Chemical Cleaning and Membrane Aging in MBR for Textile Wastewater Treatment
Abstract
:1. Introduction
2. Material and Methods
2.1. Polit-Scale MBRs for Textile Wastewater Treatment
2.2. Membrane Aging Batch Test
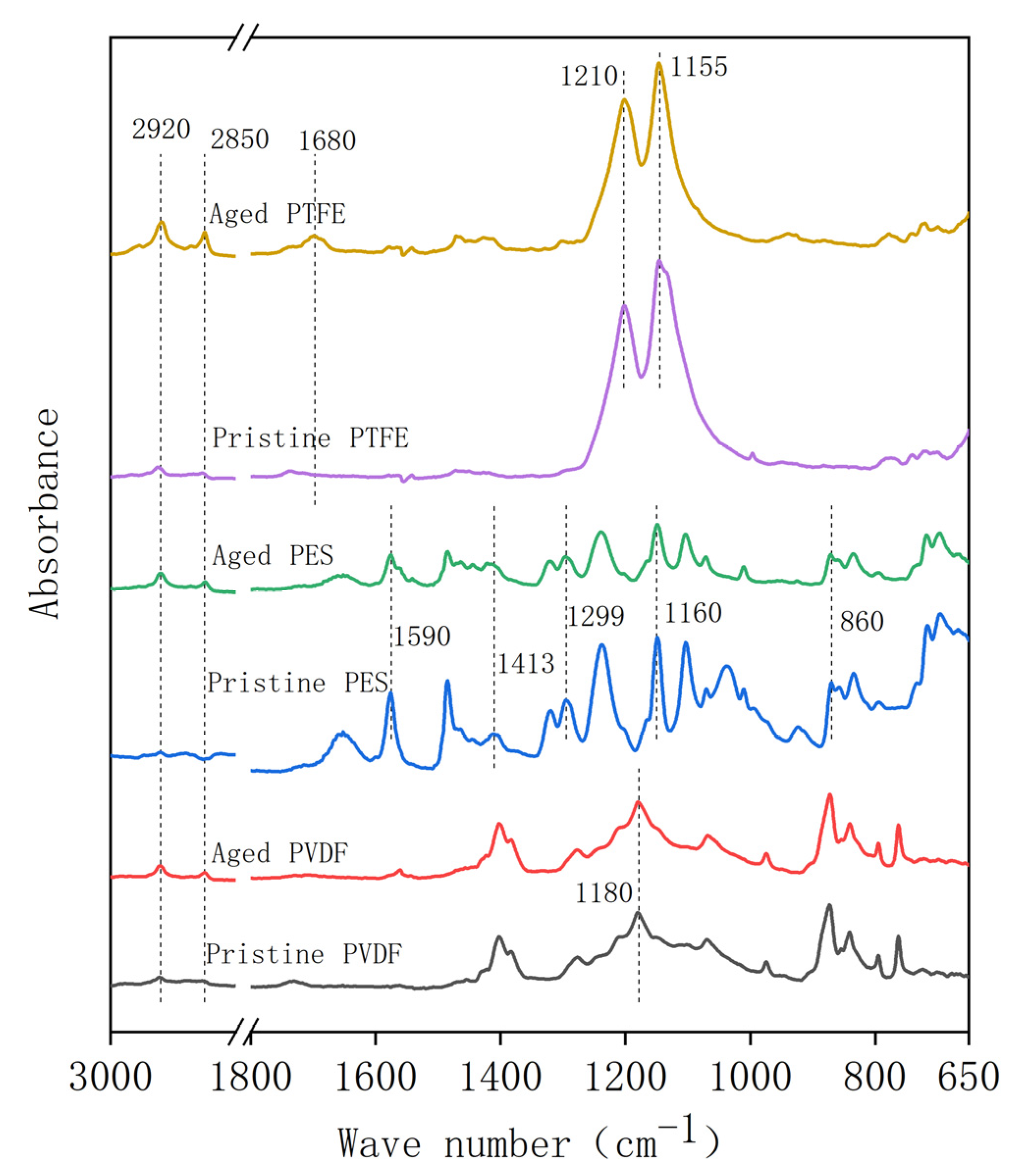
2.3. Membrane Characterization
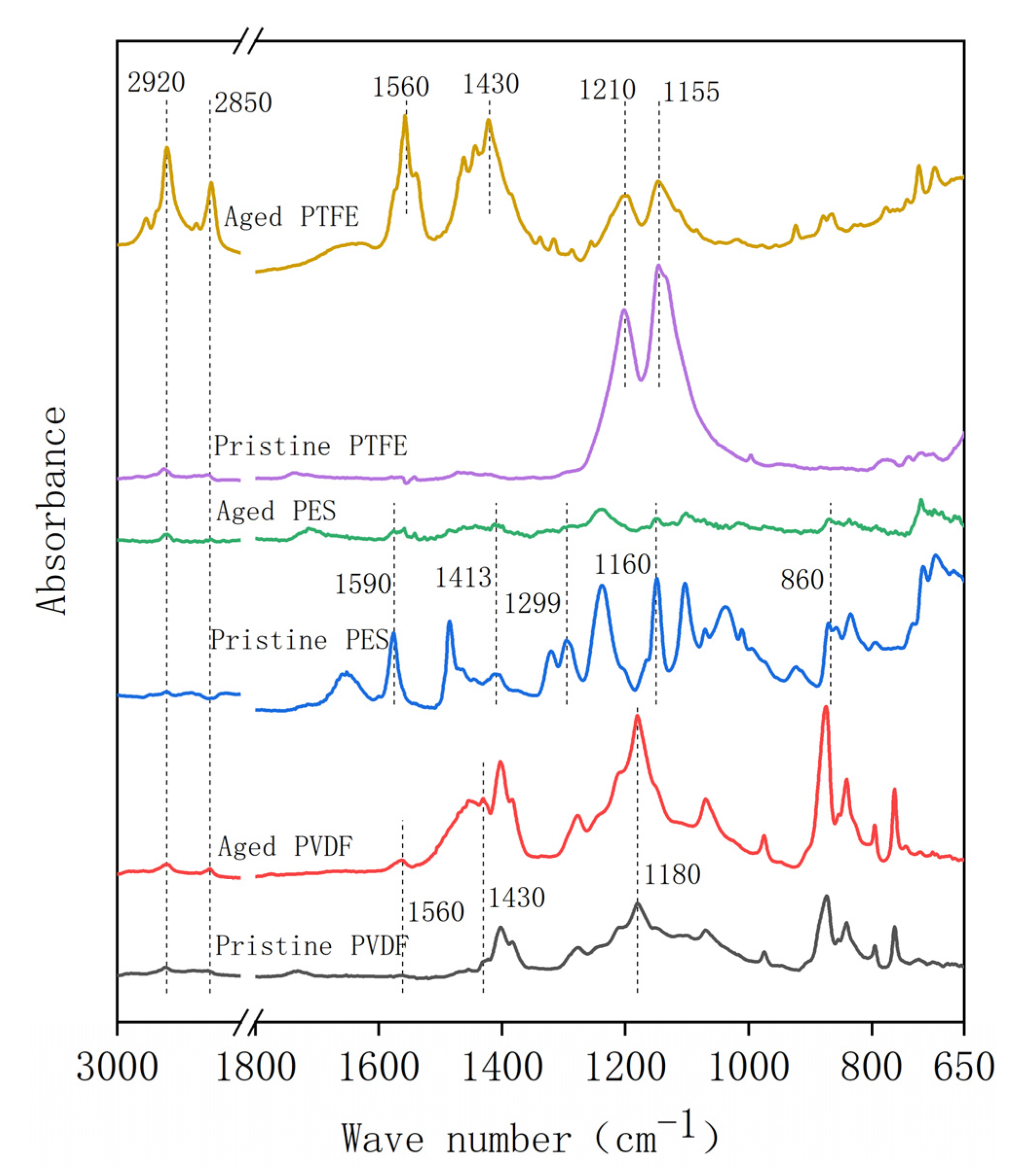
3. Results and Discussion
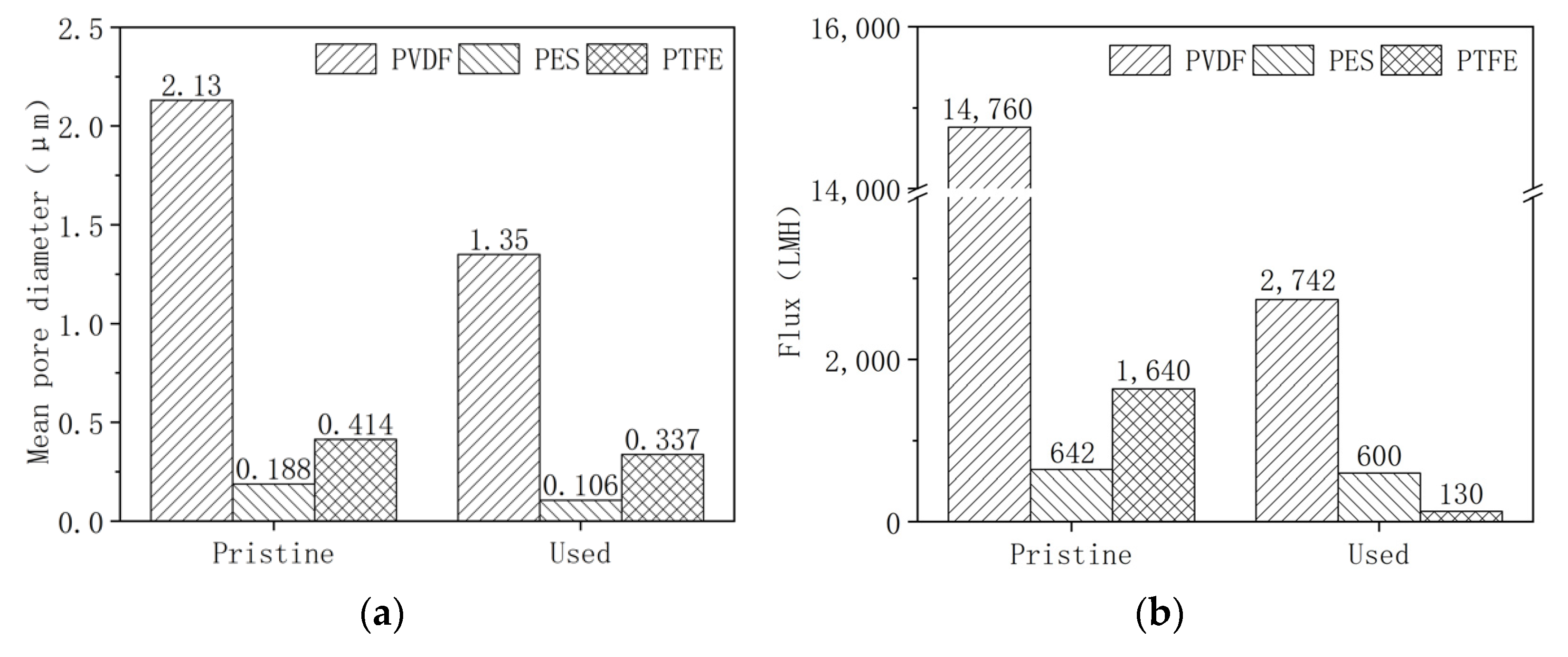
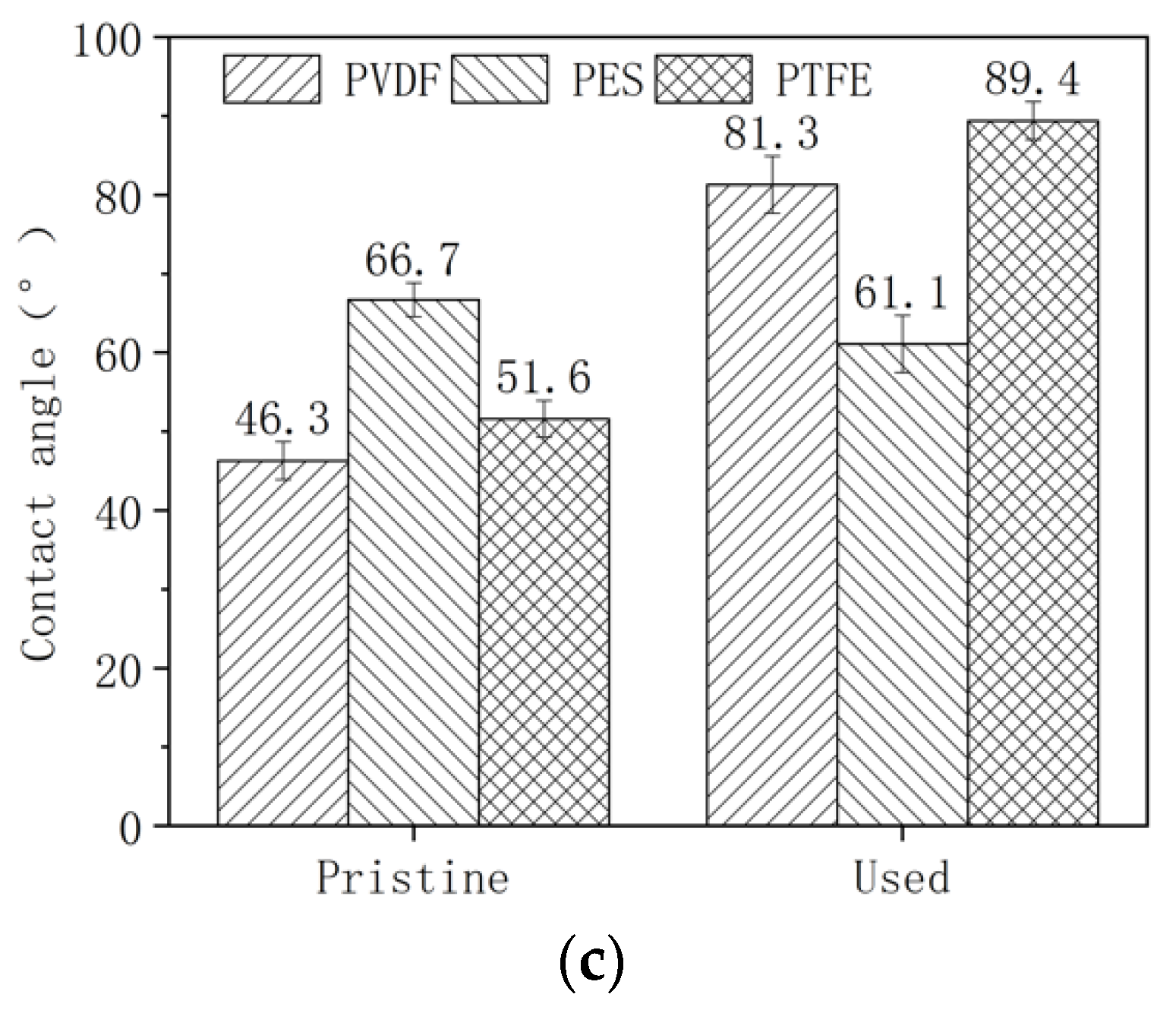
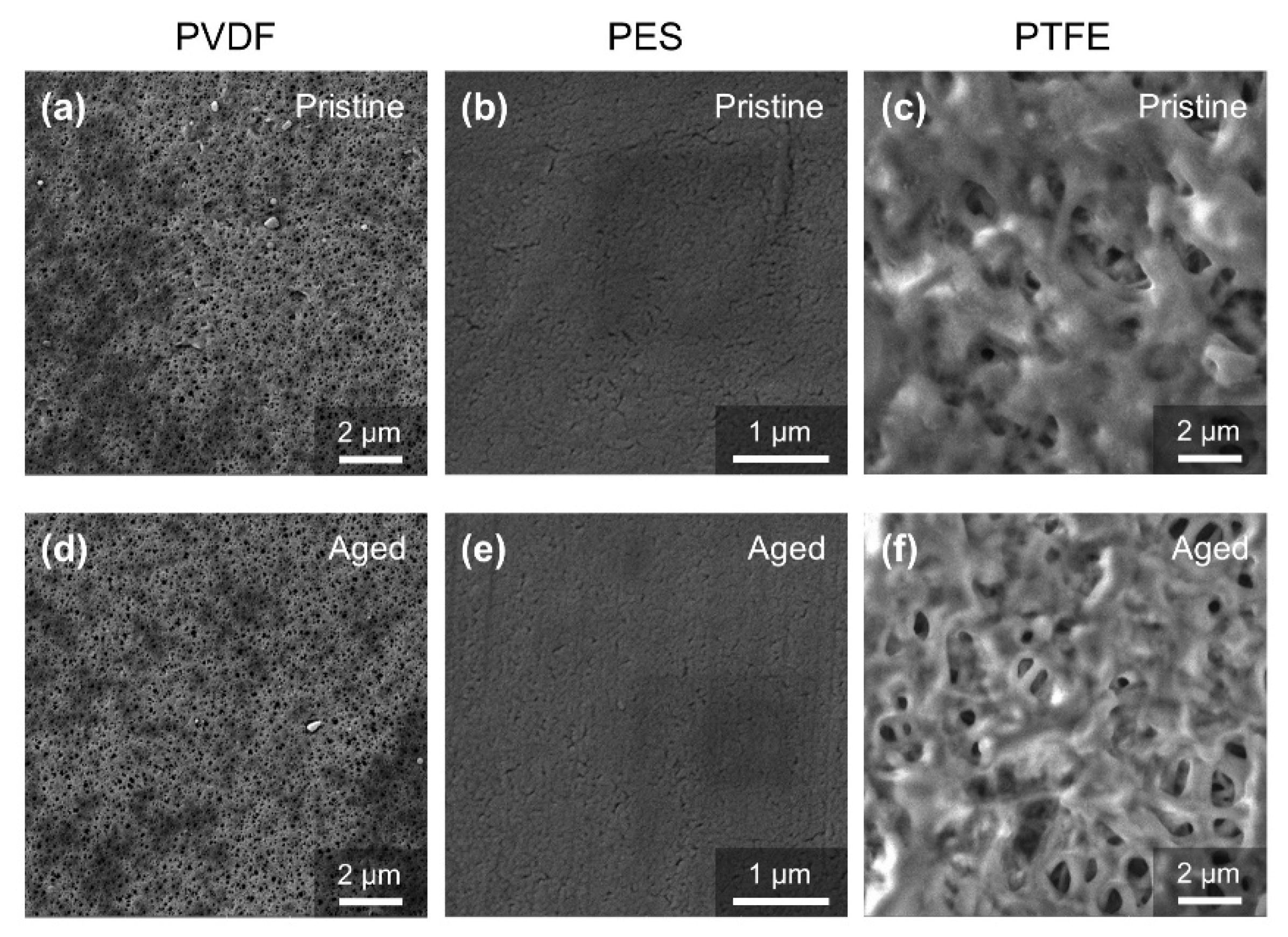
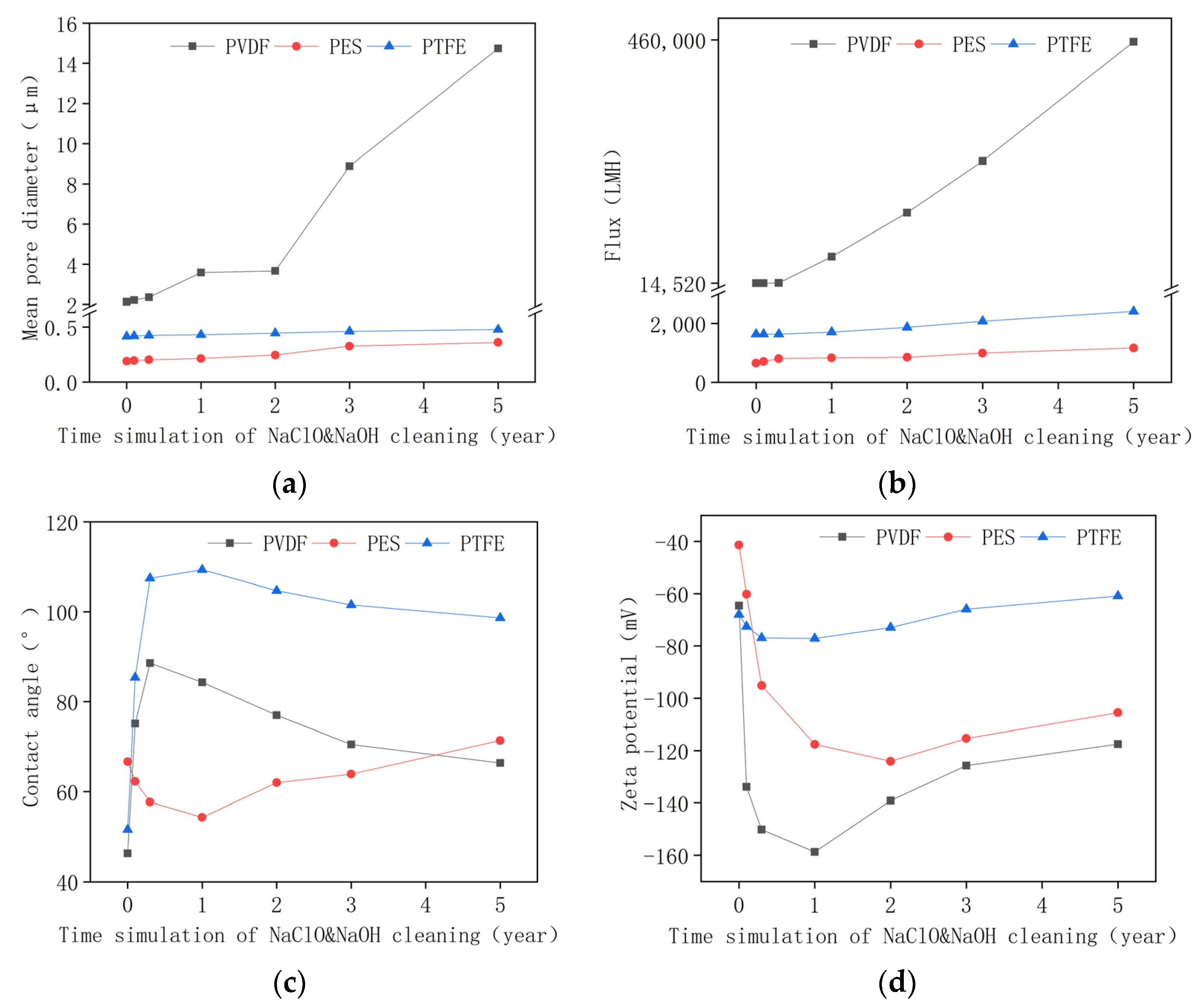
3.1. Change of Membrane Properties in the Pilot-Scale MBR Experiment
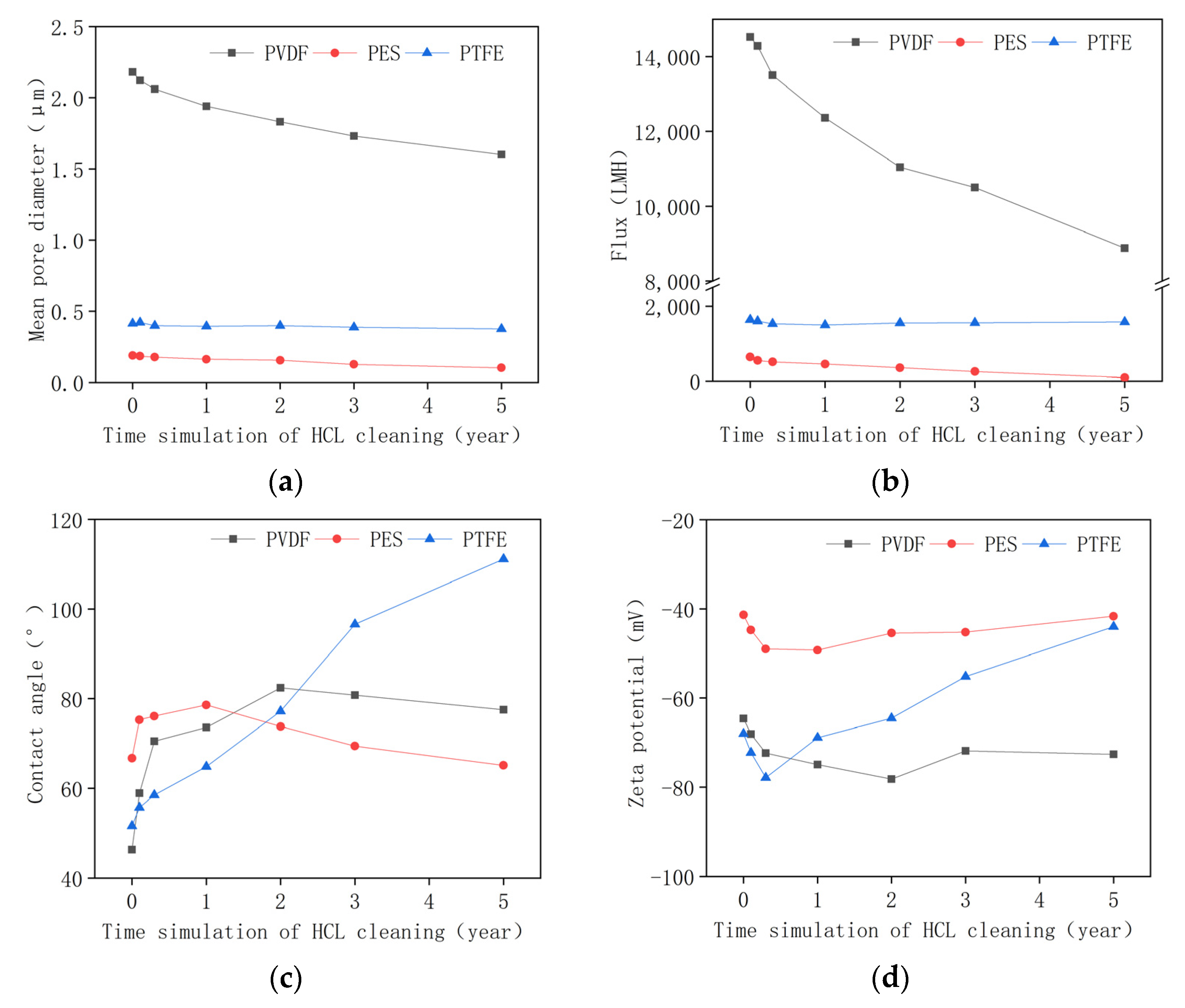
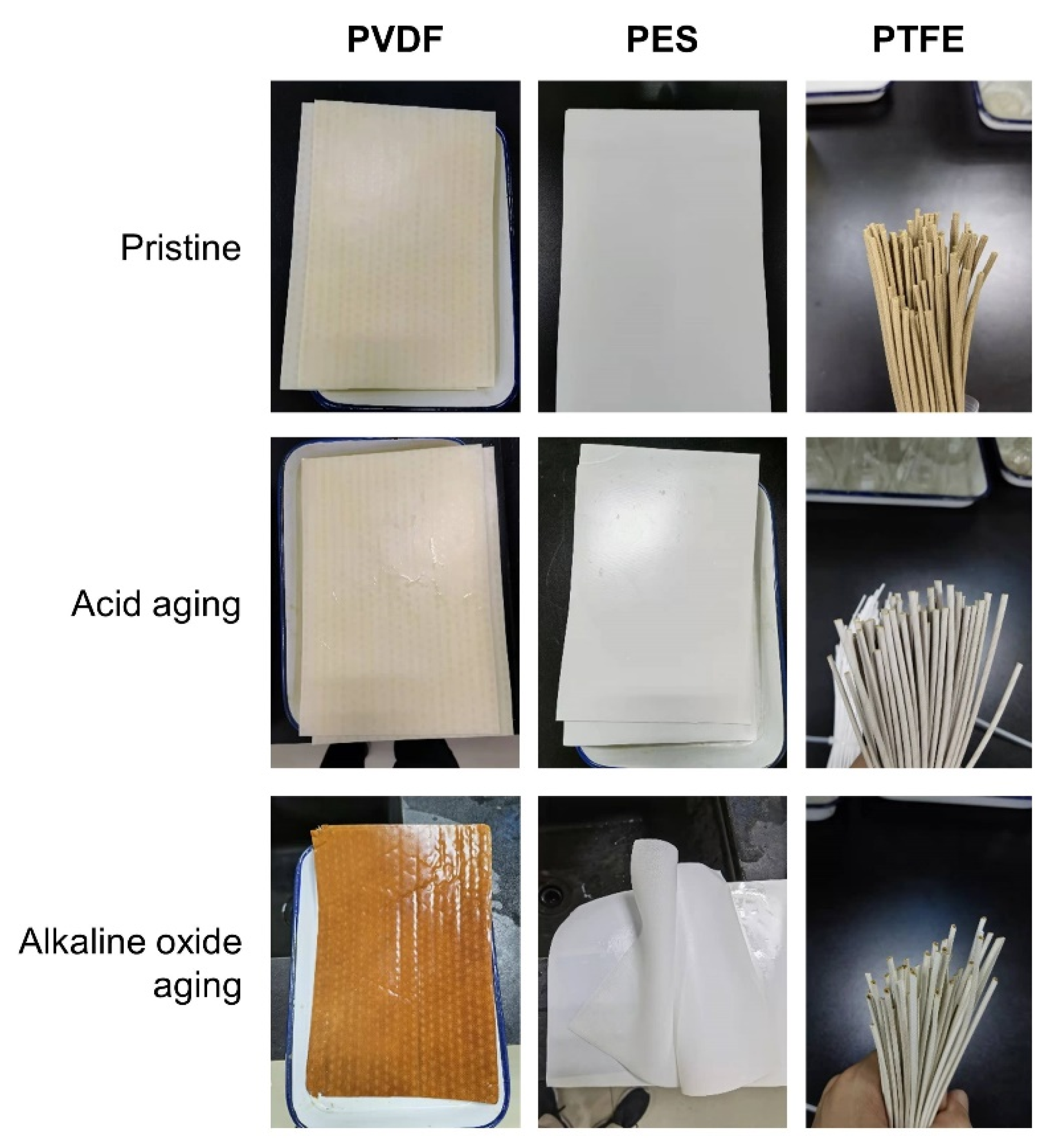
3.2. Membrane Properties in the Acid Aging Test
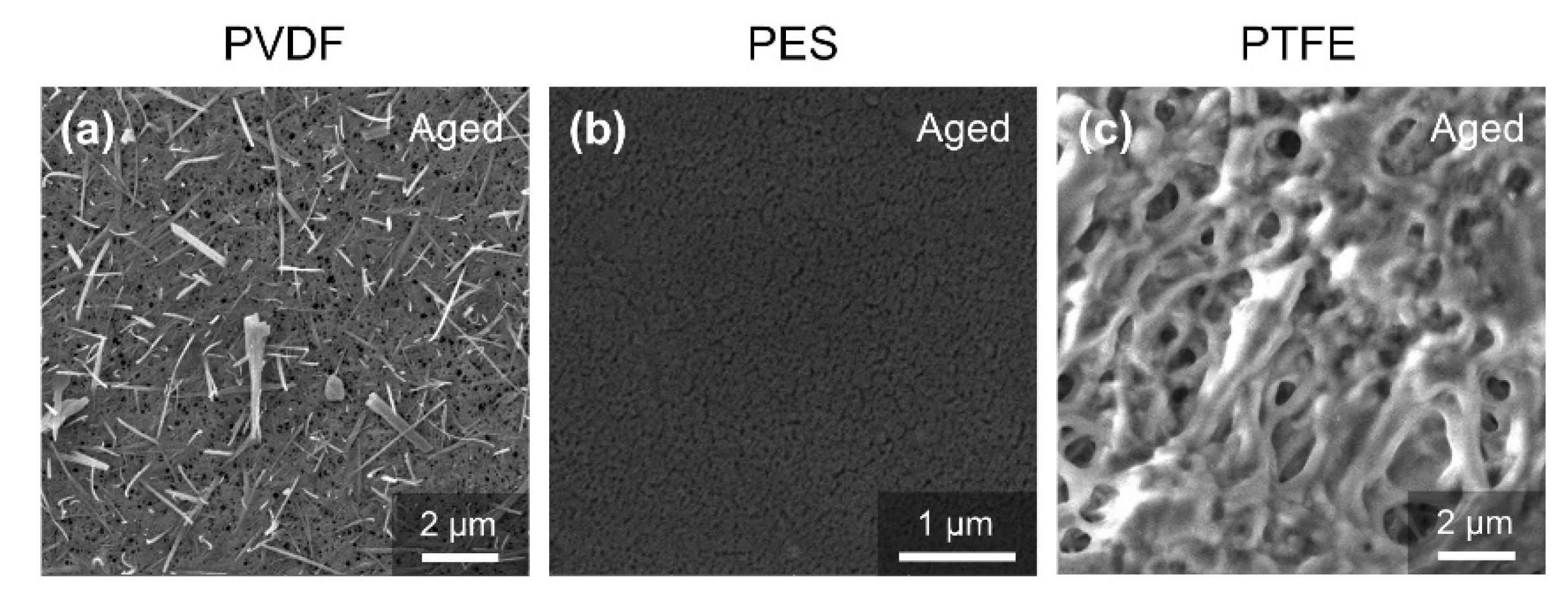
3.3. Membrane Properties in the Alkaline Oxide Aging Test
4. Conclusions
- The PVDF membrane was most susceptible to chemical cleaning. In the acid aging test, the surface hydrophobicity of PVDF increased, and the pore size and the pure water flux decreased due to the elevated hydrophobic effect. Alkaline oxide aging destructed the PVDF membrane’s structure, enlarged pore size, and increased pure water flux. The fluoro-substitution reaction and the dehydrofluorination reaction may occur in the aging.
- The PES and PTFE membranes were rather stable. Chemical cleaning barely changed the surface structure of the membrane specimens, although the interfacial properties (hydrophobicity and surface zeta potential) were altered. The chain scission of PES molecules and the dehydrofluorination of the PTFE were observed in aging.
