Chemical Vapour Deposition Graphene–PMMA Nanolaminates for Flexible Gas Barrier
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Thickness Measurements for the Evaluation of Graphene Volume Fraction
2.3. Raman Spectroscopy
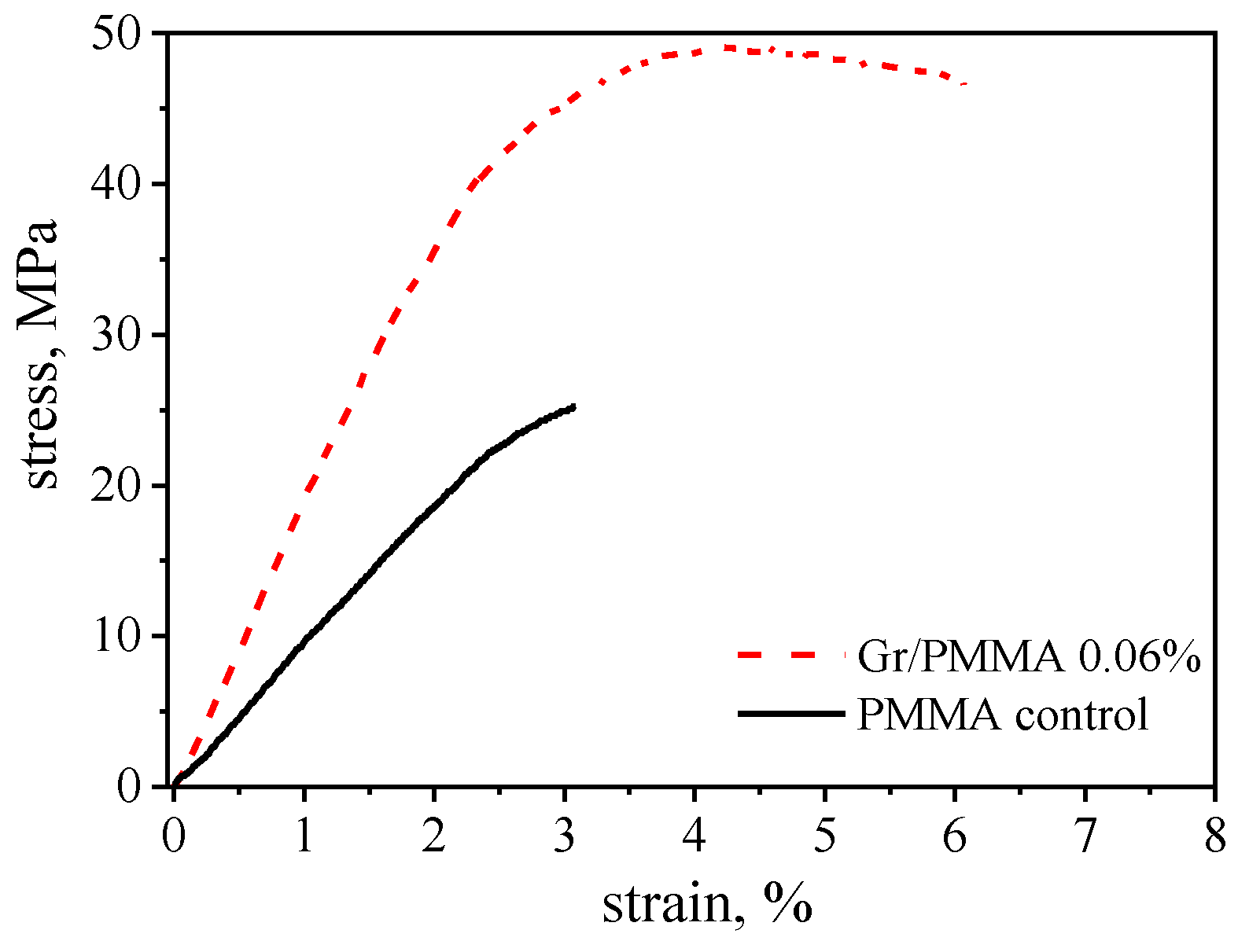
2.4. Uniaxial Tensile Testing
2.5. Scanning Electron Microscopy
2.6. Permeability Measurements
3. Results and Discussion
3.1. Characterization of Materials
3.2. Results of Permeability Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Bonaccorso, F.; Fal’ko, V.; Novoselov, K.S.; Roche, S.; Bøggild, P.; Borini, S.; Koppens, F.H.L.; Palermo, V.; Pugno, N.; et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 2015, 7, 4598–4810. [Google Scholar] [PubMed] [Green Version]
- Palermo, V. Not a molecule, not a polymer, not a substrate...the many faces of graphene as a chemical platform. Chem. Commun. 2013, 49, 2848–2857. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Liu, G.; Zhao, J.; Jin, W. Two-dimensional-material membranes: Manipulating the transport pathway for molecular separation. Acc. Mater. Res. 2021, 2, 114–128. [Google Scholar] [CrossRef]
- Bunch, J.S.; Verbridge, S.S.; Alden, J.S.; van der Zande, A.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Impermeable atomic membranes from graphene sheets. Nano Lett. 2008, 8, 2458–2462. [Google Scholar] [CrossRef] [Green Version]
- Terrones, M.; Martín, O.; González, M.; Pozuelo, J.; Serrano, B.; Cabanelas, J.C.; Vega-Díaz, S.M.; Baselga, J. Interphases in graphene polymer-based nanocomposites: Achievements and challenges. Adv. Mat. 2011, 23, 5302–5310. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Kundalwal, S.I.; Kumar, S. Gas barrier performance of graphene/polymer nanocomposites. Carbon 2016, 98, 313–333. [Google Scholar] [CrossRef] [Green Version]
- Pierleoni, D.; Xia, Z.Y.; Christian, M.; Ligi, S.; Minelli, M.; Morandi, V.; Doghieri, F.; Palermo, V. Graphene-based coatings on polymer films for gas barrier applications. Carbon 2016, 96, 503–512. [Google Scholar] [CrossRef]
