Progress in the Degradability of Biodegradable Film Materials for Packaging
Abstract
:1. Introduction
2. Degradation Mechanism of Degradable Packaging Film Materials
2.1. Photodegradation
2.2. Hydrodegradation
| Material | Conditions | Weight Loss % | Number-Average Molecular Weight (Mn) | Mechanical Properties |
|---|---|---|---|---|
| Polylactic acid (PLA) | Seawater | <2 | 96.60 × 103 to 83.85 × 103 | No significant change |
| Germicidal water | <2 | 96.60 × 103 to 67.98 × 103 | ||
| Poly (butyleneadipate-co-terephthalate) (PBAT) | Seawater | <2 | 46.67 × 103 to 20.31 × 103 | Total loss |
| Germicidal water | <2 | 46.67 × 103 to 16.02 × 103 | ||
| Poly (butylene succinate) (PBS) | Seawater | <2 | 41.56 × 103 to 30.11 × 103 | Total loss |
| Germicidal water | <2 | 41.56 × 103 to 18.63 × 103 | ||
| Polycaprolactone (PCL) | Seawater | 32 | 77.79 × 103 to 77.09 × 103 | Total loss |
| Germicidal water | <2 | 77.79 × 103 to 14.82 × 103 |
2.3. Thermal Oxidative Degradation
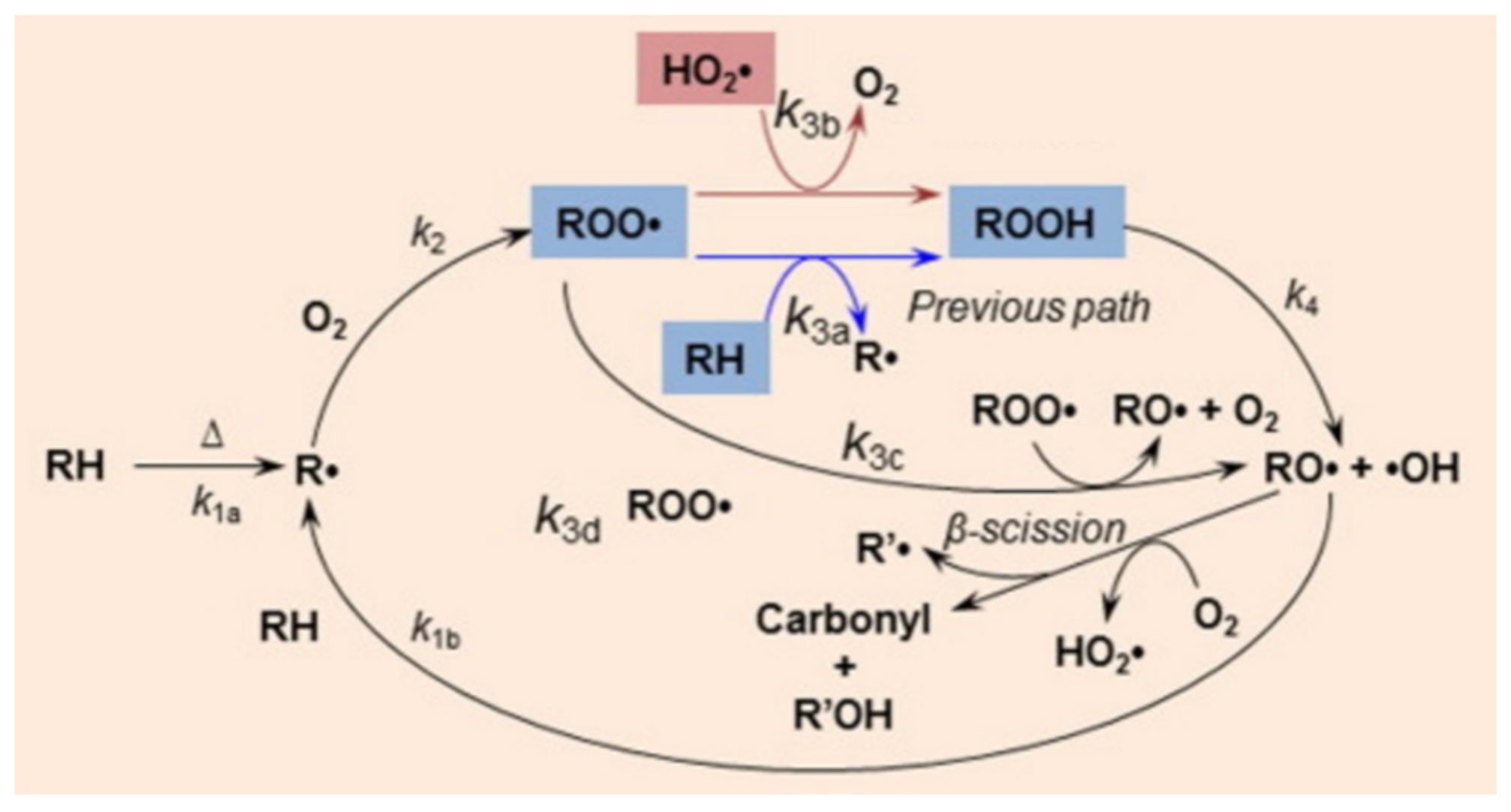
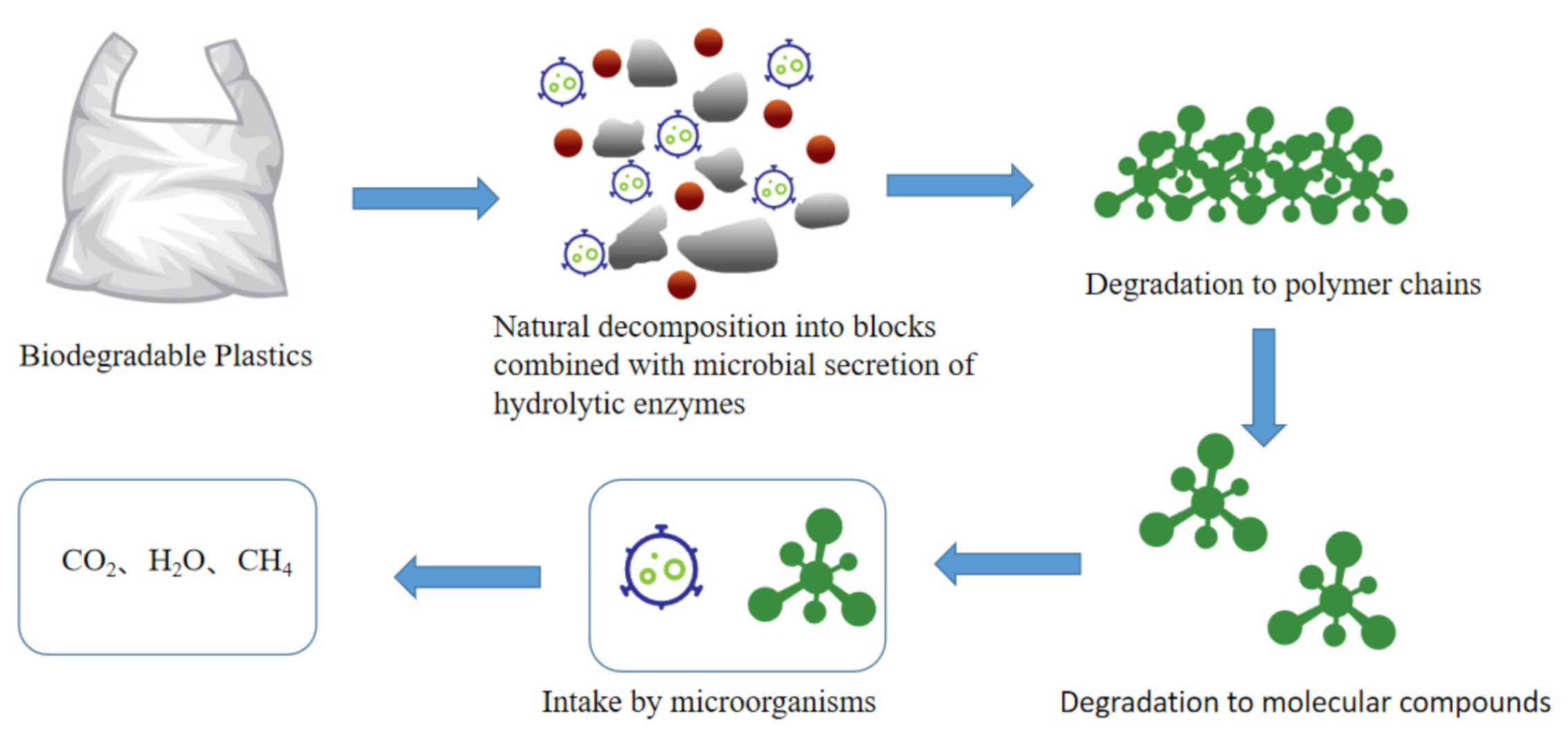
2.4. Biodegradable
| Material | Conditions | The Result of Degradation | References |
|---|---|---|---|
| Polyethylene | Degradation of high-density polyethylene with Aspergillus flavus PEDX3 strain for 28 days | Molecular weight reduction | [76] |
| Polypropylene | Degradation of polypropylene with microalgae Spirulina sp. for 112 days | Decrease in mechanical strength and relative molecular weight | [77] |
| Polystyrene | Degradation of polystyrene with Achatina fulica for 4 weeks | The mass loss was 30.7% on average, forming a functional group of oxidation intermediates | [78] |
| Polyethylene terephthalate | Degradation of polyethylene terephthalate with microalgae Spirulina sp. for 112 days | Decrease in mechanical strength | [77] |
| Polylactic acid | Degradation in accordance with ISO 17556 | 15% of Polylactic acid is degraded | [79] |
3. Biodegradable Film Materials
3.1. Natural Polymer-Based Films
3.1.1. Starch-Based Film Materials
| Modification | Conditions | Result | References |
|---|---|---|---|
| Blending with other polymers | Modified starch-based film materials with natural fibers in blends | Tensile strength and modulus of elasticity were improved, but the elongation at break was not as good as that of ordinary starch-based films | [92] |
| Blending with other polymers | Modified barley hulls (BH) by grafting palmitic acid and then blended with cross-linked polyvinyl alcohol (PVA)/starch | The physical properties of the composite film could be effectively improved, and the air and water resistance were substantially enhanced | [93] |
| Surface modification | Acetylated corn starch (AS), acetylated sugarcane fiber (AcSF) and glycerol were used to make biodegradable film materials | Mechanical properties and water resistance have been improved | [87] |
| Blending with reinforcement fillers | Different contents of metakaolin were blended with cassava starch to make film materials | The mechanical tensile strength and properties increased significantly and the elongation at break decreased | [91] |
3.1.2. Cellulose-Based Film Materials
| Material | Conditions | The Result of Degradation | References |
|---|---|---|---|
| Cellulose acetate (CA) | The film material was produced by mixing CA, sodium alginate (SA) and carrageenan (CG) by solution casting method | The tensile strength, thermal stability and antimicrobial activity of the films were improved | [102] |
| Nanocellulose (NC) | Nanocellulose is used as filler for melt blending and blown film with PLA | The mechanical strength, crystallinity and wettability are improved | [103] |
| Cellulose nanocrystals (CNC)/ Carboxymethyl cellulose (CMC) | CMC films containing various contents of CNC were prepared by solution casting method | Compared with pure CMC films, CMC/CNC composite films have better UV barrier, mechanical strength, water vapor barrier and thermal stability | [104] |
| Ethyl cellulose (EC) | Preparation of PVA/EC/tea polyphenol (TP) nanofiber films by blending electrospinning technique | The thermal stability, surface hydrophobicity, water resistance, water vapor barrier capacity and tensile properties of the composite nanofiber films were improved | [105] |
3.1.3. Chitosan-Based Film Materials
| Modification | Conditions | Result | References |
|---|---|---|---|
| Cross-linking | Preparation of a chitosan/bacterial cellulose membrane treated by multiple cross-linking methods | Mechanical strength and elongation at break increase, but its antimicrobial efficiency decreases | [112] |
| Graft copolymerization | Chitosan (CS) was grafted with caffeic acid (CA-g-CS) through carbodiimide coupling and cast into films | CA-g-CS films have higher tensile strength, elongation at break and oxidation activity, and better barrier properties to water vapor and oxygen | [114] |
| Blending with reinforcement fillers | Nickel oxide nanoparticles (NiONPs) were doped into chitosan-based films to fabricate composite films | The composite film has improved water resistance, tensile strength, thermal properties and surface hydrophobicity, and has ideal photocatalytic and antibacterial activity | [116] |
| Blending with other polymers | Biodegradable chitosan-based film containing micro ramie fiber and lignin was prepared by the casting method | Significant improvement in mechanical, water resistance, thermal and oxidation resistance properties | [117] |
3.2. Petroleum-Based Film Materials
3.2.1. Poly (Butylene Succinate) Film Materials
| Modification | Conditions | Result | References |
|---|---|---|---|
| Blending with other polymers | The PBS and plasticized whey protein (PWP) blend makes the film | Significant increase in modulus of elasticity, tensile strength and elongation at break | [124] |
| Blending with other polymers | Preparation of PCL/PBS co-blended film by immersion precipitation | Improved hydrophilicity and biodegradability, in addition to higher pollution inhibition index | [127] |
| Synthetic copolymers | Synthetic poly (butylene succinate-co-diethylene glycol succinate) (P(BS-co-DEGS)) copolymer | Crystallinity, tensile modulus, thermal stability slightly reduced and water degradation rate increased. | [129] |
| Blending with reinforcement fillers | Preparation of PBS/graphene nanoplatelets (GnP) nanocomposites | Improved barrier properties to water and oxygen | [131] |
3.2.2. Poly (Butyleneadipate-co-Terephthalate) Film Materials
| Modification | Conditions | Result | References |
|---|---|---|---|
| Blending with reinforcement fillers | Starch/PBAT nanocomposite films with high starch content were prepared by extrusion blow molding | Significant increase in mechanical strength, flexibility and hydrophobicity | [141] |
