Preparation of Nano-TiO2-Modified PVDF Membranes with Enhanced Antifouling Behaviors via Phase Inversion: Implications of Nanoparticle Dispersion Status in Casting Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Membrane Preparation
2.3. Membrane Characterization
2.4. Antifouling Performance Evaluation
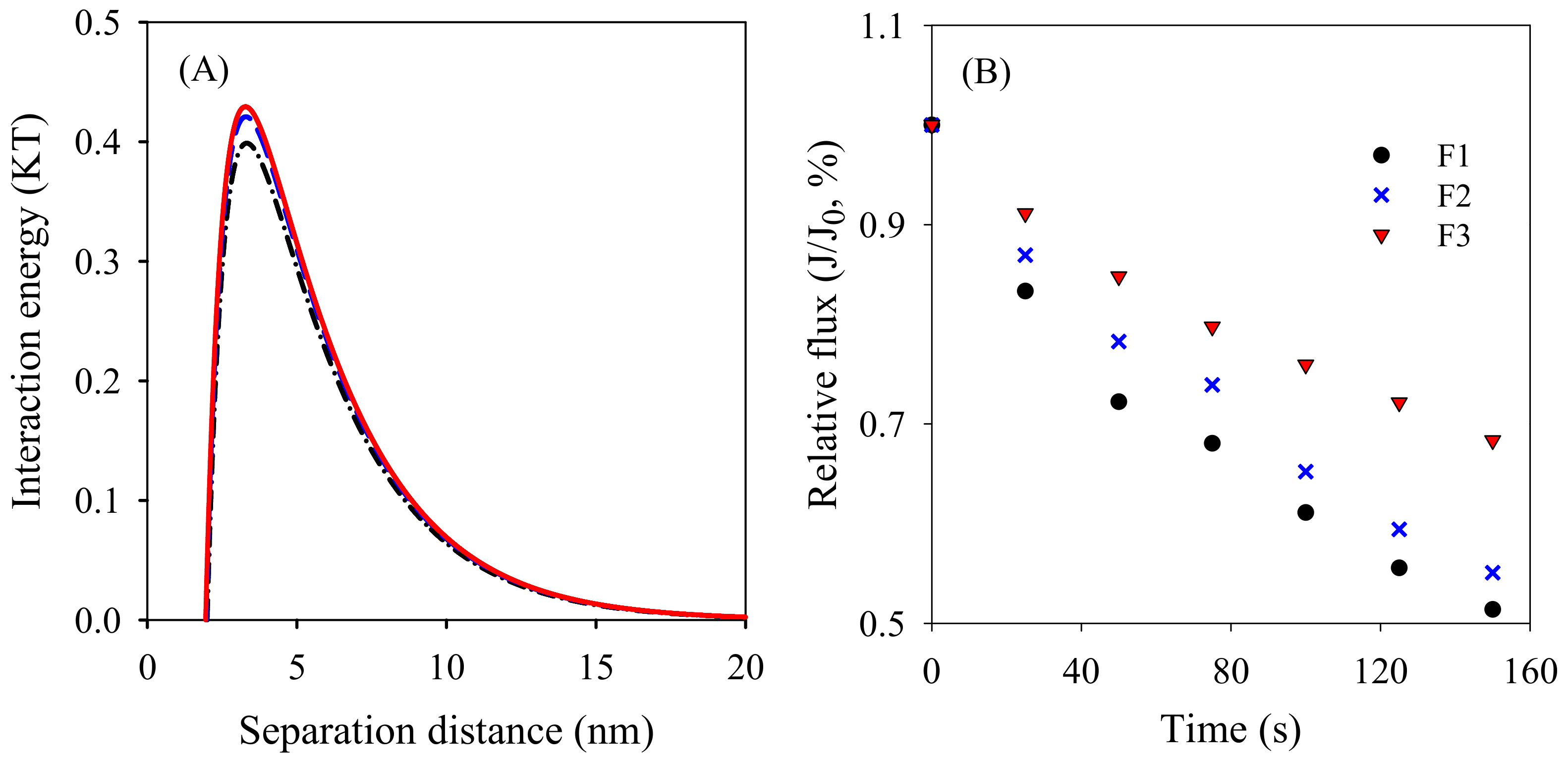
2.4.1. XDLVO Theory Analysis
2.4.2. Filtration Performance
2.5. Casting Solution Stability
3. Results and Discussion
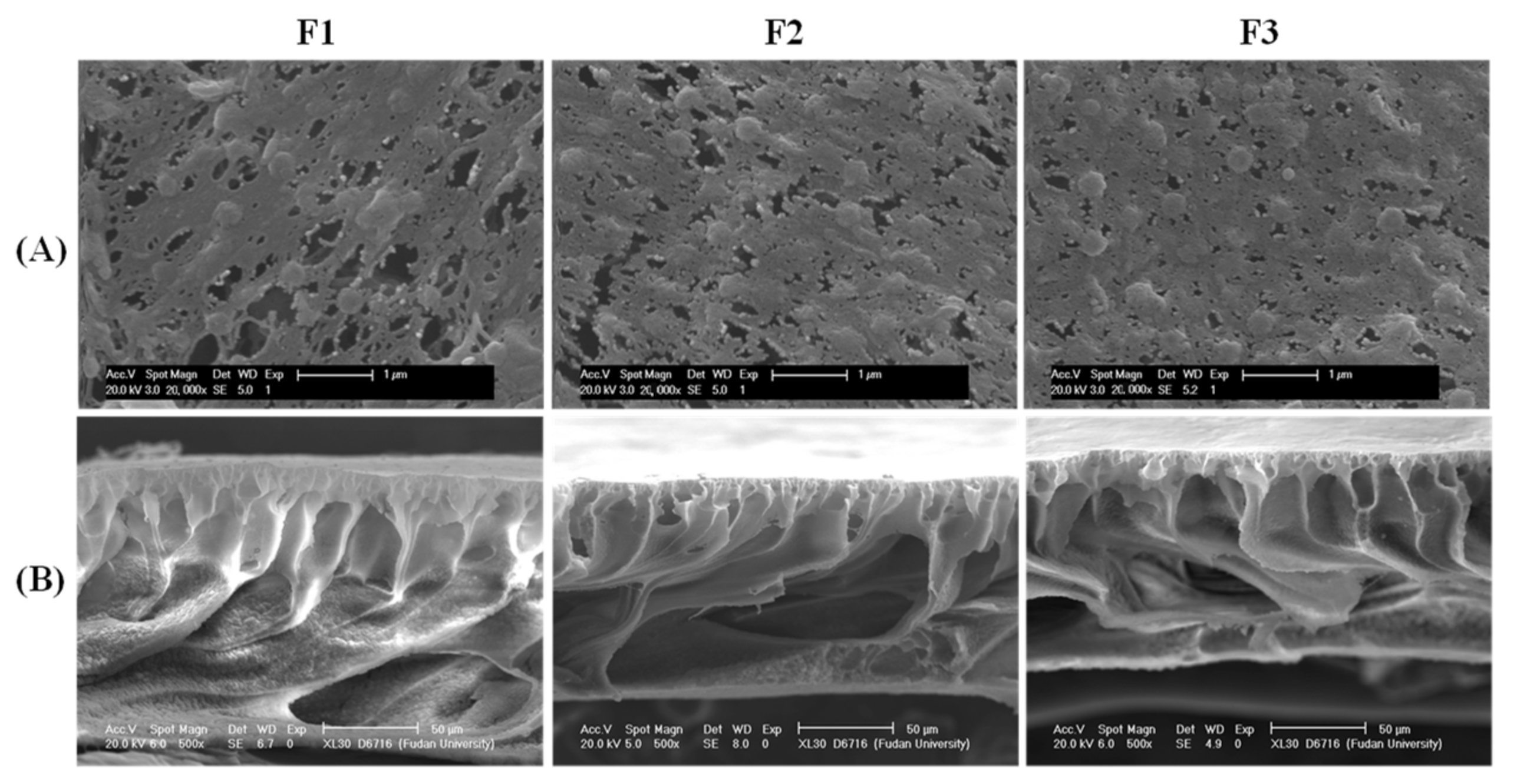
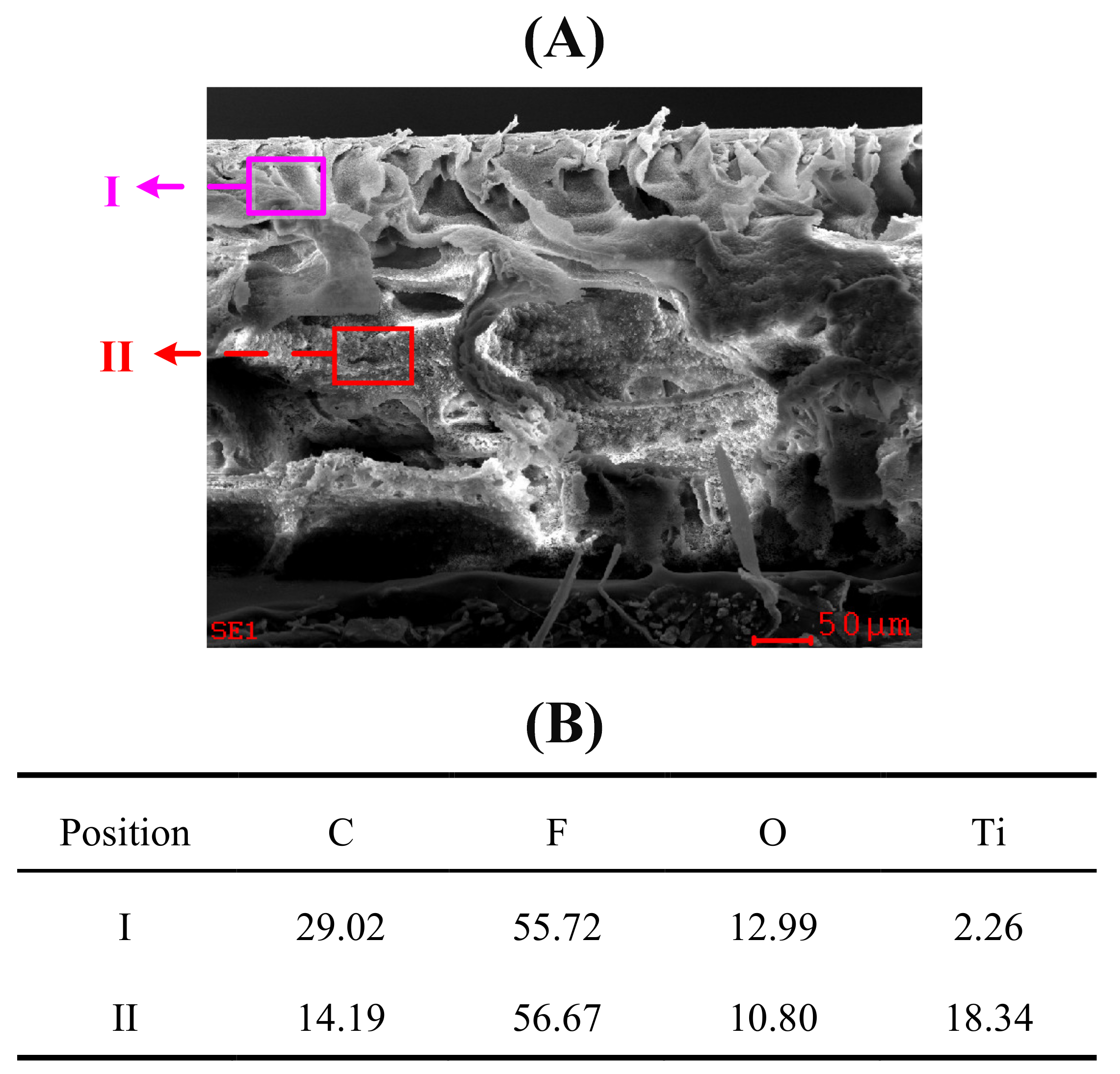
3.1. Membrane Morphologies
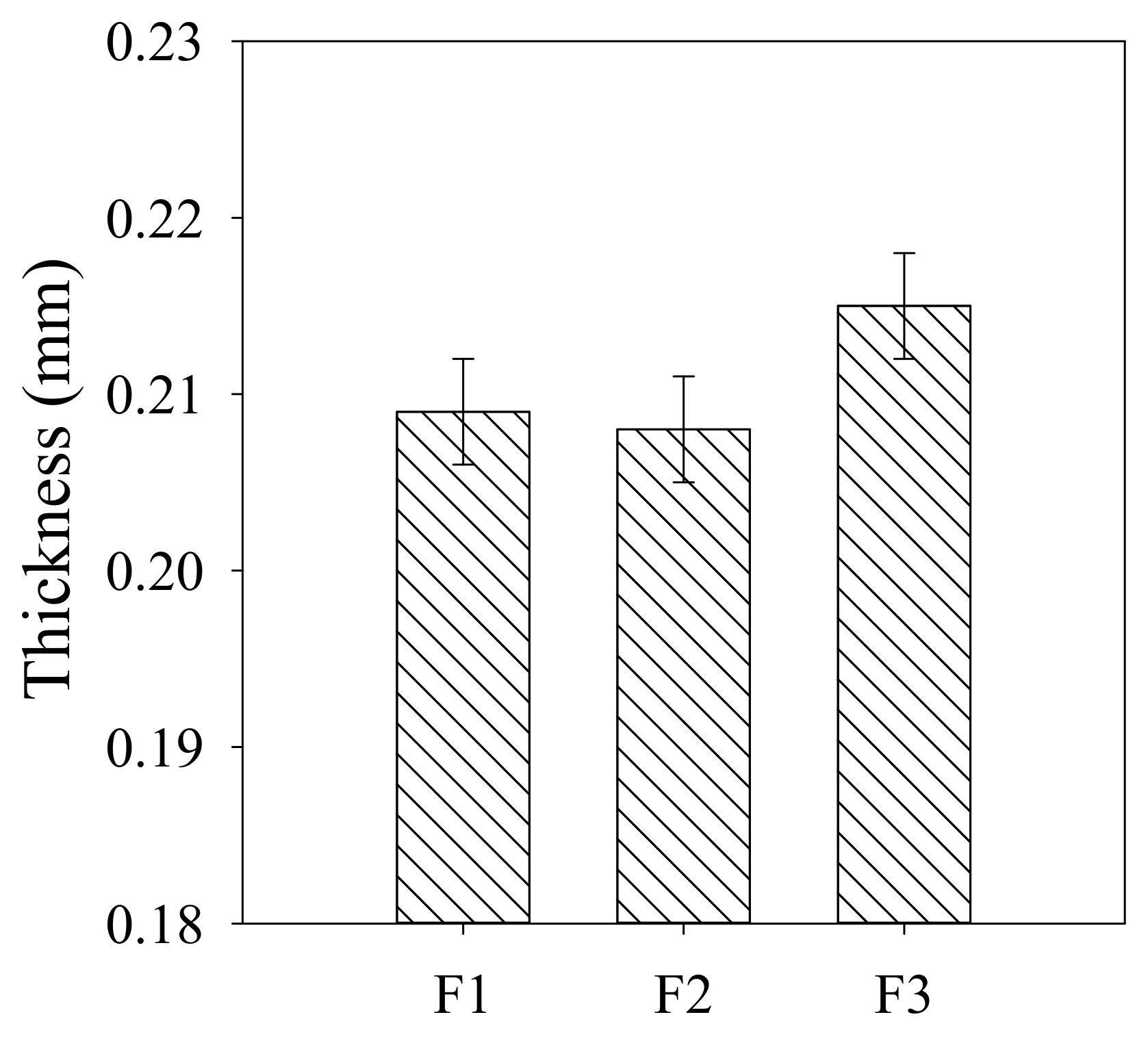
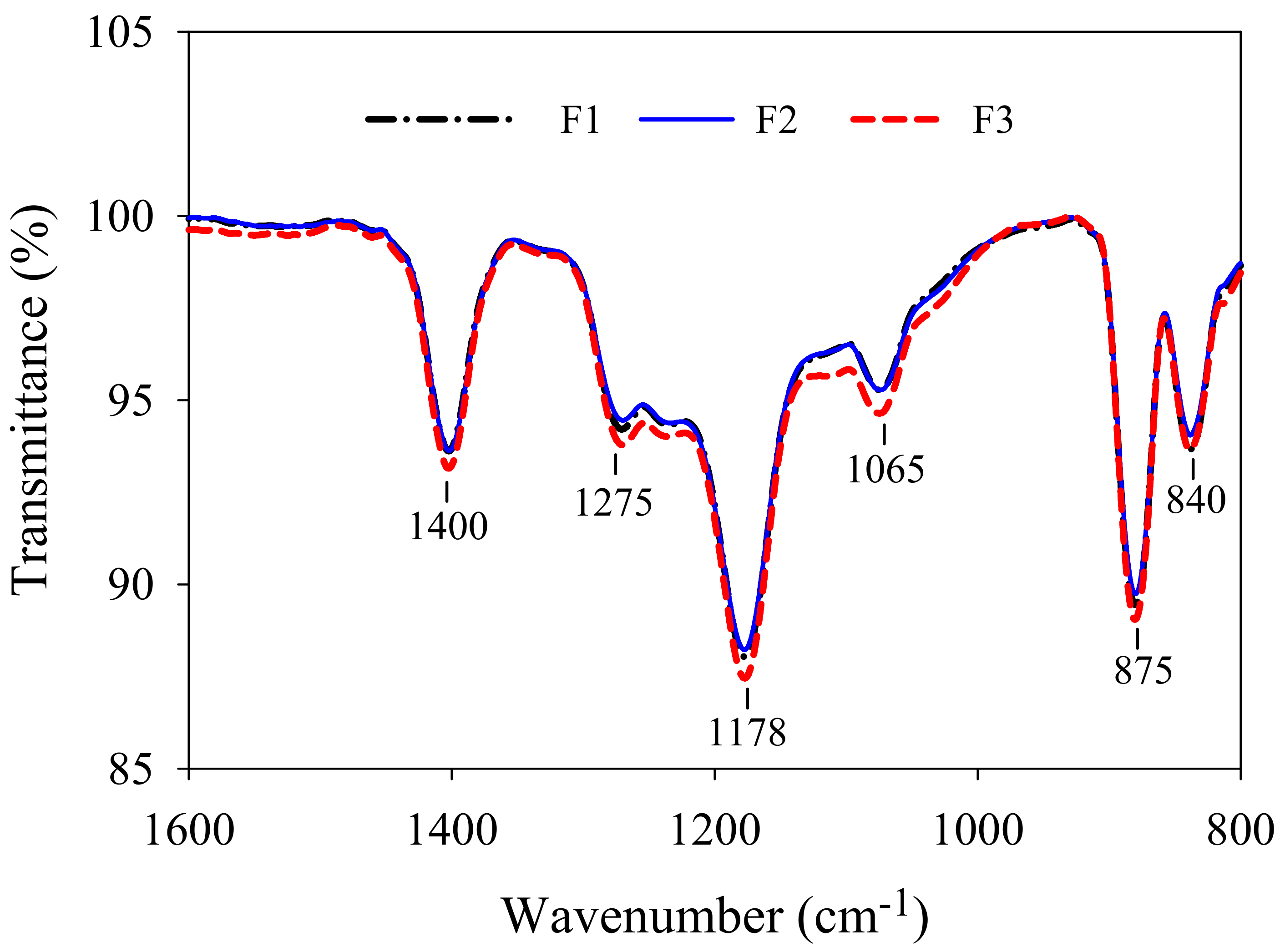
3.2. Membrane Properties
3.3. Membrane Antifouling Performance Evaluation
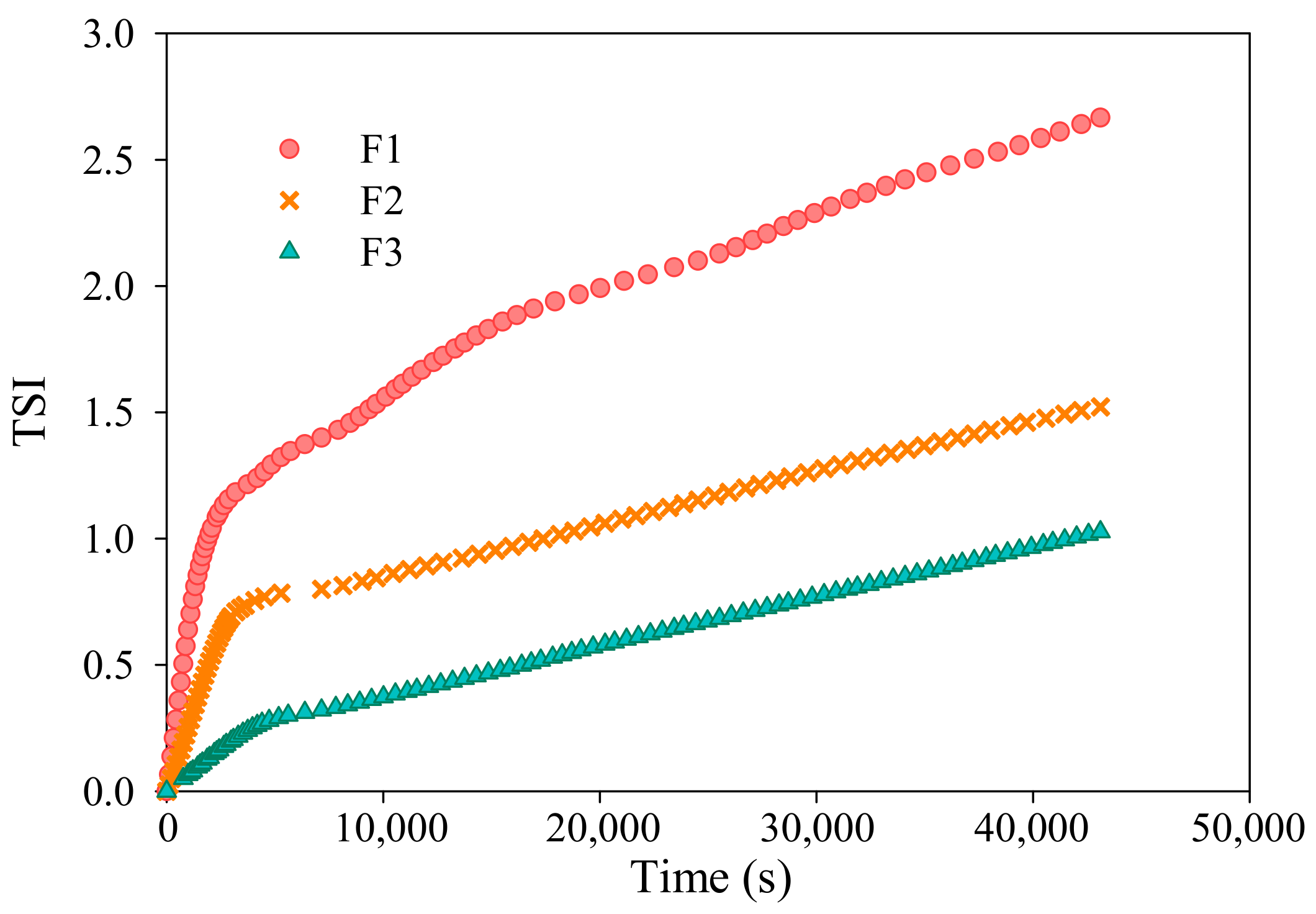
3.4. Stability of Casting Solutions for Membranes F1–F3
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 16018. [Google Scholar] [CrossRef]
- Khoo, Y.S.; Lau, W.J.; Liang, Y.Y.; Karaman, M.; Gürsoy, M.; Ismail, A.F. A green approach to modify surface properties of polyamide thin film composite membrane for improved antifouling resistance. Sep. Purif. Technol. 2020, 250, 116976. [Google Scholar] [CrossRef]
- Deng, W.; Fan, T.; Li, Y. In situ biomineralization-constructed superhydrophilic and underwater superoleophobic PVDF-TiO2 membranes for superior antifouling separation of oil-in-water emulsions. J. Membr. Sci. 2021, 622, 119030. [Google Scholar] [CrossRef]
