Dual-Phase Mixed Protonic-Electronic Conducting Hydrogen Separation Membranes: A Review
Abstract
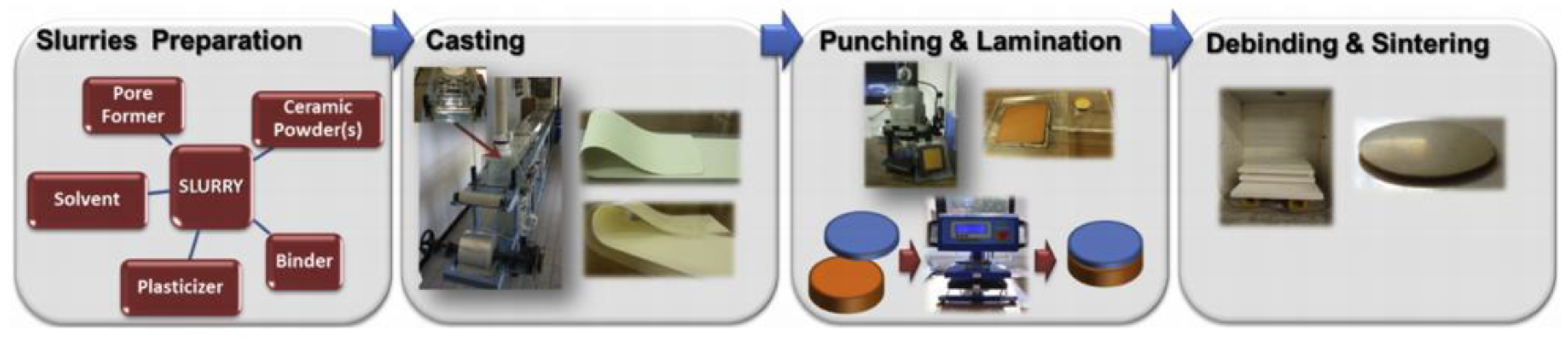
:1. Introduction
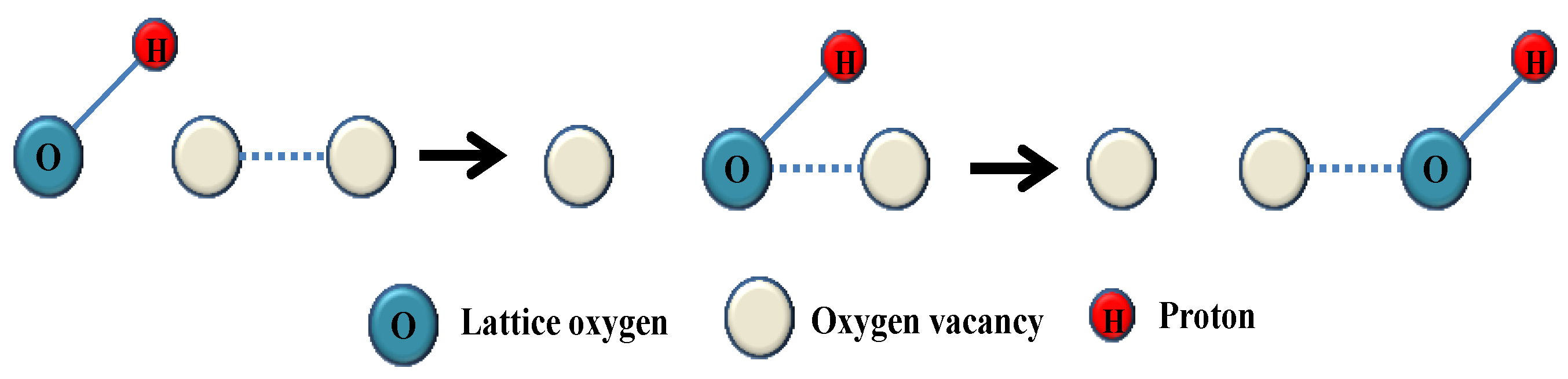
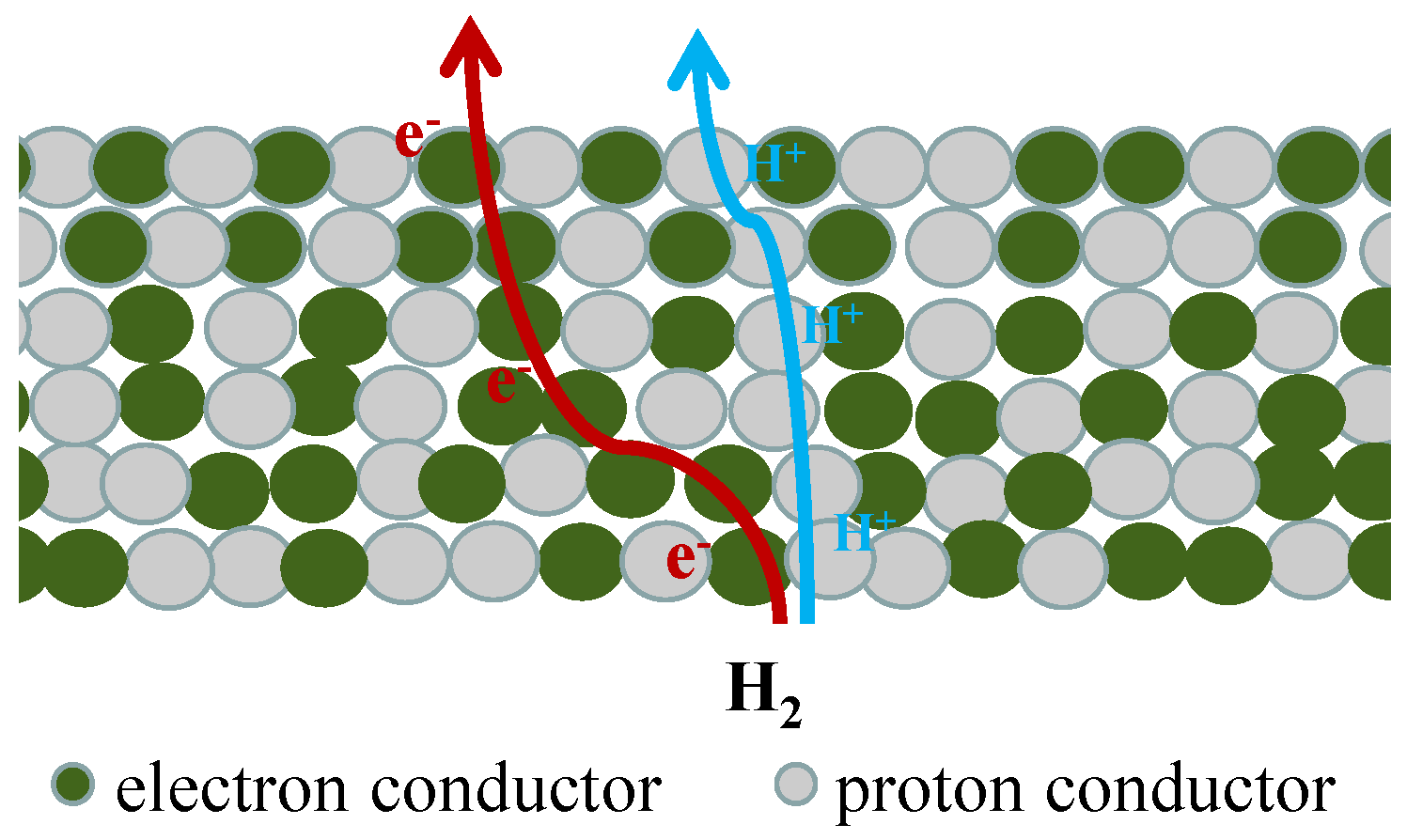
2. Transport Mechanisms
3. Cermet Dual-Phase Membrane
3.1. Pd-Based Cermet Membranes
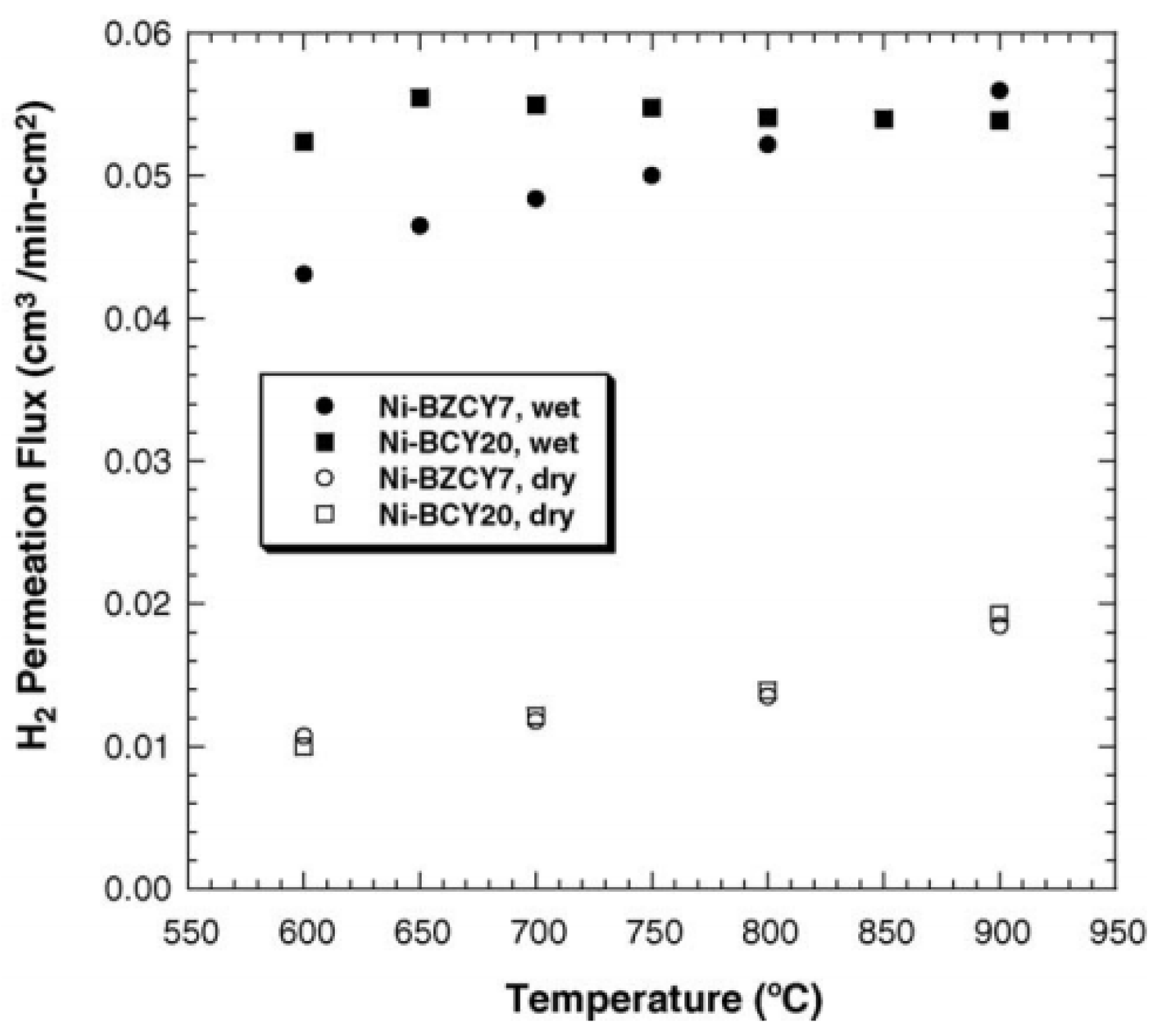
3.2. Ni-Based Cermet Membranes
4. Cercer Dual-Phase Membrane
4.1. Barium Cerate-Based Cercer Membranes
4.2. Lanthanum Tungstate Cercer Membranes
4.3. Elimination of the Inter-Phase Reaction
4.4. Phase Composition Ratio
5. Combination Mode of the Protonic and Electronic Phases
5.1. Powder Blending
5.2. Automatic Phase Separation
5.3. Independent Distribution
6. Permeability Enhancement through Membrane Configuration Optimization
7. Challenges and Future Directions
7.1. Challenges
7.2. Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, M.; He, F.; Zhou, C.; Dong, F.; Yang, G.; Zhou, W.; Shao, Z. New perovskite membrane with improved sintering and self-reconstructed surface for efficient hydrogen permeation. J. Memb. Sci. 2021, 620, 118980. [Google Scholar] [CrossRef]
- Saini, N.; Awasthi, K. Insights into the progress of polymeric nano-composite membranes for hydrogen separation and purification in the direction of sustainable energy resources. Sep. Purif. Technol. 2022, 282, 120029. [Google Scholar] [CrossRef]
- Pal, N.; Agarwal, M. Advances in materials process and separation mechanism of the membrane towards hydrogen separation. Int. J. Hydrogen Energy 2021, 46, 27062–27087. [Google Scholar] [CrossRef]
- Winter, C.J. Hydrogen energy-Abundant, efficient, clean: A debate over the energy-system-of-change. Int. J. Hydrogen Energy 2009, 34, S1–S52. [Google Scholar] [CrossRef]
- Singla, S.; Shetti, N.P.; Basu, S.; Mondal, K.; Aminabhavi, T.M. Hydrogen production technologies-Membrane based separation, storage and challenges. J. Environ. Manag. 2022, 302, 113963. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, Y.; Caro, J.; Huang, A. Fabrication of a flexible hydrogen-bonded organic framework based mixed matrix membrane for hydrogen separation. J. Memb. Sci. 2022, 643, 120021. [Google Scholar] [CrossRef]
- Zadorozhnyy, V.; Soprunyuk, V.; Klyamkin, S.; Zadorozhnyy, M.; Berdonosova, E.; Savvotin, I.; Stepashkin, A.; Korol, A.; Kvaratskheliya, A.; Semenov, D.; et al. Mechanical spectroscopy of metal/polymer composite membranes for hydrogen separation. J. Alloys Compd. 2021, 866, 159014. [Google Scholar] [CrossRef]
- Han, Z.; Xu, K.; Liao, N.; Xue, W. Theoretical investigations of permeability and selectivity of Pd-Cu and Pd-Ni membranes for hydrogen separation. Int. J. Hydrogen Energy 2021, 46, 23715–23722. [Google Scholar] [CrossRef]
- Duran, M.; Tüzün, F.N. Exploration of ceramic supports to be used in membrane reactors for hydrogen production and separation. Int. J. Hydrogen Energy 2021, 46, 29216–29229. [Google Scholar] [CrossRef]
- Mamivand, S.; Binazadeh, M.; Sohrabi, R. Applicability of membrane reactor technology in industrial hydrogen producing reactions: Current effort and future directions. J. Ind. Eng. Chem. 2021, 104, 212–230. [Google Scholar] [CrossRef]
