The Role of Cholesterol in the Interaction of the Lipid Monolayer with the Endocrine Disruptor Bisphenol-A
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Langmuir Films
2.3. PM–IRRAS
3. Results and Discussion
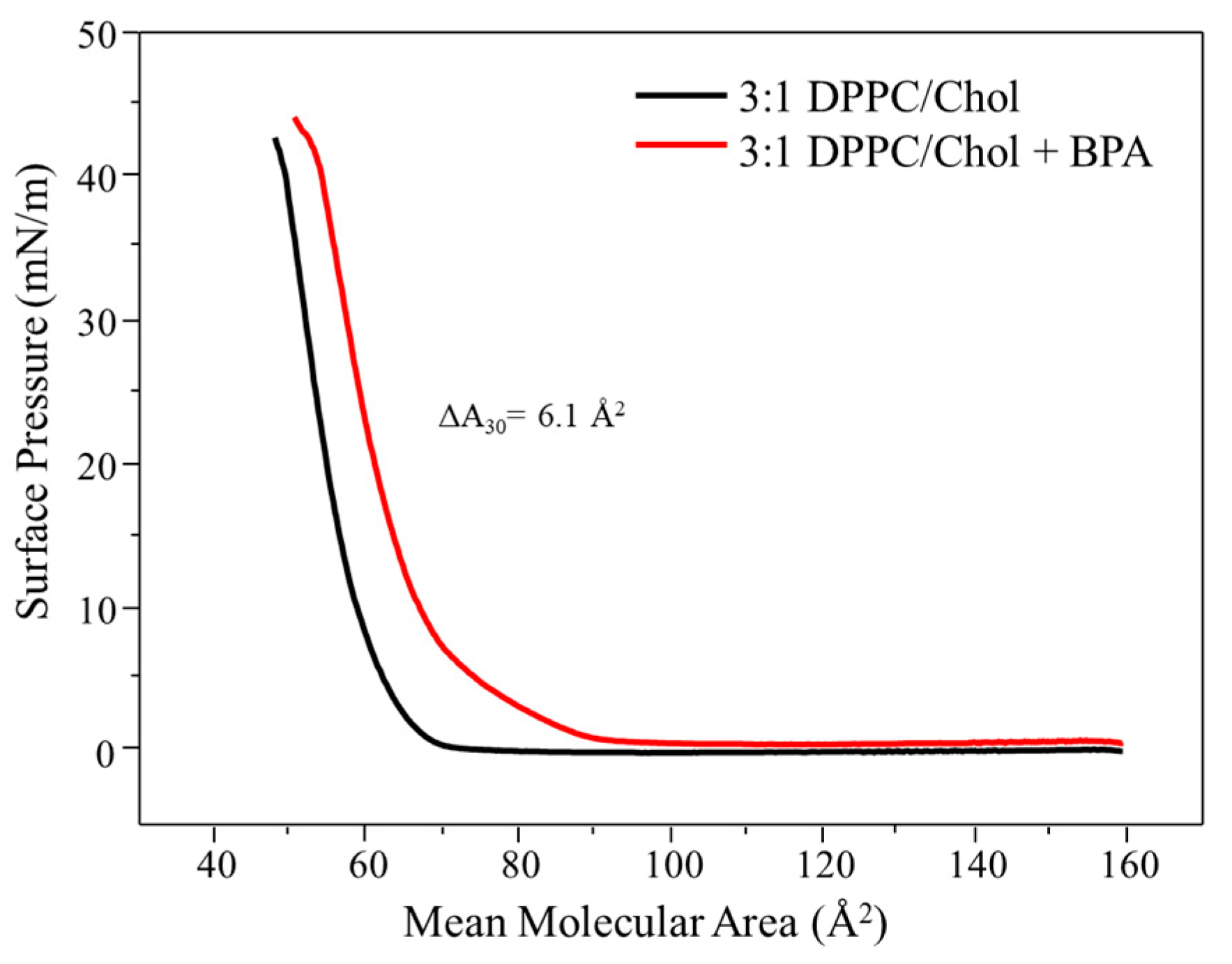
3.1. Langmuir Monolayers
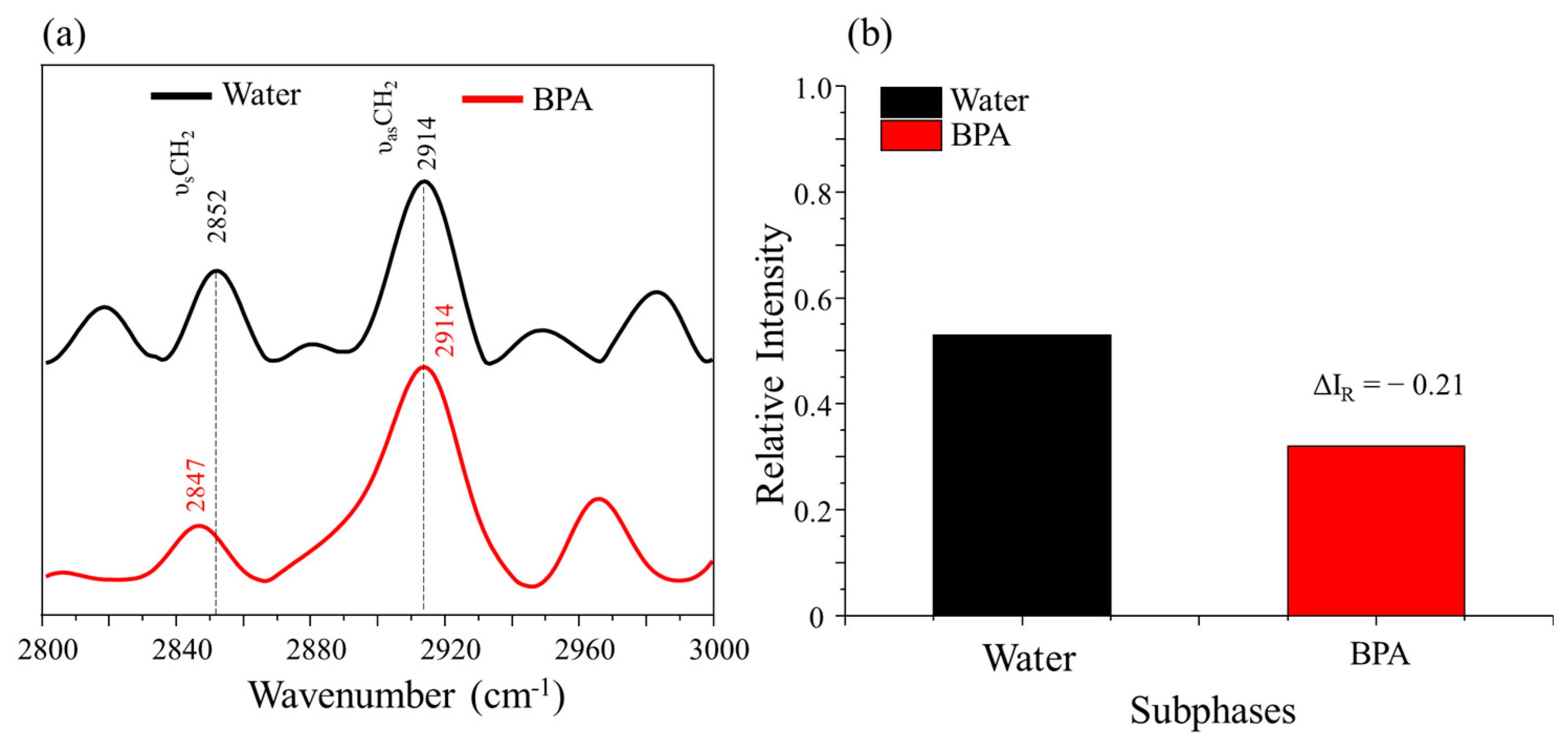
3.2. PM–IRRAS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deblonde, T.; Cossu-Leguille, C.; Hartemann, P. Emerging Pollutants in Wastewater: A Review of the Literature. Int. J. Hyg. Environ. Health 2011, 214, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Naushad, M.; Govarthanan, M.; Iqbal, J.; Alfadul, S.M. Emerging Contaminants of High Concern for the Environment: Current Trends and Future Research. Environ. Res. 2022, 207, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yin, M.; Yang, W.; Li, H.; Zhong, Y.; Mo, L.; Liang, Y.; Ma, X.; Sun, X. Emerging Pollutants in Water Environment: Occurrence, Monitoring, Fate, and Risk Assessment. Water Environ. Res. 2019, 91, 984–991. [Google Scholar] [CrossRef] [Green Version]
- Barrios-Estrada, C.; de Jesús Rostro-Alanis, M.; Muñoz-Gutiérrez, B.D.; Iqbal, H.M.N.; Kannan, S.; Parra-Saldívar, R. Emergent Contaminants: Endocrine Disruptors and Their Laccase-Assisted Degradation—A Review. Sci. Total Environ. 2018, 612, 1516–1531. [Google Scholar] [CrossRef] [PubMed]
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A Review on Endocrine Disruptors and Their Possible Impacts on Human Health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258. [Google Scholar] [CrossRef]
- Rubin, B.S. Bisphenol A: An Endocrine Disruptor with Widespread Exposure and Multiple Effects. J. Steroid Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef]