- Sakarkar, S.; Muthukumaran, S.; Jegatheesan, V. Evaluation of polyvinyl alcohol (PVA) loading in the PVA/titanium dioxide (TiO2) thin film coating on polyvinylidene fluoride (PVDF) membrane for the removal of textile dyes. Chemosphere 2020, 257, 127144. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, X.; Li, F.; Zhao, X. Poly(vinyl pyrrolidone) modified poly(vinylidene fluoride) ultrafiltration membrane via a two-step surface grafting for radioactive wastewater treatment. Sep. Purif. Technol. 2018, 194, 404–409. [Google Scholar] [CrossRef]
- Kakihana, Y.; Cheng, L.; Fang, L.F.; Wang, S.Y.; Jeon, S.; Saeki, D.; Rajabzadeh, S.; Matsuyama, H. Preparation of positively charged PVDF membranes with improved antibacterial activity by blending modification: Effect of change in membrane surface material properties. Colloids Surf. A 2017, 533, 133–139. [Google Scholar] [CrossRef]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.R.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Sandu, T.; Sârbu, A.; Damian, C.M.; Marin, A.; Vulpe, S.; Budinova, T.; Tsyntsarski, B.; Yardim, M.F.; Sirkecioglu, A. Preparation and characterization of membranes obtained from blends of acrylonitrile copolymers with poly(vinyl alcohol). J. Appl. Polym. Sci. 2014, 131, 41013. [Google Scholar] [CrossRef]
