Synthesis and Characterization of Amino Acid Decyl Esters as Early Membranes for the Origins of Life
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
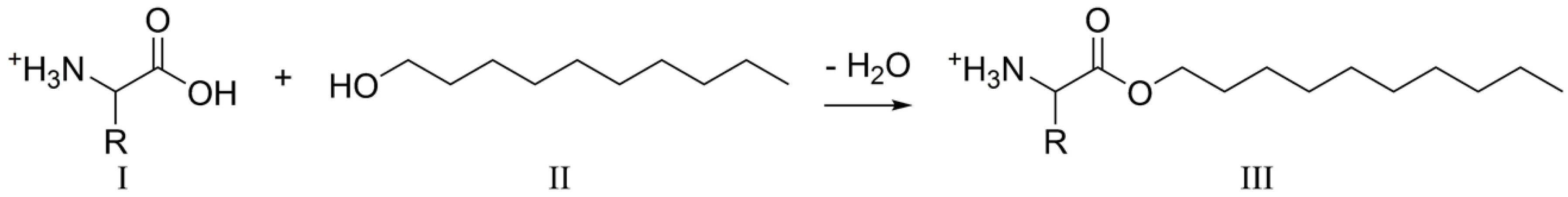
2.2. Prebiotic Ester Synthesis
2.3. Vesicle Analysis
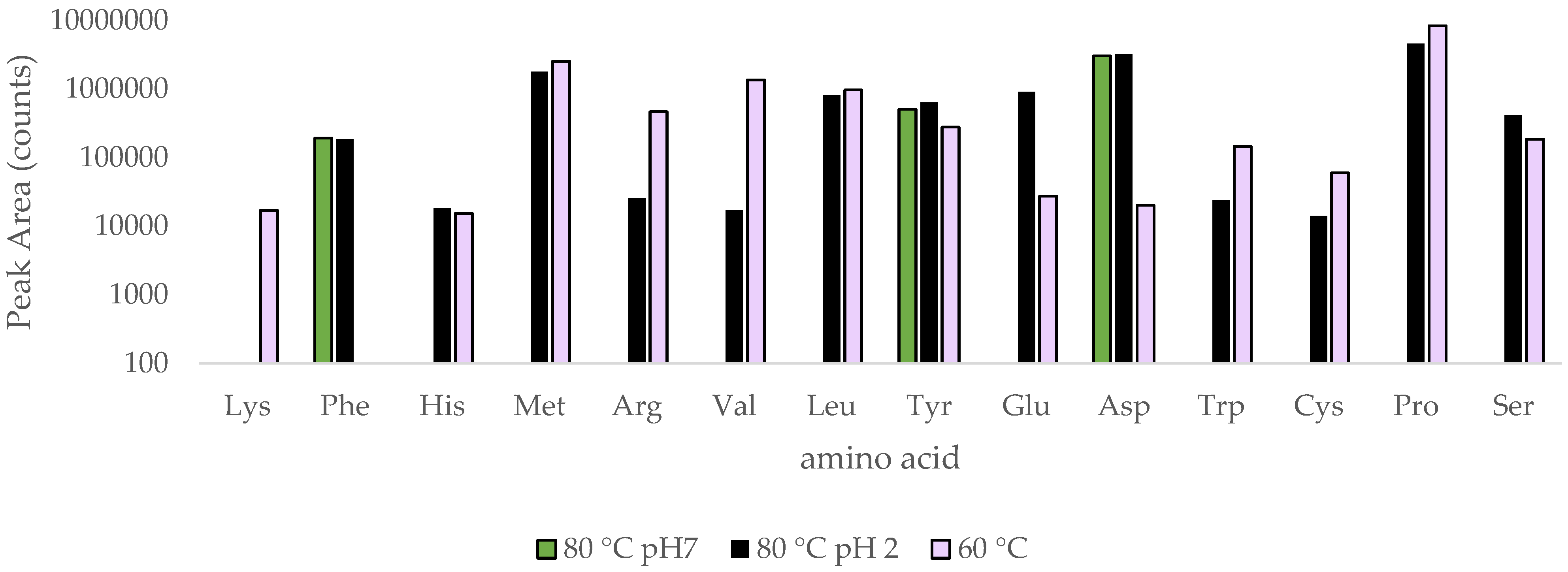
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dworkin, L.P.; Deamer, D.W.; Sandford, S.A.; Allamandola, L.J. Self-assembling amphiphilic molecules: Synthesis in simulated interstellar/precometary ices. Proc. Natl. Acad. Sci. USA 2001, 98, 815–819. [Google Scholar] [CrossRef]
- Lai, J.C.-Y.; Pearce, B.K.D.; Pudritz, R.E.; Lee, D. Meteoritic abundances of fatty acids and potential reaction pathways in planetesimals. Icarus 2019, 319, 685–700. [Google Scholar] [CrossRef]
- Konn, C.; Charlou, J.L.; Donval, J.P.; Holm, N.G.; Dehairs, F.; Bouillon, S. Hydrocarbons and oxidized organic compounds in hydrothermal fluids from Rainbow and Lost City ultramafic-hosted vents. Chem. Geol. 2009, 258, 299–314. [Google Scholar] [CrossRef]
- Rapf, R.J.; Perkins, R.J.; Yang, H.; Miyake, G.M.; Carpenter, B.K.; Vaida, V. Photochemical Synthesis of Oligomeric Amphiphiles from Alkyl Oxoacids in Aqueous Environments. J. Am. Chem. Soc. 2017, 139, 6946–6959. [Google Scholar] [CrossRef]
- Rasmussen, S.; Chen, L.H.; Deamer, D.W.; Krakauer, D.C.; Packard, N.H.; Stadler, P.F.; Bedau, M.A. Transitions from nonliving to living matter. Science 2004, 303, 963–965. [Google Scholar] [CrossRef]
- Segre, D.; Ben-Eli, D.; Deamer, D.W.; Lancet, D. The Lipid World. Orig. Life Evol. Biosph. 2001, 31, 27. [Google Scholar] [CrossRef]
- Walde, P. Surfactant assemblies and their various possible roles for the Origin(S) of life. Orig. Life Evol. Biosph. 2006, 36, 109–150. [Google Scholar] [CrossRef]
- Sutherland, J.D. The Origin of Life—Out of the Blue. Angew. Chemie-Int. Ed. 2016, 55, 104–121. [Google Scholar] [CrossRef]
- Preiner, M.; Xavier, J.C.; Do Nascimento Vieira, A.; Kleinermanns, K.; Allen, J.F.; Martin, W.F. Catalysts, autocatalysis and the origin of metabolism. Interface Focus 2019, 9, 20190072. [Google Scholar] [CrossRef]