- Pierleoni, D.; Minelli, M.; Ligi, S.; Christian, M.; Funke, S.; Reineking, N.; Morandi, V.; Doghieri, F.; Palermo, V. Selective gas permeation in graphene oxide-polymer self-assembled multilayers. Appl. Mater. Interfaces 2018, 10, 11242–11250. [Google Scholar] [CrossRef]
- Nielsen, L.E. Models for the permeability of filled polymer systems. J. Macromol. Sci. Chem. 1967, 1, 929–942. [Google Scholar] [CrossRef]
- Kalaitzidou, K.; Fukushima, H.; Drzal, L.T. Multifunctional polypropylene composites produced by incorporation of exfoliated graphite nanoplatelets. Carbon 2007, 45, 1446–1452. [Google Scholar] [CrossRef]
- Berean, K.J.; Ou, J.Z.; Nour, M.; Field, M.R.; Alsaif, M.M.Y.A.; Wang, Y.; Ramanathan, R.; Bansal, V.; Kentish, S.; Doherty, C.M.; et al. Enhanced gas permeation through graphene nanocomposites. J. Phys. Chem. C 2015, 119, 13700–13712. [Google Scholar] [CrossRef]
- Althumayri, K.; Harrison, W.J.; Shin, Y.; Gardiner, J.M.; Casiraghi, C.; Budd, P.M.; Bernardo, P.; Clarizia, G.; Jansen, J.C. The influence of few-layer graphene on the gas permeability of the high-free-volume polymer PIM-1. Philos. Trans. R. Soc. A 2016, 374, 20150031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díez-Betriu, X.; Mompeán, F.J.; Munuera, C.; Rubio-Zuazo, J.; Menéndez, R.; Castro, G.R.; de Andrés, A. Graphene-oxide stacking and defects in few-layer films: Impact of thermal and chemical reduction. Carbon 2014, 80, 40–49. [Google Scholar] [CrossRef]
- Liu, P.; Cottrill, A.L.; Kozawa, D.; Koman, V.B.; Parviz, D.; Liu, A.T.; Yang, J.; Tran, T.Q.; Wong, M.H.; Wang, S.; et al. Emerging trends in 2D nanotechnology that are redefining our understanding of “Nanocomposites”. Nano Today 2018, 21, 18–40. [Google Scholar] [CrossRef]
- Koenig, S.P.; Wang, L.; Pellegrino, J.; Bunch, J.S. Selective molecular sieving through porous graphene. Nat. Nanotechnol. 2012, 7, 728–732. [Google Scholar] [CrossRef] [Green Version]