- Căprărescu, S.; Zgarian, R.G.; Tihan, G.T.; Purcar, V.; EftimieTotu, E.; Modrogan, C.; Chiriac, A.L.; Nicolae, C.A. Biopolymeric membrane enriched with chitosan and silver for metallic ions removal. Polymers 2020, 12, 1792. [Google Scholar] [CrossRef]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Hashim, N.A.; Liu, Y.; Li, K. Preparation of PVDF hollow fiber membranes using SiO2 particles: The effect of acid and alkali treatment on the membrane performances. Ind. Eng. Chem. Res. 2011, 50, 3035–3040. [Google Scholar] [CrossRef]
- Căprărescu, S.; Modrogan, C.; Purcar, V.; Dancila, A.M.; Orbulet, O.D. Study of polyvinyl alcohol-SiO2 nanoparticles polymeric membrane in wastewater treatment containing zinc ions. Polymers 2021, 13, 1875. [Google Scholar] [CrossRef]
- Liang, S.; Xiao, K.; Mo, Y.; Huang, X. A novel ZnO nanoparticle blended polyvinylidene fluoride membrane for anti-irreversible fouling. J. Membr. Sci. 2012, 394, 184–192. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, S.; Zhang, Y.; Zhao, S. Comparing the antifouling effects of activated carbon and TiO2 in ultrafiltration membrane development. J. Colloid Interface Sci. 2018, 515, 109–118. [Google Scholar] [CrossRef]