- Liu, J.; Liu, C.; Huang, A. Co-based zeolitic imidazolate framework ZIF-9 membranes prepared on α-Al2O3 tubes through covalent modification for hydrogen separation. Int. J. Hydrogen Energy 2020, 45, 703–711. [Google Scholar] [CrossRef]
- Wey, M.-Y.; Chen, H.-H.; Lin, Y.-T.; Tseng, H.-H. Thin carbon hollow fiber membrane with Knudsen diffusion for hydrogen/alkane separation: Effects of hollow fiber module design and gas flow mode. Int. J. Hydrogen Energy 2020, 45, 7290–7302. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Liang, X.; Fu, H.; Rettenmayr, M.; Liu, D. Structure and properties of niobium carbide coated vanadium composite membranes for high temperature hydrogen separation. J. Alloys Compd. 2022, 900, 163530. [Google Scholar] [CrossRef]
- Anggarini, U.; Nagasawa, H.; Kanezashi, M.; Tsuru, T. Structural two-phase evolution of aminosilica-based silver-coordinated membranes for increased hydrogen separation. J. Memb. Sci. 2022, 642, 119962. [Google Scholar] [CrossRef]
- Liang, X.; Li, X.; Chen, R.; Nagaumi, H.; Guo, J.; Liu, D. Enhancement of hydrogen permeation stability at high temperatures for Pd/Nb30Ti35Co35/Pd composite membranes by HfN intermediate layer. J. Memb. Sci. 2022, 643, 120062. [Google Scholar] [CrossRef]
- Pal, N.; Agarwal, M.; Maheshwari, K.; Solanki, Y.S. A review on types, fabrication and support material of hydrogen separation membrane. Mater. Today Proc. 2020, 28, 1386–1391. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Meng, B.; Tan, X.; Loh, K.S.; Sunarso, J.; Liu, S. Perovskite-based mixed protonic-electronic conducting membranes for hydrogen separation: Recent status and advances. J. Ind. Eng. Chem. 2018, 60, 297–306. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Han, J.-J.; Huang, Y.; Chen, Y.; Yan, X.; Lang, W.-Z. Effect of Ba non-stoichiometry in Ba1-xZr0.1Ce0.7Y0.2O3-δ on its structure defect, sinterability and hydrogen permeability. Ceram. Int. 2020, 46, 19564–19573. [Google Scholar] [CrossRef]
- Zhou, C.; Sunarso, J.; Dai, J.; Ran, R.; Song, Y.; He, F.; Zhou, W.; Shao, Z. Realizing stable high hydrogen permeation flux through BaCo0.4Fe0.4Zr0.1Y0.1O3-δ membrane using a thin Pd film protection strategy. J. Memb. Sci. 2020, 596, 117709. [Google Scholar] [CrossRef]
- Xing, W.; Inge Dahl, P.; Valland Roaas, L.; Fontaine, M.-L.; Larring, Y.; Henriksen, P.P.; Bredesen, R. Hydrogen permeability of SrCe0.7Zr0.25Ln0.05O3-δ membranes (Ln = Tm and Yb). J. Memb. Sci. 2015, 473, 327–332. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Xue, J.; Zhuang, L.; Wang, H. Asymmetric membrane structure: An efficient approach to enhance hydrogen separation performance. Sep. Purif. Technol. 2018, 207, 363–369. [Google Scholar] [CrossRef]
- Iwahara, H.; Esaka, T.; Uchida, H.; Maeda, N. Proton conduction in sintered oxides and its application to steam electrolysis for hydrogen production. Solid State Ion. 1981, 3, 359–363. [Google Scholar] [CrossRef]
- Hashim, S.S.; Somalu, M.R.; Loh, K.S.; Liu, S.; Zhou, W.; Sunarso, J. Perovskite-based proton conducting membranes for hydrogen separation: A review. Int. J. Hydrogen Energy 2018, 43, 15281–15305. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, Y.; Amirkhiz, B.S.; Luo, J.-L.; Yan, N. Promoting the ambient-condition stability of Zr-doped barium cerate: Toward robust solid oxide fuel cells and hydrogen separation in syngas. Int. J. Hydrogen Energy 2018, 378, 134–138. [Google Scholar] [CrossRef]
