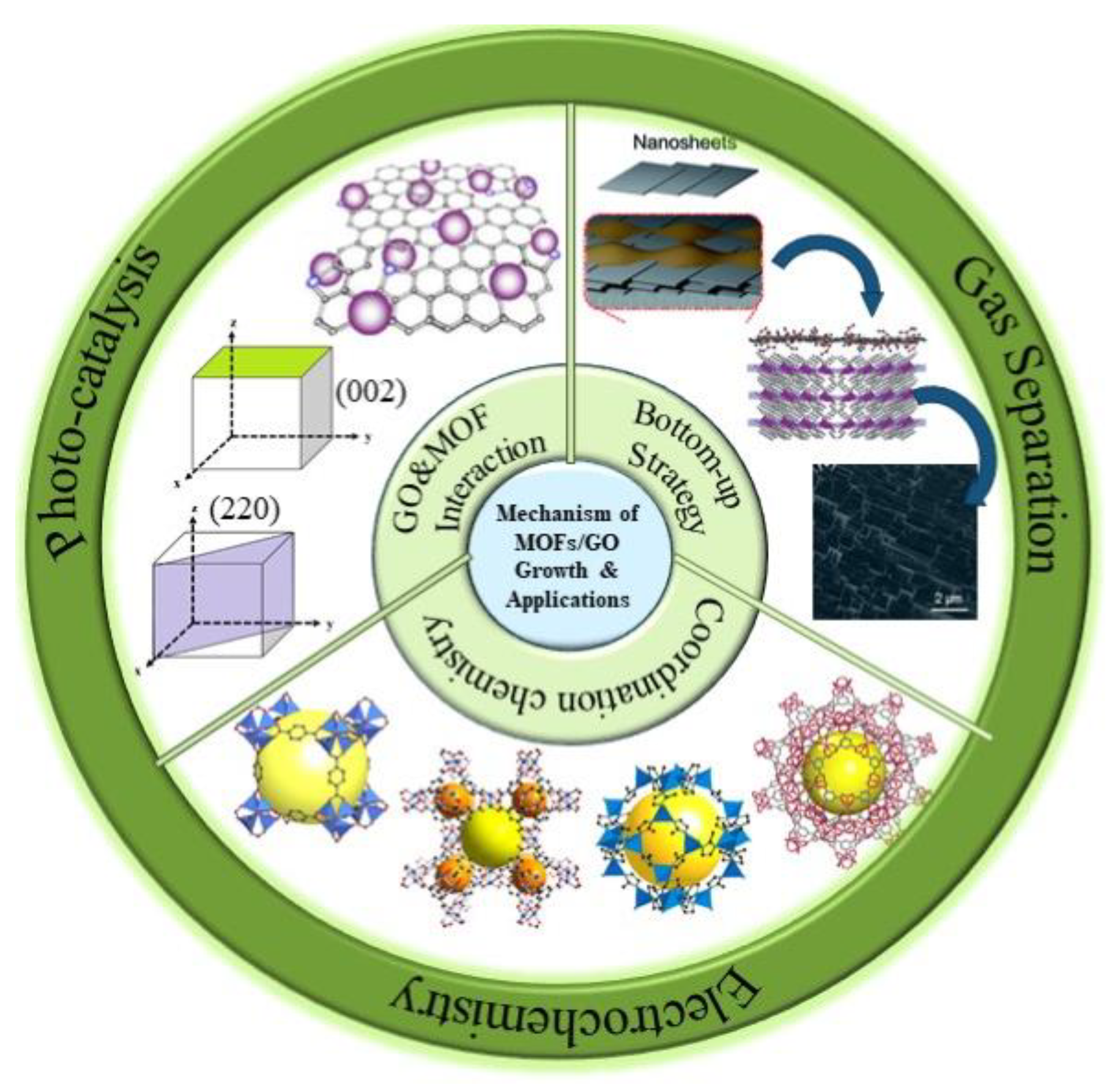
The Growth of Metal–Organic Frameworks in the Presence of Graphene Oxide: A Mini Review
Abstract
:1. Introduction
2. Factors Affecting MOF Growth on GO
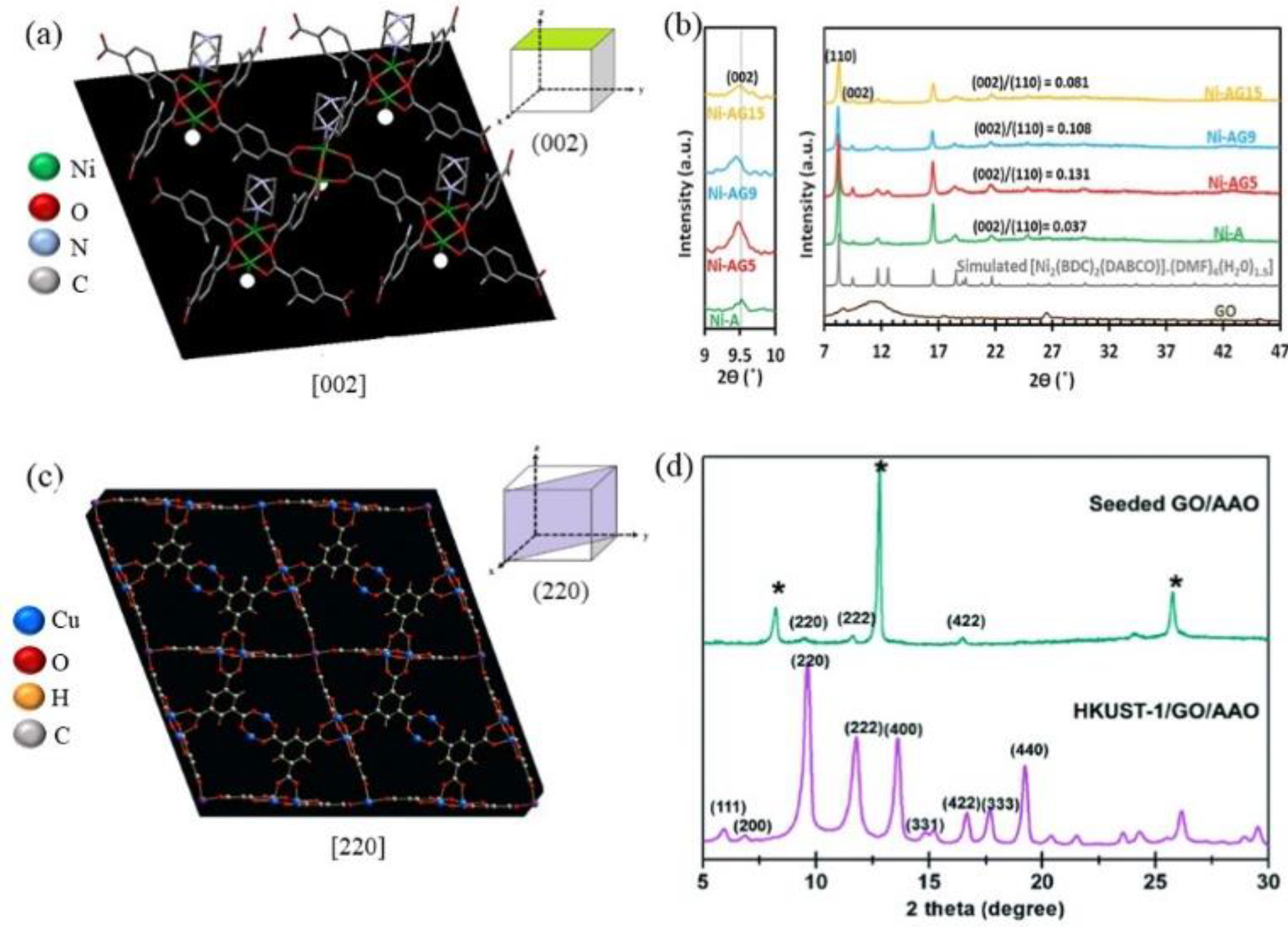
2.1. Interactions between GO Functional Groups and MOF Metal-Binding Sites
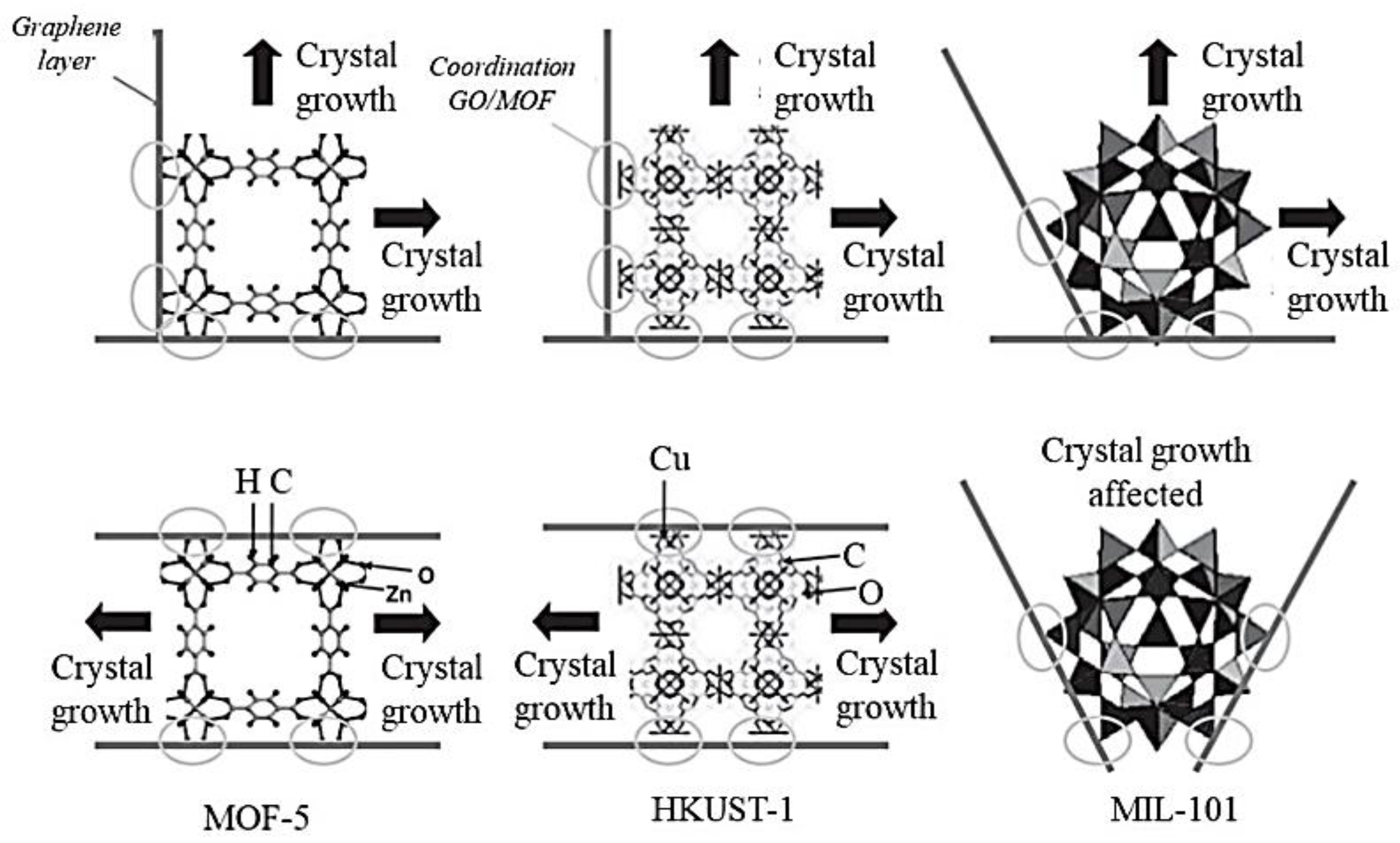
2.2. Metal Coordination Environment in Parental MOFs
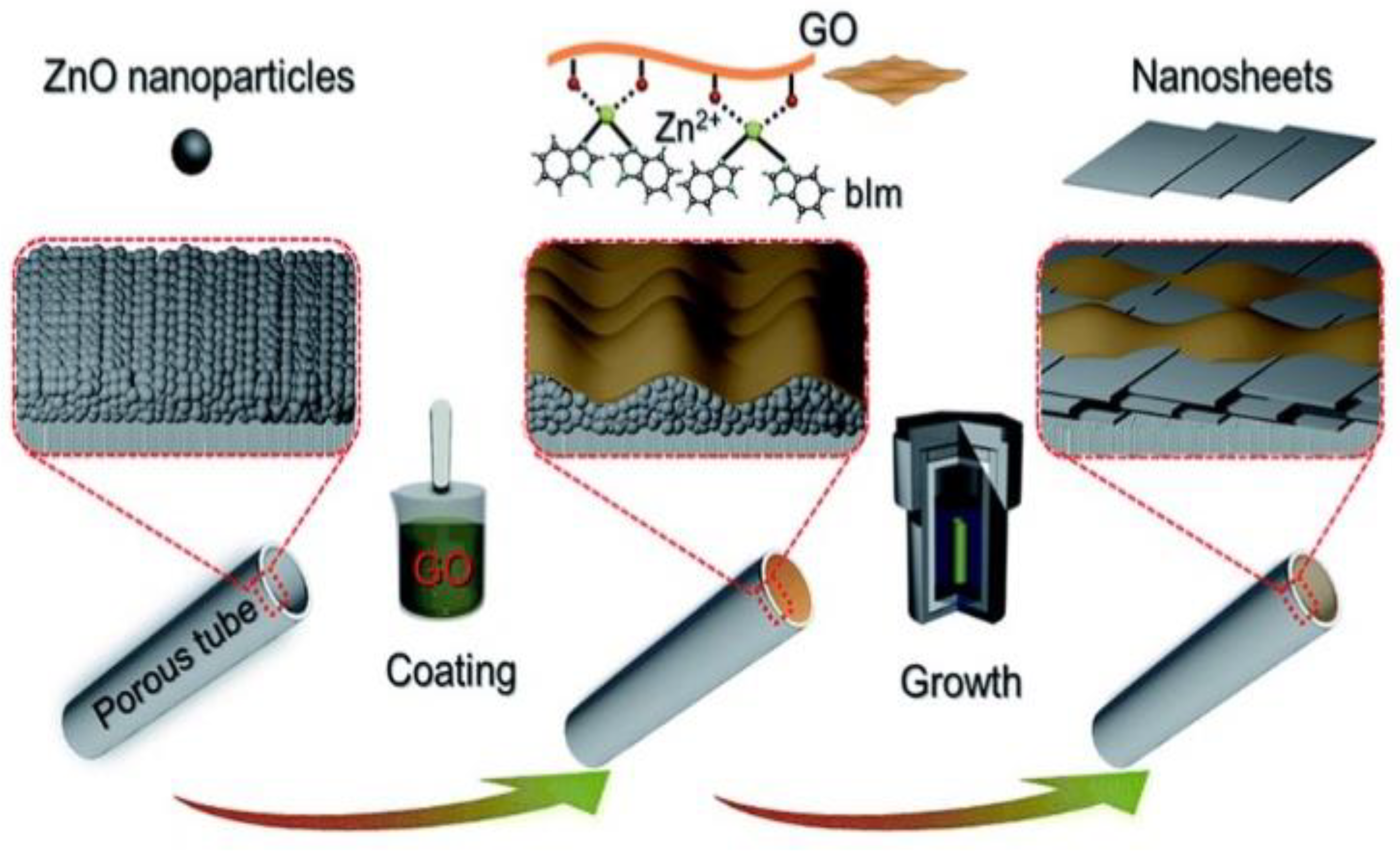
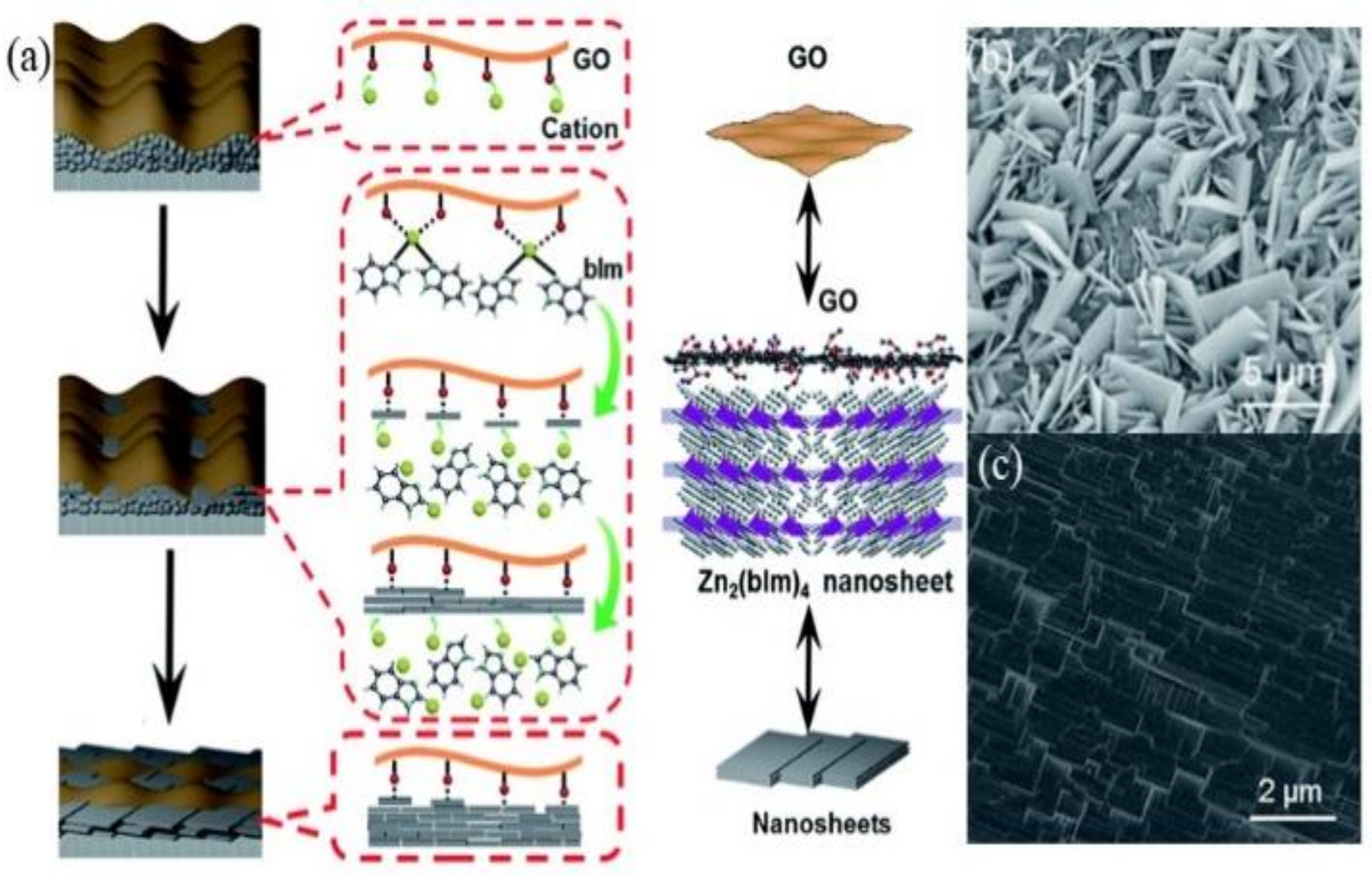
2.3. Bottom-Up Strategy for Highly Oriented MOF Formation
3. Applications of MOF/GO Composites
3.1. Gas Separation
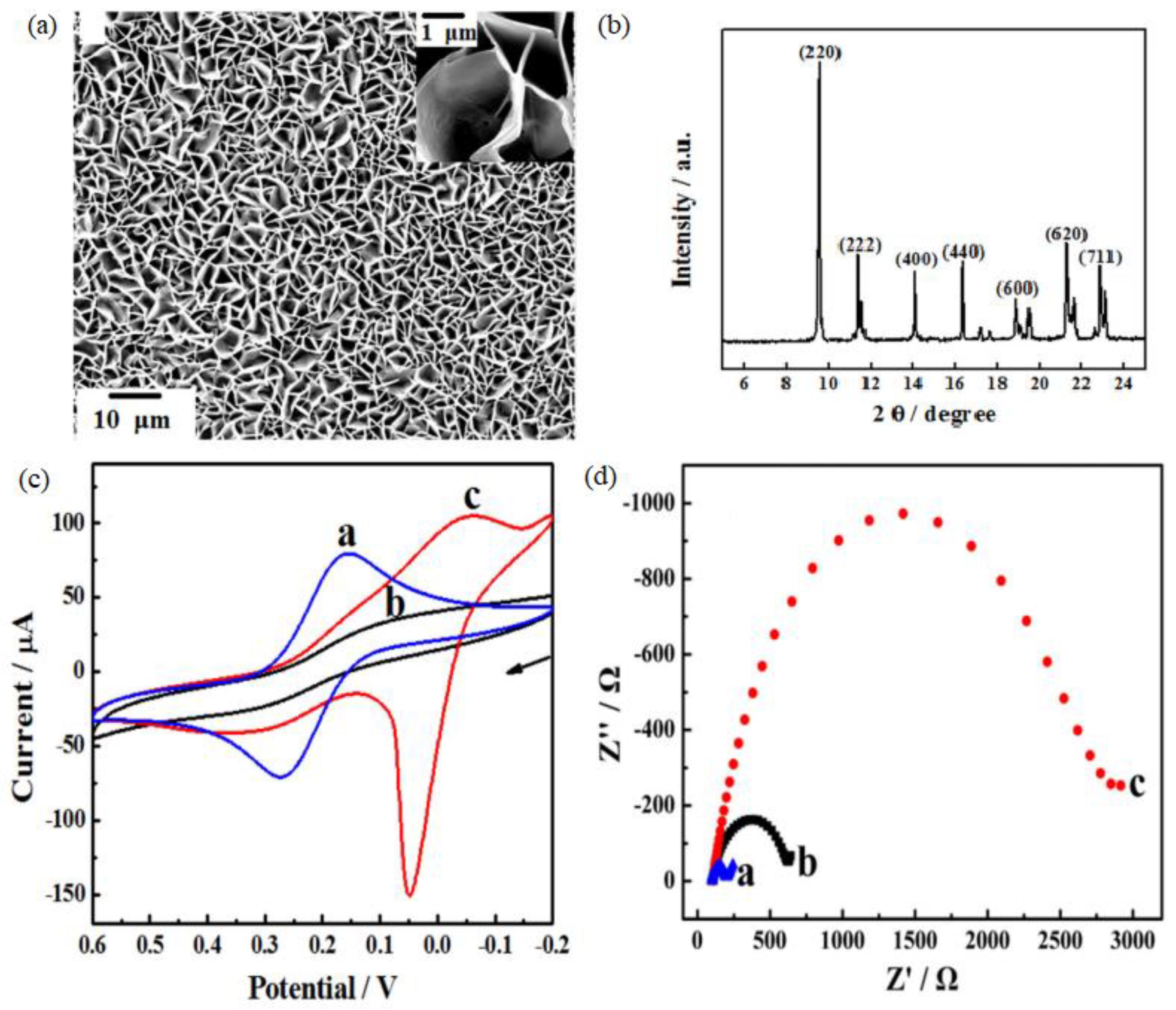
3.2. Electrochemistry
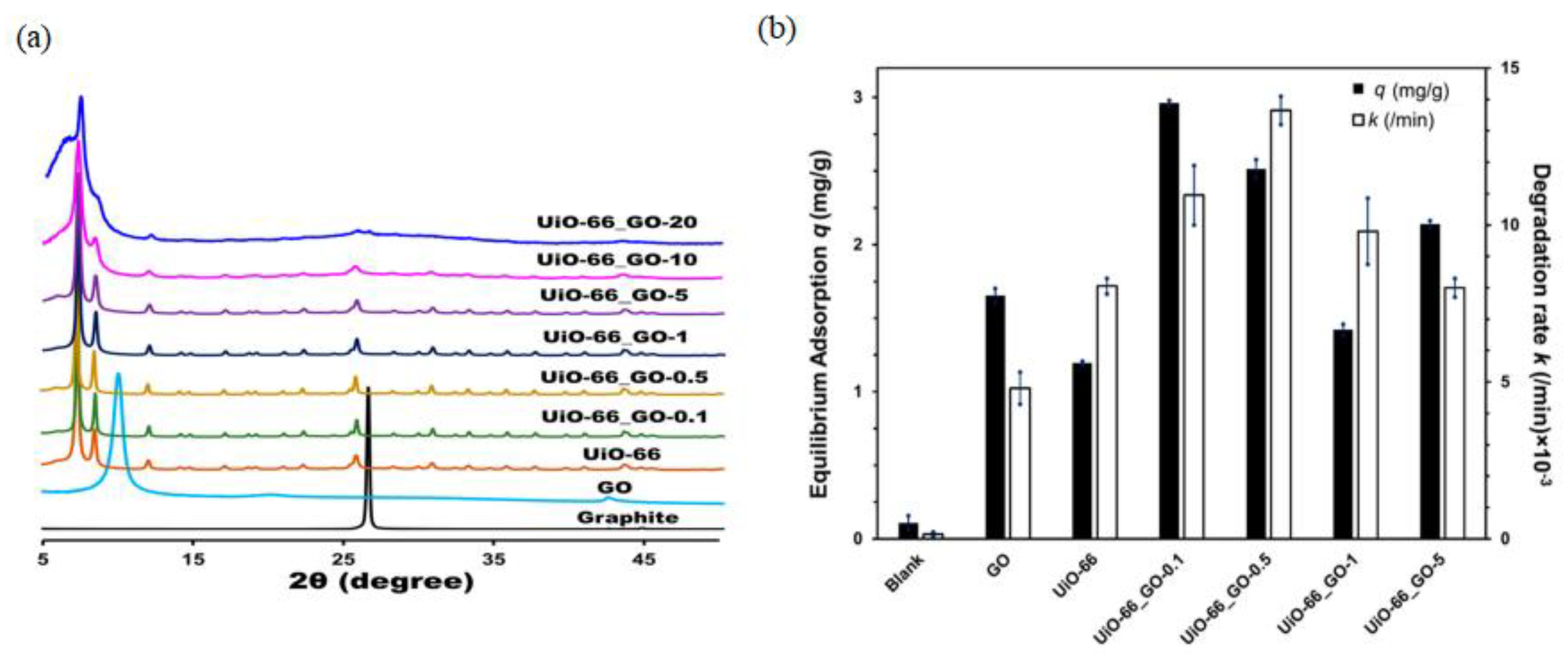
3.3. Photocatalysis
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Gangu, K.K.; Maddila, S.; Mukkamala, S.B.; Jonnalagadda, S.B. A Review on Contemporary Metal-Organic Framework Materials. Inorg. Chim. Acta 2016, 446, 61–74. [Google Scholar] [CrossRef]
- Juan-Alcaniz, J.; Gielisse, R.; Lago, A.B.; Ramos-Fernandez, E.V.; Serra-Crespo, P.; Devic, T.; Guillou, N.; Serre, C. Towards Acid MOFs-Catalytic Performance of Sulfonic Acid Functionalized Architectures. Catal. Sci. Technol. 2013, 3, 2311–2318. [Google Scholar] [CrossRef] [Green Version]
- Abdelhamid, H.N.; Mathew, A.P. Cellulose–Metal-Organic Frameworks (CelloMOFs) Hybrid Materials and Their Multifaceted Applications: A Review. Coord. Chem. Rev. 2022, 451, 214263. [Google Scholar] [CrossRef]
- Behera, P.; Subudhi, S.; Tripathy, S.P.; Parida, K. MOF Derived Nano-Materials: A Recent Progress in Strategic Fabrication, Characterization and Mechanistic Insight towards Divergent Photocatalytic Applications. Coord. Chem. Rev. 2022, 456, 214392. [Google Scholar] [CrossRef]
- Manoj, D.; Rajendran, S.; Hoang, T.K.A.; Soto-Moscoso, M. The Role of MOF Based Nanocomposites in the Detection of Phenolic Compounds for Environmental Remediation- A Review. Chemosphere 2022, 300, 134516. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, C.; Na, J.; Hossain, M.S.A.; Yan, X.; Zhang, H.; Amin, M.A.; Qi, J.; Yamauchi, Y.; Li, J. Macroscopic MOF Architectures: Effective Strategies for Practical Application in Water Treatment. Small 2022, 18, 1–22. [Google Scholar] [CrossRef]
