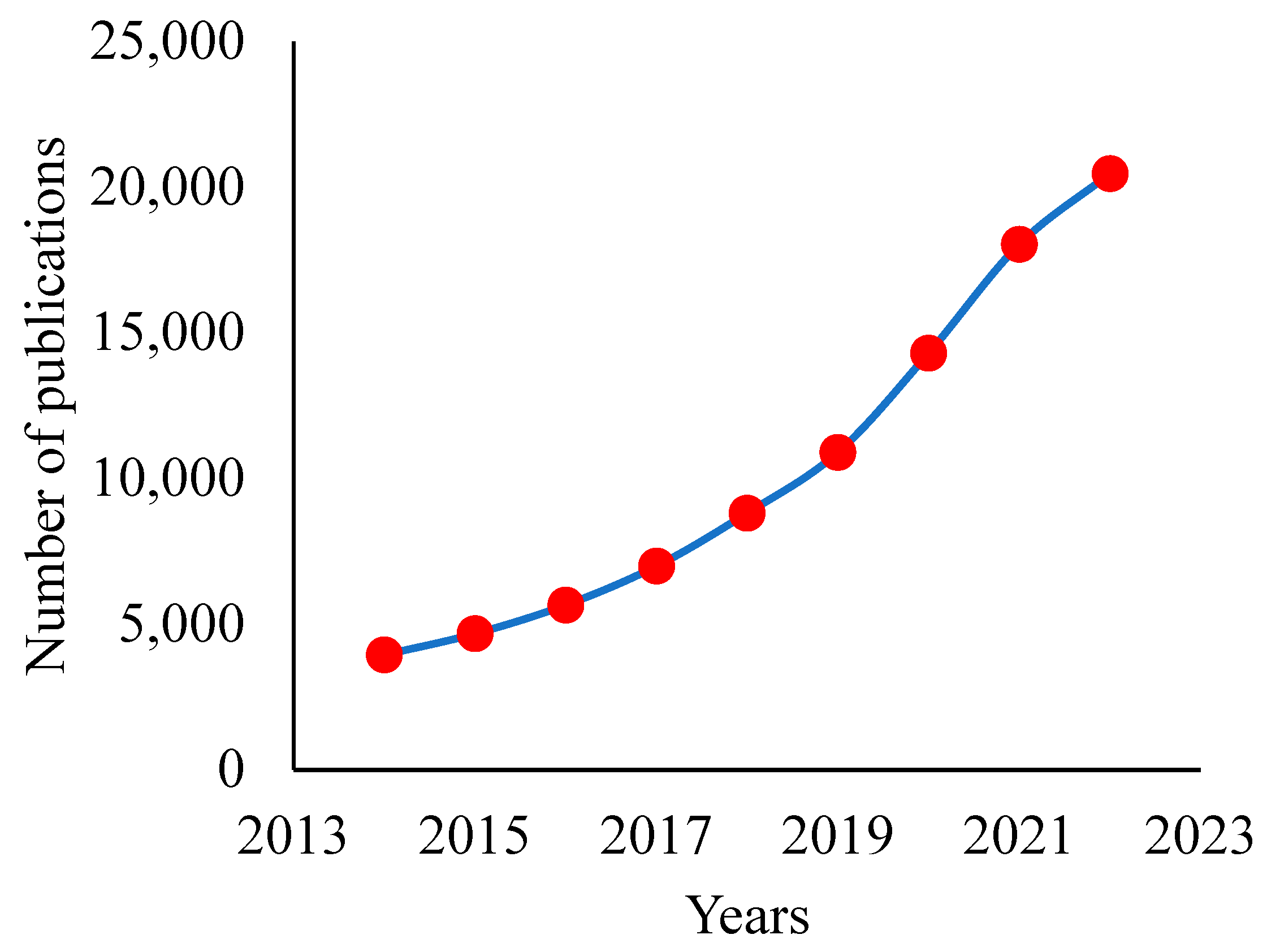










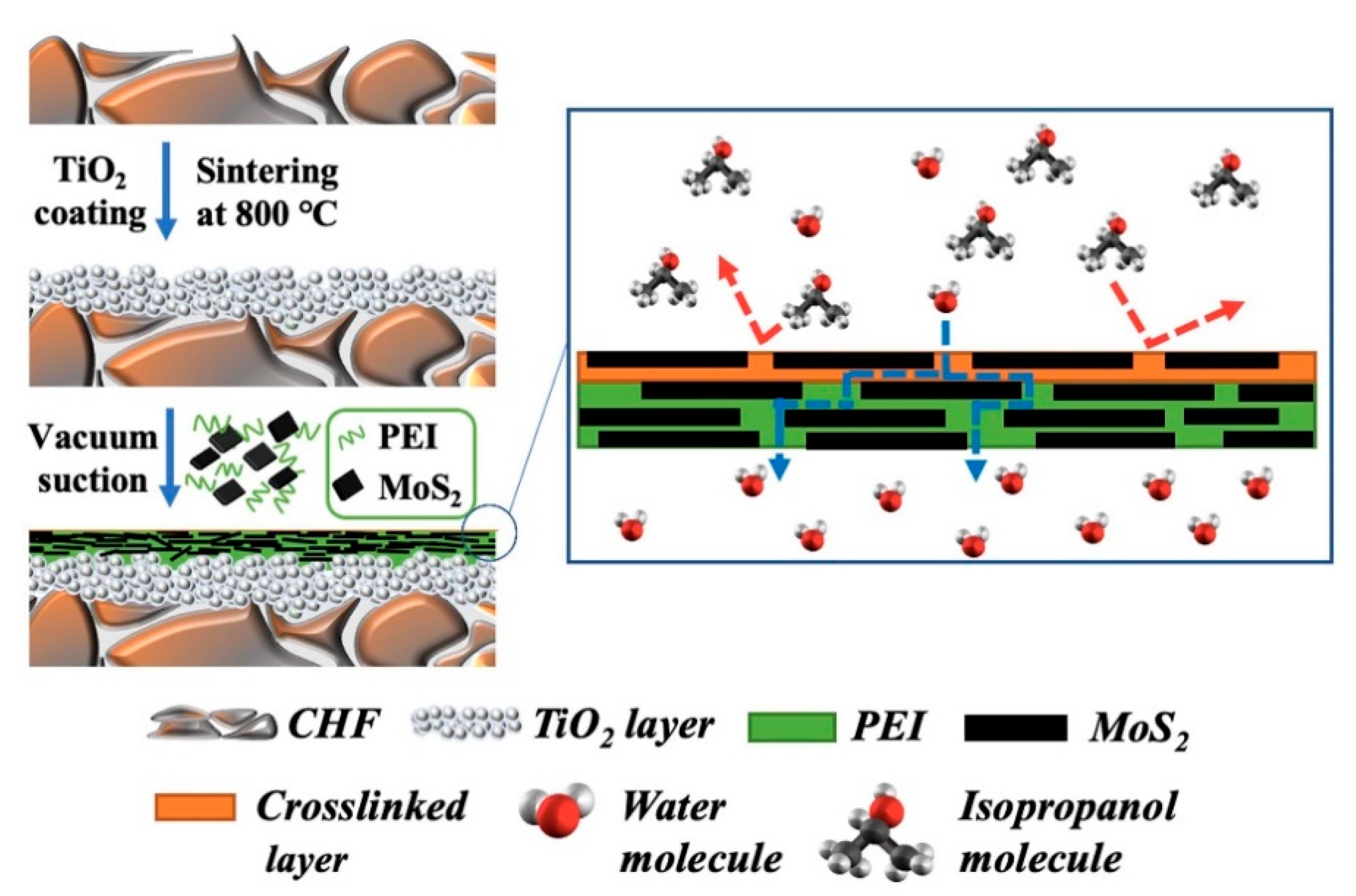
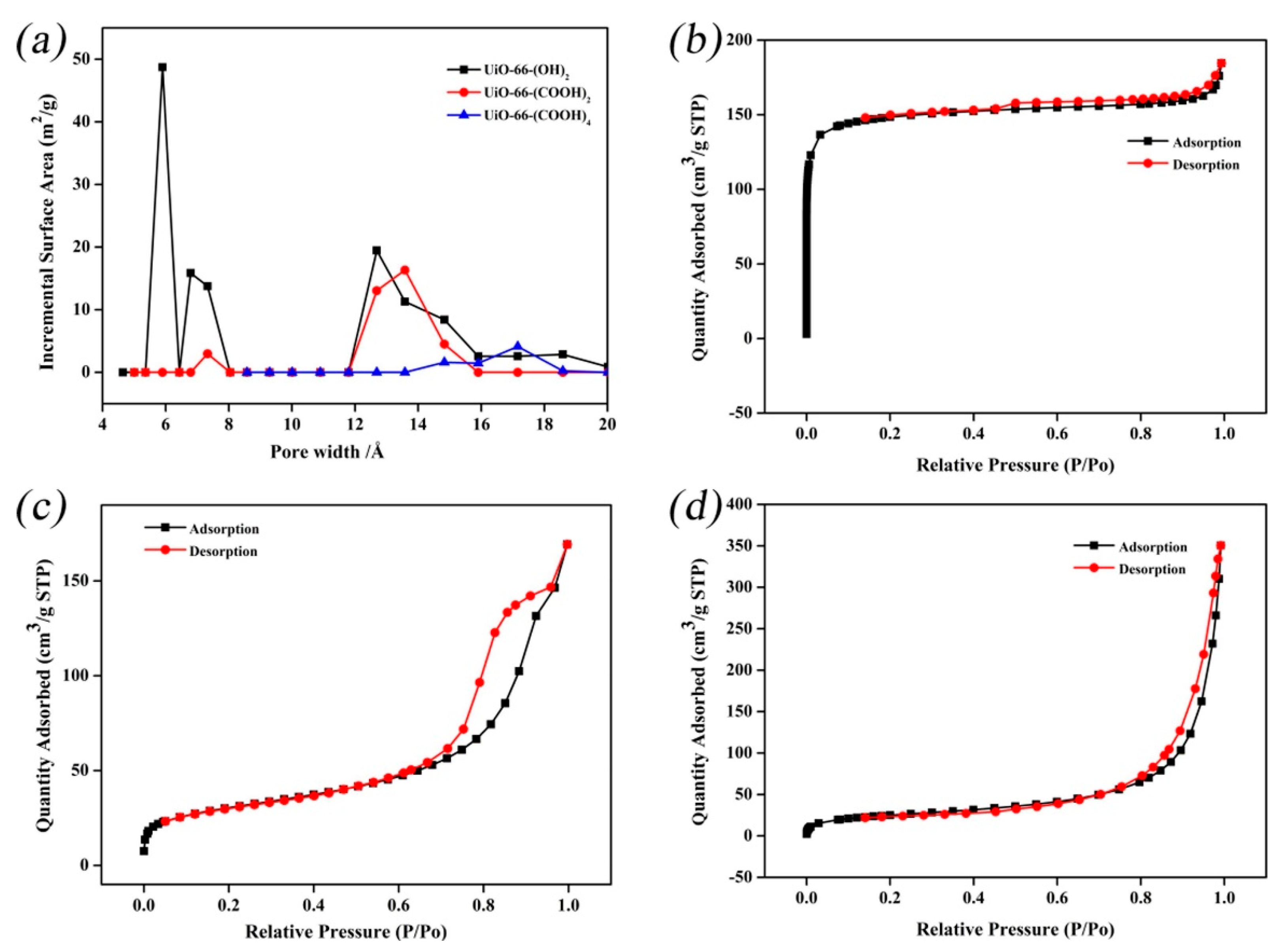
| Technology | Advantages | Disadvantages | References |
|---|---|---|---|
| Adsorption |
|
| [17,18] |
| Gas Stripping |
|
| [19,20,21] |
| Liquid-Liquid Extraction |
|
| [22] |
| Pertraction |
|
| [23,24] |
| Distillation |
|
| [2,25,26] |
| Membrane Separation |
|
| [27,28,29,30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, I.; Gupta, O. Recent Advancements in the Recovery and Reuse of Organic Solvents Using Novel Nanomaterial-Based Membranes for Renewable Energy Applications. Membranes 2023, 13, 108. https://doi.org/10.3390/membranes13010108
Gupta I, Gupta O. Recent Advancements in the Recovery and Reuse of Organic Solvents Using Novel Nanomaterial-Based Membranes for Renewable Energy Applications. Membranes. 2023; 13(1):108. https://doi.org/10.3390/membranes13010108
Chicago/Turabian StyleGupta, Indrani, and Oindrila Gupta. 2023. "Recent Advancements in the Recovery and Reuse of Organic Solvents Using Novel Nanomaterial-Based Membranes for Renewable Energy Applications" Membranes 13, no. 1: 108. https://doi.org/10.3390/membranes13010108
APA StyleGupta, I., & Gupta, O. (2023). Recent Advancements in the Recovery and Reuse of Organic Solvents Using Novel Nanomaterial-Based Membranes for Renewable Energy Applications. Membranes, 13(1), 108. https://doi.org/10.3390/membranes13010108