- Zhao, X.; Su, Y.; Cao, J.; Li, Y.; Zhang, R.; Liu, Y.; Jiang, Z. Fabrication of antifouling polymer–inorganic hybrid membranes through the synergy of biomimetic mineralization and nonsolvent induced phase separation. J. Mater. Chem. A 2015, 3, 7287–7295. [Google Scholar] [CrossRef]
- Zeng, G.; He, Y.; Yu, Z.; Zhan, Y.; Ma, L.; Zhang, L. Preparation and characterization of a novel PVDF ultrafiltration membrane by blending with TiO2-HNTs nanocomposites. Appl. Surf. Sci. 2016, 371, 624–632. [Google Scholar] [CrossRef]
- Razmjou, A.; Mansouri, J.; Chen, V. The effects of mechanical and chemical modification of TiO2 nanoparticles on the surface chemistry, structure and fouling performance of PES ultrafiltration membranes. J. Membr. Sci. 2011, 378, 73–84. [Google Scholar] [CrossRef]
- Ma, X.H.; Wen, X.; Gu, S.W.; Xu, Z.L.; Zhang, J.L. Preparation and characterization of catalytic TiO2–SPPESK–PES nanocomposite membranes and kinetics analysis in esterification. J. Membr. Sci. 2013, 430, 62–69. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Wang, Z.W.; Zhang, J.; Wang, J.; Wu, Z.C. Antifouling behaviours of PVDF/nano-TiO2 composite membranes revealed by surface energetics and quartz crystal microbalance monitoring. RSC Adv. 2014, 4, 43590–43598. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.W.; Zhang, X.R.; Zheng, X.; Wu, Z.C. Enhanced antifouling behaviours of polyvinylidene fluoride membrane modified through blending with nano-TiO2/polyethylene glycol mixture. Appl. Surf. Sci. 2015, 345, 418–427. [Google Scholar] [CrossRef]