- Ren, J.; Huang, Y.; Zhu, H.; Zhang, B.; Zhu, H.; Shen, S.; Tan, G.; Wu, F.; He, H.; Lan, S.; et al. Recent Progress on MOF-Derived Carbon Materials for Energy Storage. Carbon Energy 2020, 2, 176–202. [Google Scholar] [CrossRef] [Green Version]
- Bandosz, T.J.; Petit, C. MOF/Graphite Oxide Hybrid Materials: Exploring the New Concept of Adsorbents and Catalysts. Adsorption 2011, 17, 5–16. [Google Scholar] [CrossRef]
- Farooqui, U.R.; Ahmad, A.L.; Hamid, N.A. Graphene Oxide: A Promising Membrane Material for Fuel Cells. Renew. Sust. Energ. Rev. 2018, 82, 714–733. [Google Scholar] [CrossRef]
- An, D.; Yang, L.; Wang, T.J.; Liu, B. Separation Performance of Graphene Oxide Membrane in Aqueous Solution. Ind. Eng. Chem. Res. 2016, 55, 4803–4810. [Google Scholar] [CrossRef]
- Liu, R.; Arabale, G.; Kim, J.; Sun, K.; Lee, Y.; Ryu, C.; Lee, C. Graphene Oxide Membrane for Liquid Phase Organic Molecular Separation. Carbon 2014, 77, 933–938. [Google Scholar] [CrossRef]
- Joshi, R.K.; Alwarappan, S.; Yoshimura, M.; Sahajwalla, V.; Nishina, Y. Graphene Oxide: The New Membrane Material. Appl. Mater. Today 2015, 1, 1. [Google Scholar] [CrossRef] [Green Version]
- Petit, C.; Burress, J.; Bandosz, T.J. The Synthesis and Characterization of Copper-Based Metal-Organic Framework/Graphite Oxide Composites. Carbon 2011, 49, 563–572. [Google Scholar] [CrossRef]
- Sue, Y.C.; Wu, J.W.; Chung, S.E.; Kang, C.H.; Tung, K.L.; Wu, K.C.W.; Shieh, F.K. Synthesis of Hierarchical Micro/Mesoporous Structures via Solid-Aqueous Interface Growth: Zeolitic Imidazolate Framework-8 on Siliceous Mesocellular Foams for Enhanced Pervaporation of Water/Ethanol Mixtures. ACS Appl. Mater. Interfaces 2014, 6, 5192–5198. [Google Scholar] [CrossRef] [PubMed]
- Petit, C.; Bandosz, T.J. Exploring the Coordination Chemistry of MOF-Graphite Oxide Composites and Their Applications as Adsorbents. Dalton Trans. 2012, 41, 4027–4403. [Google Scholar] [CrossRef]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H. Preparation of a Novel Antifouling Mixed Matrix PES Membrane by Embedding Graphene Oxide Nanoplates. J. Membr. Sci. 2014, 453, 292–301. [Google Scholar] [CrossRef]
