Understanding Aging Mechanisms in the Context of UV Irradiation of a Low Fouling and Self-Cleaning PVDF-PVP-TiO2 Hollow-Fiber Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hollow-Fiber Membrane Preparation
2.2. Chemicals
2.3. BSA Adsorption-Filtration
2.4. Pure Water Permeability
2.5. Membrane Aging
2.6. Morphology Observation
2.7. Tensile Strength Tests
2.8. ATR-FTIR
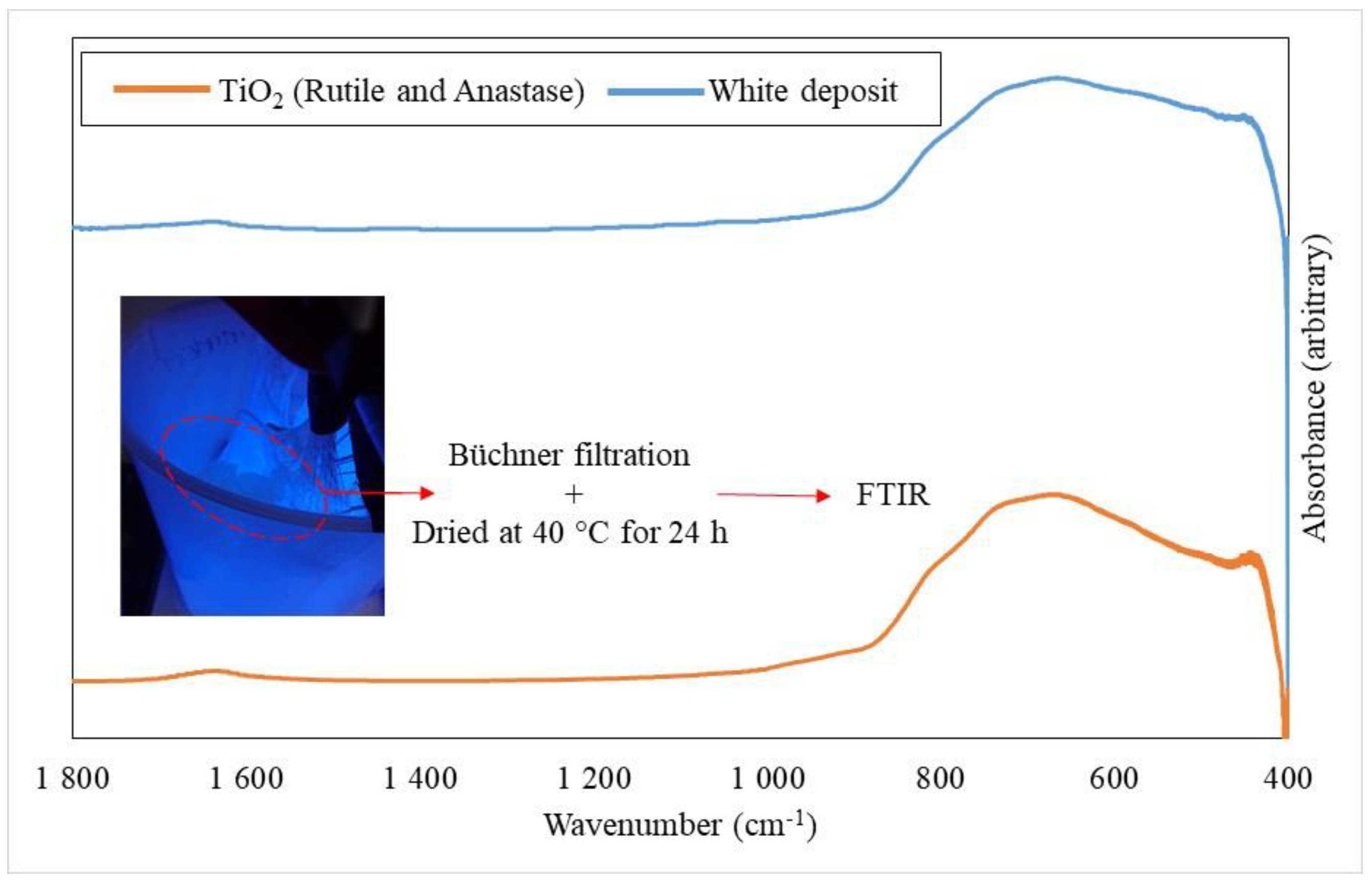
2.9. Soaking Medium Analysis
3. Results and Discussion
3.1. Aging of HFs Soaked in UP Water during Irradiation–Effect of UV Irradiation Power
3.1.1. Structural Degradation
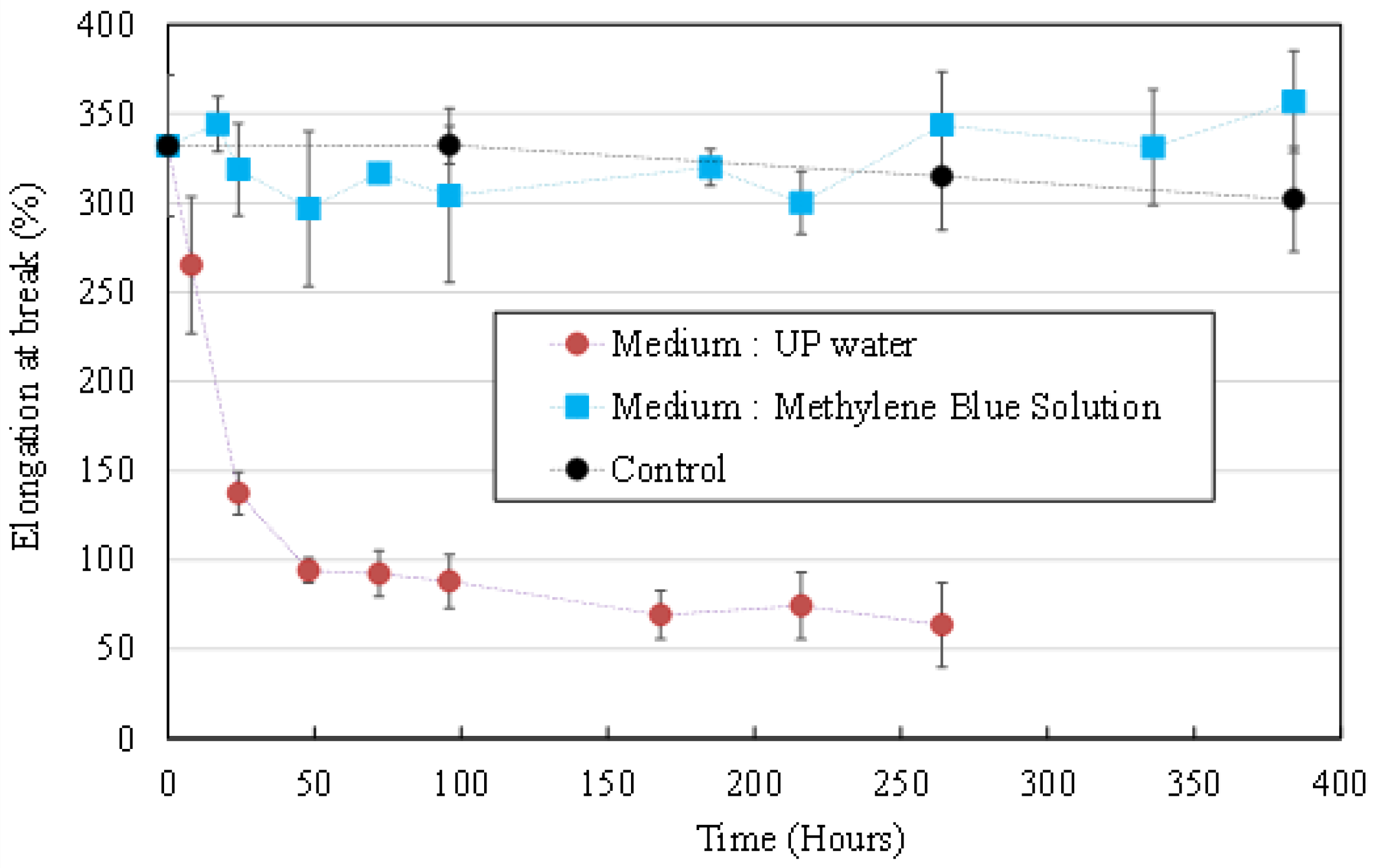
3.1.2. Mechanical Properties Evolution
3.2. Aging of HFs Soaked in MB Solution during Irradiation
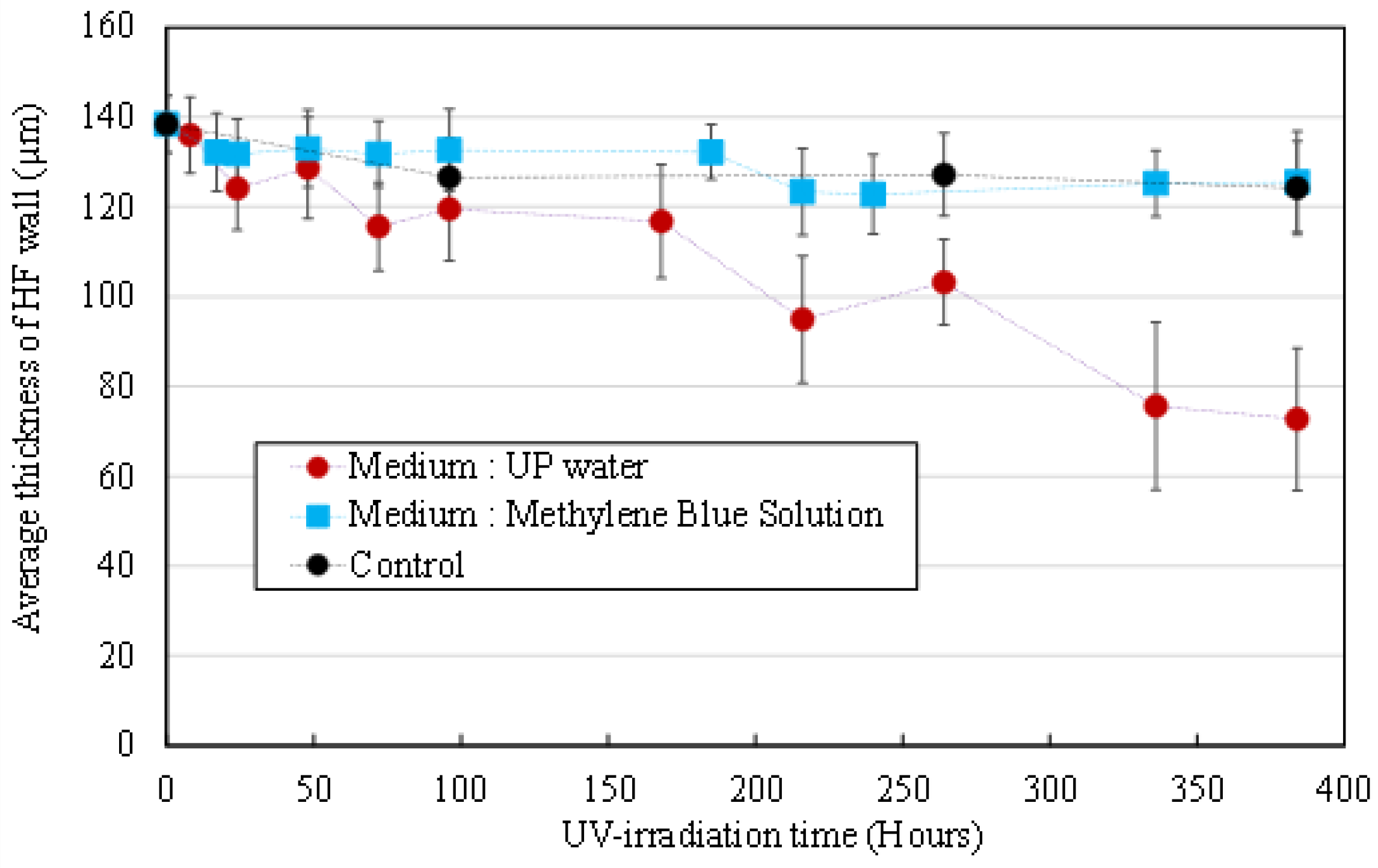
3.2.1. Morphological Degradation
3.2.2. Mechanical Properties Evolution
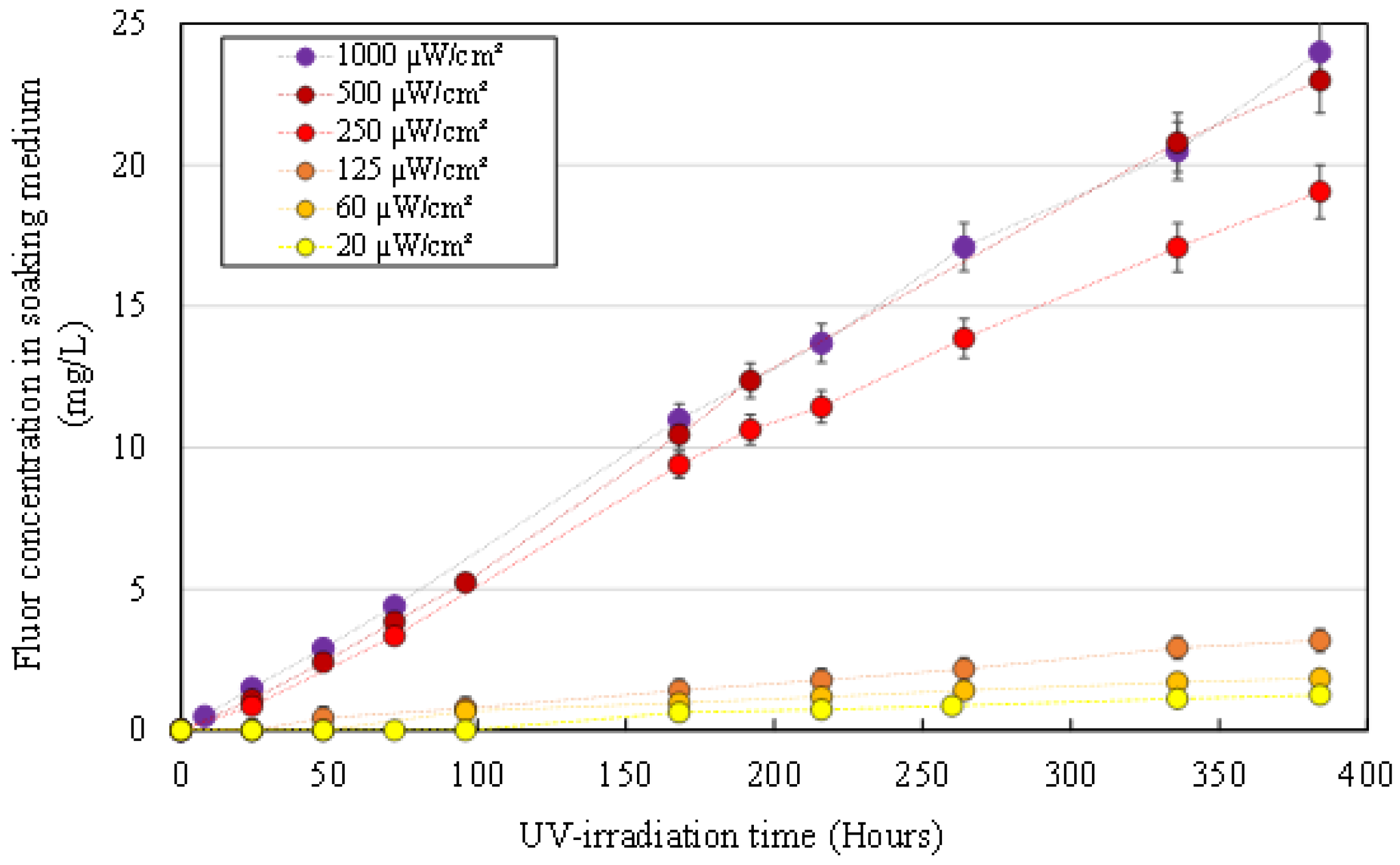
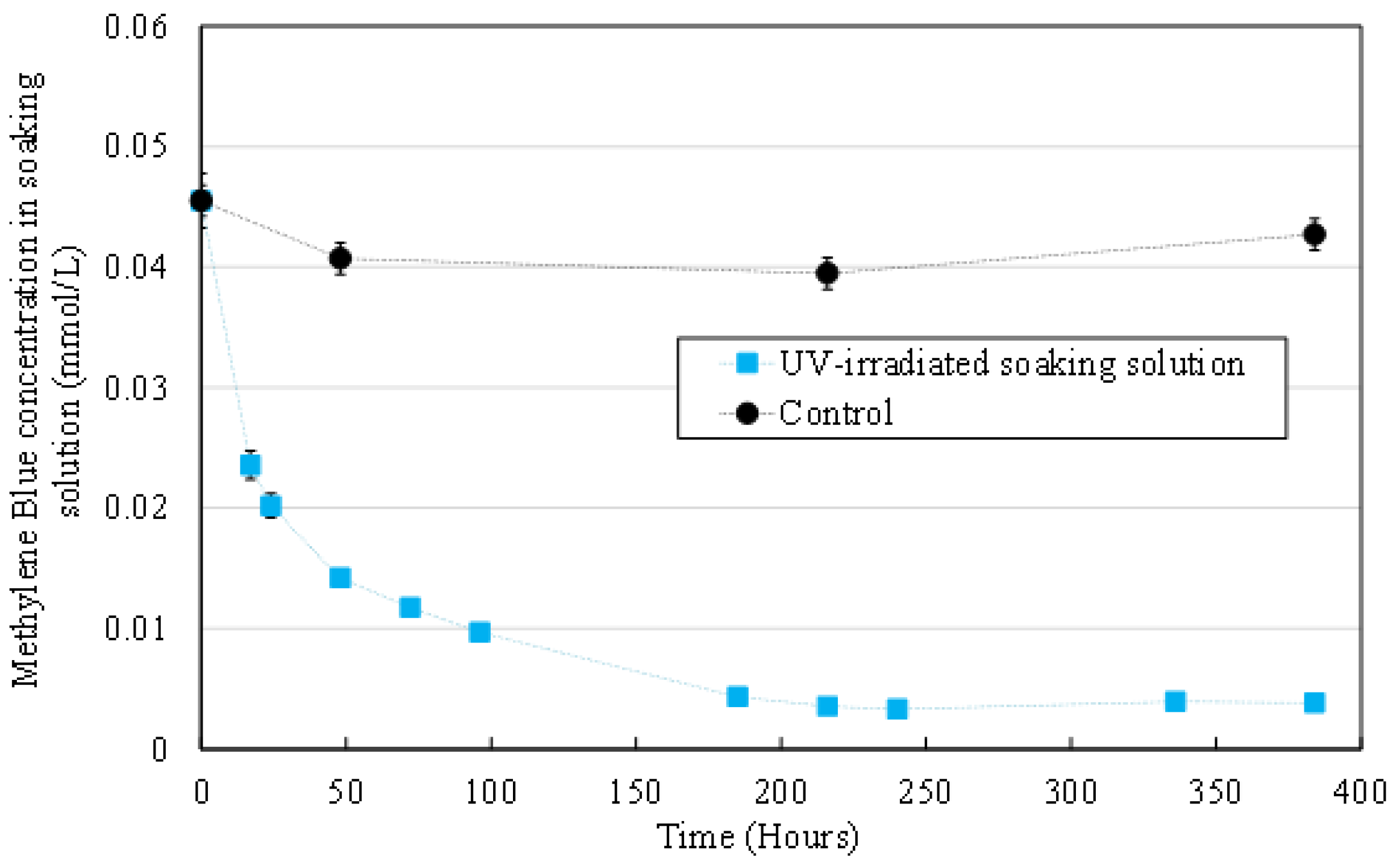
3.2.3. MB Concentration in the Aqueous Medium
3.3. Aging of BSA Pre-Fouled HFs Soaked in UP Water during Irradiation
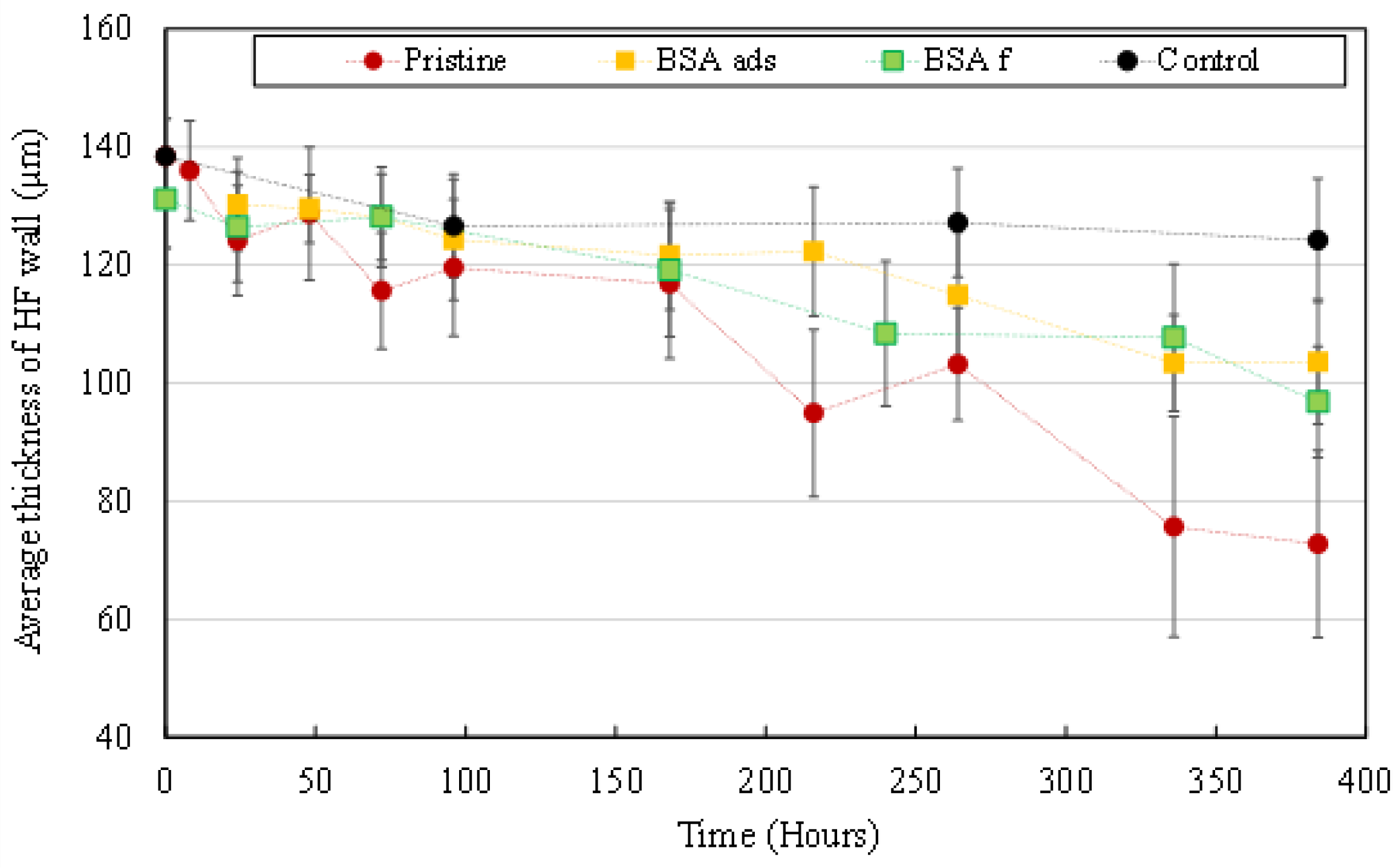
3.3.1. Morphological Degradation
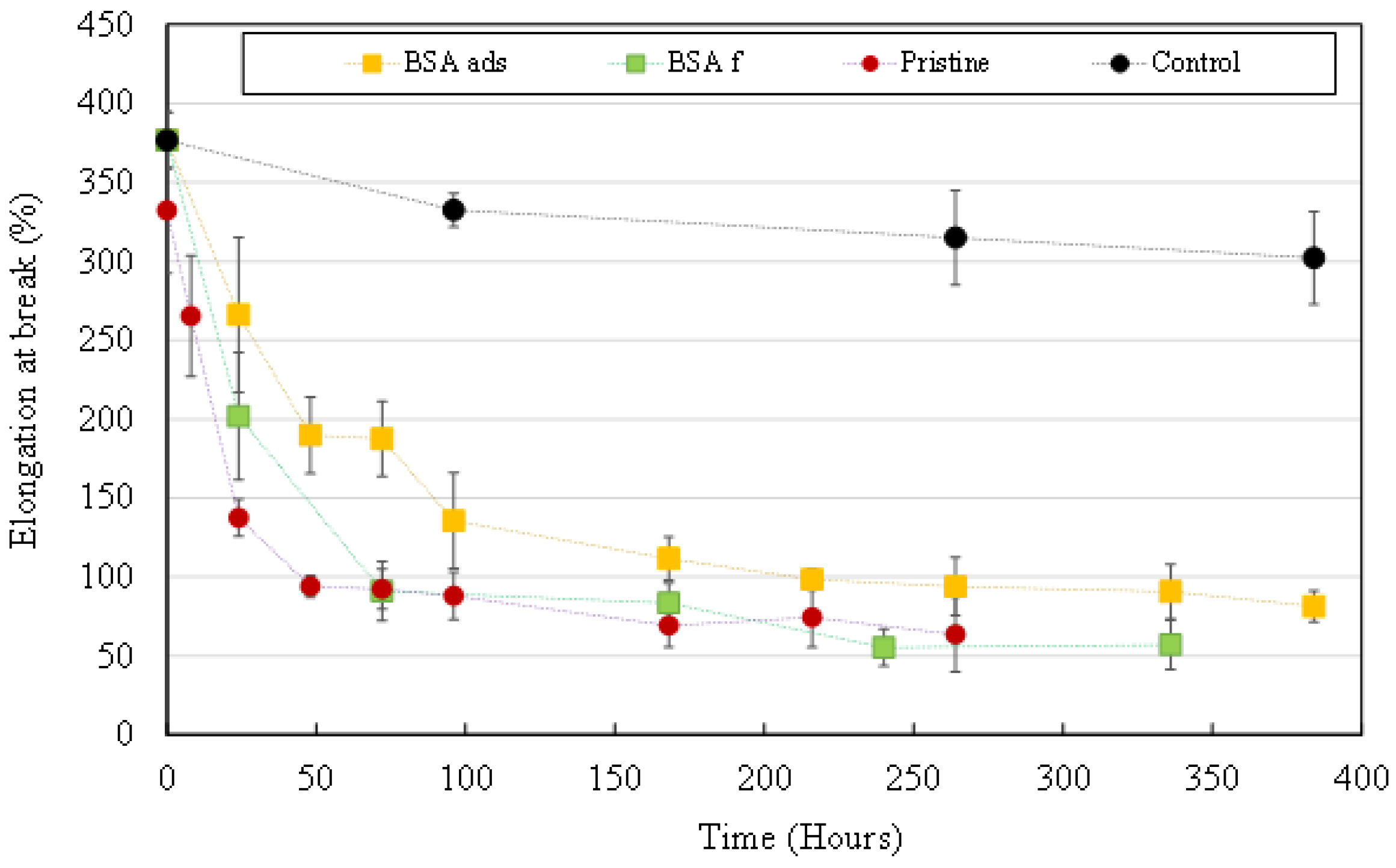
3.3.2. Mechanical Properties’ Evolution
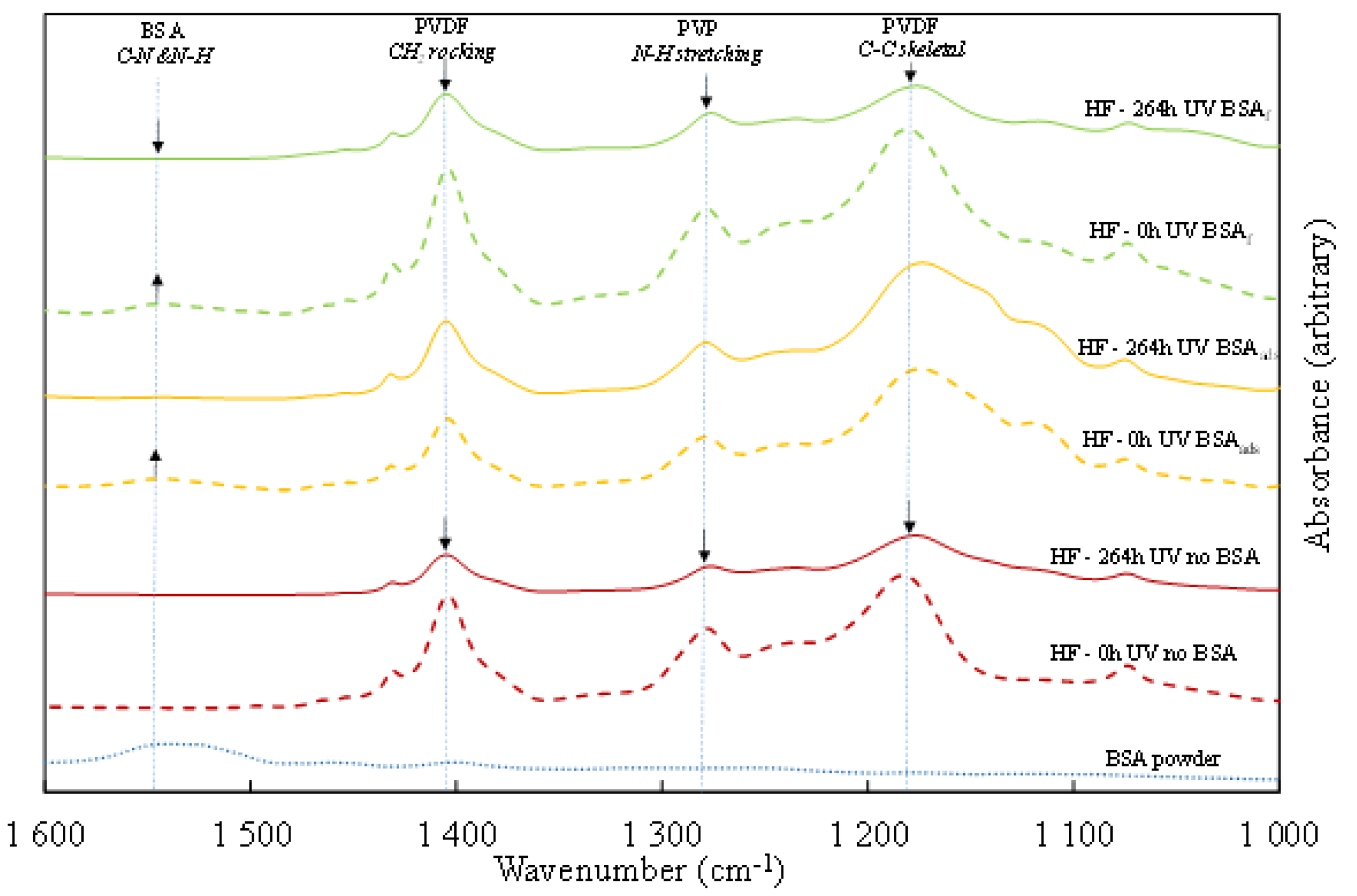
