Research Trends in the Use of Polyaniline Membrane for Water Treatment Applications: A Scientometric Analysis
Abstract
:1. Introduction
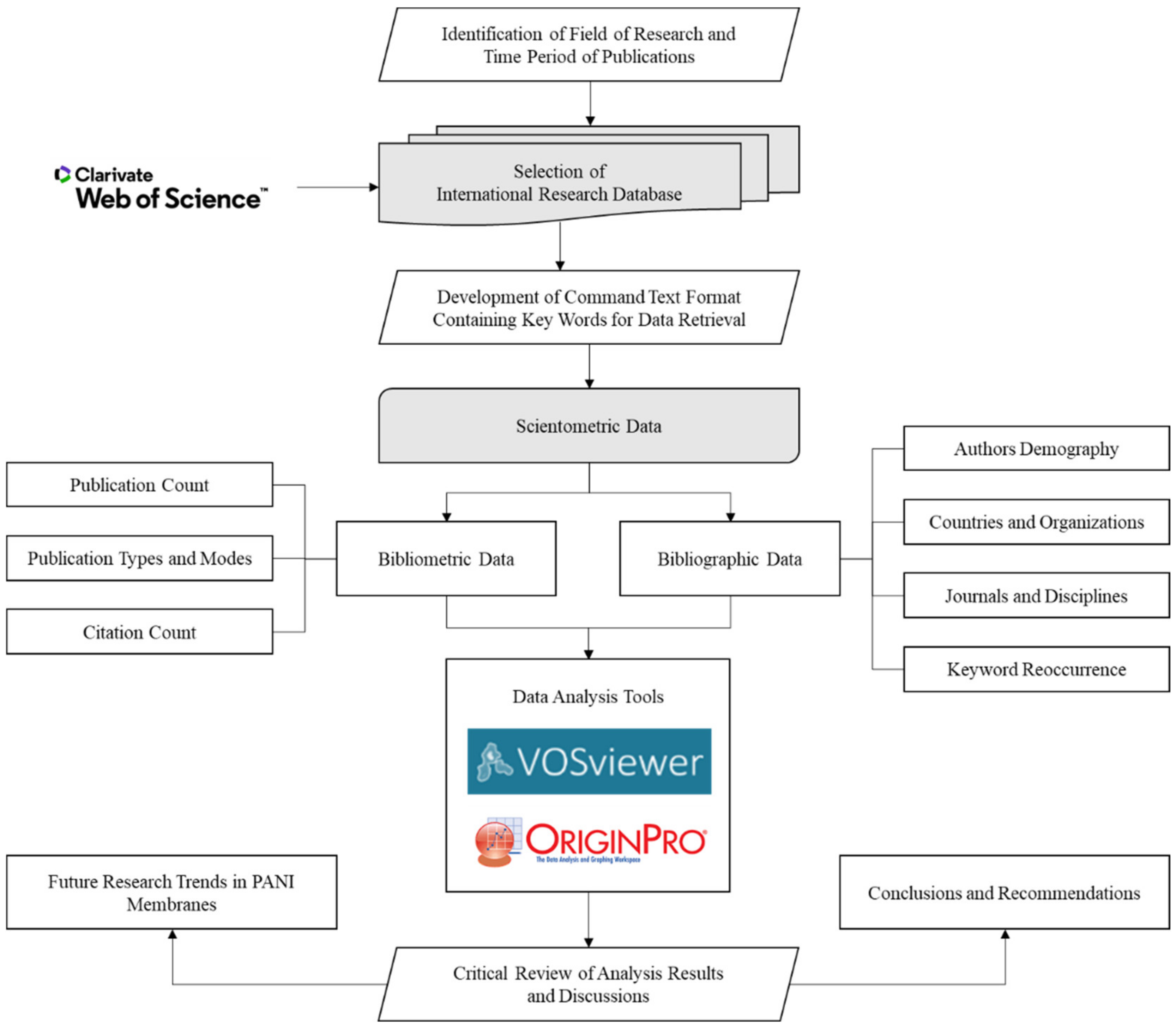
2. Research Methodology
3. Results and Discussion
3.1. Publication Count
3.2. Publication Types and Modes
3.3. Citation Count
3.4. Author Demography
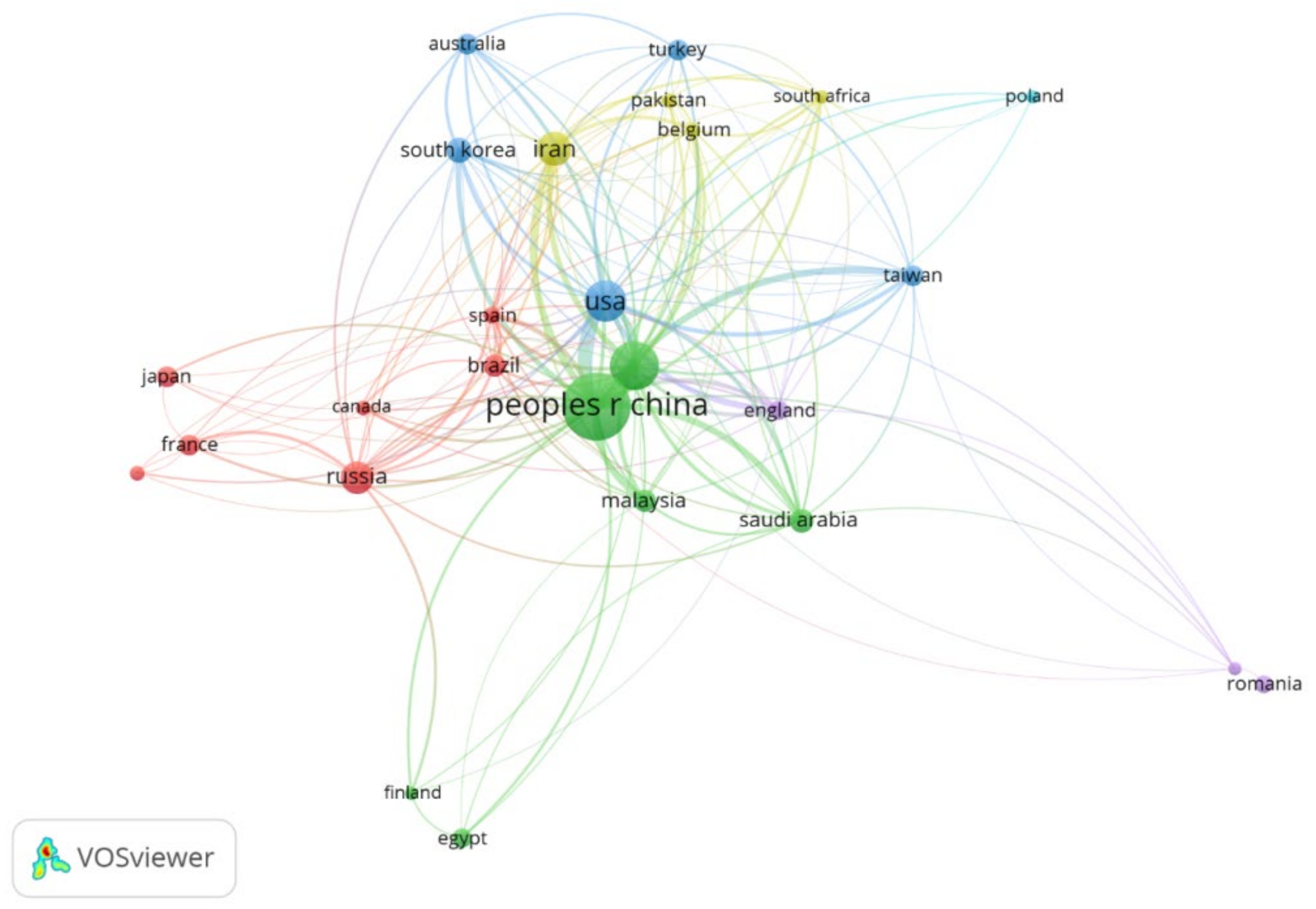
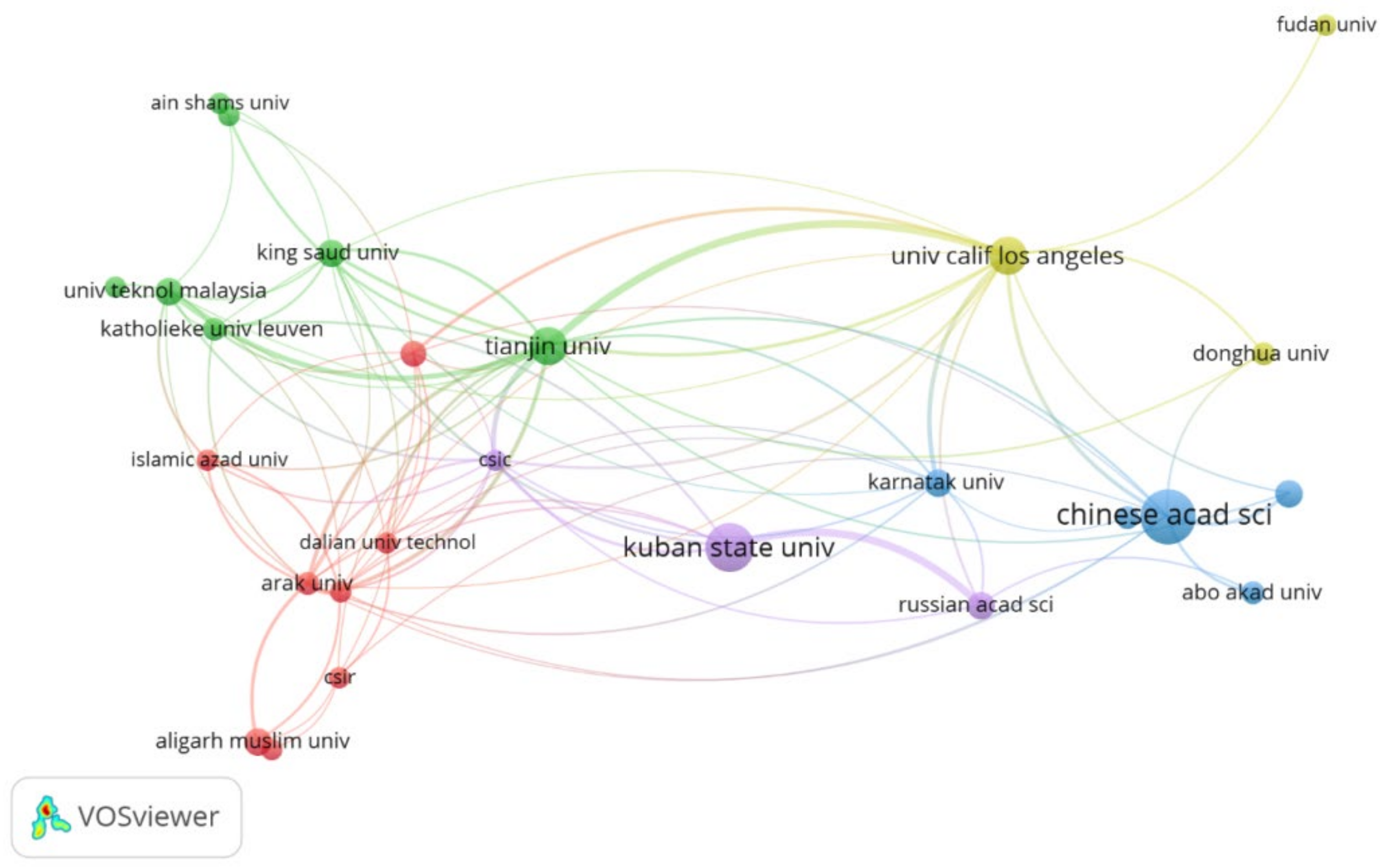
3.5. Countries and Organizations
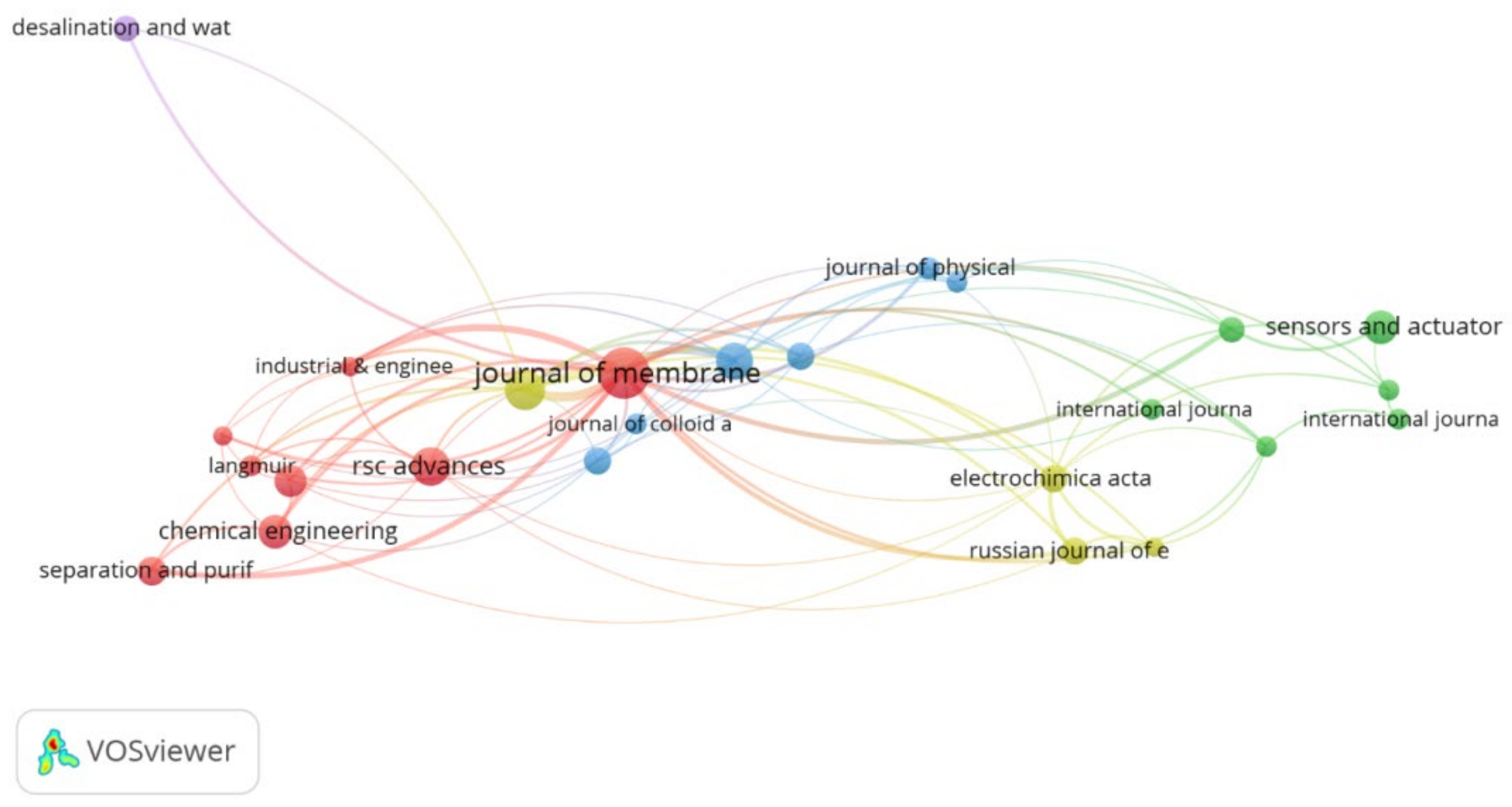
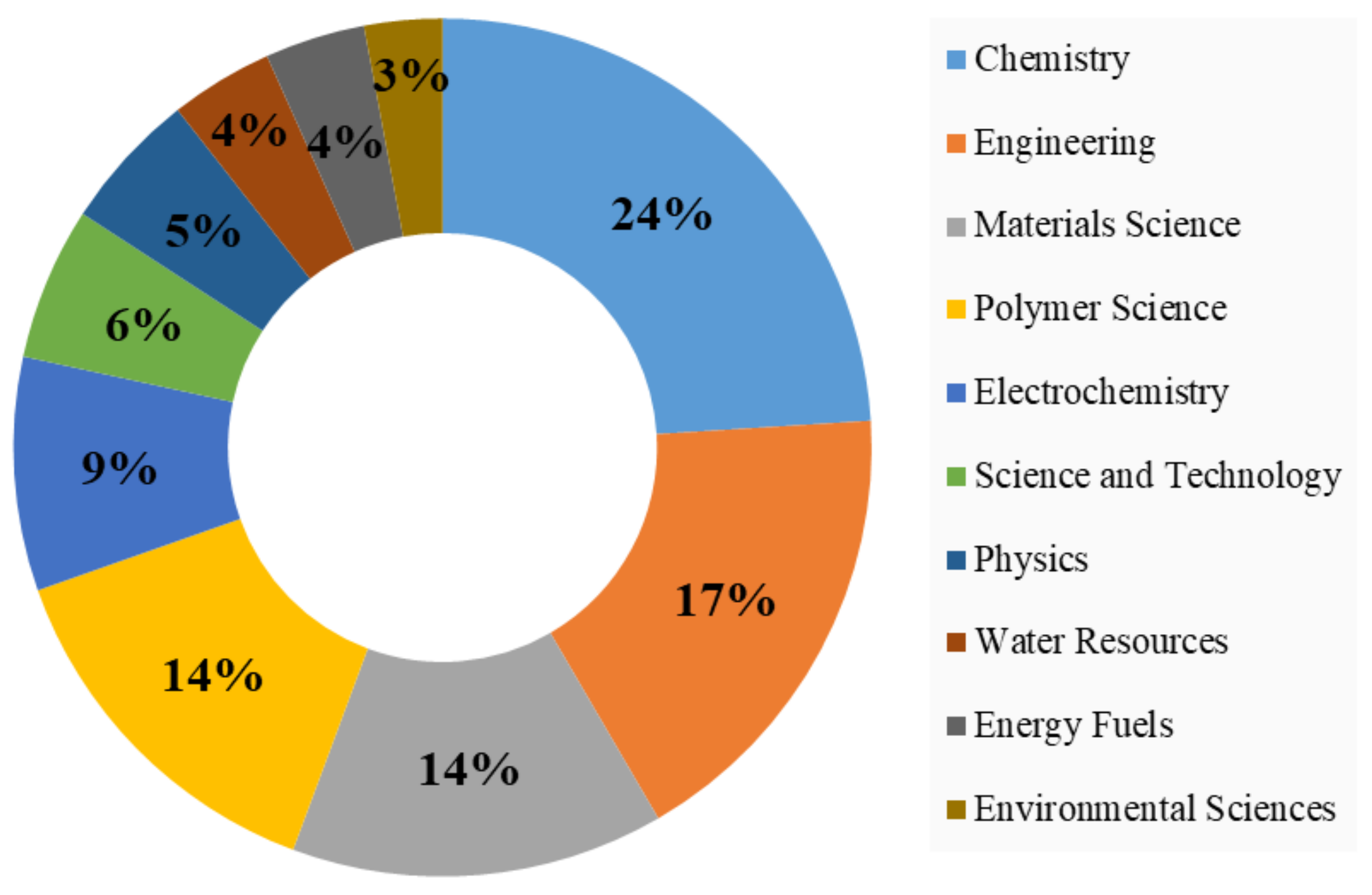
3.6. Journals and Disciplines
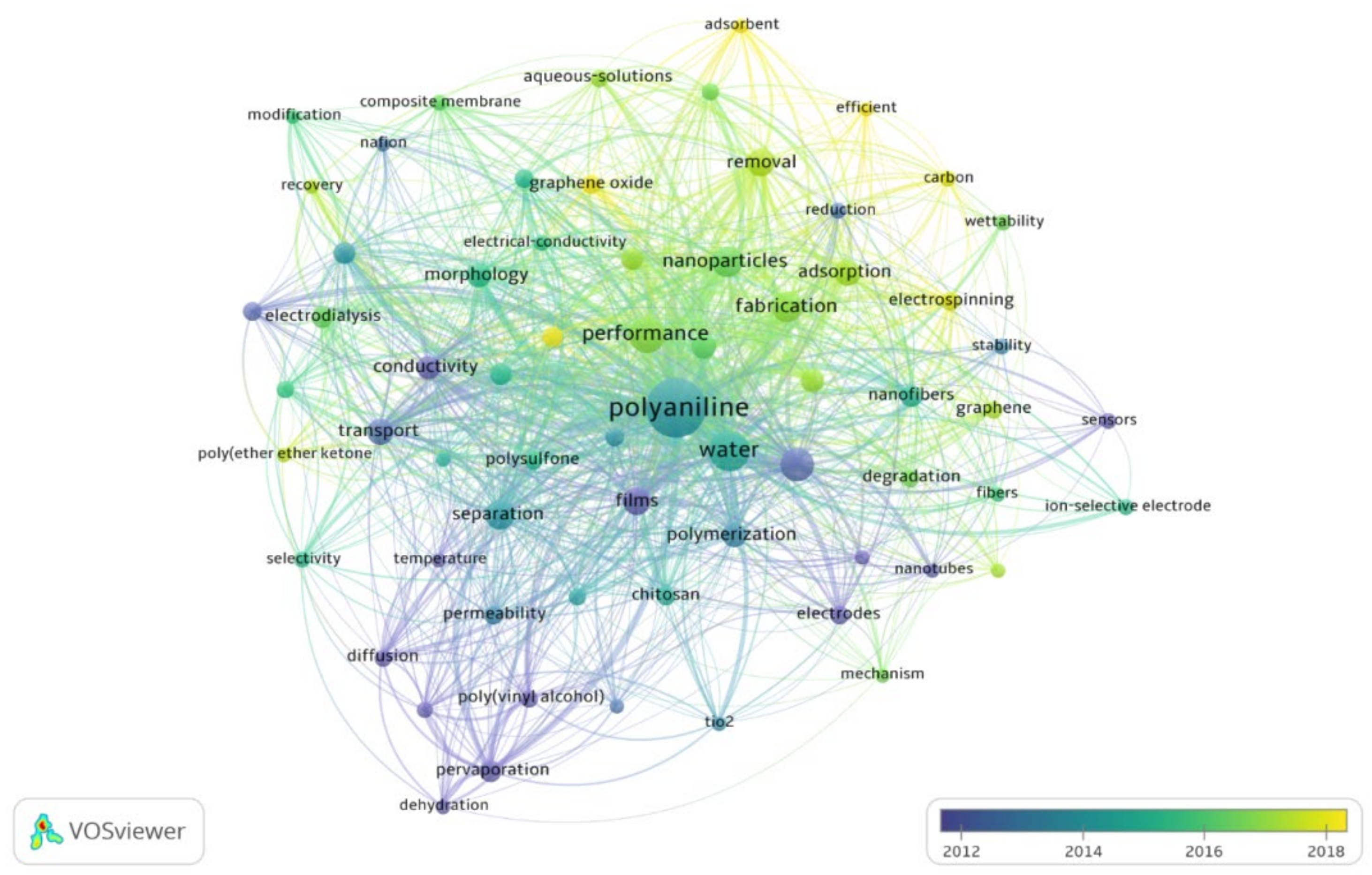
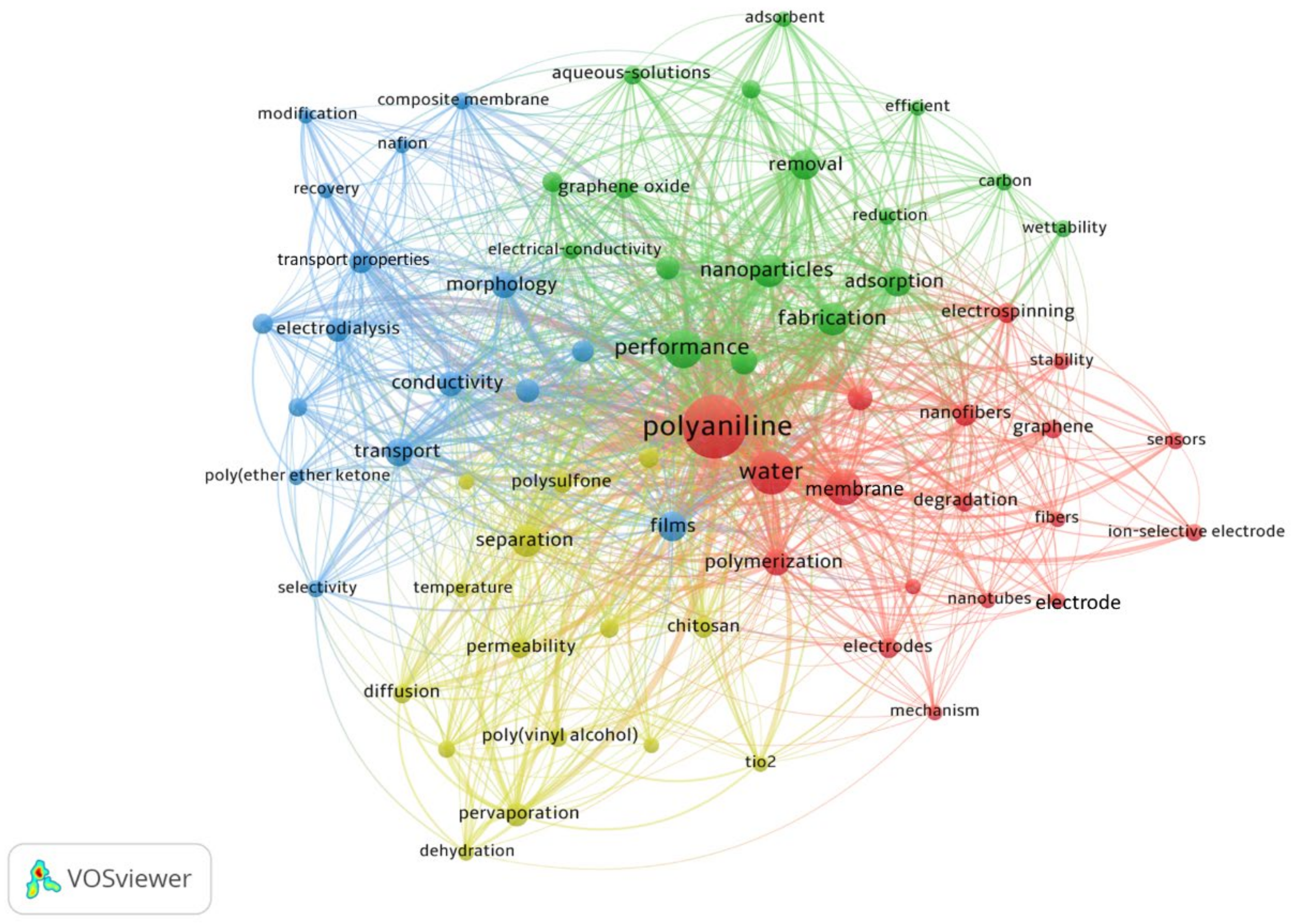
3.7. Keyword Reoccurrence
4. Future Research Trends of PANI Membranes in Water Treatment
5. Key Challenges and Way Forward
5.1. Poor Durability and Stability of PANI Membranes
5.2. Lack of Reliable Techniques to Determine PANI Concentration
5.3. PANI Degradation and Regeneration
5.4. Adverse Environmental Conditions in Wastewater Treatment
6. Conclusions
- The research growth in the application of PANI membranes for water treatment is highlighted. The analysis reveals that the concept of using PANI membranes for water treatment application was introduced during the 1990s. The era of 1995–2005 was an explorative period in which conceptual basics were developed, such as PANI membrane doping and blending, whereas, after 2005, the development era started in which this topic received steady attention.
- A total of 276 journals have published research related to this topic out of which Journal of Membrane Sciences, Desalination, and RSC Advances are the leading journals. The majority of the top journals in this field were focused on chemical aspects of membranes followed by material applications in PANI membranes.
- A total of 63 countries were found active in PANI membrane research with China, India, and the USA as top players in terms of citations and number of publications. Nevertheless, concerning the number of publications per population, China and Australia were spotted as the leaders.
- Concerning research trends, performance optimization of PANI membranes for water treatment was observed as the most discussed topic in the literature. For performance enhancement, the immensely used techniques include the use of nanoparticles and nanocomposites, modification in fabrication techniques, and the use of films.