- Nair, R.R.; Wu, H.A.; Jayaram, P.N.; Grigorieva, I.V.; Geim, A.K. Unimpeded permeation of water through helium-leak–tight graphene-based membranes. Science 2012, 335, 442–444. [Google Scholar] [CrossRef] [Green Version]
- Tian, W.; Li, W.; Yu, W.; Liu, X. A review on lattice defects in graphene: Types, generation, effects and regulation. Micromachines 2017, 8, 163. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Jin, L.; Allen, F.I.; Gao, Y.; Ci, P.; Kang, F.; Wu, J. Selective Gas Permeation in Defect-Engineered Bilayer Graphene. Nano Lett. 2021, 21, 2183–2190. [Google Scholar] [CrossRef]
- Pavlou, C.; Pastore Carbone, M.G.; Manikas, A.C.; Trakakis, G.; Koral, C.; Papari, G.; Andreone, A.; Galiotis, C. Effective EMI shielding behaviour of thin graphene/PMMA nanolaminates in the THz range. Nat. Comm. 2021, 12, 4655. [Google Scholar] [CrossRef]
- Pantano, M.F.; Pavlou, C.; Pastore Carbone, M.G.; Galiotis, C.; Pugno, N.M.; Speranza, G. Highly deformable, ultrathin large-area poly (methyl methacrylate) films. ACS Omega 2021, 6, 8308–8312. [Google Scholar] [CrossRef] [PubMed]
- Min, K.E.; Paul, D.R. Effect of tacticity on permeation properties of poly (methyl methacrylate). J. Polym. Sci. Part B Polym. Phys. 1988, 26, 1021–1033. [Google Scholar] [CrossRef]
- Raymond, P.C.; Paul, D.R. Sorption and transport of pure gases in random styrene/methyl methacrylate copolymers. J. Polym. Sci. Part B Polym. Phys. 1990, 28, 2079–2102. [Google Scholar] [CrossRef]
- Rodríguez, O.; Fornasiero, F.; Arce, A.; Radke, C.J.; Prausnitz, J.M. Solubilities and diffusivities of water vapor in poly(methylmethacrylate), poly(2-hydroxyethylmethacrylate), poly(N-vinyl-2-pyrrolidone) and poly(acrylonitrile). Polymer 2003, 44, 6323–6333. [Google Scholar] [CrossRef]
- Ushiki, I.; Hayashi, S.; Kihara, S.; Takishima, S. Solubilities and diffusion coefficients of carbon dioxide and nitrogen in poly(methyl methacrylate) at high temperatures and pressures. J. Supercrit. Fluids 2019, 152, 104565. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Vlassiouk, I.; Polizos, G.; Cooper, R.; Ivanov, I.; Keum, J.K.; Paulauskas, F.; Datskos, P.; Smirno, S. Strong and electrically conductive graphene-based composite fibers and laminates. ACS Appl. Mater. Interfaces 2015, 7, 10702–10709. [Google Scholar] [CrossRef]