| Blending with reinforcement fillers | Preparation of PBAT/lignin composite films by extrusion hot-pressing | Significantly improved flexibility and mechanical properties | [140] |
| Blending with other polymers | Compression molded biodegradable films based on PBS and PBAT at varying weights were prepared | Elongation at break increased with increasing PBAT content, and gas barrier properties decreased with increasing PBS content. | [143] |
| Blending with reinforcement fillers | Preparation of PBAT/TiO2 biodegradable films | The addition of TiO2 leads to the improvement of the overall barrier properties, thermal stability and tensile strength of PBAT composite film materials, but its elongation at break decreases | [142] |
3.2.3. Polycaprolactone Film Materials
| Modification | Conditions | Result | References |
|---|---|---|---|
| Cross-linking | Polycaprolactone (PCL) was cross-linked by adding different amounts of organic peroxides, such as di-(2-tert-butylperoxyisopropyl)-benzene (BIB) | PCL branching and cross-linking have significant effects on the mechanical properties of PCL 0.5 pbw (part by weight) BIB-modified PCL has better mechanical properties, and higher BIB content can lead to degradation and excessive cross-linking of PCL | [152] |
| Compound modification | Prepared PCL/polyvinyl chloride (PVC)/organoclay nanobioblends film | Enhanced mechanical and barrier properties, exhibiting some antibacterial activity | [153] |
| Blending with other polymers | PCL/PLA is mixed and green tea extract (GTE) is used as an antioxidant to make the film | Reduced hydrophilicity and enhanced barrier and mechanical properties | [154] |
3.3. Bio-Based Film Materials
3.3.1. Polyhydroxyalkanoates Film Materials
| Modification | Conditions | Result | References |
|---|---|---|---|
| Copolymerization modification | Four cross-linkers (citric acid, adipic acid, borax and boric acid) with polycarboxyl or polyhydroxy structures were used in the preparation of the starch/polyhydroxyalkanoate (PHA) films | With higher relative crystallinity, but hinders the formation of intercalation structures in the polymer matrix, improving light transmission and barrier properties | [163] |
| Blending with reinforcement fillers | Lignin nanoparticles homogeneously dispersed in poly-β-hydroxybutyric acid (PHB) matrix to form nanocomposites with improved properties using oil-in-water emulsion method | Improved mechanical properties, lower crystallinity, higher glass transition temperature and better barrier properties | [164] |
| Compound modification | Preparation of PHA/PLA nanocomposite films under different levels of montmorillonite | Better thermal stability and electrical conductivity | [165] |
3.3.2. Polylactic Acid Film Materials

| Modification | Conditions | Result | References |
|---|---|---|---|
| Blending with reinforcement fillers | Add bamboo cellulose nanowhiskers (BCNW) to PLA as a filler and make a film by solution casting method | Mechanical properties, glass transition temperature, cold crystallinity increase and microcrystal size increase significantly | [180] |
| Compound modification | Introduction of glass fibers (GF) modified with silane coupling agent (GF-S) into PLA to make PLA-based composites | Improved mechanical and thermodynamic properties | [179] |
| Blending with reinforcement fillers | Halloysite nanotubes (HNT) and chitosan as fillers were blended with PLA to make films | Mechanical strength and mechanical properties have been improved, with excellent barrier to water and UV light, and some antibacterial ability | [166] |
| Blending with other polymers | Cinnamic acid (CA)/PLA films obtained by casting or thermal processing | Greatly improves the mechanical properties of the film and improves the barrier to oxygen and water | [182] |
| copolymerization modification | PLA is blended with polydecalactone (PDL)-grafted cellulose copolymer (CgPD) and made into films | Improved mechanical properties and mechanical properties | [183] |
4. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Jin, T.; Zou, T.; Xu, L.; Xi, B.; Xu, D.; He, J.; Xiong, L.; Tang, C.; Peng, J.; et al. Current Progress on Plastic/Microplastic Degradation: Fact Influences and Mechanism. Environ. Pollut. 2022, 304, 119159. [Google Scholar] [CrossRef]
- Ouyang, Z.; Li, S.; Zhao, M.; Wangmu, Q.; Ding, R.; Xiao, C.; Guo, X. The Aging Behavior of Polyvinyl Chloride Microplastics Promoted by UV-Activated Persulfate Process. J. Hazard. Mater. 2022, 424, 127461. [Google Scholar] [CrossRef]
- Paletta, A.; Filho, W.L.; Balogun, A.L.; Foschi, E.; Bonoli, A. Barriers and Challenges to Plastics Valorisation in the Context of a Circular Economy: Case Studies from Italy. J. Clean. Prod. 2019, 241, 118149. [Google Scholar] [CrossRef]
- Enfrin, M.; Dumée, L.F.; Lee, J. Nano/Microplastics in Water and Wastewater Treatment Processes—Origin, Impact and Potential Solutions. Water Res. 2019, 161, 621–638. [Google Scholar] [CrossRef]
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and New Opportunities on Barrier Performance of Biodegradable Polymers for Sustainable Packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Andrady, A.L.; Pegram, J.E.; Nakatsuka, S. Studies on Enhanced Degradable Plastics: 1. The Geographic Variability in Outdoor Lifetimes of Enhanced Photodegradable Polyethylenes. J. Environ. Polym. Degrad. 1993, 1, 31–43. [Google Scholar] [CrossRef]
- Abu-Hilal, A.H.; Al-Najjar, T. Litter Pollution on the Jordanian Shores of the Gulf of Aqaba (Red Sea). Mar. Environ. Res. 2004, 58, 39–63. [Google Scholar] [CrossRef]
- Lohr, A.; Savelli, H.; Beunen, R.; Kalz, M.; Ragas, A.; Belleghem, F.V. Solutions for Global Marine Litter Pollution. Curr. Opin. Environ. Sustain. 2017, 28, 90–99. [Google Scholar] [CrossRef] [Green Version]
- Bano, K.; Kuddus, M.; RZaheer, M.; Zia, Q.; FKhan, M.; Gupta, A.; Aliev, G. Microbial Enzymatic Degradation of Biodegradable Plastics. Curr. Pharm. Biotechnol. 2017, 18, 429–440. [Google Scholar] [CrossRef]
- Ward, C.P.; Armstrong, C.J.; Walsh, A.N.; Jackson, J.H.; Reddy, C.M. Sunlight Converts Polystyrene to Carbon Dioxide and Dissolved Organic Carbon. Environ. Sci. Technol. Lett. 2019, 6, 669–674. [Google Scholar] [CrossRef]
- Dharma, H.N.C.; Jaafar, J.; Widiastuti, N.; Matsuyama, H.; Rajabsadeh, S.; Othman, M.H.D.; Rahman, M.A.; Jafri, N.N.M.; Suhaimin, N.S.; Nasir, A.M.; et al. A Review of Titanium Dioxide (TiO2)-Based Photocatalyst for Oilfield-Produced Water Treatment. Membranes 2022, 12, 345. [Google Scholar] [CrossRef]