- Using nanoparticles, carbon nanotubes, and graphene oxides; employing electrospinning, and introducing new adsorbents are hot topics in the field.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shah, A.-U.A.; Kamran, M.; Bilal, S.; Ullah, R. Cost effective chemical oxidative synthesis of soluble and electroactive polyaniline salt and its application as anticorrosive agent for steel. Materials 2019, 12, 1527. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, N.; Dutta, K.; Basu, R.K.; Kundu, P.P. Aromatic π-conjugated curcumin on surface modified polyaniline/polyhydroxyalkanoate based 3D porous scaffolds for tissue engineering applications. ACS Biomater. Sci. Eng. 2016, 2, 2365–2377. [Google Scholar] [CrossRef]
- Das, S.; Dutta, K.; Kundu, P.P. Nickel nanocatalysts supported on sulfonated polyaniline: Potential toward methanol oxidation and as anode materials for DMFCs. J. Mater. Chem. A 2015, 3, 11349–11357. [Google Scholar] [CrossRef]
- Dutta, K.; De, S. Aromatic conjugated polymers for removal of heavy metal ions from wastewater: A short review. Environ. Sci. Water Res. Technol. 2017, 3, 793–805. [Google Scholar] [CrossRef]
- Dutta, K.; Das, S.; Kundu, P.P. Highly methanol resistant and selective ternary blend membrane composed of sulfonated PV d F-co-HFP, sulfonated polyaniline and nafion. J. Appl. Polym. Sci. 2016, 133, 43294. [Google Scholar] [CrossRef]
- Huang, J.; Virji, S.; Weiller, B.H.; Kaner, R.B. Polyaniline nanofibers: Facile synthesis and chemical sensors. J. Am. Chem. Soc. 2003, 125, 314–315. [Google Scholar] [CrossRef]
- Zheng, X.; Guo, Z.; Tian, D.; Zhang, X.; Jiang, L. Electric field induced switchable wettability to water on the polyaniline membrane and oil/water separation. Adv. Mater. Interfaces 2016, 3, 1600461. [Google Scholar] [CrossRef]
- Kurada, K.V.; De, S. Polyaniline doped ultrafiltration membranes: Mechanism of membrane formation and pH response characteristics. Polymer 2018, 153, 201–213. [Google Scholar] [CrossRef]
- Xu, L.; Shahid, S.; Holda, A.K.; Emanuelsson, E.A.C.; Patterson, D. Stimuli responsive conductive polyaniline membrane: In-filtration electrical tuneability of flux and MWCO. J. Membr. Sci. 2018, 552, 153–166. [Google Scholar] [CrossRef]
- Fazullin, D.D.; Mavrin, G.V.; Sokolov, M.P. Cation-exchange membranes with polyaniline surface layer for water treatment. Am. J. Environ. Sci. 2014, 10, 424–430. [Google Scholar] [CrossRef]
- Liu, M.; Li, J.; Guo, Z. Polyaniline coated membranes for effective separation of oil-in-water emulsions. J. Colloid Interface Sci. 2016, 467, 261–270. [Google Scholar] [CrossRef]
- Wang, K.; Xu, L.; Li, K.; Liu, L.; Zhang, Y.; Wang, J. Development of polyaniline conductive membrane for electrically enhanced membrane fouling mitigation. J. Membr. Sci. 2019, 570, 371–379. [Google Scholar] [CrossRef]