- Liu, P.; Jin, Z.; Katsukis, G.; Drahushuk, L.W.; Shimizu, S.; Shih, C.; Wetzel, E.D.; Taggart-Scarff, J.K.; Qing, B.; Van Vliet, K.J.; et al. Layered and scrolled nanocomposites with aligned semi-infinite graphene inclusions at the platelet limit. Science 2016, 353, 364–367. [Google Scholar] [CrossRef] [Green Version]
- Chiou, J.S.; Paul, D.R. Sorption and transport of inert gases in PVF2/PMMA blends. J. Appl. Polym. Sci. 1986, 32, 4793–4814. [Google Scholar] [CrossRef]
- Hu, C.; Fu, Y.; Hsiao, S.; Lee, K.; Lai, J. Effect of physical aging on the gas transport properties of poly(methyl methacrylate) membranes. J. Membr. Sci. 2007, 303, 29–36. [Google Scholar] [CrossRef]
- Liu, B.; Pavlou, C.; Wang, Z.; Cang, Y.; Galiotis, C.; Fytas, G. Determination of the elastic moduli of CVD graphene by probing graphene/polymer Bragg stacks. 2D Mater. 2021, 8, 035040. [Google Scholar] [CrossRef]
- Morimune, S.; Nishino, T.; Goto, T. Ecological approach to graphene oxide reinforced poly (methyl methacrylate) nanocomposites. ACS Appl. Mater. Interfaces 2012, 4, 3596–3601. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.C.; Ji, W.F.; Lai, M.C.; Hsiao, Y.R.; Hsu, C.H.; Chuang, T.L.; Wei, Y.; Yeh, J.; Liu, W.R. Synergistic effects of hydrophobicity and gas barrier properties on the anticorrosion property of PMMA nanocomposite coatings embedded with graphene nanosheets. Polym. Chem. 2014, 5, 1049–1056. [Google Scholar] [CrossRef]
- Choi, K.; Nam, S.; Lee, Y.; Lee, M.; Jang, J.; Kim, S.J.; Jeong, Y.J.; Kim, H.; Bae, S.; Yoo, J. Reduced water vapor transmission rate of graphene gas barrier films for flexible organic field-effect transistors. ACS Nano 2015, 9, 5818–5824. [Google Scholar] [CrossRef]