- Prabhakar, P.; Sen, R.K.; Mayandi, V.; Patel, M.; Swathi, B.; Vishwakarma, J.; Gowri, V.S.; Lakshminarayanan, R.; Mondal, D.P.; Srivastava, A.K.; et al. Mussel-Inspired Chemistry to Design Biodegradable Food Packaging Films with Antimicrobial Properties. Process Saf. Environ. Prot. 2022, 162, 17–29. [Google Scholar] [CrossRef]
- Jing, X.; Wen, H.; Gong, X.; Xu, Z.; Kajetanowicz, A. Recycling Waste Plastics Packaging to Value-Added Products by Two-Step Microwave Cracking with Different Heating Strategies. Fuel Process. Technol. 2020, 201, 106346. [Google Scholar] [CrossRef]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. An Overview of Plasticwaste Generation and Management in Food Packaging Industries. Recycling 2021, 6, 12. [Google Scholar] [CrossRef]
- Webb, H.K.; Arnott, J.; Crawford, R.J.; Ivanova, E.P. Plastic Degradation and Its Environmental Implications with Special Reference to Poly(Ethylene Terephthalate). Polymers 2013, 5, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Rhim, J.-W.; Park, H.-M.; Ha, C.-S. Bio-Nanocomposites for Food Packaging Applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Al-Thawadi, S. Microplastics and Nanoplastics in Aquatic Environments: Challenges and Threats to Aquatic Organisms. Arab. J. Sci. Eng. 2020, 45, 4419–4440. [Google Scholar] [CrossRef]
- Mao, R.; Hu, Y.; Zhang, S.; Wu, R.; Guo, X. Microplastics in the Surface Water of Wuliangsuhai Lake, Northern China. Sci. Total Environ. 2020, 723, 137820. [Google Scholar] [CrossRef]
- Ngo, P.L.; Pramanik, B.K.; Shah, K.; Roychand, R. Pathway, Classification and Removal Efficiency of Microplastics in Wastewater Treatment Plants. Environ. Pollut. 2019, 255, 113326. [Google Scholar] [CrossRef]
- Galloway, T.S.; Cole, M.; Lewis, C. Interactions of Microplastic Debris throughout the Marine Ecosystem. Nat. Ecol. Evol. 2017, 1, 116. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Jung, S.W.; Shim, W.J. Combined Effects of UV Exposure Duration and Mechanical Abrasion on Microplastic Fragmentation by Polymer Type. Environ. Sci. Technol. 2017, 51, 4368–4376. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Zhou, X.; Tian, Y.; Lin, H. Microplastic Abundance, Distribution and Composition in the Mid-West Pacific Ocean. Environ. Pollut. 2020, 264, 114125. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, Y.; Zhong, S.; Liu, J.; Qin, Y.; Gao, P. Microplastics in Freshwater and Wild Fishes from Lijiang River in Guangxi, Southwest China. Sci. Total Environ. 2021, 755, 142428. [Google Scholar] [CrossRef]
- Cutroneo, L.; Reboa, A.; Besio, G.; Borgogno, F.; Canesi, L.; Canuto, S.; Dara, M.; Enrile, F.; Forioso, I.; Greco, G.; et al. Microplastics in Seawater: Sampling Strategies, Laboratory Methodologies, and Identification Techniques Applied to Port Environment. Environ. Sci. Pollut. Res. 2020, 27, 8938–8952. [Google Scholar] [CrossRef]
- Vaughan, R.; Turner, S.D.; Rose, N.L. Microplastics in the Sediments of a UK Urban Lake. Environ. Pollut. 2017, 229, 10–18. [Google Scholar] [CrossRef]
- Tibbetts, J.; Krause, S.; Lynch, I.; Smith, G.H.S. Abundance, Distribution, and Drivers of Microplastic Contamination in Urban River Environments. Water Switz. 2018, 10, 1597. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Mao, R.F.; Guo, X.; Yang, X.; Zhang, Q.; Yang, C. Microplastics in Surface Waters and Sediments of the Wei River, in the Northwest of China. Sci. Total Environ. 2019, 667, 427–434. [Google Scholar] [CrossRef]
- Wang, G.; Lu, J.; Tong, Y.; Liu, Z.; Zhou, H.; Xiayihazi, N. Occurrence and Pollution Characteristics of Microplastics in Surface Water of the Manas River Basin, China. Sci. Total Environ. 2020, 710, 136099. [Google Scholar] [CrossRef]
- Wright, S.L.; Ulke, J.; Font, A.; Chan, K.; Kelly, F.J. Atmospheric Microplastic Deposition in an Urban Environment and an Evaluation of Transport. Environ. Int. 2019, 136, 105411. [Google Scholar] [CrossRef]
- Prata, J.C.; Castro, J.L.; da Costa, J.P.; Duarte, A.C.; Cerqueira, M.; Rocha-Santos, T. An Easy Method for Processing and Identification of Natural and Synthetic Microfibers and Microplastics in Indoor and Outdoor Air. MethodsX 2020, 7, 100762. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, Y.; Du, F.; Cai, H.; Wang, G.; Shi, H. Microplastic Fallout in Different Indoor Environments. Environ. Sci. Technol. 2020, 54, 6530–6539. [Google Scholar] [CrossRef]
- Liu, M.; Lu, S.; Yang, S.; Lei, L.; Hu, J.; Lv, W.; Zhou, W.; Cao, C.; Shi, H.; Yang, X. Microplastic and Mesoplastic Pollution in Farmland Soils in Suburbs of Shanghai, China. Environ. Pollut. 2018, 242, 855–862. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, S.; Wang, X.; Yang, X.; Guo, X. The Occurrence and Distribution Characteristics of Microplastics in the Agricultural Soils of Shaanxi Province, in North-Western China. Sci. Total Environ. 2020, 720, 137525. [Google Scholar] [CrossRef]
- Payton, T.G.; Beckingham, B.A.; Dustan, P. Microplastic Exposure to Zooplankton at Tidal Fronts in Charleston Harbor, SC USA. Estuar. Coast. Shelf Sci. 2019, 232, 106510. [Google Scholar] [CrossRef]
- Wang, F.; Wong, C.S.; Chen, D.; Lu, X.; Wang, F.; Zeng, E.Y. Interaction of Toxic Chemicals with Microplastics: A Critical Review. Water Res. 2018, 139, 208–219. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, A. The Impact of Microplastics on Human Health:A Review. J. Nanjing Univ. Sci. 2020, 56, 8. [Google Scholar] [CrossRef]
- Lamberti, F.M.; Román-Ramírez, L.A.; Wood, J. Recycling of Bioplastics: Routes and Benefits. J. Polym. Environ. 2020, 28, 2551–2571. [Google Scholar] [CrossRef]
- Panchal, S.S.; Vasava, D.V. Biodegradable Polymeric Materials: Synthetic Approach. ACS Omega 2020, 5, 4370–4379. [Google Scholar] [CrossRef]
- Niaounakis, M. Recycling of Biopolymers—The Patent Perspective. Eur. Polym. J. 2019, 114, 464–475. [Google Scholar] [CrossRef]
- Dilkes-Hoffman, L.S.; Pratt, S.; Lant, P.A.; Laycock, B. 19—The Role of Biodegradable Plastic in Solving Plastic Solid Waste Accumulation. In Plastics to Energy; Al-Salem, S.M., Ed.; Plastics Design Library; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 469–505. ISBN 978-0-12-813140-4. [Google Scholar]
- Qin, Z.-H.; Mou, J.-H.; Chao, C.Y.H.; Chopra, S.S.; Daoud, W.; Leu, S.; Ning, Z.; Tso, C.Y.; Chan, C.K.; Tang, S.; et al. Biotechnology of Plastic Waste Degradation, Recycling, and Valorization: Current Advances and Future Perspectives. ChemSusChem 2021, 14, 4103–4114. [Google Scholar] [CrossRef] [PubMed]
- Zaaba, N.F.; Jaafar, M. A Review on Degradation Mechanisms of Polylactic Acid: Hydrolytic, Photodegradative, Microbial, and Enzymatic Degradation. Polym. Eng. Sci. 2020, 60, 2061–2075. [Google Scholar] [CrossRef]
