Synthesis, Characterization, and Performance of Semi-Refined Kappa Carrageenan-Based Film Incorporating Cassava Starch
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Films at Various Cassava Starch (CS) Concentrations
2.3. Characterization of Bionanocomposite Film
2.3.1. Fourier-Transform Infrared (FTIR)
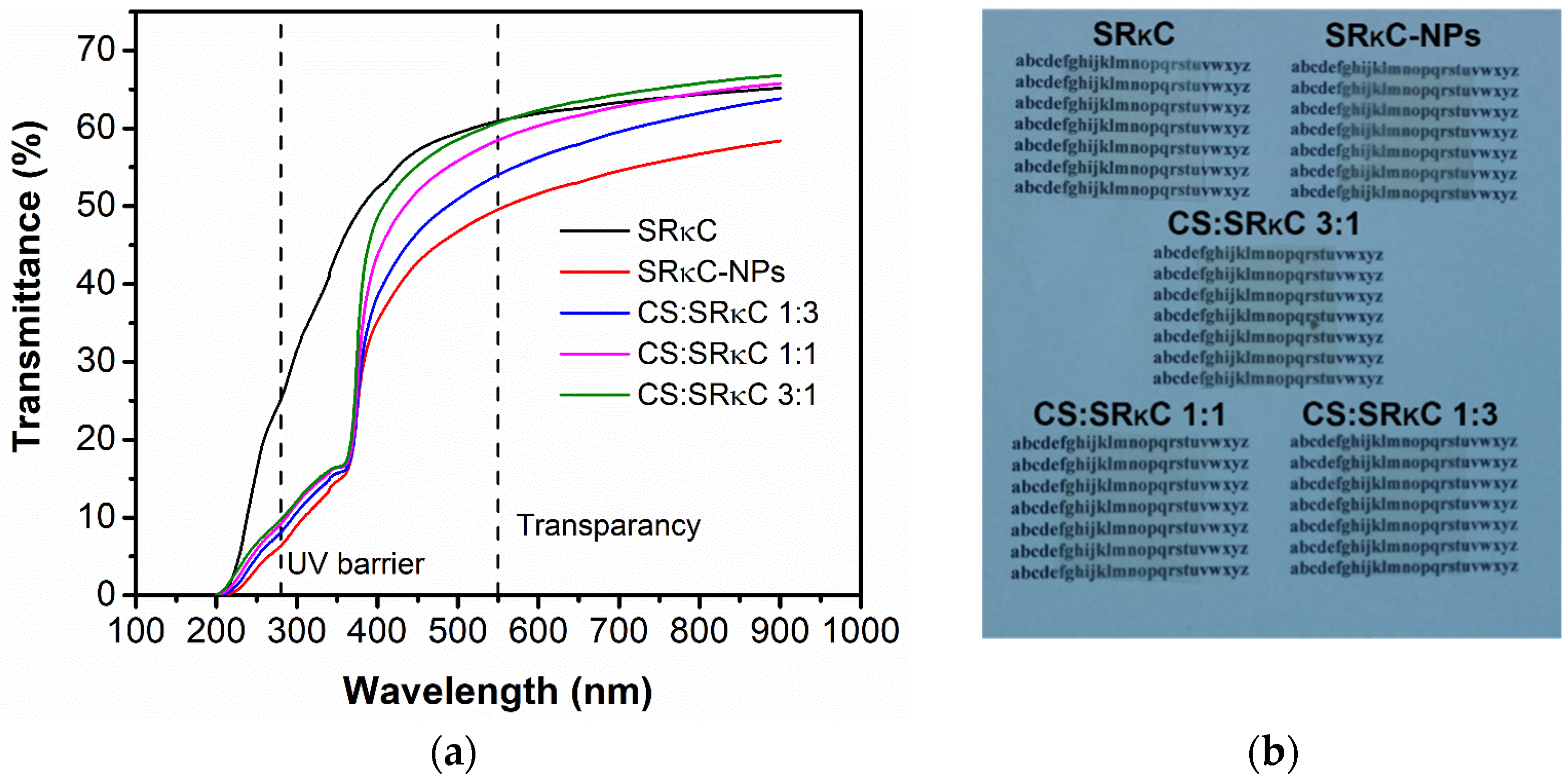
2.3.2. Optical Properties
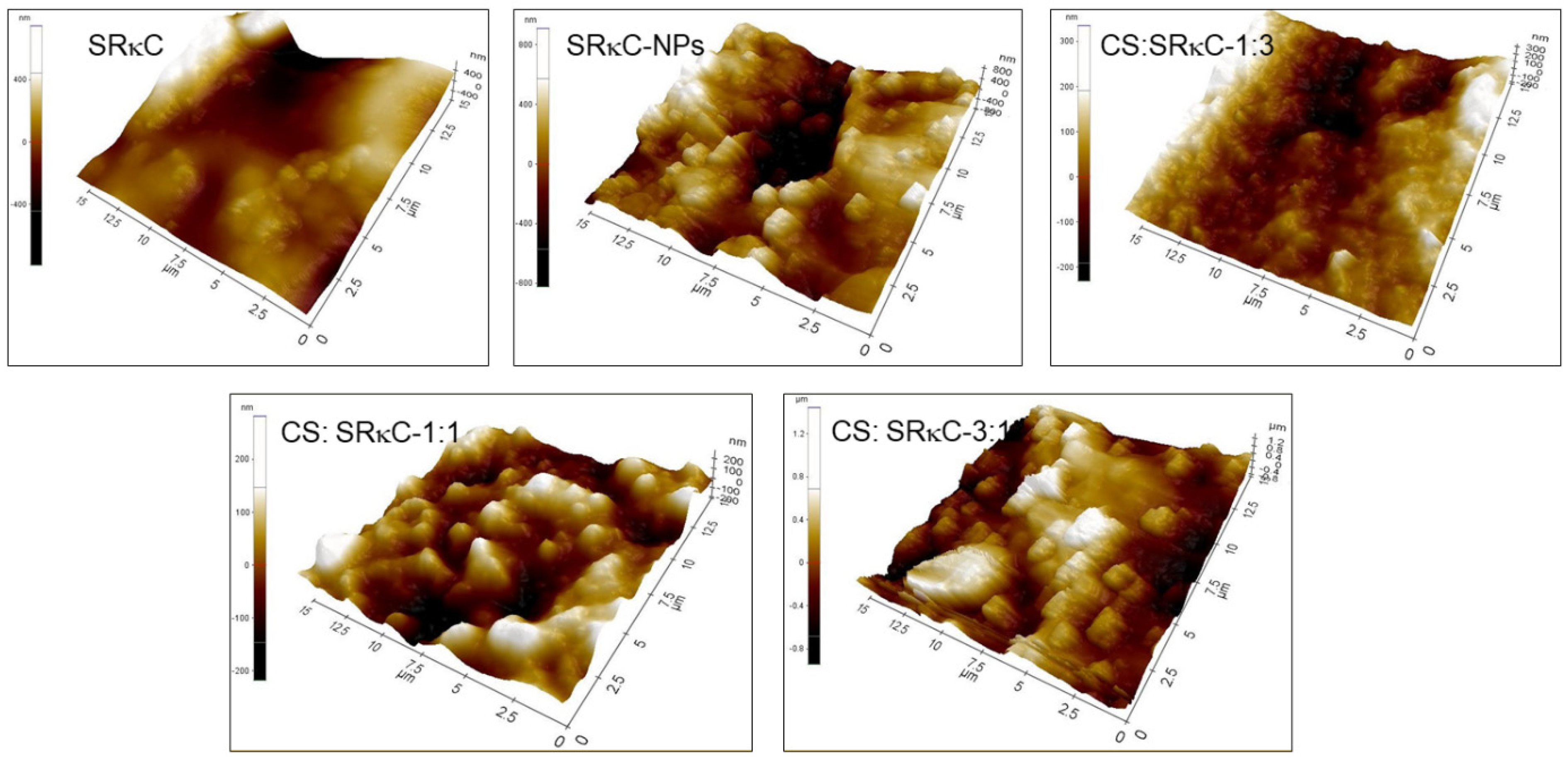
2.3.3. Surface Morphology
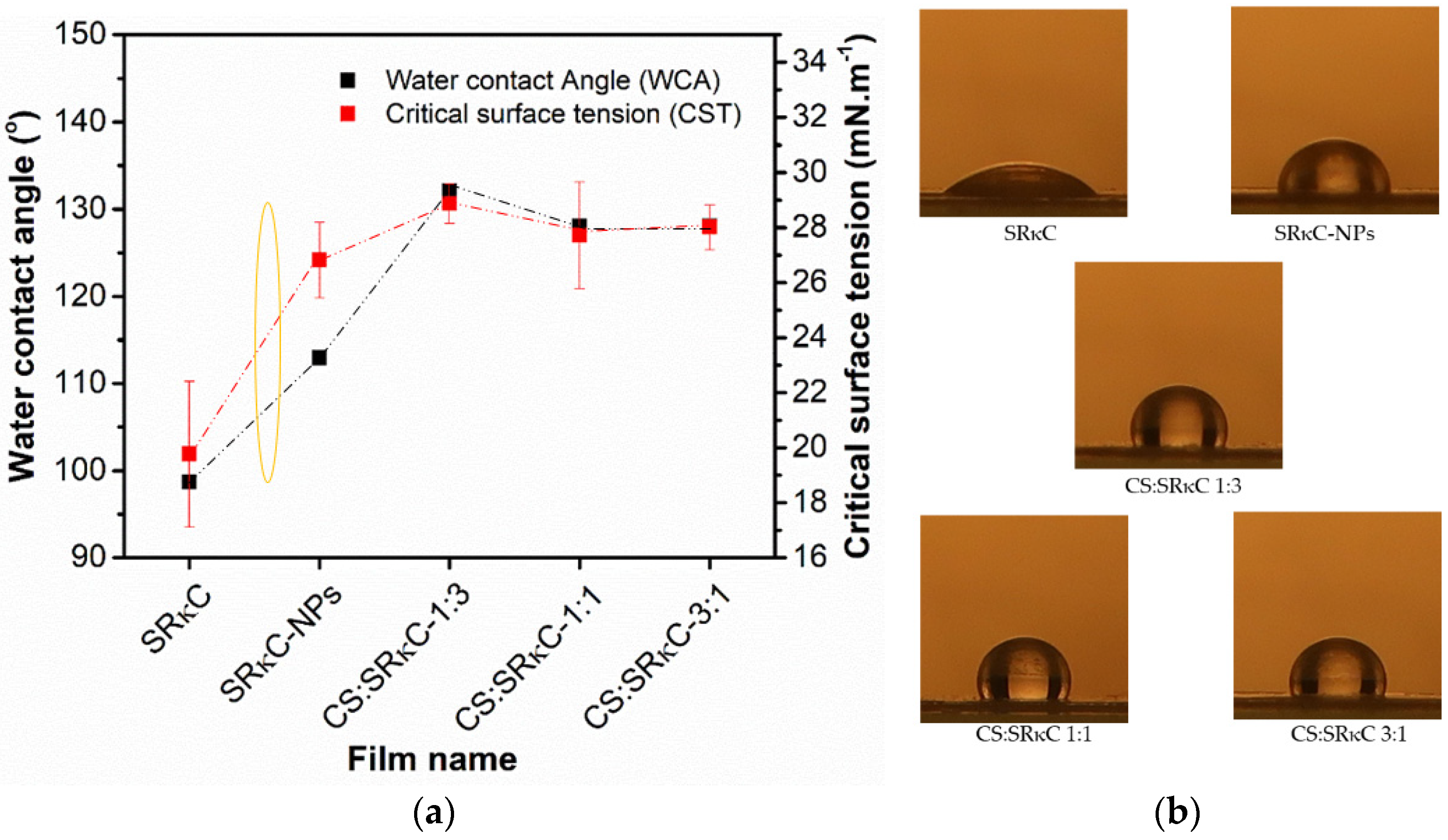
2.3.4. Wettability
2.3.5. Thickness
2.3.6. Mechanical Properties
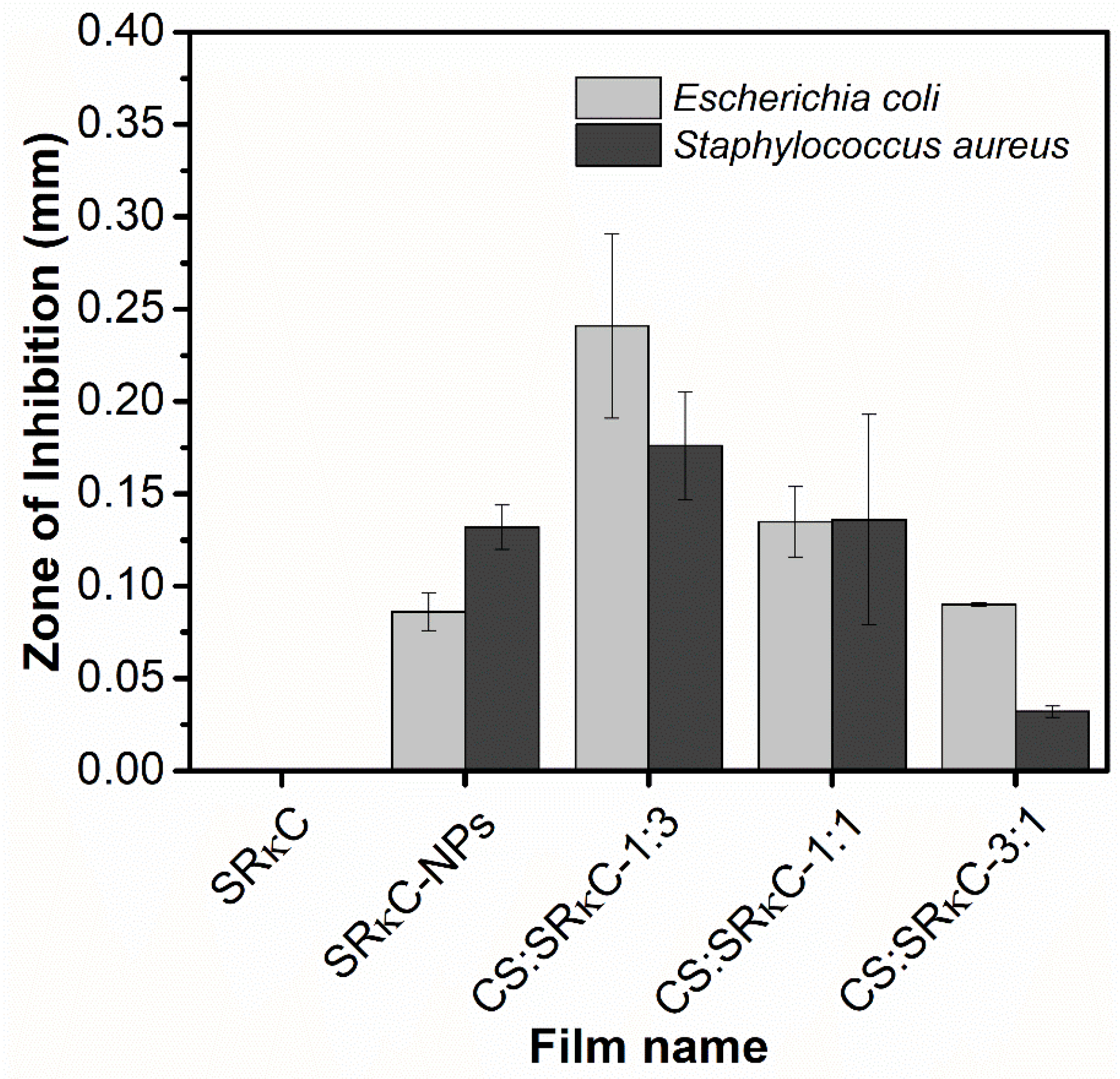
2.3.7. Antimicrobial Activity
2.3.8. Water Vapor Permeability (WVP)
2.3.9. Degradability
2.4. Performance of Minced Chicken Edible Packaging
2.4.1. Packaging of Minced Chicken Sample
2.4.2. pH Value of Packaging Sample
2.4.3. Total Volatile Base Nitrogen (TVBN)
2.4.4. Total Plate Count (TPC)
2.4.5. Thiobarbituric Acid-Reactive Substance (TBARS)
2.4.6. Weight Loss (WL)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Particle Size Distribution and Zeta Potential
3.2. Fourier-Transform Infrared (FTIR)
3.3. Surface Morphology
3.4. Wettability
3.5. Thickness
3.6. Optical Properties
3.7. Mechanical Properties
3.8. Water Vapor Permeability (WVP)
3.9. Antimicrobial Activity
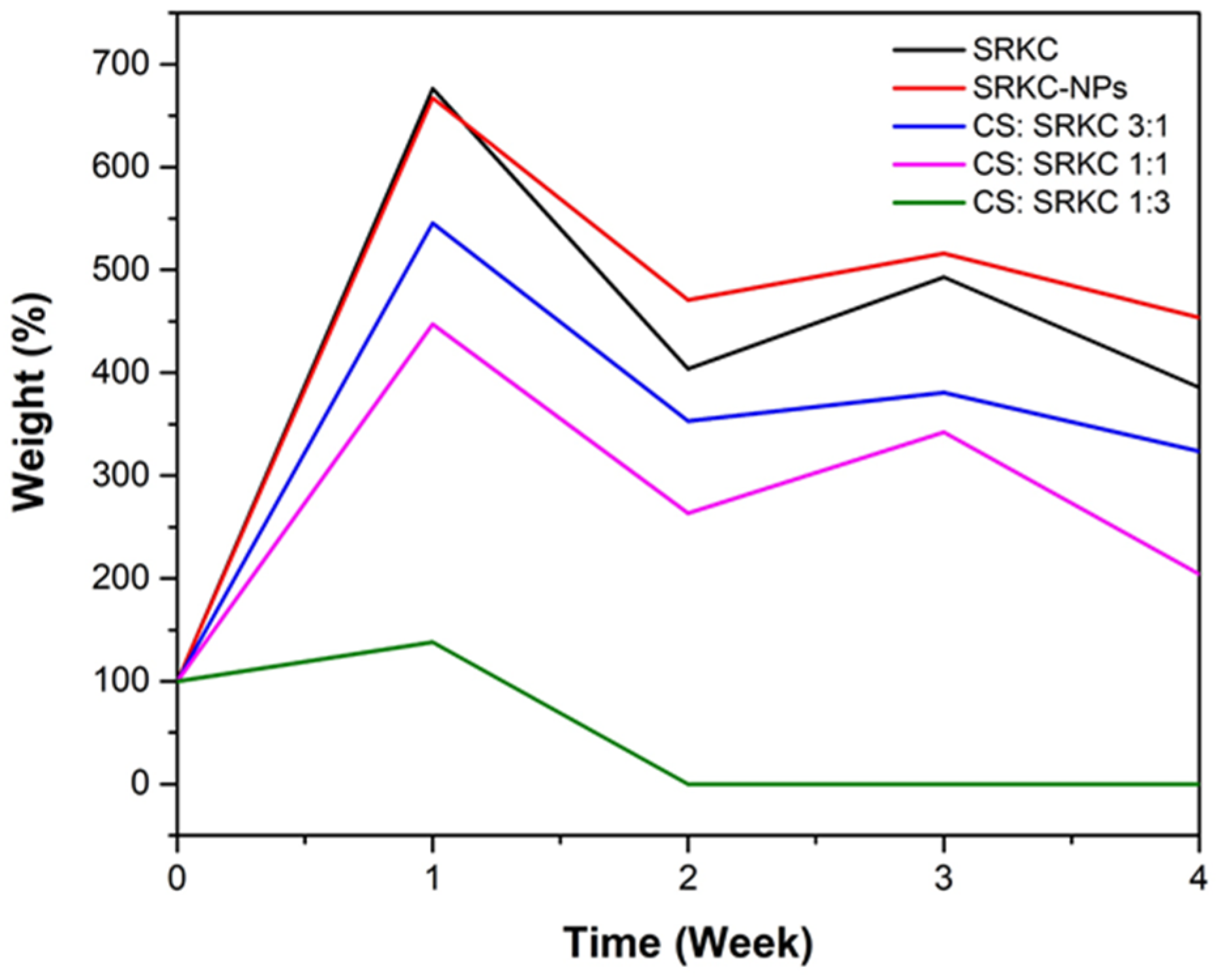
3.10. Degradability
3.11. Determination of the Best Film Type
3.12. pH Values
3.13. Total Volatile Base Nitrogen (TVBN)
3.14. Total Plate Count (TPC)
3.15. Thiobarbituric Acid-Reactive Substances (TBARS)
3.16. Weight Loss (WL)
3.17. Total Color Difference (∆E)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cardona, M.; Gorriz, A.; Barat, J.M.; Fernández-Segovia, I. Perception of fat and other quality parameters in minced and burger meat from Spanish consumer studies. Meat Sci. 2020, 166, 108138. [Google Scholar] [CrossRef] [PubMed]
- Chakanya, C.; Arnaud, E.; Muchenje, V.; Hoffman, L.C. Colour and oxidative stability of mince produced from fresh and frozen/thawed fallow deer (Dama dama) meat. Meat Sci. 2017, 126, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.; Lindström, A.; Trischler, J.; Wikström, F.; Rowe, Z. Avoiding food becoming waste in households–The role of packaging in consumers’ practices across different food categories. J. Clean. Prod. 2020, 265, 121775. [Google Scholar] [CrossRef]
- Rajendran, N.; Puppala, S.; Raj, M.S.; Angeeleena, B.R.; Rajam, C. Seaweeds Can Be a New Source for Bioplastics. J. Pharm. Res. 2012, 5, 1476–1479. [Google Scholar]