- Lin, C.-W.; Xue, S.; Ji, C.; Huang, S.-C.; Tung, V.; Kaner, R.B. Conducting polyaniline for antifouling ultrafiltration membranes: Solutions and challenges. Nano Lett. 2021, 21, 3699–3707. [Google Scholar] [CrossRef] [PubMed]
- Donthu, N.; Kumar, S.; Mukherjee, D.; Pandey, N.; Lim, W.M. How to conduct a bibliometric analysis: An overview and guidelines. J. Bus. Res. 2021, 133, 285–296. [Google Scholar] [CrossRef]
- Pritchard, A. Statistical bibliography or bibliometrics. J. Doc. 1969, 25, 348–349. [Google Scholar]
- He, L.; Fang, H.; Chen, C.; Wu, Y.; Wang, Y.; Ge, H.; Wang, L.; Wan, Y.; He, H. Metastatic castration-resistant prostate cancer: Academic insights and perspectives through bibliometric analysis. Medicine 2020, 99, e19760. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yang, Z.; Zhu, S.; Xu, B.; Li, S.; Zhang, M. A bibliometric analysis of cloud computing technology research. In Proceedings of the 2018 IEEE 3rd Advanced Information Technology, Electronic and Automation Control Conference (IAEAC), Chongqing, China, 12–14 October 2018. [Google Scholar]
- Naseer, M.N.; Zaidi, A.A.; Khan, H.; Kumar, S.; bin Owais, M.T.; Jaafar, J.; Suhaimin, N.S.; Wahab, Y.A.; Dutta, K.; Asif, M.; et al. Mapping the field of microbial fuel cell: A quantitative literature review (1970–2020). Energy Rep. 2021, 7, 4126–4138. [Google Scholar] [CrossRef]
- Youngblood, M.; Lahti, D. A bibliometric analysis of the interdisciplinary field of cultural evolution. Palgrave Commun. 2018, 4, 120. [Google Scholar] [CrossRef]
- Wu, H.; Tong, L.; Wang, Y.; Yan, H.; Sun, Z. Bibliometric analysis of global research trends on ultrasound microbubble: A quickly developing field. Front. Pharmacol. 2021, 12, 646626. [Google Scholar] [CrossRef]
- Birkle, C.; Pendlebury, D.A.; Schnell, J.; Adams, J. Web of Science as a data source for research on scientific and scholarly activity. Quant. Sci. Stud. 2020, 1, 363–376. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Guo, L.; Hong, J.; Zhao, W.; Hu, X.; Chang, C.; Liu, W.; Xiong, K. Bibliometric analysis of the inflammasome and pyroptosis in brain. Front. Pharmacol. 2020, 11, 626502. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. VOSviewer Manual; Univeristeit Leiden: Leiden, The Netherlands, 2013; Volume 1, pp. 1–53. [Google Scholar]
- Schmidt, V.; Tegtmeyer, D.; Heitbaum, J. Transport of protons and water through polyaniline membranes studied with on-line mass spectrometry. J. Electroanal. Chem. 1995, 385, 149–155. [Google Scholar] [CrossRef]
- Messina, R.; Sarazin, C.; Yu, L.T.; Buvet, R. Propriétés de membranes constituées de polymères semi-conducteurs échangeurs redox polyaniline et polypyrrole. J. Chim. Phys. 1976, 73, 919–921. [Google Scholar] [CrossRef]