- Hossain, M.K.; Yamamoto, T.; Hashizume, K. Effect of sintering conditions on structural and morphological properties of Y- and Co-doped BaZrO3 proton conductors. Ceram. Int. 2021, 47, 27177–27187. [Google Scholar] [CrossRef]
- Hossain, M.K.; Chanda, R.; El-Denglawey, A.; Emrose, T.; Rahman, M.T.; Biswas, M.C.; Hashizume, K. Recent progress in barium zirconate proton conductors for electrochemical hydrogen device applications: A review. Ceram. Int. 2021, 47, 23725–23748. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.; Zhuang, L.; Wei, Y.; Wang, H. Hydrogen permeability through Nd5.5W0.35Mo0.5Nb0.15O11.25-δ mixed protonic-electronic conducting membrane. J. Memb. Sci. 2019, 579, 33–39. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Xue, J.; Zhuang, L.; Wang, H. Tailoring hydrogen separation performance through the ceramic lanthanum tungstate membranes by chlorine doping. J. Memb. Sci. 2019, 573, 117–125. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, G.F.; Liao, Q.; Chen, Y.; Lang, W.Z. Development of Mn and Mo double-substituted La5.5WO11.25-δ based membranes with enhanced hydrogen permeation flux. J. Eur. Ceram. Soc. 2021, 41, 5711–5720. [Google Scholar] [CrossRef]
- Qi, X.; Lin, Y.S. Electrical conduction and hydrogen permeation through mixed proton-electron conducting strontium cerate membranes. Solid State Ion. 2000, 130, 149–156. [Google Scholar] [CrossRef]
- Song, S.J.; Wachsman, E.D.; Rhodes, J.; Dorris, S. Hydrogen permeability of SrCe1-xMxO3-δ(x = 0.05, M=Eu, Sm). Solid State Ion. 2004, 167, 99–105. [Google Scholar] [CrossRef]
- Phillips, R. Structural and electrical characterisation of SrCe1-xYxOξ. Solid State Ion. 1999, 125, 389–395. [Google Scholar] [CrossRef]
- Balachandran, U.; Lee, T.; Chen, L.; Song, S.; Picciolo, J.; Dorris, S. Hydrogen separation by dense cermet membranes. Fuel 2006, 85, 150–155. [Google Scholar] [CrossRef]
- Jeon, S.Y.; Lim, D.K.; Choi, M.B.; Wachsman, E.D.; Song, S.J. Hydrogen separation by Pd-CaZr0.9Y0.1O3-δ cermet composite membranes. Sep. Purif. Technol. 2011, 79, 337–341. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Lin, C.C.; Lin, W.L.; Wang, J.H.; Chen, S.Y.; Lin, P.; Wu, P.W. Palladium based cermet composite for hydrogen separation at elevated temperature. J. Power Sources 2015, 274, 965–970. [Google Scholar] [CrossRef]
- Jeon, S.-Y.; Choi, M.-B.; Singh, B.; Song, S.-J. Hydrogen separation by dual functional cermet membranes with self-repairing capability against the damage by H2S. J. Memb. Sci. 2013, 428, 46–51. [Google Scholar] [CrossRef]
- Balachandran, U.; Lee, T.H.; Park, C.Y.; Emerson, J.E.; Picciolo, J.J.; Dorris, S.E. Dense cermet membranes for hydrogen separation. Sep. Purif. Technol. 2014, 121, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Jeon, S.Y.; Choi, M.B.; Park, C.N.; Wachsman, E.D.; Song, S.J. High sulfur tolerance dual-functional cermet hydrogen separation membranes. J. Memb. Sci. 2011, 382, 323–327. [Google Scholar] [CrossRef]