- Abdul Khalil, H.P.S.; Saurabh, C.K.; Tye, Y.Y.; Lai, T.K.; Easa, A.M.; Rosamah, E.; Fazita, M.R.N.; Syakir, M.I.; Adnan, A.S.; Fizree, H.M.; et al. Seaweed based sustainable films and composites for food and pharmaceutical applications: A review. Renew. Sustain. Energy Rev. 2017, 77, 353–362. [Google Scholar] [CrossRef]
- Liang, W.; Mao, X.; Peng, X.; Tang, S. Effects of sulfate group in red seaweed polysaccharides on anticoagulant activity and cytotoxicity. Carbohydr. Polym. 2014, 101, 776–785. [Google Scholar] [CrossRef]
- Larotonda, F.D.S.; Hilliou, L.; Sereno, A.M.C. Green Edible Films Obtained from Starch-Domestic Carrageenan Mixtures. In Proceedings of the 2nd Mercosur Congress on Chemical Engineering and 4th Mercosur Congress on Process Systems Engineering, ENPROMER, Rio de Janeiro, Brazil, 14–18 August 2005; pp. 1–10. [Google Scholar]
- Aji, A.I.; Praseptiangga, D.; Rochima, E.; Joni, I.M.; Panatarani, C. Optical transparency and mechanical properties of semi-refined iota carrageenan film reinforced with SiO2 as food packaging material. AIP Conf. Proc. 2018, 1927, 030039. [Google Scholar] [CrossRef]
- Praseptiangga, D.; Zahara, H.L.; Widjanarko, P.I.; Joni, I.M.; Panatarani, C. Preparation and FTIR spectroscopic studies of SiO2-ZnO nanoparticles suspension for the development of carrageenan-based bio-nanocomposite film. AIP Conf. Proc. 2020, 2219, 100005. [Google Scholar] [CrossRef]
- Khoirunnisa, A.R.; Joni, I.M.; Panatarani, C.; Rochima, E.; Praseptiangga, D. UV-screening, transparency and water barrier properties of semi refined iota carrageenan packaging film incorporated with ZnO nanoparticles. AIP Conf. Proc. 2018, 1927, 030041. [Google Scholar] [CrossRef]
- Joni, I.M.; Vanitha, M.; Panatarani, C.; Faizal, F. Dispersion of amorphous silica nanoparticles via beads milling process and their particle size analysis, hydrophobicity and anti-bacterial activity. Adv. Powder Technol. 2020, 31, 370–380. [Google Scholar] [CrossRef]
- Praseptiangga, D.; Mufida, N.; Panatarani, C.; Joni, I.M. Enhanced multi functionality of semi-refined iota carrageenan as food packaging material by incorporating SiO2 and ZnO nanoparticles. Heliyon 2021, 7, e06963. [Google Scholar] [CrossRef] [PubMed]
- Panatarani, C.; Mufida, N.; Widyaastuti, D.; Praseptiangga, D.; Joni, I.M. Dispersion of SiO2 and ZnO nanoparticles by bead milling in the preparation of carrageenan bio-nanocomposite film. AIP Conf. Proc. 2020, 2219, 100002. [Google Scholar] [CrossRef]