3.3.3. Chemical Degradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Gao, Y.; Hu, M.; Mi, B. Membrane Surface Modification with TiO2-Graphene Oxide for Enhanced Photocatalytic Performance. J. Membr. Sci. 2014, 455, 349–356. [Google Scholar] [CrossRef]
- Abid, H.S.; Johnson, D.J.; Hashaikeh, R.; Hilal, N. A Review of Efforts to Reduce Membrane Fouling by Control of Feed Spacer Characteristics. Desalination 2017, 420, 384–402. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, M.; Kilduff, J.E.; Belfort, G. Low Fouling Synthetic Membranes by UV-Assisted Graft Polymerization: Monomer Selection to Mitigate Fouling by Natural Organic Matter. J. Membr. Sci. 2003, 222, 59–70. [Google Scholar] [CrossRef]
- Kull, K.R.; Steen, M.L.; Fisher, E.R. Surface Modification with Nitrogen-Containing Plasmas to Produce Hydrophilic, Low-Fouling Membranes. J. Membr. Sci. 2005, 246, 203–215. [Google Scholar] [CrossRef]
- Zhu, X.; Tu, W.; Wee, K.H.; Bai, R. Effective and Low Fouling Oil/Water Separation by a Novel Hollow Fiber Membrane with Both Hydrophilic and Oleophobic Surface Properties. J. Membr. Sci. 2014, 466, 36–44. [Google Scholar] [CrossRef]
- Achilli, A.; Cath, T.Y.; Marchand, E.A.; Childress, A.E. The Forward Osmosis Membrane Bioreactor: A Low Fouling Alternative to MBR Processes. Desalination 2009, 239, 10–21. [Google Scholar] [CrossRef]