- Zhu, Z.; Sun, W.; Dong, Y.; Wang, Z.; Shi, Z.; Zhang, Q.; Liu, W. Evaluation of hydrogen permeation properties of Ni-Ba(Zr0.7Pr0.1Y0.2)O3-δ cermet membranes. Int. J. Hydrogen Energy 2014, 39, 11683–11689. [Google Scholar] [CrossRef]
- Wei, Y.; Xue, J.; Fang, W.; Chen, Y.; Wang, H.; Caro, J. Enhanced stability of Zr-doped Ba(CeTb)O(3-δ)-Ni cermet membrane for hydrogen separation. Chem. Comm. 2015, 51, 11619–11621. [Google Scholar] [CrossRef] [Green Version]
- Zuo, C.; Lee, T.H.; Dorris, S.E.; Balachandran, U.; Liu, M. Composite Ni-Ba(Zr0.1Ce0.7Y0.2)O3 membrane for hydrogen separation. J. Power Sources 2006, 159, 1291–1295. [Google Scholar] [CrossRef]
- Yan, L.; Sun, W.; Bi, L.; Fang, S.; Tao, Z.; Liu, W. Influence of fabrication process of Ni-BaCe0.7Zr0.1Y0.2O3-δ cermet on the hydrogen permeation performance. J. Alloys Compd. 2010, 508, L5–L8. [Google Scholar] [CrossRef]
- Zuo, C.; Dorris, S.E.; Balachandran, U.; Liu, M. Effect of Zr-doping on the chemical stability and hydrogen permeation of the Ni-BaCe0.8Y0.2O3-δ mixed protonic-electronic conductor. Chem. Mater. 2006, 18, 4647–4650. [Google Scholar] [CrossRef]
- Fang, S.; Brinkman, K.; Chen, F. Unprecedented CO2-Promoted Hydrogen Permeation in Ni-BaZr0.1Ce0.7Y0.1Yb0.1O3-δ Membrane. ACS Appl. Mater. Interfaces 2014, 6, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Brinkman, K.S.; Chen, F. Hydrogen permeability and chemical stability of Ni-BaZr0.1Ce0.7Y0.1Yb0.1O3-δ membrane in concentrated H2O and CO2. J. Memb. Sci. 2014, 467, 85–92. [Google Scholar] [CrossRef]
- Meng, X.; Song, J.; Yang, N.; Meng, B.; Tan, X.; Ma, Z.; Li, K. Ni-BaCe0.95Tb0.05O3−δ cermet membranes for hydrogen permeation. J. Memb. Sci. 2012, 401, 300–305. [Google Scholar] [CrossRef]
- Song, S.; Moon, J.; Lee, T.; Dorris, S.; Balachandran, U. Thickness dependence of hydrogen permeability for Ni-BaCe0.8Y0.2O3-δ. Solid State Ion. 2008, 179, 1854–1857. [Google Scholar] [CrossRef]
- Kim, H.; Kim, B.; Lee, J.; Ahn, K.; Kim, H.-R.; Yoon, K.J.; Kim, B.-K.; Cho, Y.W.; Lee, H.-W.; Lee, J.-H. Microstructural adjustment of Ni-BaCe0.9Y0.1O3-δ cermet membrane for improved hydrogen permeation. Ceram. Int. 2014, 40, 4117–4126. [Google Scholar] [CrossRef]
- Zhuang, L.; Li, J.; Xue, J.; Jiang, Z.; Wang, H. Evaluation of hydrogen separation performance of Ni-BaCe0.85Fe0.15O3-δ cermet membranes. Ceram. Int. 2019, 45, 10120–10125. [Google Scholar] [CrossRef]
- Ma, X.; Yang, C.; Chen, H.; Lv, Q.; Sun, K.; Li, W. Hydrogen permeation and chemical stability of Ni-BaCe0.7In0.2Ta0.1O3-δ cermet membrane. Sep. Purif. Technol. 2020, 236, 116276. [Google Scholar] [CrossRef]
- Yang, C.; Ma, X.; Chen, H.; Lv, Q.; Sun, K.; Chen, J.; Yun, S. Chemical stability and hydrogen permeation performance of Ni-BaCe0.7Y0.3-xInxO3-δ cermet membranes. J. Alloys Compd. 2018, 762, 409–414. [Google Scholar] [CrossRef]
- Song, S.J.; Lee, T.H.; Wachsman, E.D.; Chen, L.; Dorris, S.E.; Balachandran, U. Defect Structure and Transport Properties of Ni-SrCeO3-δ Cermet for Hydrogen Separation Membrane. J. Electrochem. Soc. 2005, 152, J125. [Google Scholar] [CrossRef]
- Zhu, Z.; Sun, W.; Wang, Z.; Cao, J.; Dong, Y.; Liu, W. A high stability Ni-La0.5Ce0.5O2-δ asymmetrical metal-ceramic membrane for hydrogen separation and generation. J. Power Sources 2015, 281, 417–424. [Google Scholar] [CrossRef]