- Lin, S.; Li, Y.; Zhang, L.; Chen, S.; Hou, L. Zwitterion-like, charge-balanced ultrathin layers on polymeric membranes for antifouling property. Environ. Sci. Technol. 2018, 52, 4457–4463. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.Y.; Wang, Z.W.; Zhu, C.W.; Wu, Z.C. Modification of poly(vinylidene fluoride)/polyethersulfone blend membrane with polyvinyl alcohol for improving antifouling ability. J. Membr. Sci. 2014, 466, 293–301. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, J.; Wang, Q.Y.; Zhang, H.; Wang, Z.W.; Wu, Z.C. Evaluating of the performance of natural mineral vermiculite modified PVDF membrane for oil/water separation by membrane fouling model and xdlvo theory. J. Membr. Sci. 2022, 641, 119886. [Google Scholar] [CrossRef]
- Chen, L.; Tian, Y.; Cao, C.Q.; Zhang, J.; Li, Z.N. Interaction energy evaluation of soluble microbial products (SMP) on different membrane surfaces: Role of the reconstructed membrane topology. Water Res. 2012, 46, 2693–2704. [Google Scholar] [CrossRef]
- Delforce, L.; Hofmann, E.; Nardello-Rataj, V.; Aubry, J.M. TiO2 nanoparticle dispersions in water and nonaqueous solvents studied by gravitational sedimentation analysis: Complementarity of hansen parameters and dlvo interpretations. Colloid. Surf. A 2021, 628, 127333. [Google Scholar] [CrossRef]