- Sagade, A.A.; Aria, A.I.; Edge, S.; Melgari, P.; Gieseking, B.; Bayer, B.C.; Meyer, J.C.; Bird, D.; Brewer, P.; Hofmann, S. Graphene-based nanolaminates as ultra-high permeation barriers. NPJ 2D Mater. Appl. 2017, 1, 35. [Google Scholar] [CrossRef] [Green Version]
- Kelman, S.D.; Matteucci, S.; Bielawski, C.W.; Freeman, B.D. Crosslinking poly(1-trimethylsilyl-1-propyne) and its effect on solvent resistance and transport properties. Polymer 2007, 48, 6881–6892. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: Bristol, UK, 1975. [Google Scholar]
- Poling, B.E.; Prausnitz, J.M.; O’Connell, J.P. The Properties of Gases and Liquids, 15th ed.; McGraw-Hill: New York, NY, USA, 2000. [Google Scholar]
- Petropoulos, J.H. Some fundamental approaches to membrane gas permeability and permselectivity. J. Membr. Sci. 1990, 53, 229–258. [Google Scholar] [CrossRef]
- Mensitieri, G.; Del Nobile, M.A.; Monetta, T.; Nicodemo, L.; Bellucci, F. The effect of film thickness on oxygen sorption and transport in dry and water-saturated Kapton® polyimide. J. Membr. Sci. 1994, 89, 131–141. [Google Scholar] [CrossRef]
- Deng, S.; Berry, V. Wrinkled, rippled and crumpled graphene: An overview of formation mechanism, electronic properties, and applications. Mater. Today 2016, 19, 197–212. [Google Scholar] [CrossRef]
- Pastore Carbone, M.G.; Manikas, A.C.; Souli, I.; Pavlou, C.; Galiotis, C. Mosaic pattern formation in exfoliated graphene by mechanical deformation. Nat. Commun. 2019, 10, 1572. [Google Scholar] [CrossRef] [Green Version]


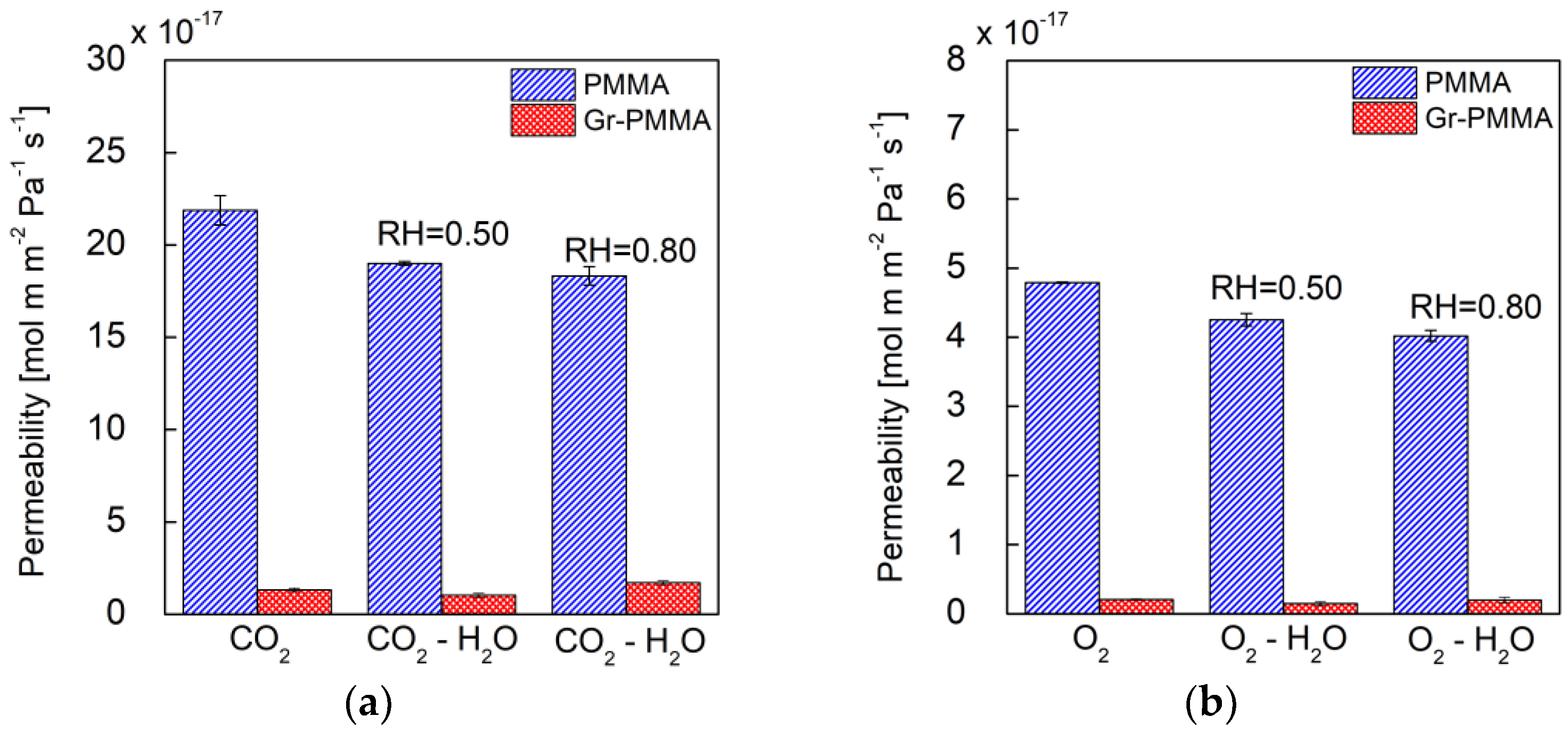
| Nanolaminate/Permeating Gas | P [mol∙m∙m−2∙Pa−1∙s−1] | P [Barrer] |
|---|---|---|
| PMMA/CO2 | 21.9 (±0.8) × 10−17 | 6.5 (±0.2) × 10−1 |
| Gr-PMMA/CO2 | 1.30 (±0.1) × 10−17 | 0.39 (±0.03) × 10−1 |
| PMMA/CO2-H2O @RH = 0.50 | 19.0 (±0.1) × 10−17 | 5.67 (±0.03) × 10−1 |
| Gr-PMMA/CO2-H2O @RH = 0.50 | 1.0 (±0.1) × 10−17 | 0.30 (±0.03) × 10−1 |
| PMMA/CO2-H2O @RH = 0.80 | 18.3 (±0.5) × 10−17 | 5.5 (±0.2) × 10−1 |
| Gr-PMMA/CO2-H2O @RH = 0.80 | 1.7 (±0.1) × 10−17 | 0.51 (±0.03) × 10−1 |
| Nanolaminate/Permeating Gas | P [mol∙m∙m−2∙Pa−1∙s−1] | P [Barrer] |