- Bhowmik, S.; Krishnamurthy, R. The role of sugar-backbone heterogeneity and chimeras in the simultaneous emergence of RNA and DNA. Nat. Chem. 2019, 11, 1009–1018. [Google Scholar] [CrossRef]
- Suárez-Marina, I.; Abul-Haija, Y.M.; Turk-MacLeod, R.; Gromski, P.S.; Cooper, G.J.T.; Olivé, A.O.; Colón-Santos, S.; Cronin, L. Integrated synthesis of nucleotide and nucleosides influenced by amino acids. Commun. Chem. 2019, 2, 28. [Google Scholar] [CrossRef]
- Kitadai, N.; Maruyama, S. Origins of building blocks of life: A review. Geosci. Front. 2018, 9, 1117–1153. [Google Scholar] [CrossRef]
- Cleaves, H.J. The origin of the biologically coded amino acids. J. Theor. Biol. 2010, 263, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Cleaves, H.J.; Chalmers, J.H.; Lazcano, A.; Miller, S.L.; Bada, J.L. A Reassessment of Prebiotic Organic Synthesis in Neutral Planetary Atmospheres. Orig. Life Evol. Biosph. 2008, 38, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, W.R.; Deamer, D.W. Liposomes from Ionic, Single-Chain Amphiphiles. Biochemistry 1978, 17, 3759–3768. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, W.R.; Mulvihill, S.J.; Deamer, D.W. Synthesis of Phospholipids and Membranes in Prebiotic Conditions. Nature 1977, 266, 78–80. [Google Scholar] [CrossRef]
- Deamer, D.W. Sources and Syntheses of Prebiotic Amphiphiles. Self-Prod. Supramol. Struct. 1994, 446, 217–229. [Google Scholar]
- Mißbach, H.; Schmidt, B.C.; Duda, J.P.; Lünsdorf, N.K.; Goetz, W.; Thiel, V. Assessing the diversity of lipids formed via Fischer-Tropsch-type reactions. Org. Geochem. 2018, 119, 110–121. [Google Scholar] [CrossRef]
- Monnard, P.-A.; Apel, C.L.; Kanavarioti, A.; Deamer, D.W. Influence of ionic inorganic solutes on self-assembly and polymerization processes related to early forms of life: Implications for a prebiotic aqueous medium. Astrobiology 2002, 2, 139–152. [Google Scholar] [CrossRef]