- Wisniewska, M.; Szewczuk-Karpisz, K. Removal possibilities of colloidal chromium (III) oxide from water using polyacrylic acid. Environ. Sci. Pollut. Res. 2013, 20, 3657–3669. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Chen, J.; Zhang, X.; Xiao, J.; An, S.; Luan, Z.; Liu, F.; Zhang, B. Dynamic demulsification of oil-in-water emulsions with electrocoalescence: Diameter distribution of oil droplets. Sep. Purif. Technol. 2021, 254, 117631. [Google Scholar] [CrossRef]
- Chen, F.; Shi, X.; Chen, X.; Chen, W. An iron (ii) phthalocyanine/poly(vinylidene fluoride) composite membrane with antifouling property and catalytic self-cleaning function for high-efficiency oil/water separation. J. Membr. Sci. 2018, 552, 295–304. [Google Scholar] [CrossRef]
- Farahani, M.H.D.A.; Vatanpour, V. A comprehensive study on the performance and antifouling enhancement of the PVDF mixed matrix membranes by embedding different nanoparticulates: Clay, functionalized carbon nanotube, SiO2 and TiO2. Sep. Purif. Technol. 2018, 197, 372–381. [Google Scholar] [CrossRef]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H. Preparation of a novel antifouling mixed matrix PES membrane by embedding graphene oxide nanoplates. J. Membr. Sci. 2014, 453, 292–301. [Google Scholar] [CrossRef]