| PMMA/O2 | 4.79 (±0.01) × 10−17 | 1.434 (±0.003) × 10−1 |
| Gr-PMMA/O2 | 0.21 (±0.01) × 10−17 | 0.063 (±0.003) × 10−1 |
| PMMA/O2-H2O @RH = 0.50 | 4.25 (±0.09) × 10−17 | 1.27 (±0.03) × 10−1 |
| Gr-PMMA/O2-H2O @RH = 0.50 | 0.15 (±0.03) × 10−17 | 0.045 (±0.009) × 10−1 |
| PMMA/O2-H2O @RH = 0.80 | 4.02 (±0.08) × 10−17 | 1.20 (±0.02) × 10−1 |
| Gr-PMMA/O2-H2O @RH = 0.80 | 0.20 (±0.04) × 10−17 | 0.06 (±0.01) × 10−1 |
| Permeating Gas | P [mol∙m∙m−2∙Pa−1∙s−1] | P [Barrer] |
|---|---|---|
| CO2 | 8.5 (±0.8) × 10−21 | 2.5 (±0.2) × 10−5 |
| CO2 @ RH = 0.5 | 6.6 (±0.9) × 10−21 | 2.1 (±0.3) × 10−5 |
| CO2 @ RH = 0.8 | 11.0 (±0.9) × 10−21 | 3.3 (±0.3) × 10−5 |
| O2 | 1.3 (±0.1) × 10−21 | 0.40 (±0.03) × 10−5 |
| O2 @ RH = 0.5 | 1.0 (±0.2) × 10−21 | 0.28 (±0.06) × 10−5 |
| O2 @ RH = 0.8 | 1.3 (±0.2) × 10−21 | 0.38 (±0.06) × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldanza, A.; Pastore Carbone, M.G.; Brondi, C.; Manikas, A.C.; Mensitieri, G.; Pavlou, C.; Scherillo, G.; Galiotis, C. Chemical Vapour Deposition Graphene–PMMA Nanolaminates for Flexible Gas Barrier. Membranes 2022, 12, 611. https://doi.org/10.3390/membranes12060611
Baldanza A, Pastore Carbone MG, Brondi C, Manikas AC, Mensitieri G, Pavlou C, Scherillo G, Galiotis C. Chemical Vapour Deposition Graphene–PMMA Nanolaminates for Flexible Gas Barrier. Membranes. 2022; 12(6):611. https://doi.org/10.3390/membranes12060611
Chicago/Turabian StyleBaldanza, Antonio, Maria Giovanna Pastore Carbone, Cosimo Brondi, Anastasios C. Manikas, Giuseppe Mensitieri, Christos Pavlou, Giuseppe Scherillo, and Costas Galiotis. 2022. "Chemical Vapour Deposition Graphene–PMMA Nanolaminates for Flexible Gas Barrier" Membranes 12, no. 6: 611. https://doi.org/10.3390/membranes12060611
APA StyleBaldanza, A., Pastore Carbone, M. G., Brondi, C., Manikas, A. C., Mensitieri, G., Pavlou, C., Scherillo, G., & Galiotis, C. (2022). Chemical Vapour Deposition Graphene–PMMA Nanolaminates for Flexible Gas Barrier. Membranes, 12(6), 611. https://doi.org/10.3390/membranes12060611