- Liu, L.; Xu, M.; Ye, Y.; Zhang, B. On the Degradation of (Micro)Plastics: Degradation Methods, Influencing Factors, Environmental Impacts. Sci. Total Environ. 2022, 806, 151312. [Google Scholar] [CrossRef]
- Bakbolat, B.; Daulbayev, C.; Sultanov, F.; Beissenov, R.; Umirzakov, A.; Mereke, A.; Bekbaev, A.; Chuprakov, I. Recent Developments of TiO2-Based Photocatalysis in the Hydrogen Evolution and Photodegradation: A Review. Nanomaterials 2020, 10, 1790. [Google Scholar] [CrossRef]
- Jin, L.; He, S.; Li, D.; Zhang, C. Status of Degradable Materials and Their Progress in Marine Research. Packag. Eng. 2020, 41, 108–115. [Google Scholar] [CrossRef]
- Li, J.; Deng, J.; Liang, L. Application Progress of Degradable Plastics in Packaging Products. Plast. Sci. Technol. 2021, 49, 94–98. [Google Scholar] [CrossRef]
- Christensen, P.A.; Egerton, T.A.; Martins-Franchetti, S.M.; Jin, C.; White, J.R. Photodegradation of Polycaprolactone/Poly(Vinyl Chloride) Blend. Polym. Degrad. Stab. 2008, 93, 305–309. [Google Scholar] [CrossRef]
- Najafi, V.; Ahmadi, E.; Ziaee, F.; Omidian, H.; Sedaghat, H. Polyaniline-Modified TiO2, a Highly Effective Photo-Catalyst for Solid-Phase Photocatalytic Degradation of PVC. J. Polym. Environ. 2019, 27, 784–793. [Google Scholar] [CrossRef]
- Krzan, A.; Hemjinda, S.; Miertus, S.; Corti, A.; Chiellini, E. Standardization and Certification in the Area of Environmentally Degradable Plastics. Polym. Degrad. Stab. 2006, 91, 2819–2833. [Google Scholar] [CrossRef]
- Solaro, R.; Corti, A.; Chiellini, E. Biodegradation of poly(vinyl alcohol) with different molecular weights and degree of hydrolysis. Polym. Adv. Technol. 2000, 11, 873–878. [Google Scholar] [CrossRef]
- Lucas, N.; Bienaime, C.; Belloy, C.; Queneudec, M.; Silvestre, F.; Nava-Saucedo, J.-E. Polymer Biodegradation: Mechanisms and Estimation Techniques—A Review. Chemosphere 2008, 73, 429–442. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.; Guo, H. Research Progress of Polyvinyl Alcohol Water-Resistant Film Materials. Membranes 2022, 12, 347. [Google Scholar] [CrossRef]
- Saini, I.; Sharma, A.; Dhiman, R.; Aggarwal, S.; Ram, S.; Sharma, P.K. Grafted SiC Nanocrystals: For Enhanced Optical, Electrical and Mechanical Properties of Polyvinyl Alcohol. J. Alloys Compd. 2017, 714, 172–180. [Google Scholar] [CrossRef]
- Panda, P.K.; Yang, J.-M.; Chang, Y.-H. Water-Induced Shape Memory Behavior of Poly (Vinyl Alcohol) and p-Coumaric Acid-Modified Water-Soluble Chitosan Blended Membrane. Carbohydr. Polym. 2021, 257, 117633. [Google Scholar] [CrossRef]
- Yang, J.; Panda, P.K.; Jie, C.J.; Dash, P.; Chang, Y. Poly (Vinyl Alcohol)/Chitosan/Sodium Alginate Composite Blended Membrane: Preparation, Characterization, and Water-induced Shape Memory Phenomenon. Polym. Eng. Sci. 2022, 62, 1526–1537. [Google Scholar] [CrossRef]
- Moulay, S. Review: Poly(Vinyl Alcohol) Functionalizations and Applications. Polym.-Plast. Technol. Eng. 2015, 54, 1289–1319. [Google Scholar] [CrossRef]
- Abdullah, Z.W.; Dong, Y.; Davies, I.J.; Barbhuiya, S. PVA, PVA Blends, and Their Nanocomposites for Biodegradable Packaging Application. Polym.-Plast. Technol. Eng. 2017, 56, 1307–1344. [Google Scholar] [CrossRef] [Green Version]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of Poly(Vinyl Alcohol) and Natural Polymers. Polym. Rev. 2018, 58, 247–287. [Google Scholar] [CrossRef]
- Liu, B.; Huang, X.; Wang, S.; Wang, D.; Guo, H. Performance of Polyvinyl Alcohol/Bagasse Fibre Foamed Composites as Cushion Packaging Materials. Coatings 2021, 11, 1094. [Google Scholar] [CrossRef]
- Lv, S.; Liu, C.; Li, H.; Zhang, Y. Assessment of Structural Modification and Time-Dependent Behavior of Poly (Lactic Acid) Based Composites upon Hydrolytic Degradation. Eur. Polym. J. 2022, 166, 111058. [Google Scholar] [CrossRef]
- Wang, G.; Huang, D.; Zhang, W.; Ji, J. Degradation Performance of Typical Biodegradable Polyesters in Seawater. J. Funct. Polym. 2020, 33, 492–499. [Google Scholar] [CrossRef]
- Shi, L.; Zhu, J.; Shi, J.; Zhao, X. Classification and Identification of Degradable Plastic Products: Current Situation and Prospect. Plast. Addit. 2021, 3, 1–5. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, W.; Xing, R.; Xie, S.; Yang, X.; Cui, P.; Lü, J.; Liao, H.; Yu, Z.; Wang, S.; et al. Enhanced in Situ Biodegradation of Microplastics in Sewage Sludge Using Hyperthermophilic Composting Technology. J. Hazard. Mater. 2020, 384, 121271. [Google Scholar] [CrossRef]
- Ammala, A.; Bateman, S.; Dean, K.; Petinakis, E.; Sangwan, P.; Wong, S.; Yuan, Q.; Yu, L.; Patrick, C.; Leong, K.H. An Overview of Degradable and Biodegradable Polyolefins. Prog. Polym. Sci. 2011, 36, 1015–1049. [Google Scholar] [CrossRef]
- Chiellini, E.; Corti, A.; D’Antone, S.; Baciu, R. Oxo-Biodegradable Carbon Backbone Polymers—Oxidative Degradation of Polyethylene under Accelerated Test Conditions. Polym. Degrad. Stab. 2006, 91, 2739–2747. [Google Scholar] [CrossRef]
- Chen, L.; Yamane, S.; Sago, T.; Hagihara, H.; Kutsuna, S.; Uchimaru, T.; Suda, H.; Sato, H.; Mizukado, J. Experimental and Modeling Approaches for the Formation of Hydroperoxide during the Auto-Oxidation of Polymers: Thermal-Oxidative Degradation of Polyethylene Oxide. Chem. Phys. Lett. 2016, 657, 83–89. [Google Scholar] [CrossRef]
- Madhu, G.; Bhunia, H.; Bajpai, P.K.; Nando, G.B. Physico-Mechanical Properties and Biodegradation of Oxo-Degradable HDPE/PLA Blends. Polym. Sci. Ser. A 2016, 58, 57–75. [Google Scholar] [CrossRef]
- Amaral-Zettler, L.A.; Zettler, E.R.; Mincer, T.J. Ecology of the Plastisphere. Nat. Rev. Microbiol. 2020, 18, 139–151. [Google Scholar] [CrossRef]
- Elahi, A.; Bukhari, D.A.; Shamim, S.; Rehman, A. Plastics Degradation by Microbes: A Sustainable Approach. J. King Saud Univ.-Sci. 2021, 33, 101538. [Google Scholar] [CrossRef]
- Kyrikou, I.; Briassoulis, D. Biodegradation of Agricultural Plastic Films: A Critical Review. J. Polym. Environ. 2007, 15, 125–150. [Google Scholar] [CrossRef]
- Reddy, R.L.; Reddy, V.S.; Gupta, G.A. Study of Bio-Plastics as Green & Sustainable Alternative to Plastics. Int. J. Emerg. Technol. Adv. Eng. 2013, 3, 82–89. [Google Scholar]
- Qin, M.; Chen, C.; Song, B.; Shen, M.; Gong, J. A Review of Biodegradable Plastics to Biodegradable Microplastics: Another Ecological Threat to Soil Environments? J. Clean. Prod. 2021, 312, 127816. [Google Scholar] [CrossRef]
- Liwarska-Bizukojc, E. Effect of (Bio)Plastics on Soil Environment: A Review. Sci. Total Environ. 2021, 795, 148889. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, D.; Li, Q.; Zhao, Y.; Li, L.; Lin, H.; Bi, Q.; Zhao, Y. Biodegradation of Polyethylene Microplastic Particles by the Fungus Aspergillus flavus from the Guts of Wax Moth Galleria mellonella. Sci. Total Environ. 2020, 704, 135931. [Google Scholar] [CrossRef]