- Romay, M.; Diban, N.; Rivero, M.J.; Urtiaga, A.; Ortiz, I. Critical Issues and Guidelines to Improve the Performance of Photocatalytic Polymeric Membranes. Catalysts 2020, 10, 570. [Google Scholar] [CrossRef]
- Mannina, G.; Cosenza, A. The Fouling Phenomenon in Membrane Bioreactors: Assessment of Different Strategies for Energy Saving. J. Membr. Sci. 2013, 444, 332–344. [Google Scholar] [CrossRef] [Green Version]
- Drews, A. Membrane Fouling in Membrane Bioreactors-Characterisation, Contradictions, Cause and Cures. J. Membr. Sci. 2010, 363, 1–28. [Google Scholar] [CrossRef]
- Méricq, J.P.; Mendret, J.; Brosillon, S.; Faur, C. High Performance PVDF-TiO2 Membranes for Water Treatment. Chem. Eng. Sci. 2015, 123, 283–291. [Google Scholar] [CrossRef]
- Oh, S.J.; Kim, N.; Lee, Y.T. Preparation and Characterization of PVDF/TiO2 Organic-Inorganic Composite Membranes for Fouling Resistance Improvement. J. Membr. Sci. 2009, 345, 13–20. [Google Scholar] [CrossRef]
- Bet-Moushoul, E.; Mansourpanah, Y.; Farhadi, K.; Tabatabaei, M. TiO2 Nanocomposite Based Polymeric Membranes: A Review on Performance Improvement for Various Applications in Chemical Engineering Processes. Chem. Eng. J. 2016, 283, 29–46. [Google Scholar] [CrossRef]
- Shi, F.; Ma, Y.; Ma, J.; Wang, P.; Sun, W. Preparation and Characterization of PVDF/TiO2 Hybrid Membranes with Ionic Liquid Modified Nano-TiO2 Particles. J. Membr. Sci. 2013, 427, 259–269. [Google Scholar] [CrossRef]