- Fang, S.; Lei, B.; Yan, L.; Sun, W.; Wei, L. CO2-Resistant Hydrogen Permeation Membranes Based on Doped Ceria and Nickel. J. Phys. Chem. C 2010, 114, 10986–10991. [Google Scholar] [CrossRef]
- Yan, L.; Sun, W.; Bi, L.; Fang, S.; Tao, Z.; Liu, W. Effect of Sm-doping on the hydrogen permeation of Ni-La2Ce2O7 mixed protonic-electronic conductor. Int. J. Hydrogen Energy 2010, 35, 4508–4511. [Google Scholar] [CrossRef]
- Xie, H.; Zhuang, L.; Wei, Y.; Xue, J.; Wang, H. CO2-tolerant Ni-La5.5WO11.25-δ dual-phase membranes with enhanced H2 permeability. Ceram. Int. 2017, 43, 14608–14615. [Google Scholar] [CrossRef]
- Rosensteel, W.A.; Sullivan, N.P. Fabrication and hydrogen permeation through novel BaZr0.9Y0.1O3-δ-Cu composite ceramic-metallic membranes. Int. J. Hydrogen Energy 2017, 42, 4216–4223. [Google Scholar] [CrossRef]
- Park, J.H.; Jeon, S.I.; Sim, W.J.; Baek, I.H.; Choi, S.H. Stability of Ta/YSZ cermet membrane for hydrogen separation. Energy Procedia 2011, 4, 756–762. [Google Scholar] [CrossRef] [Green Version]
- Unemoto, A.; Kaimai, A.; Sato, K.; Yashiro, K.; Matsumoto, H.; Mizusaki, J.; Amezawa, K.; Kawada, T. Hydrogen permeability and electrical properties in oxide composites. Solid State Ion. 2008, 178, 1663–1667. [Google Scholar] [CrossRef]
- Song, J.; Meng, B.; Tan, X. Stability and electrical conductivity of BaCe0.85Tb0.05M0.1O 3-δ (M=Co, Fe, Y, Zr, Mn) high temperature proton conductors. Ceram. Int. 2016, 42, 13278–13284. [Google Scholar] [CrossRef]
- Rosensteel, W.A.; Ricote, S.; Sullivan, N.P. Hydrogen permeation through dense BaCe0.8Y0.2O3-δ-Ce0.8Y0.2O2-δ composite-ceramic hydrogen separation membranes. Int. J. Hydrogen Energy 2016, 41, 2598–2606. [Google Scholar] [CrossRef]
- Ivanova, M.E.; Escolastico, S.; Balaguer, M.; Palisaitis, J.; Sohn, Y.J.; Meulenberg, W.A.; Guillon, O.; Mayer, J.; Serra, J.M. Hydrogen separation through tailored dual phase membranes with nominal composition BaCe0.8Eu0.2O3-δ:Ce0.8Y0.2O2-δ at intermediate temperatures. Sci. Rep. 2016, 6, 34773–34785. [Google Scholar] [CrossRef] [PubMed]
- Fish, J.S.; Ricote, S.; Lenrick, F.; Wallenberg, L.R.; Holgate, T.C.; O’hayre, R.; Bonanos, N. Synthesis by spark plasma sintering of a novel protonic/electronic conductor composite: BaCe0.2Zr0.7Y0.1O3-δ /Sr0.95Ti0.9Nb0.1O3-δ (BCZY27/STN95). J. Mater. Sci. 2013, 48, 6177–6185. [Google Scholar] [CrossRef]
- Sun, W.; Shi, Z.; Liu, W. Considerable Hydrogen Permeation Behavior through a Dense Ce0.8Sm0.2O2-δ(SDC) Asymmetric Thick Film. J. Electrochem. Soc. 2013, 160, F585–F590. [Google Scholar] [CrossRef]
- Ricote, S.; Manerbino, A.; Sullivan, N.P.; Coors, W.G. Preparation of dense mixed electron- and proton-conducting ceramic composite materials using solid-state reactive sintering: BaCe0.8Y0.1M0.1O3-δ-Ce0.8Y0.1M0.1O2-δ (M=Y, Yb, Er, Eu). J. Mater. Sci. 2014, 49, 4332–4340. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, L.; Zhang, W.; Zhou, H.; Li, Y.; Wang, L. Preparation of dual-phase composite BaCe0.8Y0.2O3/Ce0.8Y0.2O2 and its application for hydrogen permeation. Ceram. Int. 2016, 42, 6391–6398. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, X.; Meng, X.; Meng, B.; Sunarso, J.; Tan, X.; Liu, L.; Liu, S. Dual-layer BaCe0.8Y0.2O3-δ-Ce0.8Y0.2O2-δ/BaCe0.8Y0.2O3-δ-Ni hollow fiber membranes for H2 separation. J. Memb. Sci. 2020, 601, 117801. [Google Scholar] [CrossRef]