- Khoironi, A.; Anggoro, S.; Sudarno, S. Evaluation of the Interaction Among Microalgae Spirulina sp., Plastics Polyethylene Terephthalate and Polypropylene in Freshwater Environment. J. Ecol. Eng. 2019, 20, 161–173. [Google Scholar] [CrossRef]
- Song, Y.; Qiu, R.; Hu, J.; Li, X.; He, D. Biodegradation and Disintegration of Expanded Polystyrene by Land Snails Achatina fulica. Sci. Total Environ. 2020, 746, 141289. [Google Scholar] [CrossRef]
- Cucina, M.; De Nisi, P.; Trombino, L.; Tambone, F.; Adani, F. Degradation of Bioplastics in Organic Waste by Mesophilic Anaerobic Digestion, Composting and Soil Incubation. Waste Manag. 2021, 134, 67–77. [Google Scholar] [CrossRef]
- Edaes, F.S.; De Souza, C.B. Conventional Plastics’ Harmful Effects and Biological and Molecular Strategies for Biodegradable Plastics’ Production. Curr. Biotechnol. 2020, 9, 242–254. [Google Scholar] [CrossRef]
- Yu, X.; Chen, L.; Jin, Z.; Jiao, A. Research Progress of Starch-Based Biodegradable Materials: A Review. J. Mater. Sci. 2021, 56, 11187–11208. [Google Scholar] [CrossRef]
- Niranjana Prabhu, T.; Prashantha, K. A Review on Present Status and Future Challenges of Starch Based Polymer Films and Their Composites in Food Packaging Applications. Polym. Compos. 2018, 39, 2499–2522. [Google Scholar] [CrossRef]
- Cheng, J.; Wu, X.; Tao, Q.; Jiang, N. Research Progress of Starch-Based Plastics. Shanghai Plast. 2020, 1, 4. [Google Scholar] [CrossRef]
- Xie, X.; Cui, S.W.; Li, W.; Tsao, R. Isolation and Characterization of Wheat Bran Starch. Food Res. Int. 2008, 41, 882–887. [Google Scholar] [CrossRef]
- Martins, I.; Magina, S.P.; Oliveira, L.; Freire, C.; Silvestre, A.; Neto, C.P.; Gandini, A. New Biocomposites Based on Thermoplastic Starch and Bacterial Cellulose. Compos. Sci. Technol. 2009, 69, 2163–2168. [Google Scholar] [CrossRef]
- Punia, S. Barley Starch Modifications: Physical, Chemical and Enzymatic—A Review. Int. J. Biol. Macromol. 2020, 144, 578–585. [Google Scholar] [CrossRef]
- Fitch-Vargas, P.R.; Camacho-Hernández, I.L.; Martínez-Bustos, F.; Islas-Rubio, A.R.; Carrillo-Cañedo, K.I.; Calderón-Castro, A.; Jacobo-Valenzuela, N.; Carrillo-López, A.; Delgado-Nieblas, C.I.; Aguilar-Palazuelos, E. Mechanical, Physical and Microstructural Properties of Acetylated Starch-Based Biocomposites Reinforced with Acetylated Sugarcane Fiber. Carbohydr. Polym. 2019, 219, 378–386. [Google Scholar] [CrossRef]
- Lauer, M.K.; Smith, R.C. Recent Advances in Starch-Based Films toward Food Packaging Applications: Physicochemical, Mechanical, and Functional Properties. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3031–3083. [Google Scholar] [CrossRef]
- Hammache, Y.; Serier, A.; Chaoui, S. The Effect of Thermoplastic Starch on the Properties of Polypropylene/High Density Polyethylene Blend Reinforced by Nano-Clay. Mater. Res. Express 2020, 7, 025308. [Google Scholar] [CrossRef]
- Wu, H.; Lei, Y.; Lu, J.; Zhu, R.; Xiao, D.; Jiao, C.; Xia, R.; Zhang, Z.; Shen, G.; Liu, Y.; et al. Effect of Citric Acid Induced Crosslinking on the Structure and Properties of Potato Starch/Chitosan Composite Films. Food Hydrocoll. 2019, 97, 105208. [Google Scholar] [CrossRef]
- Méité, N.; Konan, L.K.; Tognonvi, M.T.; Oyetola, S. Effect of Metakaolin Content on Mechanical and Water Barrier Properties of Cassava Starch Films. S. Afr. J. Chem. Eng. 2022, 40, 186–194. [Google Scholar] [CrossRef]
- Mittal, A.; Garg, S.; Bajpai, S. Fabrication and Characteristics of Poly (Vinyl Alcohol)-Starch-Cellulosic Material Based Biodegradable Composite Film for Packaging Application. Mater. Today Proc. 2020, 21, 1577–1582. [Google Scholar] [CrossRef]
- Guo, B.; Wang, L.-J.; Yin, P.; Li, B.-G.; Li, P.-X. Ultra-High Molecular Weight Polyethylene Fiber-Reinforced Thermoplastic Corn Starch Composite. J. Thermoplast. Compos. Mater. 2017, 30, 564–577. [Google Scholar] [CrossRef]
- Liu, Y.; Sui, Y.; Liu, C.; Liu, C.; Wu, M.; Li, B.; Li, Y. A Physically Crosslinked Polydopamine/Nanocellulose Hydrogel as Potential Versatile Vehicles for Drug Delivery and Wound Healing. Carbohydr. Polym. 2018, 188, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Long, K.; Mi, H.; Cha, R.; Jiang, X. High-Efficiency Transfer of Fingerprints from Various Surfaces Using Nanofibrillated Cellulose. Nanoscale Horiz. 2019, 4, 953–959. [Google Scholar] [CrossRef]
- Wang, H.; Xie, H.; Du, H.; Wang, X.; Liu, W.; Duan, Y.; Zhang, X.; Sun, L.; Zhang, X.; Si, C. Highly Efficient Preparation of Functional and Thermostable Cellulose Nanocrystals via H2SO4 Intensified Acetic Acid Hydrolysis. Carbohydr. Polym. 2020, 239, 116233. [Google Scholar] [CrossRef]
- Jaffar, S.S.; Saallah, S.; Misson, M.; Siddiquee, S.; Roslan, J.; Saalah, S.; Lenggoro, W. Recent Development and Environmental Applications of Nanocellulose-Based Membranes. Membranes 2022, 12, 287. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A Review of Cellulose and Its Derivatives in Biopolymer-Based for Food Packaging Application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Pooresmaeil, M.; Behzadi Nia, S.; Namazi, H. Green Encapsulation of LDH(Zn/Al)-5-Fu with Carboxymethyl Cellulose Biopolymer; New Nanovehicle for Oral Colorectal Cancer Treatment. Int. J. Biol. Macromol. 2019, 139, 994–1001. [Google Scholar] [CrossRef]
- Zhang, M.; Biesold, G.M.; Choi, W.; Yu, J.; Deng, Y.; Silvestre, C.; Lin, Z. Recent Advances in Polymers and Polymer Composites for Food Packaging. Mater. Today 2022, 53, 134–161. [Google Scholar] [CrossRef]
- David, G.; Gontard, N.; Angellier-Coussy, H. Mitigating the Impact of Cellulose Particles on the Performance of Biopolyester-Based Composites by Gas-Phase Esterification. Polymers 2019, 11, 200. [Google Scholar] [CrossRef] [Green Version]
- Rajeswari, A.; Christy, E.J.S.; Swathi, E.; Pius, A. Fabrication of Improved Cellulose Acetate-Based Biodegradable Films for Food Packaging Applications. Environ. Chem. Ecotoxicol. 2020, 2, 107–114. [Google Scholar] [CrossRef]
- Ariffin, H.; Norrrahim, M.N.F.; Yasim-Anuar, T.A.T.; Nishida, H.; Hassan, M.A.; Ibrahim, N.A.; Yunus, W.M.Z.W. Oil Palm Biomass Cellulose-Fabricated Polylactic Acid Composites for Packaging Applications. In Bionanocomposites for Packaging Applications; Jawaid, M., Swain, S.K., Eds.; Springer: Cham, Switzerland, 2018; pp. 95–105. ISBN 978-3-319-67319-6. [Google Scholar]