- Albu, S.P.; Ghicov, A.; Macak, J.M.; Hahn, R.; Schmuki, P. Self-Organized, Free-Standing TiO2 Nanotube Membrane for Flow-through Photocatalytic Applications. Nano Lett. 2007, 7, 1286–1289. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.T.; Mendret, J.; Méricq, J.P.; Faur, C.; Brosillon, S. Study of Permeate Flux Behavior during Photo-Filtration Using Photocatalytic Composite Membranes. Chem. Eng. Processing-Process Intensif. 2020, 148, 107781. [Google Scholar] [CrossRef]
- Fox, M.A.; Dulay, M.T. Heterogeneous Photocatalysis. Chem. Rev. 1993, 341–357. [Google Scholar] [CrossRef]
- Chin, S.S.; Chiang, K.; Fane, A.G. The Stability of Polymeric Membranes in a TiO2 Photocatalysis Process. J. Membr. Sci. 2006, 275, 202–211. [Google Scholar] [CrossRef]
- Prulho, R.; Therias, S.; Rivaton, A.; Gardette, J.L. Ageing of Polyethersulfone/Polyvinylpyrrolidone Blends in Contact with Bleach Water. Polym. Degrad. Stab. 2013, 98, 1164–1172. [Google Scholar] [CrossRef]
- Pellegrin, B.; Prulho, R.; Rivaton, A.; Thérias, S.; Gardette, J.L.; Gaudichet-Maurin, E.; Causserand, C. Multi-Scale Analysis of Hypochlorite Induced PES/PVP Ultrafiltration Membranes Degradation. J. Membr. Sci. 2013, 447, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhao, X.; Zhang, H. Aging Retardation Strategy of PVDF Membranes: Evaluation of Free Radical Scavenging Effect of Nano-Particles. New J. Chem. 2021, 45, 6108–6119. [Google Scholar] [CrossRef]
- Lee, M.J.; Ong, C.S.; Lau, W.J.; Ng, B.C.; Ismail, A.F.; Lai, S.O. Degradation of PVDF-Based Composite Membrane and Its Impacts on Membrane Intrinsic and Separation Properties. J. Polym. Eng. 2016, 36, 261–268. [Google Scholar] [CrossRef]
- Ong, C.S.; Lau, W.J.; Al-Anzi, B.; Ismail, A.F. Photodegradation Stability Study of PVDF- and PEI-Based Membranes for Oily Wastewater Treatment Process. Membr. Water Treat. 2017, 8, 211–223. [Google Scholar] [CrossRef]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Matsuura, T.; Puteh, M.H.; Rahman, M.A.; Jaafar, J. Stability Study of Extruded Dual Layer Hollow Fibre Membranes in a Long Operation Photocatalysis Process. Polym. Test. 2018, 68, 53–60. [Google Scholar] [CrossRef]
- Lee, O.O.; Wang, Y.; Tian, R.; Zhang, W.; Shek, C.S.; Bougouffa, S.; Al-Suwailem, A.; Batang, Z.B.; Xu, W.; Wang, G.C.; et al. In Situ Environment Rather than Substrate Type Dictates Microbial Community Structure of Biofilms in a Cold Seep System. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [Green Version]