- Indumathi, M.P.; Saral Sarojini, K.; Rajarajeswari, G.R. Antimicrobial and biodegradable chitosan/cellulose acetate phthalate/ZnO nano composite films with optimal oxygen permeability and hydrophobicity for extending the shelf life of black grape fruits. Int. J. Biol. Macromol. 2019, 132, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Joni, I.M.; Ogi, T.; Purwanto, A.; Okuyama, K.; Saitoh, T.; Takeuchi, K. Decolorization of beads-milled TiO2 nanoparticles suspension in an organic solvent. Adv. Powder Technol. 2012, 23, 55–63. [Google Scholar] [CrossRef]
- Joni, I.M.; Panatarani, C.; Maulana, D.W. Dispersion of fine phosphor particles by newly developed beads mill. AIP Conf. Proc. 2016, 1712, 50019. [Google Scholar] [CrossRef]
- Joni, I.M.; Panatarani, C.; Hidayat, D.; Setianto; Wibawa, B.M.; Rianto, A.; Thamrin, H. Synthesis and dispersion of nanoparticles, and Indonesian graphite processing. AIP Conf. Proc. 2013, 1554, 20–26. [Google Scholar] [CrossRef]
- Ogi, T.; Zulhijah, R.; Iwaki, T.; Okuyama, K. Recent Progress in Nanoparticle Dispersion Using Bead Mill. KONA Powder Part. J. 2017, 34, 23. [Google Scholar] [CrossRef]
- Panatarani, C.; Rochima, E.; Ayunani; Yoga, S.; Joni, I.M. Reinforcement of Carrageenan/Starch Based Bio-Composite by Beads-Milled Chitosan. Adv. Eng. Res. 2020, 194, 272–276. [Google Scholar] [CrossRef]
- Prachayawarakorn, J.; Pomdage, W. Effect of carrageenan on properties of biodegradable thermoplastic cassava starch/low-density polyethylene composites reinforced by cotton fibers. Mater. Des. 2014, 61, 264–269. [Google Scholar] [CrossRef]
- Beigomi, M.; Mohsenzadeh, M.; Salari, A. Characterization of a novel biodegradable edible film obtained from Dracocephalum moldavica seed mucilage. Int. J. Biol. Macromol. 2018, 108, 874–883. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Properties and characterization of bionanocomposite films prepared with various biopolymers and ZnO nanoparticles. Carbohydr. Polym. 2014, 106, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Farhan, A.; Hani, N.M. Characterization of edible packaging films based on semi-refined kappa-carrageenan plasticized with glycerol and sorbitol. Food Hydrocoll. 2017, 64, 48–58. [Google Scholar] [CrossRef]
- Lamour, G.; Hamraoui, A.; Buvailo, A.; Xing, Y.; Keuleyan, S.; Prakash, V.; Eftekhari-Bafrooei, A.; Borguet, E. Contact angle measurements using a simplified experimental setup. J. Chem. Educ. 2010, 87, 1403–1407. [Google Scholar] [CrossRef]