- Membrane aging in the MBR for textile wastewater treatment should be carefully considered due to the possible intensive chemical cleaning process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jegatheesan, V.; Pramanik, B.K.; Chen, J.; Navaratna, D.; Chang, C.-Y.; Shu, L. Treatment of textile wastewater with membrane bioreactor: A critical review. Bioresour. Technol. 2016, 204, 202–212. [Google Scholar] [CrossRef]
- Brik, M.; Schoeberl, P.; Chamam, B.; Braun, R.; Fuchs, W. Advanced treatment of textile wastewater towards reuse using a membrane bioreactor. Process. Biochem. 2006, 41, 1751–1757. [Google Scholar] [CrossRef]
- Hai, F.I.; Yamamoto, K.; Nakajima, F.; Fukushi, K. Bioaugmented membrane bioreactor (MBR) with a GAC-packed zone for high rate textile wastewater treatment. Water Res. 2011, 45, 2199–2206. [Google Scholar] [CrossRef]
- Paździor, K.; Bilińska, L.; Ledakowicz, S. review of the existing and emerging technologies in the combination of AOPs and biological processes in industrial textile wastewater treatment. Chem. Eng. J. 2019, 376, 120597. [Google Scholar] [CrossRef]
- De Jager, D.; Sheldon, M.; Edwards, W. Colour removal from textile wastewater using a pilot-scale dual-stage MBR and subsequent RO system. Sep. Purif. Technol. 2014, 135, 135–144. [Google Scholar] [CrossRef]
- Keskin, B.; Ersahin, M.E.; Ozgun, H.; Koyuncu, I. Pilot and full-scale applications of membrane processes for textile wastewater treatment: A critical review. J. Water Process. Eng. 2021, 42, 102172. [Google Scholar] [CrossRef]
- Tavangar, T.; Jalali, K.; Shahmirzadi, M.A.A.; Karimi, M. Toward real textile wastewater treatment: Membrane fouling control and effective fractionation of dyes/inorganic salts using a hybrid electrocoagulation—Nanofiltration process. Sep. Purif. Technol. 2019, 216, 115–125. [Google Scholar] [CrossRef]