- Li, H.; Shi, H.; He, Y.; Fei, X.; Peng, L. Preparation and Characterization of Carboxymethyl Cellulose-Based Composite Films Reinforced by Cellulose Nanocrystals Derived from Pea Hull Waste for Food Packaging Applications. Int. J. Biol. Macromol. 2020, 164, 4104–4112. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shi, Y.; Cao, X.; Liu, Q.; Wang, H.; Kong, B. Preparation and Functional Properties of Poly(Vinyl Alcohol)/Ethyl Cellulose/Tea Polyphenol Electrospun Nanofibrous Films for Active Packaging Material. Food Control 2021, 130, 108331. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan Based Nanocomposite Films and Coatings: Emerging Antimicrobial Food Packaging Alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Panda, P.K.; Dash, P.; Yang, J.-M.; Chang, Y.-H. Development of Chitosan, Graphene Oxide, and Cerium Oxide Composite Blended Films: Structural, Physical, and Functional Properties. Cellulose 2022, 29, 2399–2411. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Wu, W.; Yang, J.; Yang, Q. Preparation and Characterization of Chitosan/Nano-ZnO Composite Film with Antimicrobial Activity. Bioprocess Biosyst. Eng. 2021, 44, 1193–1199. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Li, J.; Du, N.; Li, D.; Li, F.; Man, J. Application Status and Technical Analysis of Chitosan-Based Medical Dressings: A Review. RSC Adv. 2020, 10, 34308–34322. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, Y.; Chen, M.; Xiao, N.; Zhang, J.; Liu, C. Development of Functional Chitosan-Based Composite Films Incorporated with Hemicelluloses: Effect on Physicochemical Properties. Carbohydr. Polym. 2020, 246, 116489. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-hindi, R. Antimicrobial Food Packaging Based on Sustainable Bio-Based Materials for Reducing Foodborne Pathogens: A Review. Food Chem. 2020, 310, 125915. [Google Scholar] [CrossRef]
- Liang, J.; Wang, R.; Chen, R. The Impact of Cross-Linking Mode on the Physical and Antimicrobial Properties of a Chitosan/Bacterial Cellulose Composite. Polymers 2019, 11, 491. [Google Scholar] [CrossRef] [Green Version]
- Khouri, J.; Penlidis, A.; Moresoli, C. Viscoelastic Properties of Crosslinked Chitosan Films. Processes 2019, 7, 157. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Du, H.; Xie, M.; Ma, G.; Yang, W.; Hu, Q.; Pei, F. Characterization of the Physical Properties and Biological Activity of Chitosan Films Grafted with Gallic Acid and Caffeic Acid: A Comparison Study. Food Packag. Shelf Life 2019, 22, 100401. [Google Scholar] [CrossRef]
- Argüelles-Monal, W.M.; Lizardi-Mendoza, J.; Fernández-Quiroz, D.; Recillas-Mota, M.T.; Montiel-Herrera, M. Chitosan Derivatives: Introducing New Functionalities with a Controlled Molecular Architecture for Innovative Materials. Polymers 2018, 10, 342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ardebilchi Marand, S.; Almasi, H.; Ardebilchi Marand, N. Chitosan-Based Nanocomposite Films Incorporated with NiO Nanoparticles: Physicochemical, Photocatalytic and Antimicrobial Properties. Int. J. Biol. Macromol. 2021, 190, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Li, J.; Li, F.; Wang, X.; Man, J.; Li, J.; Zhang, C.; Peng, S. A Biodegradable Chitosan-Based Composite Film Reinforced by Ramie Fibre and Lignin for Food Packaging. Carbohydr. Polym. 2022, 281, 119078. [Google Scholar] [CrossRef] [PubMed]
- Peñas, M.I.; Pérez-Camargo, R.A.; Hernández, R.; Müller, A.J. A Review on Current Strategies for the Modulation of Thermomechanical, Barrier, and Biodegradation Properties of Poly (Butylene Succinate) (PBS) and Its Random Copolymers. Polymers 2022, 14, 1025. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, H.J.; Lee, J.W.; Choi, I.G. Biodegradability of Bio-Flour Filled Biodegradable Poly(Butylene Succinate) Bio-Composites in Natural and Compost Soil. Polym. Degrad. Stab. 2006, 91, 1117–1127. [Google Scholar] [CrossRef]
- Aliotta, L.; Seggiani, M.; Lazzeri, A.; Gigante, V.; Cinelli, P. A Brief Review of Poly (Butylene Succinate) (PBS) and Its Main Copolymers: Synthesis, Blends, Composites, Biodegradability, and Applications. Polymers 2022, 14, 844. [Google Scholar] [CrossRef]
- Zini, E.; Scandola, M. Green Composites: An Overview. Polym. Compos. 2011, 32, 1905–1915. [Google Scholar] [CrossRef]
- Platnieks, O.; Gaidukovs, S.; Kumar Thakur, V.; Barkane, A.; Beluns, S. Bio-Based Poly (Butylene Succinate): Recent Progress, Challenges and Future Opportunities. Eur. Polym. J. 2021, 161, 110855. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Dufresne, A.; Pinheiro, I.F.; Souza, D.H.S.; Gouveia, R.F.; Mei, L.H.I.; Lona, L.M.F. How Do Cellulose Nanocrystals Affect the Overall Properties of Biodegradable Polymer Nanocomposites: A Comprehensive Review. Eur. Polym. J. 2018, 108, 274–285. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Aliotta, L.; Gigante, V.; Bellusci, M.; Cinelli, P.; Bugnicourt, E.; Schmid, M.; Staebler, A.; Lazzeri, A. Preparation and Compatibilization of PBS/Whey Protein Isolate Based Blends. Molecules 2020, 25, 3313. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Kopitzky, R.; Tolga, S.; Kabasci, S. Polylactide (PLA) and Its Blends with Poly(Butylene Succinate) (PBS): A Brief Review. Polymers 2019, 11, 1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barletta, M.; Puopolo, M. Thermoforming of Compostable PLA/PBS Blends Reinforced with Highly Hygroscopic Calcium Carbonate. J. Manuf. Process. 2020, 56, 1185–1192. [Google Scholar] [CrossRef]
- Sadeghi, A.; Mousavi, S.M.; Saljoughi, E.; Kiani, S. Biodegradable Membrane Based on Polycaprolactone/Polybutylene Succinate: Characterization and Performance Evaluation in Wastewater Treatment. J. Appl. Polym. Sci. 2021, 138, 50332. [Google Scholar] [CrossRef]
- Srithep, Y.; Veang-in, O.; Pholharn, D.; Turng, L.-S.; Morris, J. Improving Polylactide Toughness by Plasticizing with Low Molecular Weight Polylactide-Poly(Butylene Succinate) Copolymer. J. Renew. Mater. 2021, 9, 1267. [Google Scholar] [CrossRef]
- Zeng, J.-B.; Huang, C.-L.; Jiao, L.; Lu, X.; Wang, Y.-Z.; Wang, X.-L. Synthesis and Properties of Biodegradable Poly(Butylene Succinate-Co-Diethylene Glycol Succinate) Copolymers. Ind. Eng. Chem. Res. 2012, 51, 12258–12265. [Google Scholar] [CrossRef]
- Mochane, M.J.; Magagula, S.I.; Sefadi, J.S.; Mokhena, T.C. A Review on Green Composites Based on Natural Fiber-Reinforced Polybutylene Succinate (PBS). Polymers 2021, 13, 1200. [Google Scholar] [CrossRef]
- Cosquer, R.; Pruvost, S.; Gouanvé, F. Improvement of Barrier Properties of Biodegradable Polybutylene Succinate/Graphene Nanoplatelets Nanocomposites Prepared by Melt Process. Membranes 2021, 11, 151. [Google Scholar] [CrossRef]
- Popa, M.S.; Frone, A.N.; Panaitescu, D.M. Polyhydroxybutyrate Blends: A Solution for Biodegradable Packaging? Int. J. Biol. Macromol. 2022, 207, 263–277. [Google Scholar] [CrossRef]