- Li, Y.; Wee, L.H.; Martens, J.A.; Vankelecom, I.F.J. ZIF-71 as a Potential Filler to Prepare Pervaporation Membranes for Bio-Alcohol Recovery. J. Mater. Chem. A 2014, 2, 10034–10040. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Guo, X.; Ying, Y.; Liu, D.; Zhong, C. Composite Ultrafiltration Membrane Tailored by MOF@GO with Highly Improved Water Purification Performance. Chem. Eng. J. 2017, 313, 890–898. [Google Scholar] [CrossRef] [Green Version]
- Petit, C.; Bandosz, T.J. MOF-Graphite Oxide Nanocomposites: Surface Characterization and Evaluation as Adsorbents of Ammonia. J. Mater. Chem. 2009, 19, 6521–6528. [Google Scholar] [CrossRef]
- Petit, C.; Bandosz, T.J. Engineering the Surface of a New Class of Adsorbents: Metal-Organic Framework/Graphite Oxide Composites. J. Colloid Interface Sci. 2015, 447, 139–151. [Google Scholar] [CrossRef]
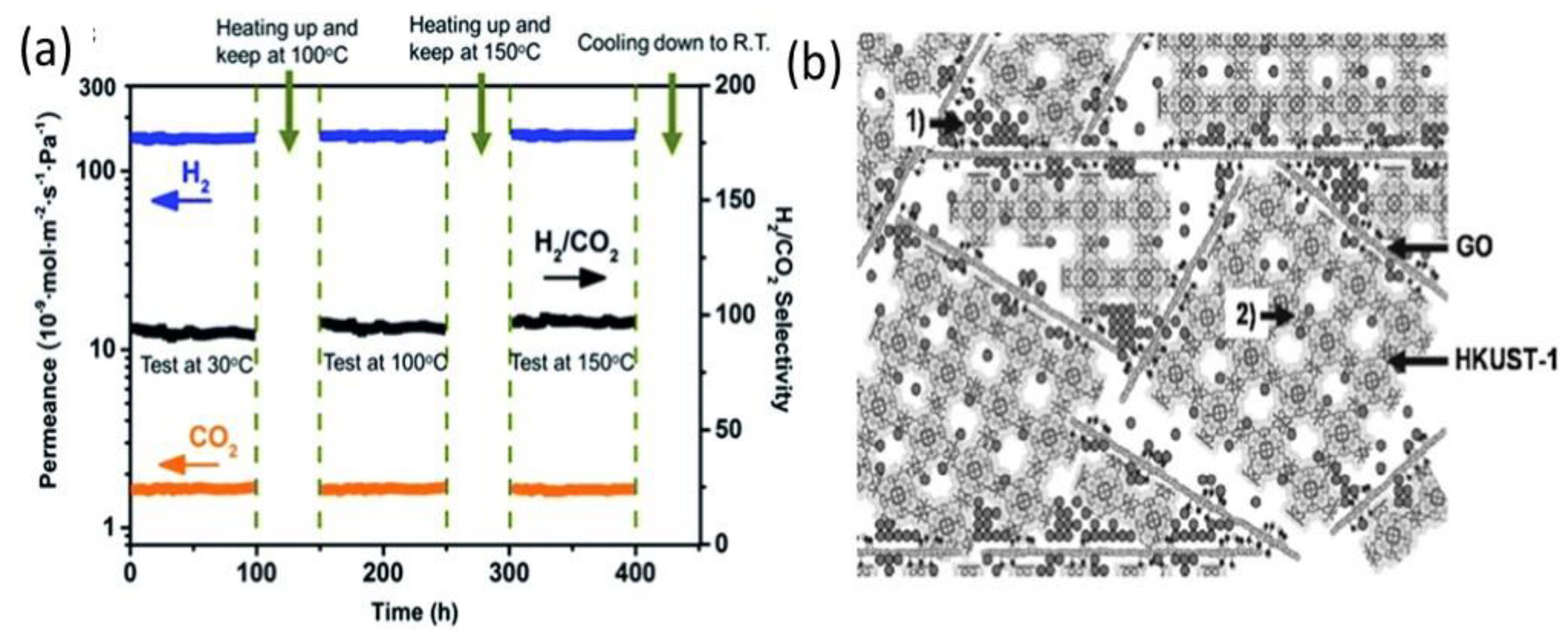
- Li, Y.; Liu, H.; Wang, H.; Qiu, J.; Zhang, X. GO-Guided Direct Growth of Highly Oriented Metal-Organic Framework Nanosheet Membranes for H2/CO2 Separation. Chem. Sci. 2018, 9, 4132–4141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Lin, L.; Tu, M.; Nian, P.; Howarth, A.J.; Farha, O.K.; Qiu, J.; Zhang, X. Growth of ZnO Self-Converted 2D Nanosheet Zeolitic Imidazolate Framework Membranes by an Ammonia-Assisted Strategy. Nano Res. 2018, 11, 1850–1860. [Google Scholar] [CrossRef]
- Nian, P.; Liu, H.; Zhang, X. Bottom-up Fabrication of Two-Dimensional Co-Based Zeolitic Imidazolate Framework Tubular Membranes Consisting of Nanosheets by Vapor Phase Transformation of Co-Based Gel for H2/CO2 Separation. J. Membr. Sci. 2019, 573, 200–209. [Google Scholar] [CrossRef]
- Dong, Y.; Yu, M.; Wang, Z.; Liu, Y.; Wang, X.; Zhao, Z. A Top-Down Strategy toward 3D Carbon Nanosheet Frameworks Decorated with Hollow Nanostructures for Superior Lithium Storage. Adv. Funct. Mater. 2016, 26, 7590–7598. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, C.; Hao, Y.; Ye, B.; Xu, M. Oriented Growth of Cross-Linked Metal-Organic Framework Film on Graphene Surface for Non-Enzymatic Electrochemical Sensor of Hydrogen Peroxide in Disinfectant. Talanta 2018, 188, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Q.; Chen, Y.; Lv, S.; Liao, X.; Yao, Y. Size Effects of Ag Nanoparticle for N2 Photofixation over Ag/g-C3N4: Built-in Electric Fields Determine Photocatalytic Performance. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127053. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, L.; Tan, X.; Sang, X.; Zhang, J.; Liu, C.; Zhang, B.; Han, B.; Yang, G. Pickering Emulsions Stabilized by a Metal-Organic Framework ( MOF ) and Graphene Oxide ( GO ) for Producing MOF / GO Composites. Soft Matter. 2017, 13, 7365–7370. [Google Scholar] [CrossRef]
- Yang, S.; Zou, Q.; Wang, T.; Zhang, L. Effects of GO and MOF@ GO on the Permeation and Antifouling Properties of Cellulose Acetate Ultrafiltration Membrane. J. Membr. Sci. 2019, 569, 48–59. [Google Scholar] [CrossRef]
- Al-Naddaf, Q.; Al-Mansour, M.; Thakkar, H.; Rezaei, F. MOF-GO Hybrid Nanocomposite Adsorbents for Methane Storage. Ind. Eng. Chem. Res. 2018, 57, 17470–17479. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Y.; Gao, F.; Ni, J.; Zhang, Y.; Lin, Z. Graphene Oxide Directed One-Step Synthesis of Flowerlike Graphene @ HKUST—1 for Enzyme-Free Detection of Hydrogen Peroxide in Biological Samples. ACS Appl. Mater. Interfaces 2016, 8, 32477–32487. [Google Scholar] [CrossRef]
- Jahan, M.; Bao, Q.; Yang, J.X.; Loh, K.P. Structure-Directing Role of Graphene in the Synthesis of Metal-Organic Framework Nanowire. J. Am. Chem. Soc. 2010, 132, 14487–14495. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lv, D.; Wu, J.; Xiao, J.; Xi, H.; Xia, Q.; Li, Z. A New MOF-505@GO Composite with High Selectivity for CO2/CH4 and CO2/N2 Separation. Chem. Eng. J. 2017, 308, 1065–1072. [Google Scholar] [CrossRef]
- Yang, C.; Wu, S.; Cheng, J.; Chen, Y. Indium-Based Metal-Organic Framework/Graphite Oxide Composite as an Efficient Adsorbent in the Adsorption of Rhodamine B from Aqueous Solution. J. Alloys Compd. 2016, 687, 804–812. [Google Scholar] [CrossRef]
- Sun, X.; Xia, Q.; Zhao, Z.; Li, Y.; Li, Z. Synthesis and Adsorption Performance of MIL-101(Cr)/Graphite Oxide Composites with High Capacities of n-Hexane. Chem. Eng. J. 2014, 239, 226–232. [Google Scholar] [CrossRef]
- Daraee, M.; Ghasemy, E.; Rashidi, A. Synthesis of Novel and Engineered UiO-66/Graphene Oxide Nanocomposite with Enhanced H2S Adsorption Capacity. J. Environ. Chem. Eng. 2020, 8, 104351. [Google Scholar] [CrossRef]