- Yang, X.; López-Grimau, V. Reduction of Cost and Environmental Impact in the Treatment of Textile Wastewater Using a Combined MBBR-MBR System. Membranes 2021, 11, 892. [Google Scholar] [CrossRef]
- Cinperi, N.C.; Ozturk, E.; Yigit, N.O.; Kitis, M. Treatment of woolen textile wastewater using membrane bioreactor, nanofiltration and reverse osmosis for reuse in production processes. J. Clean. Prod. 2019, 223, 837–848. [Google Scholar] [CrossRef]
- Ćurić, I.; Dolar, D.; Bošnjak, J. Reuse of textile wastewater for dyeing cotton knitted fabric with hybrid treatment: Coagulation/sand filtration/UF/NF-RO. J. Environ. Manag. 2021, 295, 113133. [Google Scholar] [CrossRef]
- Robinson, S.; Abdullah, S.Z.; Bérubé, P.; Le-Clech, P. Ageing of membranes for water treatment: Linking changes to performance. J. Membr. Sci. 2016, 503, 177–187. [Google Scholar] [CrossRef]
- Regula, C.; Carretier, E.; Wyart, Y.; Gésan-Guiziou, G.; Vincent, A.; Boudot, D.; Moulin, P. Chemical cleaning/disinfection and ageing of organic UF membranes: A review. Water Res. 2014, 56, 325–365. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Gao, F.; Chen, Y.; Zhang, H. A comparison study: The different impacts of sodium hypochlorite on PVDF and PSF ultrafiltration (UF) membranes. Water Res. 2017, 109, 227–236. [Google Scholar] [CrossRef]
- You, S.-J.; Damodar, R.A.; Hou, S.-C. Degradation of Reactive Black 5 dye using anaerobic/aerobic membrane bioreactor (MBR) and photochemical membrane reactor. J. Hazard. Mater. 2010, 177, 1112–1118. [Google Scholar] [CrossRef]