- Jian, J.; Xiangbin, Z.; Xianbo, H. An Overview on Synthesis, Properties and Applications of Poly(Butylene-Adipate-Co-Terephthalate)–PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Coiai, S.; Di Lorenzo, M.L.; Cinelli, P.; Righetti, M.C.; Passaglia, E. Binary Green Blends of Poly(Lactic Acid) with Poly(Butylene Adipate-Co-Butylene Terephthalate) and Poly(Butylene Succinate-Co-Butylene Adipate) and Their Nanocomposites. Polymers 2021, 13, 2489. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, H.; Youssef, A.M.; Darwish, N.A.; Abou-Kandil, A.I. Eco-Friendly Polymer Composites for Green Packaging: Future Vision and Challenges. Compos. Part B Eng. 2019, 172, 16–25. [Google Scholar] [CrossRef]
- Rodrigues, B.V.M.; Silva, A.S.; Melo, G.F.S.; Vasconscellos, L.M.R.; Marciano, F.R.; Lobo, A.O. Influence of Low Contents of Superhydrophilic MWCNT on the Properties and Cell Viability of Electrospun Poly (Butylene Adipate-Co-Terephthalate) Fibers. Mater. Sci. Eng. C 2016, 59, 782–791. [Google Scholar] [CrossRef] [Green Version]
- Rios, J.; Lebeau, J.; Yang, T.; Li, S.; Lynch, M.D. A Critical Review on the Progress and Challenges to a More Sustainable, Cost Competitive Synthesis of Adipic Acid. Green Chem. 2021, 23, 3172–3190. [Google Scholar] [CrossRef]
- Pérez-Camargo, R.A.; Fernández-d’Arlas, B.; Cavallo, D.; Debuissy, T.; Pollet, E.; Avérous, L.; Müller, A.J. Tailoring the Structure, Morphology, and Crystallization of Isodimorphic Poly(Butylene Succinate-Ran-Butylene Adipate) Random Copolymers by Changing Composition and Thermal History. Macromolecules 2017, 50, 597–608. [Google Scholar] [CrossRef]
- Nunes, M.A.B.S.; Marinho, V.A.D.; Falcão, G.A.M.; Canedo, E.L.; Bardi, M.A.G.; Carvalho, L.H. Rheological, Mechanical and Morphological Properties of Poly(Butylene Adipate-Co-Terephthalate)/Thermoplastic Starch Blends and Its Biocomposite with Babassu Mesocarp. Polym. Test. 2018, 70, 281–288. [Google Scholar] [CrossRef]
- Xiong, S.-J.; Pang, B.; Zhou, S.-J.; Li, M.-K.; Yang, S.; Wang, Y.-Y.; Shi, Q.; Wang, S.-F.; Yuan, T.-Q.; Sun, R.-C. Economically Competitive Biodegradable PBAT/Lignin Composites: Effect of Lignin Methylation and Compatibilizer. ACS Sustain. Chem. Eng. 2020, 8, 5338–5346. [Google Scholar] [CrossRef]
- Zhai, X.; Wang, W.; Zhang, H.; Dai, Y.; Dong, H.; Hou, H. Effects of High Starch Content on the Physicochemical Properties of Starch/PBAT Nanocomposite Films Prepared by Extrusion Blowing. Carbohydr. Polym. 2020, 239, 116231. [Google Scholar] [CrossRef]
- Raja, V.; Natesan, R. TiO2 Nanoparticles/Poly(Butylene Adipate-co-terephthalate) Bionanocomposite Films for Packaging Applications. Polym. Adv. Technol. 2017, 28, 1699–1706. [Google Scholar] [CrossRef]
- de Matos Costa, A.R.; Crocitti, A.; Hecker de Carvalho, L.; Carroccio, S.C.; Cerruti, P.; Santagata, G. Properties of Biodegradable Films Based on Poly(Butylene Succinate) (PBS) and Poly(Butylene Adipate-Co-Terephthalate) (PBAT) Blends. Polymers 2020, 12, 2317. [Google Scholar] [CrossRef] [PubMed]
- Labet, M.; Thielemans, W. Synthesis of Polycaprolactone: A Review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The Return of a Forgotten Polymer—Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Elzubair, A.; Elias, C.N.; Suarez, J.C.M.; Lopes, H.P.; Vieira, M.V.B. The Physical Characterization of a Thermoplastic Polymer for Endodontic Obturation. J. Dent. 2006, 34, 784–789. [Google Scholar] [CrossRef]
- Bouakaz, B.S.; Habi, A.; Grohens, Y.; Pillin, I. Organomontmorillonite/Graphene-PLA/PCL Nanofilled Blends: New Strategy to Enhance the Functional Properties of PLA/PCL Blend. Appl. Clay Sci. 2017, 139, 81–91. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Mendieta, J.R.; Ortega-Toro, R. In-Depth Study from Gluten/PCL-Based Food Packaging Films Obtained under Reactive Extrusion Conditions Using Chrome Octanoate as a Potential Food Grade Catalyst. Food Hydrocoll. 2021, 111, 106255. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Yusoh, K. A Review on the Recent Research of Polycaprolactone (PCL). Adv. Mater. Res. 2016, 1134, 249–255. [Google Scholar] [CrossRef]
- Thakur, M.; Majid, I.; Hussain, S.; Nanda, V. Poly(ε-Caprolactone): A Potential Polymer for Biodegradable Food Packaging Applications. Packag. Technol. Sci. 2021, 34, 449–461. [Google Scholar] [CrossRef]
- Dorati, R.; Pisani, S.; Maffeis, G.; Conti, B.; Modena, T.; Chiesa, E.; Bruni, G.; Musazzi, U.M.; Genta, I. Study on Hydrophilicity and Degradability of Chitosan/Polylactide-Co-Polycaprolactone Nanofibre Blend Electrospun Membrane. Carbohydr. Polym. 2018, 199, 150–160. [Google Scholar] [CrossRef]
- Przybysz, M.; Hejna, A.; Haponiuk, J.; Formela, K. Structural and Thermo-Mechanical Properties of Poly(ε-Caprolactone) Modified by Various Peroxide Initiators. Polymers 2019, 11, 1101. [Google Scholar] [CrossRef] [Green Version]
- Hadj-Hamou, A.S.; Yahiaoui, F. Performances of PCL/PVC/Organoclay Nanobioblends Films for Packaging Applications. Macromol. Symp. 2019, 386, 1800239. [Google Scholar] [CrossRef]
- Sadeghi, A.; Razavi, S.M.A.; Shahrampour, D. Fabrication and Characterization of Biodegradable Active Films with Modified Morphology Based on Polycaprolactone-Polylactic Acid-Green Tea Extract. Int. J. Biol. Macromol. 2022, 205, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Aragosa, A.; Specchia, V.; Frigione, M. PHB Produced by Bacteria Present in the Argan Field Soil: A New Perspective for the Synthesis of the Bio-Based Polymer. Proceedings 2020, 69, 5. [Google Scholar] [CrossRef]
- Muneer, F.; Rasul, I.; Azeem, F.; Siddique, M.H.; Zubair, M.; Nadeem, H. Microbial Polyhydroxyalkanoates (PHAs): Efficient Replacement of Synthetic Polymers. J. Polym. Environ. 2020, 28, 2301–2323. [Google Scholar] [CrossRef]
- Sharma, V.; Sehgal, R.; Gupta, R. Polyhydroxyalkanoate (PHA): Properties and Modifications. Polymer 2021, 212, 123161. [Google Scholar] [CrossRef]
- Tripathi, A.D.; Mishra, P.K.; Darani, K.K.; Agarwal, A.; Paul, V. Hydrothermal Treatment of Lignocellulose Waste for the Production of Polyhydroxyalkanoates Copolymer with Potential Application in Food Packaging. Trends Food Sci. Technol. 2022, 123, 233–250. [Google Scholar] [CrossRef]
- Kumar, V.; Sehgal, R.; Gupta, R. Blends and Composites of Polyhydroxyalkanoates (PHAs) and Their Applications. Eur. Polym. J. 2021, 161, 110824. [Google Scholar] [CrossRef]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, Production, Recent Developments and Applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Pakalapati, H.; Chang, C.-K.; Show, P.L.; Arumugasamy, S.K.; Lan, J.C.-W. Development of Polyhydroxyalkanoates Production from Waste Feedstocks and Applications. J. Biosci. Bioeng. 2018, 126, 282–292. [Google Scholar] [CrossRef]
- Tan, D.; Wang, Y.; Tong, Y.; Chen, G.-Q. Grand Challenges for Industrializing Polyhydroxyalkanoates (PHAs). Trends Biotechnol. 2021, 39, 953–963. [Google Scholar] [CrossRef]