- Hong, J.; He, Y. Effects of nano sized zinc oxide on the performance of PVDF microfiltration membranes. Desalination 2012, 302, 71–79. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.W.; Wang, Q.Y.; Pan, C.; Wu, Z.C. Comparison of antifouling behaviours of modified PVDF membranes by TiO2 sols with different nanoparticle size: Implications of casting solution stability. J. Membr. Sci. 2017, 525, 378–386. [Google Scholar] [CrossRef]
- Song, H.; Shao, J.; He, Y.; Liu, B.; Zhong, X. Natural organic matter removal and flux decline with PEG–TiO2-doped PVDF membranes by integration of ultrafiltration with photocatalysis. J. Membr. Sci. 2012, 405, 48–56. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Hou, J.W.; Uliana, A.; Zhang, Y.T.; Tian, M.M.; van der Bruggen, B. The rapid emergence of two-dimensional nanomaterials for high-performance separation membranes. J. Mater. Chem. A 2018, 6, 3773–3792. [Google Scholar] [CrossRef]




| Membrane No. | Porosity (%) | Water Permeability (L/(m2·h·kPa) | Contact Angle (°) | Zeta Potential (mV) |
|---|---|---|---|---|
| F1 | 35.27 ± 3.71 | 62.91 ± 0.77 | 88.07 ± 0.97 | −25.22 ± 1.83 |
| F2 | 41.21 ± 4.67 | 65.74 ± 1.77 | 87.22 ± 0.23 | −26.66 ± 0.36 |
| F3 | 37.01 ± 4.67 | 64.96 ± 0.27 | 82.81 ± 0.51 | −27.18 ± 0.33 |
| Membrane No. | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| F1 | 32.06 ± 0.68 | 17.65 ± 0.57 |