- Miyoshi, T.; Nguyen, T.P.; Tsumuraya, T.; Tanaka, H.; Morita, T.; Itokawa, H.; Hashimoto, T. Energy reduction of a submerged membrane bioreactor using a polytetrafluoroethylene (PTFE) hollow-fiber membrane. Front. Environ. Sci. Eng. 2018, 12, 1. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, S.; Oh, Y.; Zhou, Z.; Shin, H.-S.; Chae, S.-R. Fouling in membrane bioreactors: An updated review. Water Res. 2017, 114, 151–180. [Google Scholar] [CrossRef]
- Li, K.; Su, Q.; Li, S.; Wen, G.; Huang, T. Aging of PVDF and PES ultrafiltration membranes by sodium hypochlorite: Effect of solution pH. J. Environ. Sci. 2021, 104, 444–455. [Google Scholar] [CrossRef]
- Abdullah, S.Z.; Bérubé, P.R. Assessing the effects of sodium hypochlorite exposure on the characteristics of PVDF based membranes. Water Res. 2013, 47, 5392–5399. [Google Scholar] [CrossRef]
- Abdullah, S.Z.; Bérubé, P.R. Filtration and cleaning performances of PVDF membranes aged with exposure to sodium hypochlorite. Sep. Purif. Technol. 2018, 195, 253–259. [Google Scholar] [CrossRef]
- Puspitasari, V.; Granville, A.; Le-Clech, P.; Chen, V. Cleaning and ageing effect of sodium hypochlorite on polyvinylidene fluoride (PVDF) membrane. Sep. Purif. Technol. 2010, 72, 301–308. [Google Scholar] [CrossRef]
- Lebron, Y.A.R.; Moreira, V.R.; da Costa, P.R.; Alkmin, A.R.; Neta, L.S.D.F.; Cerqueira, A.C.; Amaral, M.C.S. Chemical cleaning procedures on permeability recovery and lifespan of MBR membranes treating petroleum refinery wastewater: From bench- to pilot-scale applications. J. Water Process. Eng. 2021, 44, 102411. [Google Scholar] [CrossRef]