- Tatykayev, B.; Donat, F.; Alem, H.; Balan, L.; Medjahdi, G.; Uralbekov, B.; Schneider, R. Synthesis of Core/Shell ZnO/RGO Nanoparticles by Calcination of ZIF-8/RGO Composites and Their Photocatalytic Activity. ACS Omega 2017, 2, 4946–4954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petit, C.; Bandosz, T.J. MOF-Graphite Oxide Composites: Combining the Uniqueness of Graphene Layers and Metal-Organic Frameworks. Adv. Mater. 2009, 21, 4753–4757. [Google Scholar] [CrossRef]
- Pokhrel, J.; Bhoria, N.; Wu, C.; Kumar, K.S.; Margetis, H.; Anastasiou, S.; George, G.; Mittal, V.; Romanos, G.; Karonis, D. Cu- and Zr-Based Metal Organic Frameworks and Their Composites with Graphene Oxide for Capture of Acid Gases at Ambient Temperature. J. Solid State Chem. 2018, 266, 233–243. [Google Scholar] [CrossRef]
- Asgharnejad, L.; Abbasi, A.; Shakeri, A. Ni-Based Metal-Organic Framework/GO Nanocomposites as Selective Adsorbent for CO2 over N2. Microporous Mesoporous Mater. 2018, 262, 227–234. [Google Scholar] [CrossRef]
- Liu, L.; Yan, Y.; Cai, Z.; Lin, S.; Hu, X. Growth-Oriented Fe-based MOFs Synergized with Graphene Aerogels for High-Performance Supercapacitors. Adv. Mater. Interfaces 2018, 5, 1701548. [Google Scholar] [CrossRef]
- Liu, S.; Sun, L.; Xu, F.; Zhang, J.; Jiao, C.; Li, F.; Li, Z.; Wang, S.; Wang, Z.; Jiang, X.; et al. Nanosized Cu-MOFs induced by graphene oxide and enhanced gas storage capacity. Energy Environ. Sci. 2013, 6, 818–822. [Google Scholar] [CrossRef]
- Gu, Z.G.; Zhang, J. Epitaxial Growth and Applications of Oriented Metal-Organicamework Thin Films. Coord. Chem. Rev. 2019, 378, 513–532. [Google Scholar] [CrossRef]
- Kim, D.; Coskun, A. Graphene Oxide-Templated Preferential Growth of Continuous MOF Thin Films. CrystEngComm 2016, 18, 4013–4017. [Google Scholar] [CrossRef]
- Zhuang, J.L.; Terfort, A.; Wöll, C. Formation of Oriented and Patterned Films of Metal-Organic Frameworks by Liquid Phase Epitaxy: A Review. Coord. Chem. Rev. 2016, 307, 391–424. [Google Scholar] [CrossRef]
- Petit, C.; Bandosz, T.J. Synthesis, Characterization, and Ammonia Adsorption Properties of Mesoporous Metal-Organic Framework (MIL(Fe))-Graphite Oxide Composites: Exploring the Limits of Materials Fabrication. Adv. Funct. Mater. 2011, 21, 2108–2117. [Google Scholar] [CrossRef]