- Rebollo, E.; Mortalò, C.; Escolástico, S.; Boldrini, S.; Barison, S.; Serra, J.M.; Fabrizio, M. Exceptional hydrogen permeation of all-ceramic composite robust membranes based on BaCe0.65Zr0.20Y0.15O3-δ and Y-or Gd-doped ceria. Energy Environ. Sci. 2015, 8, 3675–3686. [Google Scholar] [CrossRef]
- Mercadelli, E.; Gondolini, A.; Montaleone, D.; Pinasco, P.; Escolástico, S.; Serra, J.M.; Sanson, A. Production strategies of asymmetric BaCe0.65Zr0.20Y0.15O3-δ -Ce0.8Gd0.2O2-δ membrane for hydrogen separation. Int. J. Hydrogen Energy 2020, 45, 7468–7478. [Google Scholar] [CrossRef]
- Fish, J.S.; Ricote, S.; O’hayre, R.; Bonanos, N. Electrical properties and flux performance of composite ceramic hydrogen separation membranes. J. Mater. Chem. A 2015, 3, 5392–5401. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, Y.; Zhuang, L.; Xue, J.; Wei, Y.; Feldhoff, A.; Caro, J.; Wang, H. A Dual-Phase Ceramic Membrane with Extremely High H2 Permeation Flux Prepared by Autoseparation of a Ceramic Precursor. Angew. Chem. Int. Ed. 2016, 55, 10895–10898. [Google Scholar] [CrossRef]
- Jia, L.; Ashtiani, S.; Liang, F.; He, G.; Jiang, H. Hydrogen permeation through dual-phase ceramic membrane derived from automatic phase-separation of SrCe0.50Fe0.50O3-δ precursor. Int. J. Hydrogen Energy 2020, 45, 4625–4634. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, H.; Meng, B.; Wang, X.; Sunarso, J.; Tan, X.; Liu, S. SrCe0.95Y0.05O3-δ-ZnO dual-phase membranes for hydrogen permeation. RSC Adv. 2016, 6, 36786–36793. [Google Scholar] [CrossRef]
- Meng, B.; Wang, H.; Cheng, H.; Wang, X.; Meng, X.; Sunarso, J.; Tan, X.; Liu, S. Hydrogen permeation performance of dual-phase protonic-electronic conducting ceramic membrane with regular and independent transport channels. Sep. Purif. Technol. 2019, 213, 515–523. [Google Scholar] [CrossRef]
- Escolástico, S.; Solís, C.; Kjølseth, C.; Serra, J.M. Outstanding hydrogen permeation through CO2-stable dual-phase ceramic membranes. Energy Environ. Sci. 2014, 7, 3736–3746. [Google Scholar] [CrossRef]
- Liang, W.; Zhang, Y.; Hu, T.; Jiang, H. Enhanced H2 production by using La5.5WO11.25-δ-La0.8Sr0.2FeO3-δ mixed oxygen ion-proton-electron triple-conducting membrane. Int. J. Hydrogen Energy 2021, 46, 33143–33151. [Google Scholar] [CrossRef]
- Escolástico, S.; Kjølseth, C.; Serra, J.M. Catalytic activation of ceramic H2 membranes for CMR processes. J. Memb. Sci. 2016, 517, 57–63. [Google Scholar] [CrossRef]
- Polfus, J.M.; Xing, W.; Fontaine, M.-L.; Denonville, C.; Henriksen, P.P.; Bredesen, R. Hydrogen separation membranes based on dense ceramic composites in the La27W5O55.5-LaCrO3 system. J. Memb. Sci. 2015, 479, 39–45. [Google Scholar] [CrossRef]
- Fontaine, M.-L.; Denonville, C.; Li, Z.; Xing, W.; Polfus, J.M.; Kvello, J.; Graff, J.S.; Dahl, P.I.; Henriksen, P.P.; Bredesen, R. Fabrication and H2 flux measurement of asymmetric La27W3.5Mo1.5O55.5-δ-La0.87Sr0.13CrO3-δ membranes. J. Eur. Ceram. Soc. 2018, 38, 1695–1701. [Google Scholar] [CrossRef]