- Bagheri, M.; Mirbagheri, S.A. Critical review of fouling mitigation strategies in membrane bioreactors treating water and wastewater. Bioresour. Technol. 2018, 258, 318–334. [Google Scholar] [CrossRef]
- Qu, F.; Yang, Z.; Li, X.; Yu, H.; Pan, Z.; Fan, G.; He, J.; Rong, H. Membrane fouling control by UV/persulfate in tertiary wastewater treatment with ultrafiltration: A comparison with UV/hydroperoxide and role of free radicals. Sep. Purif. Technol. 2020, 257, 117877. [Google Scholar] [CrossRef]
- Yu, H.; Du, C.; Qu, F.; He, J.; Rong, H. Efficient biostimulants for bacterial quorum quenching to control fouling in MBR. Chemosphere 2021, 286, 131689. [Google Scholar] [CrossRef]
- Tanis-Kanbur, M.B.; Peinador, R.I.; Calvo, J.I.; Hernández, A.; Chew, J.W. Porosimetric membrane characterization techniques: A review. J. Membr. Sci. 2021, 619, 118750. [Google Scholar] [CrossRef]
- Li, B.; Ke, X.-X.; Yuan, Z.-H.; Zhong, L.-B.; Zhao, Q.-B.; Zheng, Y.-M. High performance electrospun thin-film composite forward osmosis membrane by tailoring polyamide active layer with polydopamine interlayer for desulfulrization wastewater desalination. Desalination 2022, 534, 115781. [Google Scholar] [CrossRef]
- Liu, J.; Cui, H.; Zhang, T.; Liu, X.; Wang, L. Application of the quorum sensing inhibitor to improve ARGs removal by membrane-based household drinking water treatment process. Sep. Purif. Technol. 2021, 285, 120352. [Google Scholar] [CrossRef]
- Yan, Z.; Lu, Z.; Chen, X.; Jiang, Y.; Huang, Z.; Liu, L.; Fan, G.; Chang, H.; Qu, F.; Liang, H. Membrane distillation treatment of landfill leachate: Characteristics and mechanism of membrane fouling. Sep. Purif. Technol. 2022, 289, 289. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Suo, J.; Luo, T. Surface modification of porous poly(tetrafluoraethylene) film by a simple chemical oxidation treatment. Appl. Surf. Sci. 2010, 256, 2293–2298. [Google Scholar] [CrossRef]