- Awanis Hashim, N.; Liu, Y.; Li, K. Stability of PVDF Hollow Fibre Membranes in Sodium Hydroxide Aqueous Solution. Chem. Eng. Sci. 2011, 66, 1565–1575. [Google Scholar] [CrossRef]
- Kuhn, K.J.; Hahn, B.; Percec, V.; Urban, M.W. Structural and Quantitative Analysis of Surface Modified Poly(Vinylidene Fluoride) Films Using Atr Ft-Ir Spectroscopy. Appl. Spectrosc. 1987, 41, 843–847. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and Photostabilization of Polymers, Especially Polystyrene: Review. Springerplus 2013, 2, 2021. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Su, Q.; Li, S.; Wen, G.; Huang, T. Aging of PVDF and PES Ultrafiltration Membranes by Sodium Hypochlorite: Effect of Solution PH. J. Environ. Sci. 2021, 104, 444–455. [Google Scholar] [CrossRef]
- Ravereau, J.; Fabre, A.; Brehant, A.; Bonnard, R.; Sollogoub, C.; Verdu, J. Ageing of Polyvinylidene Fluoride Hollow Fiber Membranes in Sodium Hypochlorite Solutions. J. Membr. Sci. 2016, 505, 174–184. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.A.; Choo, K.H.; Lee, C.H.; Lee, H.I.; Hyeon, T.; Choi, W.; Kwon, H.H. Use of Ultrafiltration Membranes for the Separation of TiO2 Photocatalysts in Drinking Water Treatment. Ind. Eng. Chem. Res. 2001, 40, 1712–1719. [Google Scholar] [CrossRef]