- Shi, X.; Yu, J.; Liu, Q.; Shao, L.; Zhang, Y.; Sun, Z.; Huang, H. Metal-Organic-Framework-Derived N-, P-, and O-Codoped Nickel/Carbon Composites Homogeneously Decorated on Reduced Graphene Oxide for Energy Storage. ACS Appl. Nano Mater. 2020, 3, 5625–5636. [Google Scholar] [CrossRef]
- Xia, W.; Mahmood, A.; Zou, R.; Xu, Q. Metal-Organic Frameworks and Their Derived Nanostructures for Electrochemical Energy Storage and Conversion. Energy Environ. Sci. 2015, 8, 1837–1866. [Google Scholar] [CrossRef]
- Hong, J.; Park, S.J.; Kim, S. Synthesis and Electrochemical Characterization of Nanostructured Ni-Co-MOF/Graphene Oxide Composites as Capacitor Electrodes. Electrochim. Acta 2019, 311, 62–71. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, N.; Liang, Y. Preparation of CeO2/Cu-MOF/GO Composite for Efficient Electrocatalytic Oxygen Evolution Reaction. Ionics 2021, 27, 4347–4360. [Google Scholar] [CrossRef]
- Teixeira, M.; Campo, M.C.; Pacheco Tanaka, D.A.; Llosa Tanco, M.A.; Magen, C.; Mendes, A. Composite Phenolic Resin-Based Carbon Molecular Sieve Membranes for Gas Separation. Carbon 2011, 49, 4348–4358. [Google Scholar] [CrossRef]
- Centeno, T.A.; Fuertes, A.B. Carbon Molecular Sieve Gas Separation Membranes Based on Poly(Vinylidene Chloride-Co-Vinyl Chloride). Carbon 2000, 38, 1067–1073. [Google Scholar] [CrossRef]
- He, L.; Pan, Y.; Wang, T.; Yu, L. Molecular Simulation and Optimization on the Microporous Structure in Carbon Molecular Sieve Membrane for CO2/CH4 Separation. Chem. Phys. Lett. 2020, 738, 136910. [Google Scholar] [CrossRef]
- Hazazi, K.; Ma, X.; Wang, Y.; Ogieglo, W.; Alhazmi, A.; Han, Y.; Pinnau, I. Ultra-Selective Carbon Molecular Sieve Membranes for Natural Gas Separations Based on a Carbon-Rich Intrinsically Microporous Polyimide Precursor. J. Membr. Sci. 2019, 585, 1–9. [Google Scholar] [CrossRef]
- Fu, Y.J.; Liao, K.S.; Hu, C.C.; Lee, K.R.; Lai, J.Y. Development and Characterization of Micropores in Carbon Molecular Sieve Membrane for Gas Separation. Microporous Mesoporous Mater. 2011, 143, 78–86. [Google Scholar] [CrossRef]