- Fu, C.; Liu, S.; Gong, T.; Gu, A.; Yu, Z. Investigation on surface structure of potassium permanganate/nitric acid treated poly(tetrafluoroethylene). Appl. Surf. Sci. 2014, 317, 771–775. [Google Scholar] [CrossRef]
- Yang, L.; Wei, J.F.; Zhao, K.Y.; Luo, Z.A. Preparation of a Hydrophilic PVDF Membranes by Electron Beam Induced Grafting Polymerization of Acrylic Acid. Adv. Mater. Res. 2013, 625, 273–276. [Google Scholar] [CrossRef]
- Zhang, M.; Nguyen, Q.T.; Ping, Z. Hydrophilic modification of poly (vinylidene fluoride) microporous membrane. J. Membr. Sci. 2009, 327, 78–86. [Google Scholar] [CrossRef]
- Cai, J.; Jin, T.; Kou, J.; Zou, S.; Xiao, J.; Meng, Q. Lucas–Washburn Equation-Based Modeling of Capillary-Driven Flow in Porous Systems. Langmuir 2021, 37, 1623–1636. [Google Scholar] [CrossRef]
- Xiao, K.; Sun, J.; Mo, Y.; Fang, Z.; Liang, P.; Huang, X.; Ma, J.; Ma, B. Effect of membrane pore morphology on microfiltration organic fouling: PTFE/PVDF blend membranes compared with PVDF membranes. Desalination 2014, 343, 217–225. [Google Scholar] [CrossRef]
- Rabuni, M.; Sulaiman, N.N.; Aroua, M.; Chee, C.Y.; Hashim, N.A. Impact of in situ physical and chemical cleaning on PVDF membrane properties and performances. Chem. Eng. Sci. 2015, 122, 426–435. [Google Scholar] [CrossRef]