- Zhang, T.; Oyama, T.; Aoshima, A.; Hidaka, H.; Zhao, J.; Serpone, N. Photooxidative N-Demethylation of Methylene Blue in Aqueous TiO2 Dispersions under UV Irradiation. J. Photochem. Photobiol. A Chem. 2001, 140, 163–172; [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.M. Photocatalytic Degradation Pathway of Methylene Blue in Water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Kwon, C.H.; Shin, H.; Kim, J.H.; Choi, W.S.; Yoon, K.H. Degradation of Methylene Blue via Photocatalysis of Titanium Dioxide. Mater. Chem. Phys. 2004, 86, 78–82. [Google Scholar] [CrossRef]
- Rauf, M.A.; Meetani, M.A.; Khaleel, A.; Ahmed, A. Photocatalytic Degradation of Methylene Blue Using a Mixed Catalyst and Product Analysis by LC/MS. Chem. Eng. J. 2010, 157, 373–378. [Google Scholar] [CrossRef]
- Noubactep, C. Characterizing the Discoloration of Methylene Blue in Fe0/H2O Systems. J. Hazard. Mater. 2009, 166, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Sies, H. Strategies of Antioxidant Defense. Eur. J. Biochem. 1993, 215, 213–219. [Google Scholar] [CrossRef]
- Kuzmenko, D.; Arkhangelsky, E.; Belfer, S.; Freger, V.; Gitis, V. Chemical Cleaning of UF Membranes Fouled by BSA. Desalination 2005, 179, 323–333. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roubaud, E.; Maréchal, W.; Lorain, O.; Lamaa, L.; Peruchon, L.; Brochier, C.; Mendret, J.; Mericq, J.