- Xing, W.; Syvertsen, G.E.; Grande, T.; Li, Z.; Haugsrud, R. Hydrogen permeation, transport properties and microstructure of Ca-doped LaNbO4 and LaNb3O9 composites. J. Memb. Sci. 2012, 415, 878–885. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J.; Su, T.; Liu, W.; Qi, H.; Yang, J. Synthesis, microstructure and electrical properties of BaZr0.9Y0.1O3-δ: BaCe0.86Y0.1Zn0.04O3-δ proton conductors. Mater. Sci. Eng. B 2015, 196, 35–39. [Google Scholar] [CrossRef]
- Tan, X.; Song, J.; Meng, X.; Meng, B. Preparation and characterization of BaCe0.95Tb0.05O3-α hollow fibre membranes for hydrogen permeation. J. Eur. Ceram. Soc. 2012, 32, 2351–2357. [Google Scholar] [CrossRef]
- Zhuang, L.; Li, J.; Chen, L.; Xue, J.; Chen, X.; Wang, H. Metalloid phosphorus cation doping: An effective strategy to improve permeability and stability through the hydrogen permeable membranes. Sep. Purif. Technol. 2019, 210, 320–326. [Google Scholar] [CrossRef]


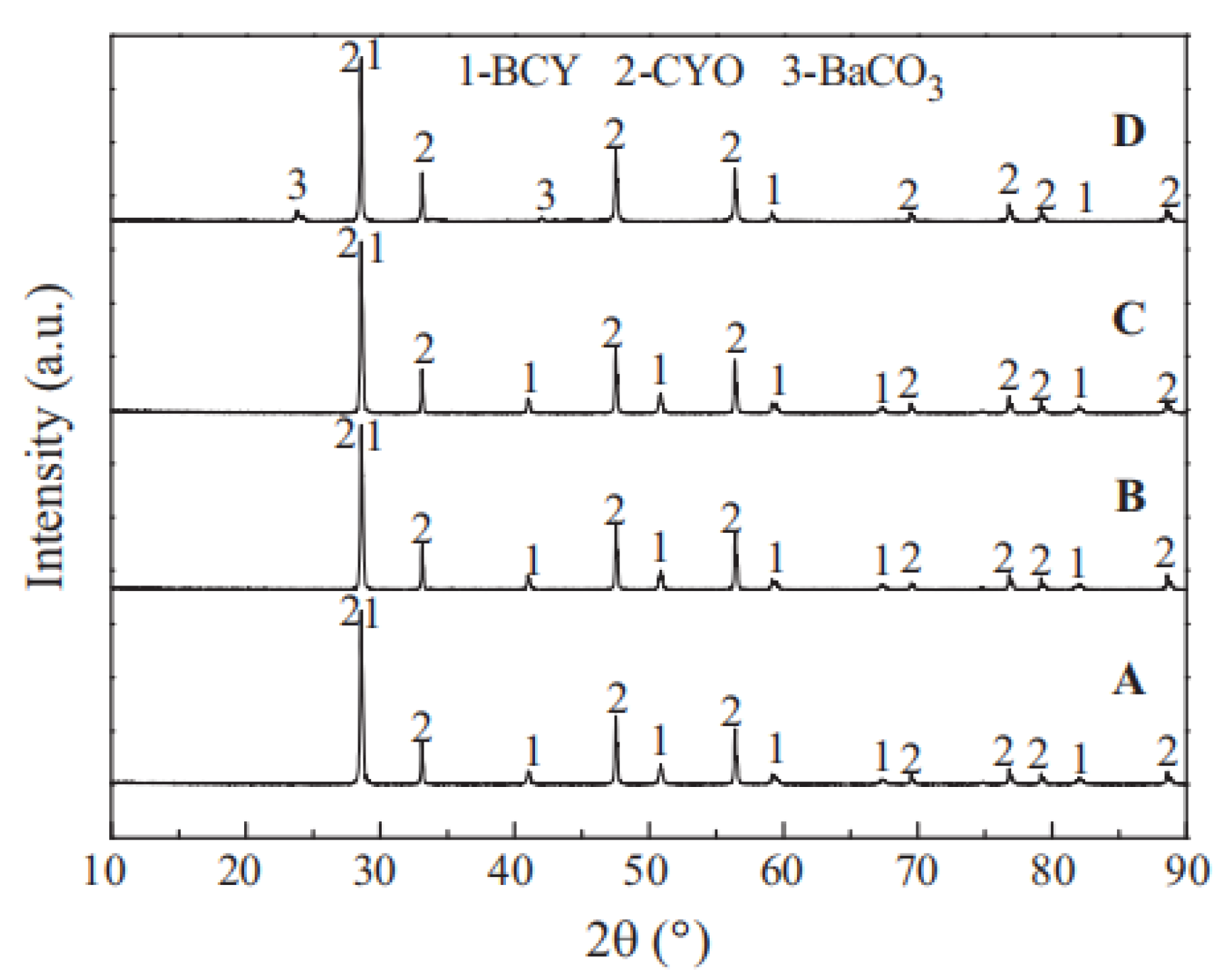
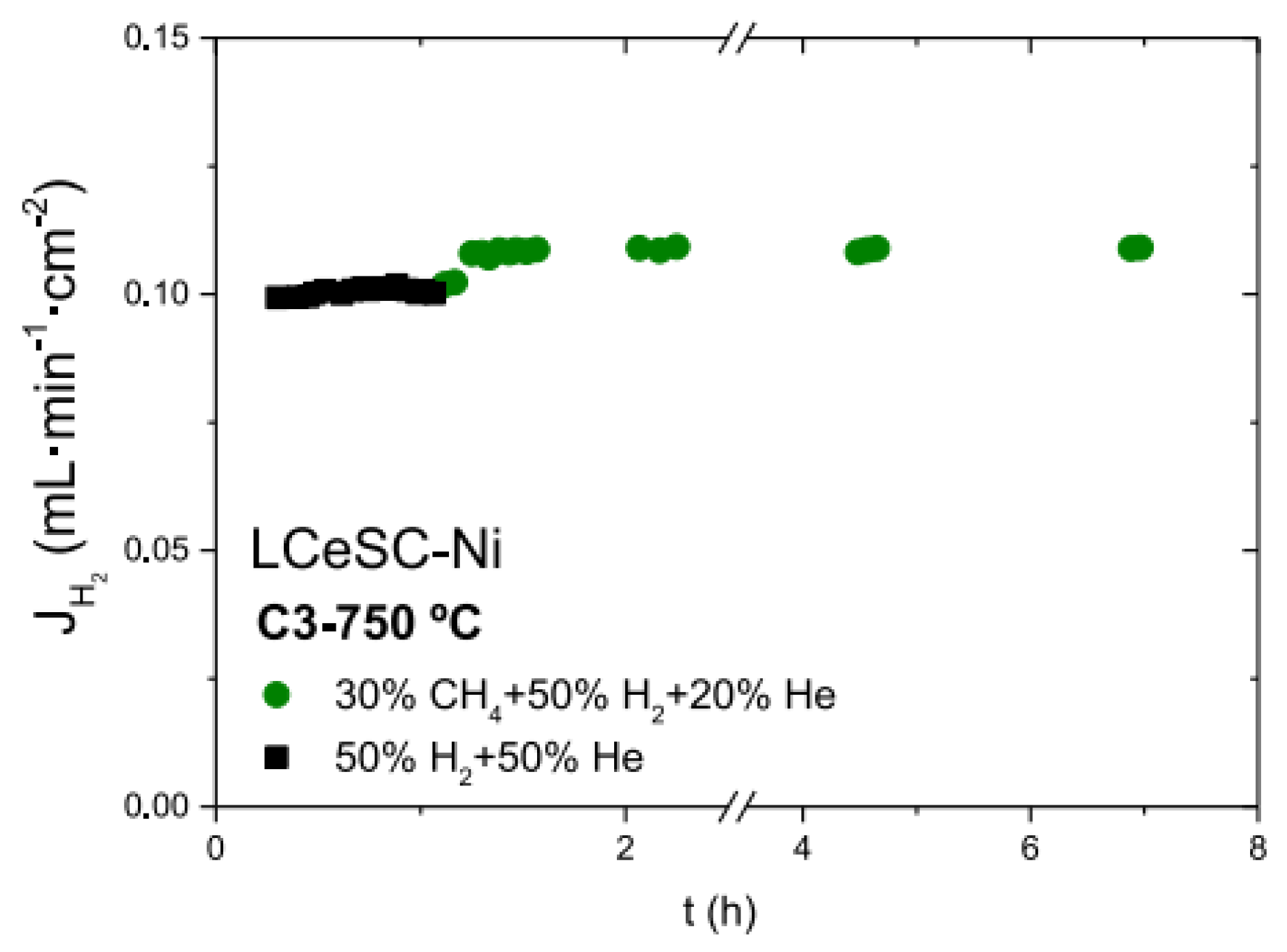

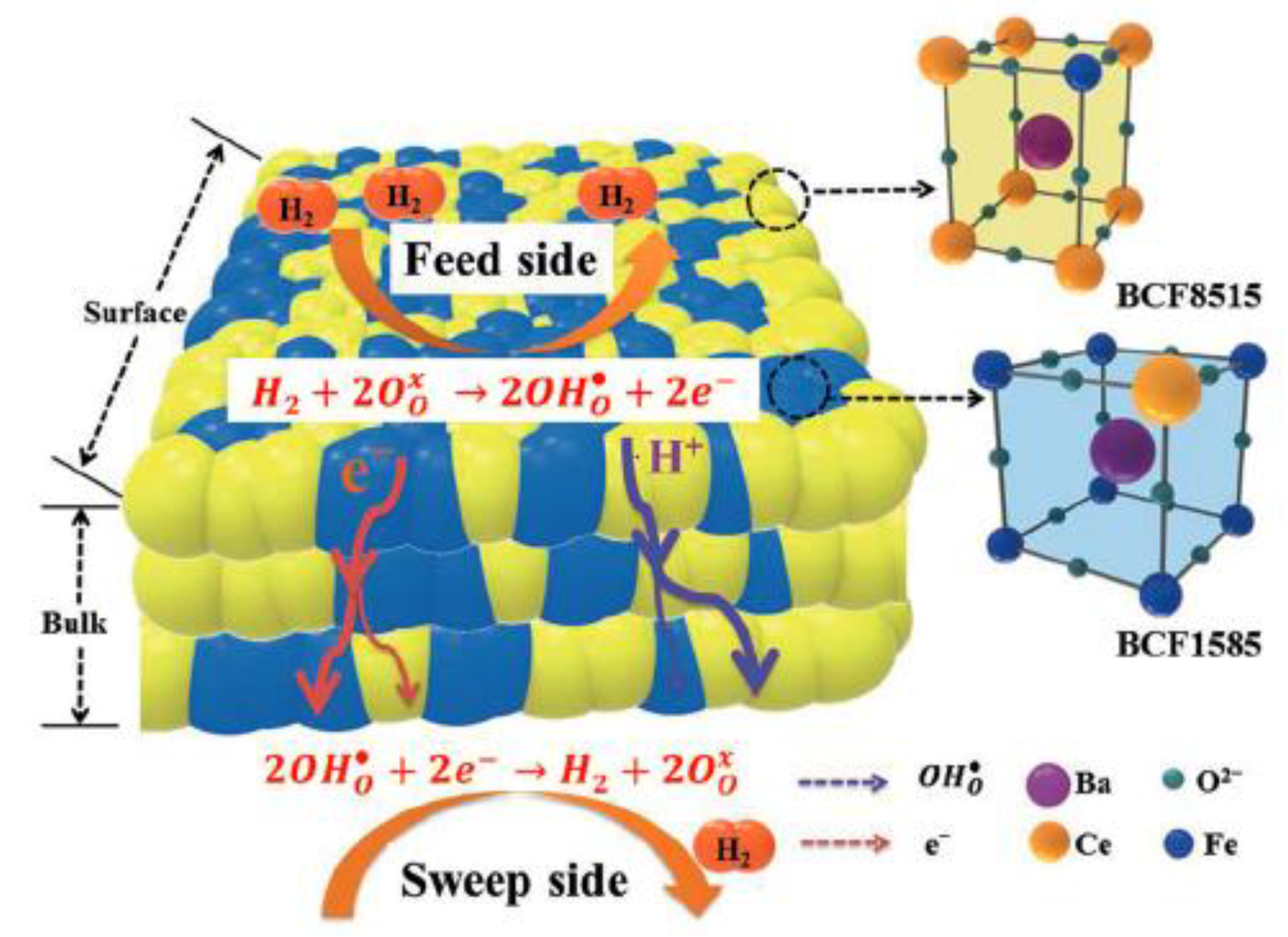

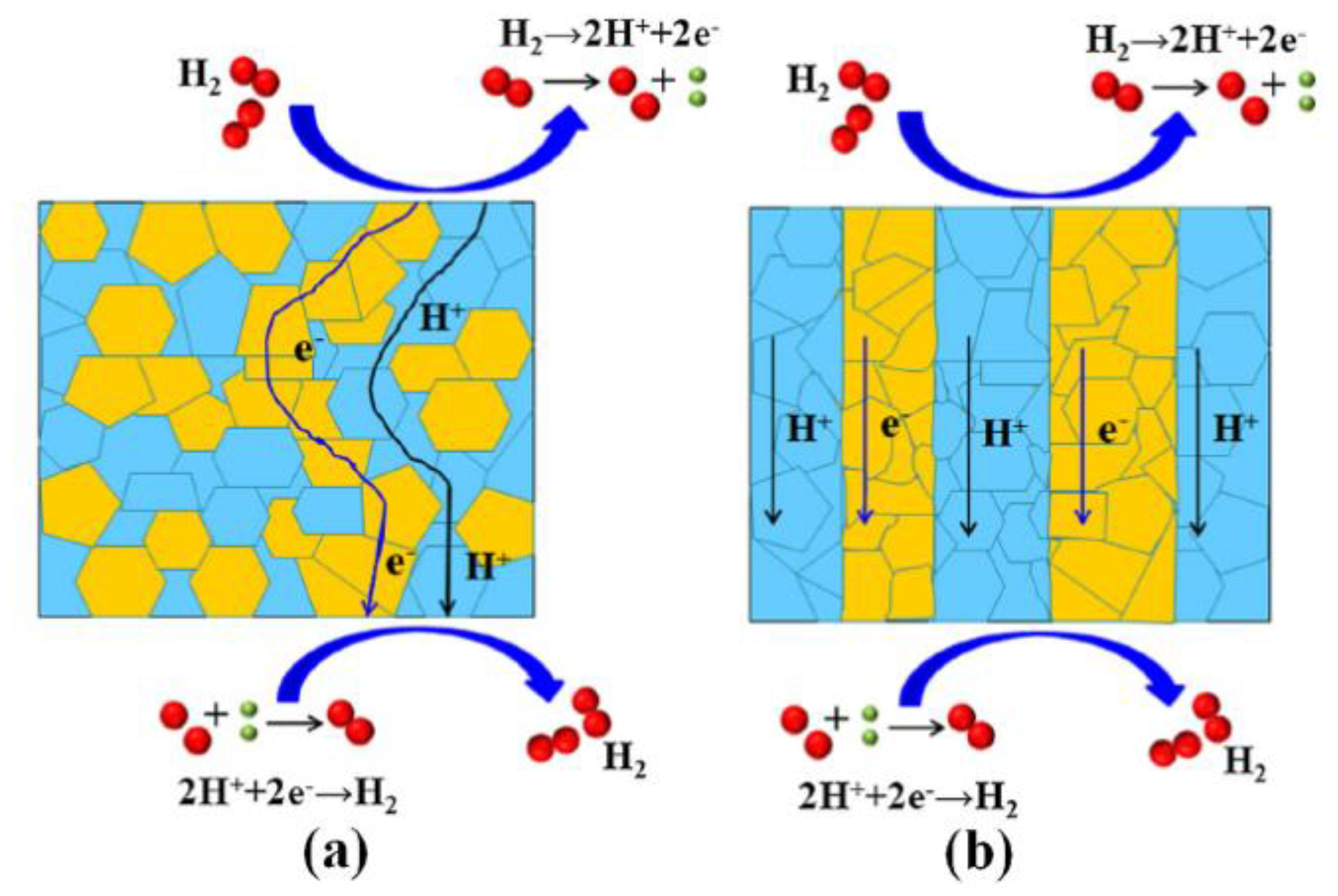
| Composition | Hydrogen Flux (mL·min−1·cm−2) | T (°C) | Thickness (mm) | Feed/Sweep Gas | Note | Ref. |
|---|---|---|---|---|---|---|
| 50Pd-50YSZ | 20 | 900 | 0.22 | 100%H2/N2 | mechanical mixing | [33] |
| 50Pd-50YSZ | 5.5 | 900 | 0.218 | 80% H2-He/N2 | ball-milling | [36] |
| 50Pd-50YSZ | 52 | 900 | 0.018 | 100%H2/N2 | mechanical mixing; asymmetric | [37] |
| 50Pd-50Gd0.2Ce0.8O2-δ | 5.5 | 900 | 0.282 | 80% H2-He/N2 | ball-milling | [38] |
| 50Pd-50CaZr0.9Y0.1O3-δ | 2.3 | 900 | 0.50 | 80% H2-He/N2 | ball-milling | [34] |
| 50Pd-50BaCe0.4Zr0.4Gd0.1Dy0.1O3-δ | 2.77 | 700 | 0.40 | 100%H2/N2 | ball-milling | [35] |
| 40Ni-60BaZr0.7Pr0.1Y0.2O3-δ | 0.0162 | 950 | 0.40 | wet50%H2-N2/Ar | mechanical mixing | [39] |
| 50Ni-50BaCe0.85Te0.05 Zr0.1O3-δ | 0.17 | 800 | 0.50 | 50%H2-He/Ar | mechanical mixing | [40] |
| 40Ni-60BaZr0.1Ce0.7Y0.2O3-δ | 0.805 | 900 | 0.266 | 100%H2/N2 | ball-milling | [41] |
| 40Ni-60BaZr0.1Ce0.7Y0.2O3-δ | 0.056 | 900 | 1 | 100%H2/N2 | ball-milling | [42] |
| 40Ni-60BaZr0.1Ce0.7Y0.2O3-δ | 0.087 | 900 | 0.5 | wet4%H2-Ar/N2 | ball-milling | [43] |
| 40Ni-60BaZr0.1Ce0.7Y0.1Yb0.1O3-δ | 0.0174 | 900 | 0.75 | 20%H2-He/N2 | mechanical mixing | [44] |
| 40Ni-60BaZr0.1Ce0.7Y0.1Yb0.1O3-δ | 0.215 | 900 | 0.40 | wet80%H2-He/N2 | mechanical mixing | [45] |
| 50Ni-50BaCe0.95Tb0.05O3-δ | 0.53 | 850 | 0.65 | 50%H2-He/N2 | ball-milling | [46] |
| 50Ni-50BaCe0.95Tb0.05O3-δ | 0.914 | 850 | 0.09 | 50%H2-He/N2 | ball-milling; asymmetric | [46] |
| 40Ni-60BaCe0.9Y0.1O3-δ | 0.24 | 900 | 0.08 | wet3.8%H2-He/N2 | ball-milling | [47] |
| 40Ni-60BaCe0.9Y0.1O3-δ | 0.76 | 800 | 0.23 | 100%H2/N2 | high-energy milling | [48] |
| 50Ni-50BaCe0.85Fe0.15O3-δ | 0.325 | 1000 | 0.50 | 50%H2-He/wet Ar | ball-milling | [49] |
| 40Ni-60BaCe0.7In0.2Ta0.1O3-δ | 0.15 | 900 | 1 | 20%H2-N2/Ar | mechanical mixing | [50] |
| 40Ni-60BaCe0.7Y0.2In0.1O3-δ | 0.013 | 850 | 0.86 | 20%H2-N2 /Ar | mechanical mixing | [51] |
| 40Ni-60SrCe0.8Yb0.2O3-δ | 0.105 | 900 | 0.25 | wet20%H2-He/N2 | ball-milling | [52] |
| 40Ni-60La0.5Ce0.5O2-δ | 0.088 | 900 | 0.048 | 20%H2-N2/Ar | mechanical mixing; asymmetric | [53] |
| 40Ni-60La0.5Ce0.5O2-δ | 0.021 | 900 | 0.6 | wet20%H2-N2/Ar | ball-milling | [54] |