- Ren, L.; Yu, S.; Yang, H.; Li, L.; Cai, L.; Xia, Q.; Shi, Z.; Liu, G. Chemical cleaning reagent of sodium hypochlorite eroding polyvinylidene fluoride ultrafiltration membranes: Aging pathway, performance decay and molecular mechanism. J. Membr. Sci. 2021, 625, 119141. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, X.; Cao, G. Impacts of sodium hydroxide and sodium hypochlorite aging on polyvinylidene fluoride membranes fabricated with different methods. J. Environ. Sci. 2018, 67, 294–308. [Google Scholar] [CrossRef]
- Hanafi, Y.; Szymczyk, A.; Rabiller-Baudry, M.; Baddari, K. Degradation of Poly(Ether Sulfone)/Polyvinylpyrrolidone Membranes by Sodium Hypochlorite: Insight from Advanced Electrokinetic Characterizations. Environ. Sci. Technol. 2014, 48, 13419–13426. [Google Scholar] [CrossRef]
| CODcr (mg/L) | Ammonia Nitrogen (mg/L) | Total Phosphorus (mg/L) | pH | |
|---|---|---|---|---|
| Low loading From the exit end of the aeration tank | 145 ± 23 | 0.26 ± 0.12 | 0.41 ± 0.31 | 7.98 ± 0.53 |
| Medium loading From the middle of the aeration tank | 187 ± 35 | 0.89 ± 0.26 | 1.94 ± 1.13 | 8.12 ± 0.29 |
| High loading From the inlet of the aeration tank | 560 ± 91 | 17.35 ± 3.84 | 6.10 ± 2.93 | 8.29 ± 0.65 |
| Membrane Material | Membrane Module | Membrane Area (m2) | Flux (LMH) | Operation Mode (min) | MLSS in MBR Tank (g/L) | |
|---|---|---|---|---|---|---|
| MBR 1 | PVDF | Flat sheet | 420 | 15 | 8 min on, 2 min off | 12 |
| MBR 2 | PES | Flat sheet | 470 | 15 | 8 min on, 2 min off | 12 |
| MBR 3 | PTFE | Hollow fiber | 360 | 15 | 8 min on, 2 min off | 12 |
| Chemical | Chemical Cleaning Procedures in Real MBR Operation | CT Value (gh/L) | Batch Tests | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Offline Cleaning (1 per Month for 12 h) | Online Cleaning (1 per Week for 15 min) | 1 Month | Chemical Concentration | Soaking Time in a Continuous Batch-Test Matched with the CT Value in Real MBRs Clean | ||||||