- Tabatabaei, R.H.; Jafari, S.M.; Mirzaei, H.; Nafchi, A.M.; Dehnad, D. Preparation and characterization of nano-SiO2 reinforced gelatin-k-carrageenan biocomposites. Int. J. Biol. Macromol. 2018, 111, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Chambi, H.; Grosso, C. Edible films produced with gelatin and casein cross-linked with transglutaminase. Food Res. Int. 2006, 39, 458–466. [Google Scholar] [CrossRef]
- Sani, M.A.; Ehsani, A.; Hashemi, M. Whey protein isolate/cellulose nanofibre/TiO2 nanoparticle/rosemary essential oil nanocomposite film: Its effect on microbial and sensory quality of lamb meat and growth of common foodborne pathogenic bacteria during refrigeration. Int. J. Food Microbiol. 2017, 251, 8–14. [Google Scholar] [CrossRef]
- Paisoonsin, S.; Pornsunthorntawee, O.; Rujiravanit, R. Preparation and characterization of ZnO-deposited DBD plasma-treated PP packaging film with antibacterial activities. Appl. Surf. Sci. 2013, 273, 824–835. [Google Scholar] [CrossRef]
- Hao, W.; Dafu, W.; Anna, Z.; Huining, X. Soil burial biodegradation of antimicrobial biodegradable PBAT films. Polym. Degrad. Stab. 2015, 116, 14–22. [Google Scholar] [CrossRef]
- Cai, J.; Chen, Q.; Wan, X.; Zhao, J. Determination of total volatile basic nitrogen (TVBN) content and Warner-Bratzler shear force (WBSF) in pork using Fourier transform near infrared (FT-NIR) spectroscopy. Food Chem. 2011, 126, 1354–1360. [Google Scholar] [CrossRef]
- Wi, G.; Bae, J.; Kim, H.; Cho, Y.; Choi, M.-J. Evaluation of the physicochemical and structural properties and sensory characteristics of meat analogues prepared with various non-animal based liquid additives. Foods 2020, 9, 461. [Google Scholar] [CrossRef]
- Šupová, M.; Martynková, G.S.; Barabaszová, K. Effect of nanofillers dispersion in polymer matrices: A Review. Sci. Adv. Mat. 2011, 3, 1–25. [Google Scholar] [CrossRef]
- Joni, I.M.; Rukiah; Panatarani, C. Synthesis of silica particles by precipitation method of sodium silicate: Effect of temperature, pH and mixing technique. AIP Conf. Proc. 2020, 2219, 080018. [Google Scholar] [CrossRef]
- Joni, I.M.; Purwanto, A.; Iskandar, F.; Okuyama, K. Dispersion Stability Enhancement of Titania Nanoparticles in Organic Solvent Using a Bead Mill Process. Ind. Eng. Chem. Res. 2009, 48, 6916–6922. [Google Scholar] [CrossRef]
- Tongdeesoontorn, W.; Mauer, L.J.; Wongruong, S.; Sriburi, P.; Rachtanapun, P. Mechanical and physical properties of cassava starch-gelatin composite films. Int. J. Polym. Mater. Polym. Biomater. 2012, 61, 778–792. [Google Scholar] [CrossRef]