| 40Ni-60La0.4875Ca0.0125Ce0.5O2-δ | 0.025 | 900 | 0.6 | wet20%H2-N2/Ar | ball-milling | [54] |
| 40Ni-60La1.95Sm0.05Ce2O7 | 0.037 | 900 | 0.6 | wet20%H2- Ar/N2 | ball-milling | [55] |
| 60Ni-40La5.5WO11.25-δ | 0.18 | 1000 | 0.5 | 50%H2-He/Ar | ball-milling | [56] |
| Cu-BaZr0.9Y0.1O3-δ | 0.00242 | 882 | 1.90 | 100%H2/Ar | molten-copper infiltration technique | [57] |
| 60Ta-40YSZ | 1.2 | 500 | 0.50 | 100%H2/Ar | mechanical mixing | [58] |
| Composition | H2 Flux (mL·min−1·cm−2) | T (°C) | Thickness (mm) | Feed/Sweep Gas | Note | Ref. |
|---|---|---|---|---|---|---|
| 50BaCe0.8Y0.2O3-δ-50Ce0.8Y0.2O2-δ | 0.0744 | 900 | 1.44 | wet50%H2-He/wet Ar | symmetric | [61] |
| 30BaCe0.8Y0.2O3-δ-70Ce0.8Y0.2O2-δ | 0.228 | 850 | 1.15 | 40%H2-N2/He | symmetric | [66] |
| 30BaCe0.8Y0.2O3-δ-70Ce0.8Y0.2O2-δ | 0.566 | 900 | 0.017 | 50%H2-He/N2 | asymmetric | [67] |
| 50BaCe0.65Zr0.2Y0.15O3-δ-50Ce0.85Gd0.15O2-δ | 2.4 | 1040 | 0.65 | 50%H2-He/wet Ar | symmetric; ZnO | [68] |
| 50BaCe0.65Zr0.2Y0.15O3-δ-50Ce0.8Gd0.2O2-δ | 0.47 | 750 | 0.77 | 50%H2-He/Ar | asymmetric; ZnO | [69] |
| 50BaCe0.8Eu0.2O3-δ-50Ce0.8Y0.2O2-δ | 0.61 | 700 | 0.5 | 50%H2-He/wet Ar | symmetric | [62] |
| 50BaCe0.2Zr0.7Y0.1O3-δ-50Sr0.95Ti0.9Nb0.1O3-δ | 0.035 | 800 | 1 | 50%H2-He/Ar | symmetric; Pd coated | [70] |
| 50BaCe0.85Fe0.15O3-δ-50BaCe0.15Fe0.85O3-δ | 0.76 | 950 | 1 | 50%H2-He/Ar | auto-decomposition | [71] |
| 50SrCe0.95Fe0.05O3-δ-50SrFe0.95Ce0.05O3-δ | 0.38 | 940 | 0.7 | 60%H2-N2/wet Ar | auto-decomposition | [72] |
| 80SrZrO3-20SrFeO3 | 0.0048 | 900 | 1 | 50%H2-He/wet Ar | symmetric | [59] |
| 90SrCe0.95Y0.05O3-δ-10ZnO | 0.039 | 900 | 1 | 21%H2-He/N2 | symmetric | [73] |
| SrCe0.9Y0.1O3-Ce0.8Sm0.2O2 | 0.163 | 900 | 1 | 20%H2-He/N2 | laminated | [74] |
| 50La5.5WO11.25-δ-50La0.87Sr0.13CrO3-δ | 0.15 | 700 | 0.37 | wet50%H2-N2/wet Ar | symmetric | [75] |
| 50La5.5WO11.25-δ-50La0.8Sr0.2FeO3-δ | 0.15 | 900 | 0.5 | 50%H2-N2/wet Ar | symmetric | [76] |
| 60La5.5WO11.25-δ-40La0.87Sr0.13CrO3-δ | 0.22 | 750 | 0.3 | wet50%H2-N2/wet Ar | symmetric; Pt coated | [77] |
| 70La27W3.5Mo1.5O55.5-δ-30La0.87Sr0.13CrO3-δ | 0.0077 | 700 | 1.43 | wet50%H2-N2/wet Ar | symmetric; Pt coated | [78] |
| 70La27W3.5Mo1.5O55.5-δ-30La0.87Sr0.13CrO3-δ | 0.014 | 900 | 0.05 | 50%H2-He/Ar | asymmetric | [79] |
| 70La0.995Ca0.005Nb4-δ-30LaNb3O9 | 0.0012 | 900 | 2 | wet H2/wet Ar | symmetric | [80] |
| 65Nd5.5WO11.25-δ-35Cu0.5Ni0.5O | 0.033 | 900 | 0.15 | 10% ethanol-40% H2O-Ar/Ar | asymmetric; catalyst coated | [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H. Dual-Phase Mixed Protonic-Electronic Conducting Hydrogen Separation Membranes: A Review. Membranes 2022, 12, 647. https://doi.org/10.3390/membranes12070647
Cheng H. Dual-Phase Mixed Protonic-Electronic Conducting Hydrogen Separation Membranes: A Review. Membranes. 2022; 12(7):647. https://doi.org/10.3390/membranes12070647
Chicago/Turabian StyleCheng, Hongda. 2022. "Dual-Phase Mixed Protonic-Electronic Conducting Hydrogen Separation Membranes: A Review" Membranes 12, no. 7: 647. https://doi.org/10.3390/membranes12070647
APA StyleCheng, H. (2022). Dual-Phase Mixed Protonic-Electronic Conducting Hydrogen Separation Membranes: A Review. Membranes, 12(7), 647. https://doi.org/10.3390/membranes12070647