- Gan, L.-M.; Chan, H.S.O.; Chew, C.-H.; Ma, L. Method of Producing Conductive Polymers in Microemulsions. U.S. Patent 5256730A, 26 October 1993. [Google Scholar]
- Hisao, H.; Kenji, M.; Tomoumi, O. Anisotropic Membrane for Gas Separation Consisting of Polyaniline and Its Production. JPH06238138A, 17 February 1993. [Google Scholar]
- Kenichi, M. Production of Functional Water with Low Surface Tension. JPH10139403A, 5 November 1996. [Google Scholar]
- Ball, I.J.; Huang, S.-C.; Su, T.M.; Kaner, R.B. Permselectivity and temperature-dependent permeability of polyaniline membranes. Synth. Met. 1997, 84, 799–800. [Google Scholar] [CrossRef]
- Su, T.M.; Ball, I.J.; Conklin, J.A.; Huang, S.-C.; Larson, R.K.; Nguyen, S.L.; Lew, B.M.; Kaner, R.B. Polyaniline/polyimide blends for pervaporation and gas separation studies. Synth. Met. 1997, 84, 801–802. [Google Scholar] [CrossRef]
- Huang, S.-C.; Ball, I.J.; Kaner, R.B. Polyaniline membranes for pervaporation of carboxylic acids and water. Macromolecules 1998, 31, 5456–5464. [Google Scholar] [CrossRef]
- Ball, I.; Huang, S.-C.; Kaner, R. Polyaniline Membranes for Liquid Separations. In Technical Papers of the Annual Technical Conference-Society of Plastics Engineers Incorporated; Society of Plastics Engineers Inc.: Danbury, CT, USA, 1998. [Google Scholar]
- Lee, Y.M.; Nam, S.Y.; Ha, S.Y. Pervaporation of water/isopropanol mixtures through polyaniline membranes doped with poly (acrylic acid). J. Membr. Sci. 1999, 159, 41–46. [Google Scholar]
- Huang, S.-C.; Huang, C.-T.; Lu, S.-Y.; Chou, K.-S. Ceramic/polyaniline composite porous membranes. J. Porous Mater. 1999, 6, 153–159. [Google Scholar] [CrossRef]
- Ball, I.J.; Huang, S.-C.; Miller, K.J.; Wolf, R.A.; Shimano, J.Y.; Kaner, R.B. The pervaporation of ethanol/water feeds with polyaniline membranes and blends. Synth. Met. 1999, 102, 1311–1312. [Google Scholar] [CrossRef]
- Ball, I.J.; Huang, S.; Wolf, R.A.; Shimano, J.Y.; Kaner, R.B. Pervaporation studies with polyaniline membranes and blends. J. Membr. Sci. 2000, 174, 161–176. [Google Scholar] [CrossRef]
- Wang, P.; Tan, K.L.; Kang, E.T.; Neoh, K.G. Preparation and characterization of semi-conductive poly (vinylidene fluoride)/polyaniline blends and membranes. Appl. Surf. Sci. 2002, 193, 36–45. [Google Scholar] [CrossRef]
- Amado, F.D.; Gondran, E.; Ferreira, J.Z.; Rodrigues, M.A.; Ferreira, C.A. Synthesis and characterisation of high impact polystyrene/polyaniline composite membranes for electrodialysis. J. Membr. Sci. 2004, 234, 139–145. [Google Scholar] [CrossRef]