- Loo, C.P.Y.; Sarbon, N.M. Chicken skin gelatin films with tapioca starch. Food Biosci. 2020, 35, 100589. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of starch type on the physico-chemical properties of edible films. Int. J. Biol. Macromol. 2017, 98, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Marvizadeh, M.M.; Oladzadabbasabadi, N.; Nafchi, A.M.; Jokar, M. Preparation and characterization of bionanocomposite film based on tapioca starch/bovine gelatin/nanorod zinc oxide. Int. J. Biol. Macromol. 2017, 99, 1–7. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Kim, S.S.; Lee, J.; Lee, J. Effect of TiO2 on highly elastic, stretchable UV protective nanocomposite films formed by using a combination of k-Carrageenan, xanthan gum and gellan gum. Int. J. Biol. Macromol. 2019, 123, 1020–1027. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Ren, J.; Chang, M.; He, B.; Zhang, C. Effects of nano-ZnO and nano-SiO2 particles on properties of PVA/xylan composite films. Int. J. Biol. Macromol. 2019, 132, 978–986. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.-W. Carrageenan-based hydrogels and films: Effect of ZnO and CuO nanoparticles on the physical, mechanical, and antimicrobial properties. Food Hydrocoll. 2017, 67, 45–53. [Google Scholar] [CrossRef]
- Nowzari, F.; Shábanpour, B.; Ojagh, S.M. Comparison of chitosan–gelatin composite and bilayer coating and film effect on the quality of refrigerated rainbow trout. Food Chem. 2013, 141, 1667–1672. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.P.N.; Lumdubwong, N. Starch behaviors and mechanical properties of starch blend films with different plasticizers. Carbohydr. Polym. 2016, 154, 112–120. [Google Scholar] [CrossRef]
- Saedi, S.; Shokri, M.; Rhim, J.-W. Preparation of carrageenan-based nanocomposite films incorporated with functionalized halloysite using AgNP and sodium dodecyl sulfate. Food Hydrocoll. 2020, 106, 105934. [Google Scholar] [CrossRef]
- SNI 01-2354.4-2006; Chemical Test Method-Part 4: Determination of Protein Content by the Total Nitrogen Method in Fishery Product. National Standardization Agency Republic of Indonesia: Jakarta, Indonesia, 2006. Available online: https://legalcentric.com/content/view/157549 (accessed on 21 November 2022).
- SNI 3924:2009; Quality of Chicken Meat. National Standardization Agency Republic of Indonesia: Jakarta, Indonesia, 2009.
- Muscat, D.; Adhikari, B.; Adhikari, R.; Chaudhary, D.S. Comparative study of film forming behaviour of low and high amylose starches using glycerol and xylitol as plasticizers. J. Food Eng. 2012, 109, 189–201. [Google Scholar] [CrossRef]