- Ma, X.; Swaidan, R.; Teng, B.; Tan, H.; Salinas, O.; Litwiller, E.; Han, Y.; Pinnau, I. Carbon Molecular Sieve Gas Separation Membranes Based on an Intrinsically Microporous Polyimide Precursor. Carbon 2013, 62, 88–96. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, H.; Zhang, S.; Zhang, F.; Jin, J. Carbon Molecular Sieve Membranes Derived from TrÖger’s Base-Based Microporous Polyimide for Gas Separation. ChemSusChem 2018, 11, 916–923. [Google Scholar] [CrossRef]
- Tseng, H.H.; Itta, A.K. Modification of Carbon Molecular Sieve Membrane Structure by Self-Assisted Deposition Carbon Segment for Gas Separation. J. Membr. Sci. 2012, 389, 223–233. [Google Scholar] [CrossRef]
- Petit, C.; Mendoza, B.; Bandosz, T.J. Hydrogen Sulfide Adsorption on MOFs and MOF/Graphite Oxide Composites. ChemPhysChem 2010, 11, 3678–3684. [Google Scholar] [CrossRef]
- Petit, C.; Mendoza, B.; Bandosz, T.J. Reactive Adsorption of Ammonia on Cu-based MOF/Graphene Composites. Langmuir 2010, 26, 15302–15309. [Google Scholar] [CrossRef] [Green Version]
- Hu, A.; Pang, Q.; Tang, C.; Bao, J.; Liu, H.; Ba, K.; Xie, S.; Chen, J.; Chen, J.; Yue, Y.; et al. Epitaxial Growth and Integration of Insulating Metal-Organic Frameworks in Electrochemistry. J. Am. Chem. Soc. 2019, 141, 11322–11327. [Google Scholar] [CrossRef]
- Heu, R.; Ateia, M.; Yoshimura, C.; Awfa, D.; Punyapalakul, P. Photocatalytic Degradation of Organic Micropollutants in Water by Zr-Mof/GO Composites. J. Compos. Sci. 2020, 4, 54. [Google Scholar] [CrossRef]
- Shan, B.; James, J.B.; Armstrong, M.R.; Close, E.C.; Letham, P.A.; Nikkhah, K.; Lin, Y.S.; Mu, B. Influences of Deprotonation and Modulation on Nucleation and Growth of UiO-66: Intergrowth and Orientation. J. Phys. Chem. C 2018, 122, 2200–2206. [Google Scholar] [CrossRef]
- Vu, H.T.; Tran, L.T.; Le, G.H.; Nguyen, Q.K.; Vu, T.M.; Vu, T.A. Synthesis and Application of Novel Fe-MIL-53/GO Nanocomposite for Photocatalytic Degradation of Reactive Dye from Aqueous Solution. Vietnam J. Chem. 2019, 57, 681–685. [Google Scholar] [CrossRef]