- Daraei, P.; Madaeni, S.S.; Ghaemi, N.; Salehi, E.; Khadivi, M.A.; Moradian, R.; Astinchap, B. Novel polyethersulfone nanocomposite membrane prepared by PANI/Fe3O4 nanoparticles with enhanced performance for Cu (II) removal from water. J. Membr. Sci. 2012, 415, 250–259. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Z.; Sun, N.; Wang, J.; Wang, S. Performance improvement of polysulfone ultrafiltration membrane by blending with polyaniline nanofibers. J. Membr. Sci. 2008, 320, 363–371. [Google Scholar] [CrossRef]
- Nagarale, R.; Gohil, G.; Shahi, V.K. Sulfonated poly (ether ether ketone)/polyaniline composite proton-exchange membrane. J. Membr. Sci. 2006, 280, 389–396. [Google Scholar] [CrossRef]
- Sairam, M.; Patil, M.B.; Veerapur, R.S.; Patil, S.A.; Aminabhavi, T.M. Novel dense poly (vinyl alcohol)–TiO2 mixed matrix membranes for pervaporation separation of water–isopropanol mixtures at 30 °C. J. Membr. Sci. 2006, 281, 95–102. [Google Scholar] [CrossRef]
- Gabrielli, C.; Keddam, M.; Nadi, N.; Perrot, H. Ions and solvent transport across conducting polymers investigated by ac electrogravimetry. Application to polyaniline. J. Electroanal. Chem. 2000, 485, 101–113. [Google Scholar] [CrossRef]
- Guillen, G.R.; Farrell, T.P.; Kaner, R.B.; Hoek, E.M.V. Pore-structure, hydrophilicity, and particle filtration characteristics of polyaniline–polysulfone ultrafiltration membranes. J. Mater. Chem. 2010, 20, 4621–4628. [Google Scholar] [CrossRef]
- Naidu, B.V.K.; Sairam, M.; Raju, K.V.S.N.; Aminabhavi, T.M. Pervaporation separation of water plus isopropanol mixtures using novel nanocomposite membranes of poly (vinyl alcohol) and polyaniline. J. Membr. Sci. 2005, 260, 142–155. [Google Scholar]
- Li, R.; Liu, L.; Yang, F. Removal of aqueous Hg (II) and Cr (VI) using phytic acid doped polyaniline/cellulose acetate composite membrane. J. Hazard. Mater. 2014, 280, 20–30. [Google Scholar] [CrossRef]
- Alcaraz-Espinoza, J.J.; Chávez-Guajardo, A.E.; Medina-Llamas, J.C.; Andrade, C.A.S.; de Melo, C.P. Hierarchical composite polyaniline–(electrospun polystyrene) fibers applied to heavy metal remediation. ACS Appl. Mater. Interfaces 2015, 7, 7231–7240. [Google Scholar] [CrossRef] [PubMed]
- Web of Science. Categories & Collections (Scope Notes) 2021. Available online: https://mjl.clarivate.com/help-center (accessed on 12 September 2021).
- Liao, Y.; Li, X.-G.; Hoek, E.M.V.; Kaner, R.B. Carbon nanotube/polyaniline nanofiber ultrafiltration membranes. J. Mater. Chem. A 2013, 1, 15390–15396. [Google Scholar] [CrossRef]
- Liao, Y.; Yu, D.-G.; Wang, X.; Chain, W.; Li, X.-G.; Hoek, E.M.V.; Kaner, R.B. Carbon nanotube-templated polyaniline nanofibers: Synthesis, flash welding and ultrafiltration membranes. Nanoscale 2013, 5, 3856–3862. [Google Scholar] [CrossRef] [PubMed]