| 1-Month Equivalent | 3-Months Equivalent | 1-Year Equivalent | 2-Years Equivalent | 3-Years Equivalent | 5-Years Equivalent | |||||
| Sodium Hypochlorite (mg/L) | 3000 | 500 | 36.5 | 3000 | 12.16 h | 36.48 h | 6.08 days | 12.16 days | 18.24 days | 30.40 days |
| Sodium hydroxide (mg/L) | 40,000 | 480 | 39,452 | 12.16 h | 36.48 h | 6.08 days | 12.16 days | 18.24 days | 30.40 days | |
| Hydrochloric acid (mg/L) | 20,000 | 500 | 240.5 | 19,767 | 12.16 h | 36.48 h | 6.08 days | 12.16 days | 18.24 days | 30.40 days |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Shangguan, S.; Xie, C.; Yang, H.; Wei, C.; Rong, H.; Qu, F. Chemical Cleaning and Membrane Aging in MBR for Textile Wastewater Treatment. Membranes 2022, 12, 704. https://doi.org/10.3390/membranes12070704
Yu H, Shangguan S, Xie C, Yang H, Wei C, Rong H, Qu F. Chemical Cleaning and Membrane Aging in MBR for Textile Wastewater Treatment. Membranes. 2022; 12(7):704. https://doi.org/10.3390/membranes12070704
Chicago/Turabian StyleYu, Huarong, Siyuan Shangguan, Chenyu Xie, Haiyang Yang, Chunhai Wei, Hongwei Rong, and Fangshu Qu. 2022. "Chemical Cleaning and Membrane Aging in MBR for Textile Wastewater Treatment" Membranes 12, no. 7: 704. https://doi.org/10.3390/membranes12070704
APA StyleYu, H., Shangguan, S., Xie, C., Yang, H., Wei, C., Rong, H., & Qu, F. (2022). Chemical Cleaning and Membrane Aging in MBR for Textile Wastewater Treatment. Membranes, 12(7), 704. https://doi.org/10.3390/membranes12070704