- Vom Saal, F.S.; Vandenberg, L.N. Update on the Health Effects of Bisphenol A: Overwhelming Evidence of Harm. Endocrinology 2021, 162, 1–25. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, H.; Wu, J.; Yuan, L.; Wang, Y.; Du, X.; Wang, R.; Marwa, P.W.; Petlulu, P.; Chen, X.; et al. The Adverse Health Effects of Bisphenol A and Related Toxicity Mechanisms. Environ. Res. 2019, 176, 108575. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human Exposure to Bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef]
- Dallio, M.; Masarone, M.; Errico, S.; Gravina, A.G.; Nicolucci, C.; di Sarno, R.; Gionti, L.; Tuccillo, C.; Persico, M.; Stiuso, P.; et al. Role of Bisphenol A as Environmental Factor in the Promotion of Non-Alcoholic Fatty Liver Disease: In Vitro and Clinical Study. Aliment. Pharmacol. Ther. 2018, 47, 826–837. [Google Scholar] [CrossRef] [Green Version]
- Wetherill, Y.B.; Akingbemi, B.T.; Kanno, J.; McLachlan, J.A.; Nadal, A.; Sonnenschein, C.; Watson, C.S.; Zoeller, R.T.; Belcher, S.M. In Vitro Molecular Mechanisms of Bisphenol A Action. Reprod. Toxicol. 2007, 24, 178–198. [Google Scholar] [CrossRef] [PubMed]
- Nobre, T.M.; Pavinatto, F.J.; Caseli, L.; Barros-Timmons, A.; Dynarowicz-Łatka, P.; Oliveira, O.N. Interactions of Bioactive Molecules & Nanomaterials with Langmuir Monolayers as Cell Membrane Models. Thin Solid Films 2015, 593, 158–188. [Google Scholar] [CrossRef]
- van Meer, G.; de Kroon, A.I.P.M. Lipid Map of the Mammalian Cell Lipid Map of the Mammalian Cell. J. Cell Sci. 2011, 124, 5–8. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.-T.; Kreutzberger, A.J.B.; Lee, J.; Kiessling, V.; Tamm, L.K. The Role of Cholesterol in Membrane Fusion. Chem. Phys. Lipids 2016, 199, 136–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, S.; Doktorova, M.; Molugu, T.R.; Heberle, F.A.; Scott, H.L.; Dzikovski, B.; Nagao, M.; Stingaciu, L.-R.; Standaert, R.F.; Barrera, F.N.; et al. How Cholesterol Stiffens Unsaturated Lipid Membranes. Proc. Natl. Acad. Sci. USA 2020, 117, 21896–21905. [Google Scholar] [CrossRef] [PubMed]