- Souza, V.; Pires, J.; Vieira, É.; Coelhoso, I.; Duarte, M.; Fernando, A. Shelf-Life Assessment of Fresh Poultry Meat Packaged in Novel Bionanocomposite of Chitosan/Montmorillonite Incorporated with Ginger Essential Oil. Coatings 2018, 8, 177. [Google Scholar] [CrossRef]
- Praseptiangga, D.; Widyaastuti, D.; Panatarani, C.; Joni, I.M. Development and characterization of semi-refined iota carrageenan/SiO2-ZnO bio-nanocomposite film with the addition of cassava starch for application on minced chicken meat packaging. Foods 2021, 10, 2776. [Google Scholar] [CrossRef]
- Japanese Industrial Standard Z 1707; General Rules of Plastic Films for Food Packaging. Japanese Standard Association: Tokyo, Japan, 1975.
- Pires, J.R.A.; de Souza, V.G.L.; Fernando, A.L. Chitosan/montmorillonite bionanocomposites incorporated with rosemary and ginger essential oil as packaging for fresh poultry meat. Food Packag. Shelf Life. 2018, 17, 142–149. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Vieira, É.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Activity of chitosan-montmorillonite bionanocomposites incorporated with rosemary essential oil: From in vitro assays to application in fresh poultry meat. Food Hydrocoll. 2019, 89, 241–252. [Google Scholar] [CrossRef]
- O’Sullivan, M.G.; Kerry, J.P. Sensory and quality properties of packaged fresh and processed meats. In Advances in Meat, Poultry and Seafood Packaging; Kerry, J.P., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 86–111. [Google Scholar] [CrossRef]
- Remya, S.; Mohan, C.O.; Bindu, J.; Sivaraman, G.K.; Venkateshwarlu, G.; Ravishankar, C.N. Effect of chitosan based active packaging film on the keeping quality of chilled stored barracuda fish. J. Food Sci. Technol. 2016, 53, 685–693. [Google Scholar] [CrossRef]
- Instruments, P. Colour Meter PCE-TCD 100 Instrument Manual; PCE Instruments: Jupiter, FL, USA, 2010. [Google Scholar]
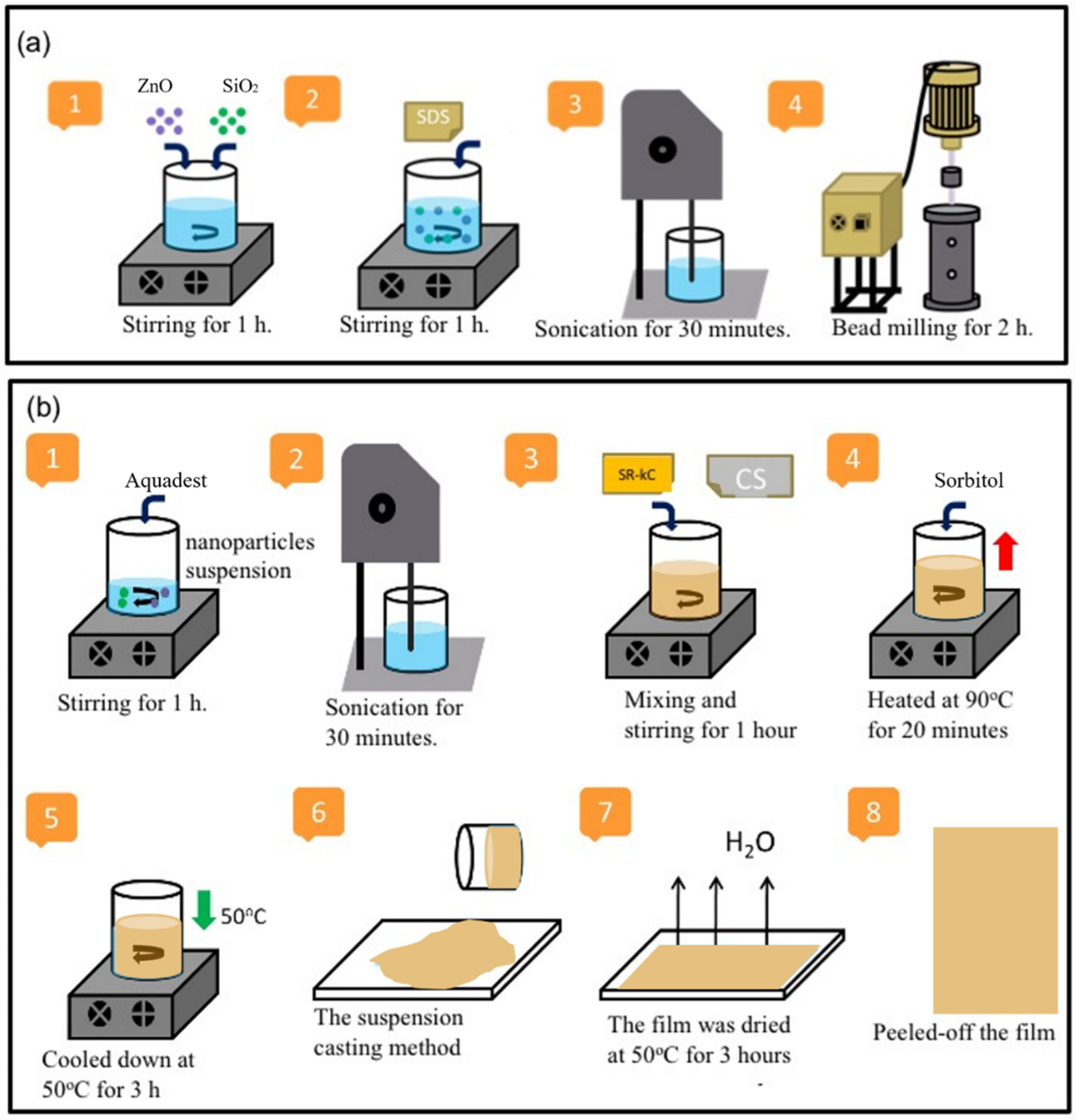
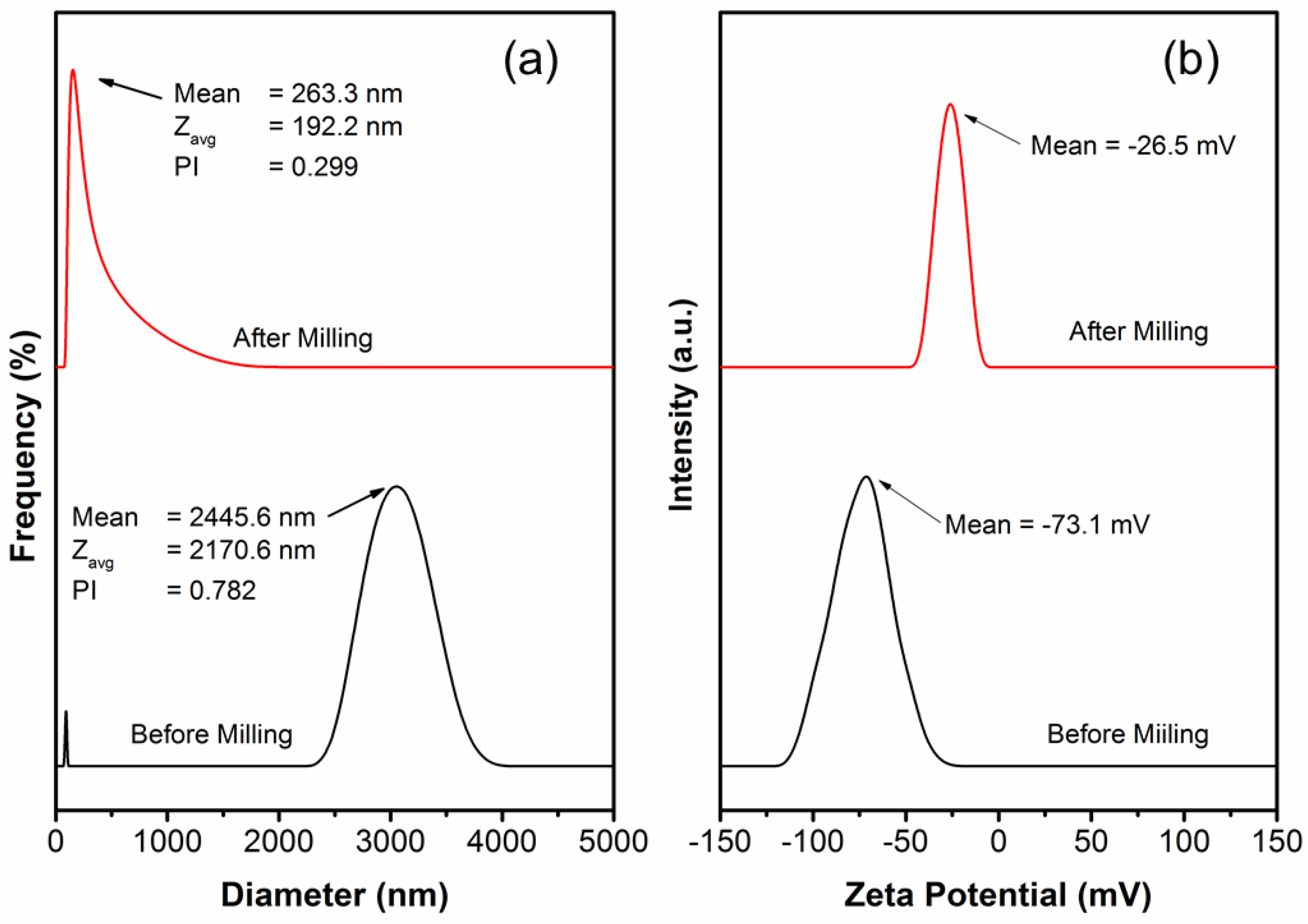
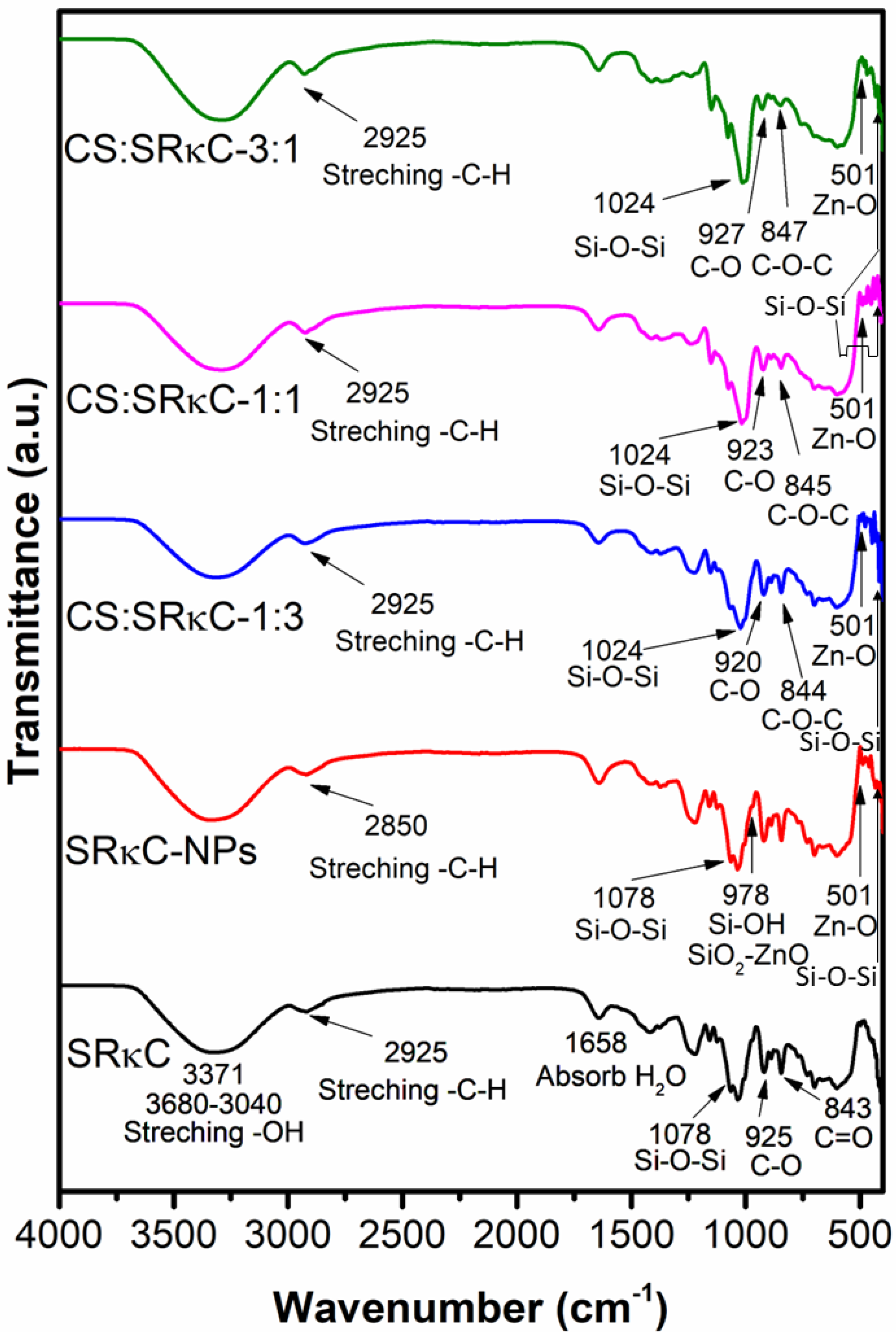
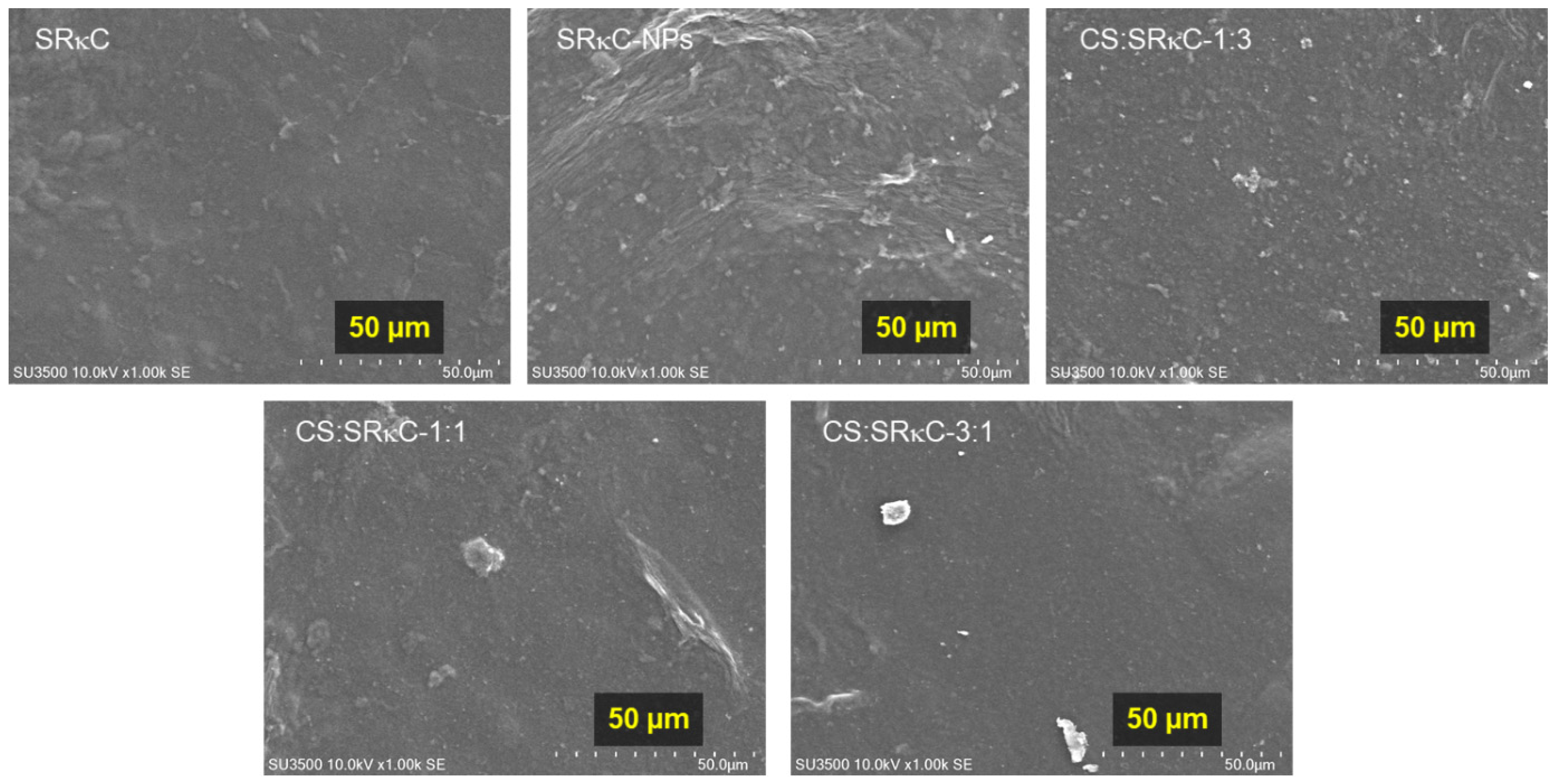
| Film Name | ZnO NPs (g) | SiO2 NPs (g) | CS (g) | SRκC (g) | Aquadest (g) | Sorbitol (g) | SDS (g) | Total (g) |
|---|---|---|---|---|---|---|---|---|
| SRκC | - | - | - | 2.0 | 97.0 | 1.0 | - | 100 |
| SRκC−NPs | 0.3 | 0.1 | - | 2.0 | 96.6 | 1.0 | 0.04 | 100 |
| CS:SRκC 1:3 | 0.3 | 0.1 | 0.5 | 1.5 | 96.6 | 1.0 | 0.04 | 100 |