- Sun, S.; Liu, P.; Ji, N.; Hou, H.; Dong, H. Effects of Various Cross-Linking Agents on the Physicochemical Properties of Starch/PHA Composite Films Produced by Extrusion Blowing. Food Hydrocoll. 2018, 77, 964–975. [Google Scholar] [CrossRef]
- Lugoloobi, I.; Li, X.; Zhang, Y.; Mao, Z.; Wang, B.; Sui, X.; Feng, X. Fabrication of Lignin/Poly(3-Hydroxybutyrate) Nanocomposites with Enhanced Properties via a Pickering Emulsion Approach. Int. J. Biol. Macromol. 2020, 165, 3078–3087. [Google Scholar] [CrossRef] [PubMed]
- Torğut, G.; Gürler, N. Nanofiller Reinforced Biodegradable PHA/PLA Composites: Physico-Chemical, Thermal and Dielectric Properties. J. Polym. Res. 2021, 28, 452. [Google Scholar] [CrossRef]
- Singh, A.A.; Sharma, S.; Srivastava, M.; Majumdar, A. Modulating the Properties of Polylactic Acid for Packaging Applications Using Biobased Plasticizers and Naturally Obtained Fillers. Int. J. Biol. Macromol. 2020, 153, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-González, M.; Ahmed, A.; Maamo, K.; Salem, M.; Jordan, C.; Harasek, M. Evaluation of Nanofiltration Membranes for Pure Lactic Acid Permeability. Membranes 2022, 12, 302. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Li, W.; Liu, D.; Yuan, M.; Li, L. Development of Active Packaging Film Made from Poly (Lactic Acid) Incorporated Essential Oil. Prog. Org. Coat. 2017, 103, 76–82. [Google Scholar] [CrossRef]
- Messin, T.; Marais, S.; Follain, N.; Guinault, A.; Gaucher, V.; Delpouve, N.; Sollogoub, C. Biodegradable PLA/PBS Multinanolayer Membrane with Enhanced Barrier Performances. J. Membr. Sci. 2020, 598, 117777. [Google Scholar] [CrossRef]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.-C. Poly (Lactic Acid) Blends: Processing, Properties and Applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, Y.; Gu, J.; Tan, H. Physicochemical Evolutions of Starch/Poly (Lactic Acid) Composite Biodegraded in Real Soil. J. Environ. Manag. 2018, 228, 223–231. [Google Scholar] [CrossRef]
- Valentina, I.; Haroutioun, A.; Fabrice, L.; Vincent, V.; Roberto, P. Poly(Lactic Acid)-Based Nanobiocomposites with Modulated Degradation Rates. Materials 2018, 11, 1943. [Google Scholar] [CrossRef] [Green Version]
- Limsukon, W.; Auras, R.; Selke, S. Hydrolytic Degradation and Lifetime Prediction of Poly(Lactic Acid) Modified with a Multifunctional Epoxy-Based Chain Extender. Polym. Test. 2019, 80, 106108. [Google Scholar] [CrossRef]
- Lv, S. Degradation Behavior and Mechanism of Poly(1actic Acid)Based Composites. Ph.D. Thesis, Northeast Forest University, Harbin, China, 2019. [Google Scholar]
- Piemonte, V.; Sabatini, S.; Gironi, F. Chemical Recycling of PLA: A Great Opportunity Towards the Sustainable Development? J. Polym. Environ. 2013, 21, 640–647. [Google Scholar] [CrossRef]
- Ainali, N.M.; Kalaronis, D.; Evgenidou, E.; Kyzas, G.Z.; Bobori, D.C.; Kaloyianni, M.; Yang, X.; Bikiaris, D.N.; Lambropoulou, D.A. Do Poly(Lactic Acid) Microplastics Instigate a Threat? A Perception for Their Dynamic towards Environmental Pollution and Toxicity. Sci. Total Environ. 2022, 832, 155014. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Laurencin, C.T. Biodegradable Polymers as Biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Li, F.; Zhang, C.; Weng, Y.; Diao, X.; Zhou, Y.; Song, X. Enhancement of Gas Barrier Properties of Graphene Oxide/Poly (Lactic Acid) Films Using a Solvent-Free Method. Materials 2020, 13, 3024. [Google Scholar] [CrossRef]
- Jing, M.; Che, J.; Xu, S.; Liu, Z.; Fu, Q. The Effect of Surface Modification of Glass Fiber on the Performance of Poly(Lactic Acid) Composites: Graphene Oxide vs. Silane Coupling Agents. Appl. Surf. Sci. 2018, 435, 1046–1056. [Google Scholar] [CrossRef]
- Qian, S.; Zhang, H.; Yao, W.; Sheng, K. Effects of Bamboo Cellulose Nanowhisker Content on the Morphology, Crystallization, Mechanical, and Thermal Properties of PLA Matrix Biocomposites. Compos. Part B Eng. 2018, 133, 203–209. [Google Scholar] [CrossRef]
- Alias, A.R.; Wan, M.K.; Sarbon, N.M. Emerging Materials and Technologies of Multi-Layer Film for Food Packaging Application: A Review. Food Control 2022, 136, 108875. [Google Scholar] [CrossRef]
- Ordoñez, R.; Atarés, L.; Chiralt, A. Properties of PLA Films with Cinnamic Acid: Effect of the Processing Method. Food Bioprod. Process. 2022, 133, 25–33. [Google Scholar] [CrossRef]
- Lee, W.; Lee, J.; Chung, J.W.; Kwak, S.-Y. Enhancement of Tensile Toughness of Poly(Lactic Acid) (PLA) through Blending of a Polydecalactone-Grafted Cellulose Copolymer: The Effect of Mesophase Transition on Mechanical Properties. Int. J. Biol. Macromol. 2021, 193, 1103–1113. [Google Scholar] [CrossRef]
- Sebastian, A.D.; Sneck, S.I.; Soderlund, M.J. Package Wrapping Including PLA Film with Moisture Barrier by Atomic Layer Deposition. WO2018013367A1, 18 January 2018. [Google Scholar]
- Huiqun, W. Biodegradable Material, Preparation Method and Use Thereof. WO/2014/056293, 17 April 2014. [Google Scholar]
- Peter, A.; Mihaly, C.A.; Nicula, C.; Mihaly, C.L.; Talaşman, C.M.; Căpriţă, F.-C.; Constantin, C.; Dumitraşcu, I.; Drazic, G.; Bele, M.; et al. Process for Producing Active Food Packaging Based on Polylactic Acid Modified with Nanocomposite. Romania Patent RO134492A0, 30 October 2020. [Google Scholar]

| Classification | Category | Features |
|---|---|---|
| By degradation principle | Biodegradable plastics | Similar performance to traditional plastics, good degradability, high safety |
| Photodegradable plastics | Simple and low cost production process | |
| Thermal oxidative degradation plastics | Requires oxygen and heat | |
| Hydrodegradable plastics | Short degradation time, no trace, no pollution, low cost | |
| By degradation characteristics | Fully degradable plastics | Completely disintegrates and leaves no trace |
| Incomplete degradable plastics | Partial degradation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, C.; Guo, H. Progress in the Degradability of Biodegradable Film Materials for Packaging. Membranes 2022, 12, 500. https://doi.org/10.3390/membranes12050500
Guo C, Guo H. Progress in the Degradability of Biodegradable Film Materials for Packaging. Membranes. 2022; 12(5):500. https://doi.org/10.3390/membranes12050500
Chicago/Turabian StyleGuo, Chuanyan, and Hongge Guo. 2022. "Progress in the Degradability of Biodegradable Film Materials for Packaging" Membranes 12, no. 5: 500. https://doi.org/10.3390/membranes12050500
APA StyleGuo, C., & Guo, H. (2022). Progress in the Degradability of Biodegradable Film Materials for Packaging. Membranes, 12(5), 500. https://doi.org/10.3390/membranes12050500