- Al-Rekabi, Z.; Contera, S. Multifrequency AFM Reveals Lipid Membrane Mechanical Properties and the Effect of Cholesterol in Modulating Viscoelasticity. Proc. Natl. Acad. Sci. USA 2018, 115, 2658–2663. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, G.C.M.; Cruz, M.A.E.; Faria, A.N.; Zancanela, D.C.; Ciancaglini, P.; Ramos, A.P. Biomimetic Collagen/Phospholipid Coatings Improve Formation of Hydroxyapatite Nanoparticles on Titanium. Mater. Sci. Eng. C 2017, 77, 102–110. [Google Scholar] [CrossRef]
- Nuraje, N.; Bai, H.; Su, K. Bolaamphiphilic Molecules: Assembly and Applications. Prog. Polym. Sci. 2012, 38, 302–343. [Google Scholar] [CrossRef]
- Maximino, M.D.; Silva, C.Y.; Cavalcante, D.G.S.M.; Martin, C.S.; Job, A.E.; Oliveira, O.N.; Aléssio, P. Consequences of the Exposure to Bisphenol A in Cell Membrane Models at the Molecular Level and Hamster Ovary Cells Viability. Colloids Surf. B Biointerfaces 2021, 203, 111762. [Google Scholar] [CrossRef]
- Ruiz, G.C.M.; Pazin, W.M.; do Carmo Morato, L.F.; Oliveira, O.N.; Constantino, C.J.L. Correlating Mono- and Bilayers of Lipids to Investigate the Pronounced Effects of Steroid Hormone 17α-Ethynylestradiol on Membrane Models of DPPC/Cholesterol. J. Mol. Liq. 2020, 311, 113324. [Google Scholar] [CrossRef]
- Wyżga, B.; Połeć, K.; Olechowska, K.; Hąc-Wydro, K. The Impact of Toxic Bisphenols on Model Human Erythrocyte Membranes. Colloids Surf. B Biointerfaces 2020, 186, 110670. [Google Scholar] [CrossRef] [PubMed]
- Marsh, D. Lateral Pressure in Membranes. Biochimica Biophysica Acta (BBA) Rev. Biomembranes 1996, 1286, 183–223. [Google Scholar] [CrossRef]
- Casares, D.; Escribá, P.V.; Rosselló, C.A. Membrane Lipid Composition: Effect on Membrane and Organelle Structure, Function and Compartmentalization and Therapeutic Avenues. Int. J. Mol. Sci. 2019, 20, 2167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoki, P.H.B.; Alessio, P.; Furini, L.N.; Constantino, C.J.L.; Neves, T.A.T.; Paulovich, F.V.; Oliveira, M.C.F. De Molecularly Designed Layer-by-Layer (LbL) Films to Detect Catechol Using Information Visualization Methods. Langmuir 2013, 29, 7542–7550. [Google Scholar] [CrossRef]