- Shawky, H.A.; Chae, S.-R.; Lin, S.; Wiesner, M.R. Synthesis and characterization of a carbon nanotube/polymer nanocomposite membrane for water treatment. Desalination 2011, 272, 46–50. [Google Scholar] [CrossRef]
- Chajanovsky, I.; Suckeveriene, R.Y. Suckeveriene, Preparation of Hybrid Polyaniline/Nanoparticle Membranes for Water Treatment Using an Inverse Emulsion Polymerization Technique under Sonication. Processes 2020, 8, 1503. [Google Scholar] [CrossRef]
- Wang, D.-W.; Li, F.; Zhao, J.; Ren, W.; Chen, Z.-G.; Tan, J.; Wu, Z.-S.; Gentle, I.; Lu, G.Q.; Cheng, H.-M. Fabrication of graphene/polyaniline composite paper via in situ anodic electropolymerization for high-performance flexible electrode. ACS Nano 2009, 3, 1745–1752. [Google Scholar] [CrossRef]
- Mao, L.; Zhang, K.; Chan, H.S.O.; Wu, J. Surfactant-stabilized graphene/polyaniline nanofiber composites for high performance supercapacitor electrode. J. Mater. Chem. 2012, 22, 80–85. [Google Scholar] [CrossRef]
- Wang, T.; Sun, H.; Peng, T.; Liu, B.; Hou, Y.; Lei, B. Preparation and characterization of polyaniline/p-phenylenediamine grafted graphene oxide composites for supercapacitors. J. Mol. Struct. 2020, 1221, 128835. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.L.; Zhao, X.S.; Wu, J. Graphene/polyaniline nanofiber composites as supercapacitor electrodes. Chem. Mater. 2010, 22, 1392–1401. [Google Scholar] [CrossRef]

| Rank | Title | Citations | Avg. Citation per Year | Ref. |
|---|---|---|---|---|
| 1 | Novel polyethersulfone nanocomposite membrane prepared by PANI/Fe3O4 nanoparticles with enhanced performance for Cu(II) removal from water | 202 | 20.2 | [39] |
| 2 | Performance improvement of polysulfone ultrafiltration membrane by blending with polyaniline nanofibers | 158 | 11.3 | [40] |
| 3 | Sulfonated poly (ether ether ketone)/polyaniline composite proton-exchange membrane | 134 | 8.38 | [41] |
| 4 | Novel dense poly (vinyl alcohol)-TiO2 mixed matrix membranes for pervaporation separation of water-isopropanol mixtures at 30 °C | 122 | 7.63 | [42] |
| 5 | Ions and solvent transport across conducting polymers investigated by AC electrogravimetry. Application to polyaniline | 108 | 4.91 | [43] |
| 6 | Pore-structure, hydrophilicity, and particle filtration characteristics of polyaniline-polysulfone ultrafiltration membranes | 86 | 7.17 | [44] |
| 7 | Pervaporation separation of water plus isopropanol mixtures using novel nanocomposite membranes of poly (vinyl alcohol) and polyaniline | 84 | 4.94 | [45] |
| 8 | Removal of aqueous Hg(II) and Cr(VI) using phytic acid doped polyaniline/cellulose acetate composite membrane | 83 | 10.4 | [46] |