- Maurer, S.E.; Deamer, D.W.; Boncella, J.M.; Monnard, P.A. Chemical evolution of amphiphiles: Glycerol monoacyl derivatives stabilize plausible prebiotic membranes. Astrobiology 2009, 9, 979–987. [Google Scholar] [CrossRef]
- Dalai, P.; Ustriyana, P.; Sahai, N. Aqueous magnesium as an environmental selection pressure in the evolution of phospholipid membranes on early earth. Geochim. Cosmochim. Acta 2018, 223, 216–228. [Google Scholar] [CrossRef]
- Maurer, S.E.; Tølbøl Sørensen, K.; Iqbal, Z.; Nicholas, J.; Quirion, K.; Gioia, M.; Monnard, P.-A.; Hanczyc, M.M. Vesicle Self-Assembly of Monoalkyl Amphiphiles under the Effects of High Ionic Strength, Extreme pH, and High Temperature Environments. Langmuir 2018, 34, 15560–15568. [Google Scholar] [CrossRef] [PubMed]
- Caschera, F.; de la Serna, J.B.; Loffler, P.M.G.; Rasmussen, T.E.; Hanczyc, M.M.; Bagatolli, L.A.; Monnard, P.A.; Bernardino de la Serna, J.; Löffler, P.M.G.; Rasmussen, T.E.; et al. Stable Vesicles Composed of Monocarboxylic or Dicarboxylic Fatty Acids and Trimethylammonium Amphiphiles. Langmuir 2011, 27, 14078–14090. [Google Scholar] [CrossRef] [PubMed]
- Namani, T.; Deamer, D.W. Stability of model membranes in extreme environments. Orig. Life Evol. Biosph. 2008, 38, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Albertsen, A.N.; Duffy, C.D.; Sutherland, J.D.; Monnard, P.A. Self-Assembly of Phosphate Amphiphiles in Mixtures of Prebiotically Plausible Surfactants. Astrobiology 2014, 14, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.P.; Sawant, A.A.; Rajamani, S. Spontaneous emergence of membrane-forming protoamphiphiles from a lipid–amino acid mixture under wet–dry cycles. Chem. Sci. 2021, 12, 2970–2978. [Google Scholar] [CrossRef] [PubMed]
- Gibard, C.; Bhowmik, S.; Karki, M.; Kim, E.-K.; Krishnamurthy, R. Phosphorylation, oligomerization and self-assembly in water under potential prebiotic conditions. Nat. Chem. 2018, 10, 212–217. [Google Scholar] [CrossRef]