| CS:SRκC 1:1 | 0.3 | 0.1 | 1.0 | 1.0 | 96.6 | 1.0 | 0.04 | 100 |
| CS:SRκC 3:1 | 0.3 | 0.1 | 1.5 | 0.5 | 96.6 | 1.0 | 0.04 | 100 |
| Film Name | Thickness (µm) | Tensile Strength (MPa) | Elongation at Break (%) | WVP (10−6 g·h−1·m−1·Pa−1) |
|---|---|---|---|---|
| SRC | 57.30 ± 8.17 a | 28.63 ± 0.61 a | 2.76 ± 1.22 a | 1.05 ± 0.05 |
| SRκC−NPs | 58.14 ± 7.85 a | 30.86 ± 0.56 ab | 4.02 ± 1.00 ab | 0.88 ± 0.11 |
| CS:SRκC 1:3 | 55.70 ± 6.21 ab | 32.44 ± 0.14 a | 3.39 ± 0.29 a | 0.84 ± 0.04 |
| CS:SRκC 1:1 | 55.10 ± 8.07 ab | 17.58 ± 0.30 ab | 4.39 ± 0.82 ab | 0.84 ± 0.04 |
| CS:SRκC 3:1 | 53.52 ± 7.33 b | 9.4 ± 0.44 b | 7.56 ± 0.39 b | 0.83 ± 0.08 |
| Film Name | % Transmittance @ λ = 280 | % Transmittance @ λ = 550 |
|---|---|---|
| SRκC | 25.14 | 62.69 |
| SRκC−NPs | 6.34 | 53.33 |
| CS:SRκC 1:3 | 8.05 | 58.26 |
| CS:SRκC 1:1 | 9.17 | 61.86 |
| CS:SRκC 3:1 | 9.72 | 63.57 |
| Film Name | Transparency | UV Barrier | Tensile Strength | Elongation at Break | WVP | Antimicrobial Activity | Total |
|---|---|---|---|---|---|---|---|
| SRκC | 62.69 | 25.14 | 28.63 | 2.76 | 1.01 | 0.00 | 0.24 |
| SRκC−NPs | 53.33 | 6.34 | 30.86 | 4.02 | 0.88 | 0.21 | 0.46 |
| CS:SRκC 1:3 | 58.26 | 8.05 | 32.44 | 3.39 | 0.84 | 0.42 | 0.64 |
| CS:SRκC 1:1 | 61.86 | 9.17 | 17.58 | 4.39 | 0.84 | 0.27 | 0.55 |
| CS:SRκC 3:1 | 63.57 | 9.72 | 9.41 | 7.56 | 0.83 | 0.42 | 0.56 |
| Parameter | Day | Unpacked Minced Chicken | SRκC Packaging | SRκC−NP Packaging | CS:SRκC 1:3 Packaging |
|---|---|---|---|---|---|
| WL (%) | 0 | 0 Aa | 0 Aa | 0 Aa | 0 Aa |
| 3 | 8.06 ± 1.21 Bc | 3.41 ± 0.31 Bab | 2.19 ± 1.15 Ba | 4.69 ± 0.63 Bb | |
| 6 | 11.52 ± 1.17 Cc | 4.01 ± 0.43 Ca | 4.16 ± 0.31 Ca | 6.25 ± 0.39 Cb | |
| 9 | 18.19 ± 1.06 Dc | 5.69 ± 0.49 Da | 6.42 ± 0.8 Da | 8.65 ± 1.13 Da | |
| 12 | 22.31 ± 0.79 Ec | 6.55 ± 0.44 Ea | 7.5 ± 0.75 Da | 10.51 ± 1.62 Ec | |
| pH | 0 | 5.99 ± 0.02 Aa | 5.99 ± 0.02 Aa | 5.99 ± 0.02 Aa | 5.99 ± 0.02 Aa |
| 3 | 5.97 ± 0.01 Aa | 5.99 ± 0.01 Aab | 6.01 ± 0.02 Aab | 5.98 ± 0.02 Aab | |
| 6 | 5.98 ± 0.01 Aa | 5.97 ± 0.04 Aa | 6.04 ± 0.02 Ab | 5.99 ± 0.03 Aa | |
| 9 | 6.43 ± 0.01 Bc | 6.38 ± 0.01 Ba | 6.41 ± 0.01 Bb | 6.39 ± 0.01 Ba | |
| 12 | 7.46 ± 0.04 Cc | 7.07 ± 0.02 Ca | 7.31 ± 0.12 Cb | 7.32 ± 0.07 Cb | |
| ΔE | 0 | - | - | - | - |
| 3 | 1.63 ± 0.75 Aa | 2.47 ± 0.38 Aab | 3.35 ± 0.49 Bb | 2.81 ± 1.33 Aab | |
| 6 | 3.29 ± 1.65 Bab | 2.48 ± 0.86 Aab | 1.70 ± 0.42 Aa | 4.70 ± 2.67 Ab | |
| 9 | 2.05 ± 0.48 ABa | 3.19 ± 2.25 Aa | 1.22 ± 0.33 Aa | 3.27 ± 1.82 Aa | |
| 12 | 9.63 ± 0.06 Ca | 9.78 ± 1.73 Ba | 9.34 ± 1.74 Ca | 9.74 ± 0.33 Ba | |