-P.; Brosillon, S.; Faur, C.; et al. Understanding Aging Mechanisms in the Context of UV Irradiation of a Low Fouling and Self-Cleaning PVDF-PVP-TiO2 Hollow-Fiber Membrane. Membranes 2022, 12, 538. https://doi.org/10.3390/membranes12050538
Roubaud E, Maréchal W, Lorain O, Lamaa L, Peruchon L, Brochier C, Mendret J, Mericq J-P, Brosillon S, Faur C, et al. Understanding Aging Mechanisms in the Context of UV Irradiation of a Low Fouling and Self-Cleaning PVDF-PVP-TiO2 Hollow-Fiber Membrane. Membranes. 2022; 12(5):538. https://doi.org/10.3390/membranes12050538
Chicago/Turabian StyleRoubaud, Emma, William Maréchal, Olivier Lorain, Lina Lamaa, Laure Peruchon, Cédric Brochier, Julie Mendret, Jean-Pierre Mericq, Stephan Brosillon, Catherine Faur, and et al. 2022. "Understanding Aging Mechanisms in the Context of UV Irradiation of a Low Fouling and Self-Cleaning PVDF-PVP-TiO2 Hollow-Fiber Membrane" Membranes 12, no. 5: 538. https://doi.org/10.3390/membranes12050538
APA StyleRoubaud, E., Maréchal, W., Lorain, O., Lamaa, L., Peruchon, L., Brochier, C., Mendret, J., Mericq, J.-P., Brosillon, S., Faur, C., & Causserand, C. (2022). Understanding Aging Mechanisms in the Context of UV Irradiation of a Low Fouling and Self-Cleaning PVDF-PVP-TiO2 Hollow-Fiber Membrane. Membranes, 12(5), 538. https://doi.org/10.3390/membranes12050538