| 9 | Hierarchical Composite Polyaniline-(Electrospun Polystyrene) Fibers Applied to Heavy Metal Remediation | 81 | 11.6 | [47] |
| 10 | Electric Field Induced Switchable Wettability to Water on the Polyaniline Membrane and Oil/Water Separation | 79 | 13.2 | [7] |
| Name | Organization | Research Area |
|---|---|---|
| Wang, Jixiao | Tianjin University | Chemical engineering, polymers |
| Wang, Zhi | Tianjin University | Chemical engineering |
| Kononenko, Natalia A | Kuban State University | Physical chemistry |
| Zhao, Song | Tianjin University | Modeling and simulation |
| Wang, Shi-Chang | Tianjin University | Chemical engineering, membrane science |
| Huang, Shin-Chin | National University Kaohsiung | Chemical engineering, membrane science |
| Kaner, Rb | University of California LA | Material science |
| Ball, I. Joseph | University of California LA | Chemical engineering, material science |
| Berezina, Ninel P. | Kuban State University | Physical chemistry |
| Van Der Bruggen, Bart | Tshwane University of Technology | Chemical engineering |
| Rank | Country | Publication Contribution | Citation Contribution | Normalized Contribution |
|---|---|---|---|---|
| 1 | China | 23.41% | 22.98% | 1.28 × 10−7 |
| 2 | India | 12.74% | 11.91% | 7.1 × 10−8 |
| 3 | USA | 9.10% | 13.23% | 2.12 × 10−7 |
| 4 | Iran | 6.11% | 5.62% | 5.6 × 10−7 |
| 5 | Russia | 5.59% | 3.08% | 2.95 × 10−7 |
| 6 | South Korea | 3.51% | 3.12% | 5.21 × 10−7 |
| 7 | Saudi Arabia | 3.12% | 1.46% | 6.89 × 10−7 |
| 8 | Brazil | 2.73% | 4.29% | 9.88 × 10−8 |
| 9 | Malaysia | 2.73% | 2.86% | 6.49 × 10−7 |
| 10 | Australia | 2.34% | 2.83% | 7.01 × 10−7 |
| Rank | Organization | Country | Publication Contribution | Citation Contribution |
|---|---|---|---|---|
| 1 | Chinese Academy of Sciences | China | 8.51% | 10.18% |
| 2 | Kuban State University | Russia | 7.09% | 3.64% |
| 3 | Tianjin University | China | 4.96% | 9.72% |
| 4 | University of California LA | USA | 4.96% | 6.66% |
| 5 | Politechnica University of Bucharest | Romania | 4.26% | 1.53% |
| 6 | Aligarh Muslim University | India | 3.19% | 3.89% |
| 7 | Karnatak University | India | 3.19% | 7.61% |
| 8 | King Saud University | Saudi Arabia | 3.19% | 1.43% |
| 9 | Russian Academy of Sciences | Russia | 3.19% | 2.02% |
| 10 | Universiti Teknologi Malaysia | Malaysia | 3.19% | 2.58% |
| Rank | Journal Name | Impact Factor | Document Contribution | Citation Contribution | Category |
|---|---|---|---|---|---|
| 1 | Journal of Membrane Science | 8.74 | 11.85% | 21.91% | Polymer science Engineering, chemical |
| 2 | Desalination | 9.5 | 7.41% | 7.01% | Water resources Engineering, chemical |