- Maurer, S.E.; Nguyen, G. Prebiotic Vesicle Formation and the Necessity of Salts. Orig. Life Evol. Biosph. 2016, 46, 215–222. [Google Scholar] [CrossRef] [PubMed]
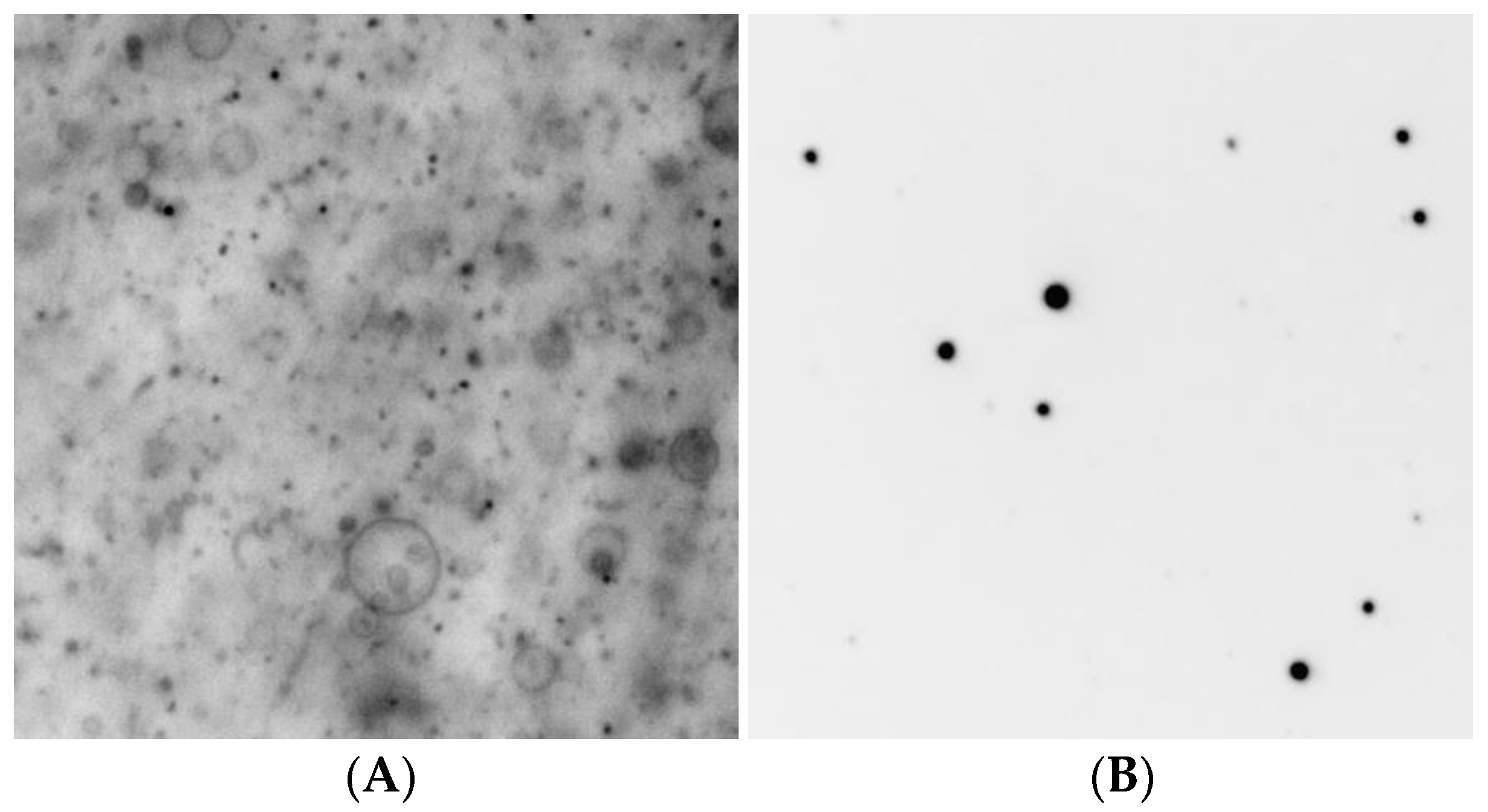
| Amino Acid | 3-Letter Abbreviation | Yield 60 °C | Microscopic Structures 60 °C | Microscopic Structures 80 °C |
|---|---|---|---|---|
| Methionine | Met | 70 | Amorphous solids | None |
| Aspartic Acid | Asp | 68 | Vesicles * | Vesicles |
| Glycine | Gly | 65 | Vesicles | Oil |
| Proline | Pro | 62 | Vesicles | None |
| Leucine | Leu | 58 | Vesicles * | Vesicles * |
| Tryptophan | Trp | 57 | Crystals | Crystals |
| Alanine | Ala | 51 | Vesicles * | Vesicles |
| Cysteine | Cys | 47 | Vesicles * | Vesicles |
| Glutamic Acid | Glu | 45 | Vesicles * | Vesicles |
| Serine | Ser | 41 | Oil, vesicles | Vesicles |
| Valine | Val | 39 | Vesicles * | Vesicles |
| Tyrosine | Tyr | 38 | Crystals | Crystals |
| Arginine | Arg | 12 | Oil | Oil |
| Histidine | His | 3 | None | Oil |
| Lysine | Lys | 3 | None | Oil |
| Phenylalanine | Phe | 2 | Oil | Crystals |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lago, I.; Black, L.; Wilfinger, M.; Maurer, S.E. Synthesis and Characterization of Amino Acid Decyl Esters as Early Membranes for the Origins of Life. Membranes 2022, 12, 858. https://doi.org/10.3390/membranes12090858
Lago I, Black L, Wilfinger M, Maurer SE. Synthesis and Characterization of Amino Acid Decyl Esters as Early Membranes for the Origins of Life. Membranes. 2022; 12(9):858. https://doi.org/10.3390/membranes12090858
Chicago/Turabian StyleLago, Isabella, Lissa Black, Maximillian Wilfinger, and Sarah E. Maurer. 2022. "Synthesis and Characterization of Amino Acid Decyl Esters as Early Membranes for the Origins of Life" Membranes 12, no. 9: 858. https://doi.org/10.3390/membranes12090858
APA StyleLago, I., Black, L., Wilfinger, M., & Maurer, S. E. (2022). Synthesis and Characterization of Amino Acid Decyl Esters as Early Membranes for the Origins of Life. Membranes, 12(9), 858. https://doi.org/10.3390/membranes12090858