- Chaturvedi, G.; Kaur, A.; Kansal, S.K. CdS-Decorated MIL-53(Fe) Microrods with Enhanced Visible Light Photocatalytic Performance for the Degradation of Ketorolac Tromethamine and Mechanism Insight. J. Phys. Chem. C 2019, 123, 16857–16867. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, H.; Wang, H. Synthesis of Iron(III)-Based Metal-Organic Framework/Graphene Oxide Composites with Increased Photocatalytic Performance for Dye Degradation. RSC Adv. 2014, 4, 40435–40438. [Google Scholar] [CrossRef]
- Liao, X.; Wang, F.; Wang, Y.; Wei, W.; Xiao, Z.; Liu, H.; Hao, Q.; Lu, S.; Li, Z. Functionalized g-C3N4 Sheets Assisted Synthesis of Growth-Oriented MIL-88B-Fe with Rod-like Structure: Upgrading Framework Photo-Catalytic Performance and Stability. Appl. Surf. Sci. 2020, 503, 144089. [Google Scholar] [CrossRef]
- Fu, Z.; Xu, G. Crystalline, Highly Oriented MOF Thin Film: The Fabrication and Application. Chem. Rec. 2017, 17, 518–534. [Google Scholar] [CrossRef]
- Pambudi, F.I.; Anderson, M.W.; Att, M.P. Crystal Growth of the Core and Rotated Epitaxial Shell of a Heterometallic Metal-Organic Framework Revealed with Atomic Force Microscopy. Faraday Discuss. 2021, 231, 112–126. [Google Scholar] [CrossRef]
- Bonakala, S.; Lalitha, A.; Shin, J.E.; Moghadam, F.; Semino, R.; Park, H.B.; Maurin, G. Understanding of the Graphene Oxide/Metal-Organic Framework Interface at the Atomistic Scale. ACS Appl. Mater. Interfaces 2018, 10, 33619–33629. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazlan, N.A.; Butt, F.S.; Lewis, A.; Yang, Y.; Yang, S.; Huang, Y. The Growth of Metal–Organic Frameworks in the Presence of Graphene Oxide: A Mini Review. Membranes 2022, 12, 501. https://doi.org/10.3390/membranes12050501
Mazlan NA, Butt FS, Lewis A, Yang Y, Yang S, Huang Y. The Growth of Metal–Organic Frameworks in the Presence of Graphene Oxide: A Mini Review. Membranes. 2022; 12(5):501. https://doi.org/10.3390/membranes12050501
Chicago/Turabian StyleMazlan, Nurul A., Fraz Saeed Butt, Allana Lewis, Yaohao Yang, Shuiqing Yang, and Yi Huang. 2022. "The Growth of Metal–Organic Frameworks in the Presence of Graphene Oxide: A Mini Review" Membranes 12, no. 5: 501. https://doi.org/10.3390/membranes12050501
APA StyleMazlan, N. A., Butt, F. S., Lewis, A., Yang, Y., Yang, S., & Huang, Y. (2022). The Growth of Metal–Organic Frameworks in the Presence of Graphene Oxide: A Mini Review. Membranes, 12(5), 501. https://doi.org/10.3390/membranes12050501