| 3 | RSC Advances | 3.36 | 7.04% | 5.24% | Chemistry, multidisciplinary |
| 4 | Journal of Applied Polymer Science | 3.13 | 6.30% | 3.43% | Polymer science |
| 5 | Chemical Engineering Journal | 13.3 | 5.19% | 3.41% | Engineering, environmental engineering, chemical |
| 6 | Sensors and Actuators B-Chemical | 7.46 | 5.19% | 7.21% | Chemistry, analytical electrochemistry instruments and instrumentation |
| 7 | ACS Applied Materials & Interfaces | 9.23 | 4.81% | 7.26% | Materials science, multidisciplinary nanoscience and nanotechnology |
| 8 | Separation and Purification Technology | 7.31 | 4.07% | 3.74% | Engineering, chemical |
| 9 | Electrochimica Acta | 6.9 | 3.70% | 2.62% | Electrochemistry |
| 10 | Journal of Materials Chemistry A | 12.7 | 3.70% | 3.60% | Materials science, multidisciplinary chemistry, physical Energy and fuels |
| Rank | Keywords | % Age Occurrence |
|---|---|---|
| 1 | Polyaniline | 16.73 |
| 2 | Water | 6.52 |
| 3 | Performance | 4.79 |
| 4 | Membranes | 4.00 |
| 5 | Nanoparticles | 3.10 |
| 6 | Fabrication | 3.05 |
| 7 | Separation | 2.68 |
| 8 | Films | 2.63 |
| 9 | Removal | 2.37 |
| 10 | Adsorption | 2.31 |
| 11 | Transport properties | 2.10 |
| 12 | Polymerization | 1.84 |
| 13 | Morphology | 1.79 |
| 14 | Nanocomposite | 1.79 |
| 15 | Conductivity | 1.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naseer, M.N.; Dutta, K.; Zaidi, A.A.; Asif, M.; Alqahtany, A.; Aldossary, N.A.; Jamil, R.; Alyami, S.H.; Jaafar, J. Research Trends in the Use of Polyaniline Membrane for Water Treatment Applications: A Scientometric Analysis. Membranes 2022, 12, 777. https://doi.org/10.3390/membranes12080777
Naseer MN, Dutta K, Zaidi AA, Asif M, Alqahtany A, Aldossary NA, Jamil R, Alyami SH, Jaafar J. Research Trends in the Use of Polyaniline Membrane for Water Treatment Applications: A Scientometric Analysis. Membranes. 2022; 12(8):777. https://doi.org/10.3390/membranes12080777
Chicago/Turabian StyleNaseer, Muhammad Nihal, Kingshuk Dutta, Asad A. Zaidi, Muhammad Asif, Ali Alqahtany, Naief A. Aldossary, Rehan Jamil, Saleh H. Alyami, and Juhana Jaafar. 2022. "Research Trends in the Use of Polyaniline Membrane for Water Treatment Applications: A Scientometric Analysis" Membranes 12, no. 8: 777. https://doi.org/10.3390/membranes12080777
APA StyleNaseer, M. N., Dutta, K., Zaidi, A. A., Asif, M., Alqahtany, A., Aldossary, N. A., Jamil, R., Alyami, S. H., & Jaafar, J. (2022). Research Trends in the Use of Polyaniline Membrane for Water Treatment Applications: A Scientometric Analysis. Membranes, 12(8), 777. https://doi.org/10.3390/membranes12080777