| TBARS (mg MDA/kg sample) | 0 | 0.23 ± 0 Aa | 0.23 ± 0 Aa | 0.23 ± 0 Aa | 0.23 ± 0 Aa |
| 3 | 0.72 ± 0.1 Bb | 0.42 ± 0.07 Ba | 0.35 ± 0.01 ABa | 0.35 ± 0.02 Aa | |
| 6 | 0.77 ± 0.24 Bb | 0.39 ± 0.03 Ba | 0.47 ± 0.08 Ba | 0.58 ± 0.05 Bab | |
| 9 | 0.8 ± 0.05 Ba | 1.02 ± 0.02 Cab | 0.82 ± 0.24 Ca | 1.15 ± 0.23 Cb | |
| 12 | 1.17 ± 0.24 Ca | 1.12 ± 0.04 Da | 1.08 ± 0.03 Da | 1.06 ± 0.16 Ca | |
| TVBN (mg N/100 g) | 0 | 5.42 ± 0.06 Aa | 5.42 ± 0.06 Aa | 5.42 ± 0.06 Aa | 5.42 ± 0.06 Aa |
| 3 | 5.54 ± 0.11 Aa | 10.66 ± 0.04 Bb | 10.94 ± 0.09 Bc | 11.06 ± 0.13 Bc | |
| 6 | 11.08 ± 0.1 Ba | 11.09 ± 0.09 Ca | 11.00 ± 0.16 Ba | 10.71 ± 0.42 Ba | |
| 9 | 31.49 ± 0.57 Cc | 29.35 ± 0.4 Db | 23.84 ± 0.26 Ca | 23.71 ± 1.06 Ca | |
| 12 | 48.62 ± 0.2 Dd | 40.73 ± 0.3 Ec | 39.6 ± 0.31 Db | 33.47 ± 0.7 Da | |
| TPC (log CFU/g) | 0 | 5.56 ± 0 Aa | 5.56 ± 0 Aa | 5.56 ± 0 Aa | 5.56 ± 0 Aa |
| 3 | 6.02 ± 0.07 Ab | 5.76 ± 0.1 Ba | 5.6 ± 0.03 Aa | 5.61 ± 0.12 Aa | |
| 6 | 6.77 ± 0.63 Ba | 6.1 ± 0.02 Ba | 6.08 ± 0.03 Ca | 6.01 ± 0.01 Ba | |
| 9 | 6.41 ± 0.12 Ba | 6.23 ± 0.04 Ca | 6.24 ± 0.08 Ca | 6.21 ± 0.01 Ca | |
| 12 | 8.07 ± 0.09 Cb | 7.21 ± 0.02 Da | 7.35 ± 0.06 Da | 7.33 ± 0.03 Da |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panatarani, C.; Praseptiangga, D.; Widjanarko, P.I.; Azhary, S.Y.; Nurlilasari, P.; Rochima, E.; Joni, I.M. Synthesis, Characterization, and Performance of Semi-Refined Kappa Carrageenan-Based Film Incorporating Cassava Starch. Membranes 2023, 13, 100. https://doi.org/10.3390/membranes13010100
Panatarani C, Praseptiangga D, Widjanarko PI, Azhary SY, Nurlilasari P, Rochima E, Joni IM. Synthesis, Characterization, and Performance of Semi-Refined Kappa Carrageenan-Based Film Incorporating Cassava Starch. Membranes. 2023; 13(1):100. https://doi.org/10.3390/membranes13010100
Chicago/Turabian StylePanatarani, Camellia, Danar Praseptiangga, Putut Ismu Widjanarko, Sundoro Yoga Azhary, Puspita Nurlilasari, Emma Rochima, and I Made Joni. 2023. "Synthesis, Characterization, and Performance of Semi-Refined Kappa Carrageenan-Based Film Incorporating Cassava Starch" Membranes 13, no. 1: 100. https://doi.org/10.3390/membranes13010100
APA StylePanatarani, C., Praseptiangga, D., Widjanarko, P. I., Azhary, S. Y., Nurlilasari, P., Rochima, E., & Joni, I. M. (2023). Synthesis, Characterization, and Performance of Semi-Refined Kappa Carrageenan-Based Film Incorporating Cassava Starch. Membranes, 13(1), 100. https://doi.org/10.3390/membranes13010100