- Pavinatto, A.; Delezuk, J.A.M.; Souza, A.L.; Pavinatto, F.J.; Volpati, D.; Miranda, P.B.; Campana-Filho, S.P.; Oliveira, O.N. Experimental Evidence for the Mode of Action Based on Electrostatic and Hydrophobic Forces to Explain Interaction between Chitosans and Phospholipid Langmuir Monolayers. Colloids Surf. B Biointerfaces 2016, 145, 201–207. [Google Scholar] [CrossRef]
- Maximino, M.D.; Martin, C.S.; Aléssio, P. Bisphenol A Exposure Induces Multiple Effects in DOPC Membrane Models. J. Mol. Liq. 2022, 359, 119253. [Google Scholar] [CrossRef]
- Hąc-Wydro, K.; Połeć, K.; Broniatowski, M. The Comparative Analysis of the Effect of Environmental Toxicants: Bisphenol A, S and F on Model Plant, Fungi and Bacteria Membranes. The Studies on Multicomponent Systems. J. Mol. Liq. 2019, 289, 111136. [Google Scholar] [CrossRef]
- Maximino, M.D.; Constantino, C.J.L.; Oliveira, O.N.; Alessio, P. Synergy in the Interaction of Amoxicillin and Methylene Blue with Dipalmitoyl Phosphatidyl Choline (DPPC) Monolayers. Appl. Surf. Sci. 2019, 476, 493–500. [Google Scholar] [CrossRef]
- Zawisza, I.; Bin, X.; Lipkowski, J. Potential-Driven Structural Changes in Langmuir-Blodgett DMPC Bilayers Determined by in Situ Spectroelectrochemical PM IRRAS. Langmuir 2007, 23, 5180–5194. [Google Scholar] [CrossRef]
- Zawisza, I.; Wittstock, G.; Boukherroub, R.; Szunerits, S. PM IRRAS Investigation of Thin Silica Films Deposited on Gold. Part 1. Theory and Proof of Concept. Langmuir 2007, 23, 9303–9309. [Google Scholar] [CrossRef]
- Levin, I.W.; Thompson, T.E.; Barenholz, Y.; Huang, C. Two Types of Hydrocarbon Chain Interdigitation in Sphingomyelin Bilayers. Biochemistry 1985, 24, 6282–6286. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.E.; Caseli, L. The Interaction of Mefloquine Hydrochloride with Cell Membrane Models at the Air-Water Interface Is Modulated by the Monolayer Lipid Composition. J. Colloid Interface Sci. 2014, 431, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Attwood, S.J.; Hoopes, M.I.; Drolle, E.; Karttunen, M.; Leonenko, Z. Melatonin Directly Interacts with Cholesterol and Alleviates Cholesterol Effects in Dipalmitoylphosphatidylcholine Monolayers. Soft Matter 2014, 10, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, D.M.; Czernel, G.; Murphy, B.; Runge, B.; Magnussen, O.M.; Gagoś, M. Effect of Cholesterol and Ergosterol on the Antibiotic Amphotericin B Interactions with Dipalmitoylphosphatidylcholine Monolayers: X-ray Reflectivity Study. Biochim. Biophys. Acta Biomembr. 2014, 1838, 2947–2953. [Google Scholar] [CrossRef]
- Flint, S.; Markle, T.; Thompson, S.; Wallace, E. Bisphenol A Exposure, Effects, and Policy: A Wildlife Perspective. J. Environ. Manag. 2012, 104, 19–34. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katata, V.M.; Maximino, M.D.; Silva, C.Y.; Alessio, P. The Role of Cholesterol in the Interaction of the Lipid Monolayer with the Endocrine Disruptor Bisphenol-A. Membranes 2022, 12, 729. https://doi.org/10.3390/membranes12080729
Katata VM, Maximino MD, Silva CY, Alessio P. The Role of Cholesterol in the Interaction of the Lipid Monolayer with the Endocrine Disruptor Bisphenol-A. Membranes. 2022; 12(8):729. https://doi.org/10.3390/membranes12080729
Chicago/Turabian StyleKatata, Victoria M., Mateus D. Maximino, Carla Y. Silva, and Priscila Alessio. 2022. "The Role of Cholesterol in the Interaction of the Lipid Monolayer with the Endocrine Disruptor Bisphenol-A" Membranes 12, no. 8: 729. https://doi.org/10.3390/membranes12080729
APA StyleKatata, V. M., Maximino, M. D., Silva, C. Y., & Alessio, P. (2022). The Role of Cholesterol in the Interaction of the Lipid Monolayer with the Endocrine Disruptor Bisphenol-A. Membranes, 12(8), 729. https://doi.org/10.3390/membranes12080729