| F2 | 33.00 ± 1.05 | 11.37 ± 0.35 |
| F3 | 35.06 ± 1.74 | 18.45 ± 0.58 |
| Membrane No. | Rq (nm) | Ra (nm) | Rmax (nm) |
|---|---|---|---|
| F1 | 25.97 ± 1.31 | 20.10 ± 1.18 | 295.50 ± 55.86 |
| F2 | 22.65 ± 1.34 | 17.80 ± 0.99 | 246.00 ± 11.31 |
| F3 | 26.30 ± 2.69 | 20.30 ± 1.13 | 245.00 ± 48.08 |
| BSA | Concentration (g/L) | pH | Size (nm) | |
|---|---|---|---|---|
| 1.0 | 7.0 | 322.9 ± 4.4 | ||
| Membrane/BSA | Zeta Potential (mV) | Contact Angle (°) | ||
| Water | Formamide | Diiodomethane | ||
| F1 | −25.2 ± 1.8 | 88.1 ± 1.0 | 55.8 ± 1.1 | 47.2 ± 0.2 |
| F2 | −26.7 ± 0.4 | 87.2 ± 0.2 | 55.5 ± 0.1 | 48.0 ± 0.1 |
| F3 | −27.2 ± 0.3 | 82.8 ± 0.5 | 47.2 ± 0.7 | 42.2 ± 0.2 |
| BSA | −10.3 ± 0.3 | 66.1 ± 2.4 | 52.7 ± 1.8 | 48.4 ± 2.0 |
| Surface Tension Parameters for Each Membrane and BSA (mJ/m2) | ||||||
| Membrane NO. | γLW | γ+ | γ− | γAB | γTOT | |
| F1 | 35.82 ± 0.10 | 1.11 ± 0.11 | 0.75 ± 0.15 | 0.91 ± 0.12 | 36.73 ± 0.20 | |
| F2 | 35.37 ± 0.03 | 1.16 ± 0.03 | 0.98 ± 0.08 | 1.07 ± 0.03 | 36.44 ± 0.03 | |
| F3 | 38.50 ± 0.10 | 1.78 ± 0.09 | 1.09 ± 0.05 | 1.39 ± 0.06 | 39.89 ± 0.16 | |
| BSA | 35.14 ± 1.13 | 0.28 ± 0.14 | 17.01 ± 1.87 | 2.14 ± 0.61 | 37.28 ± 0.79 | |
| The Free Energy of Cohesion of Membrane (mJ/m2) | The Free Energy of Adhesion of Membranes (mJ/m2) | |||||
| Membrane NO. | ΔG121LW | Δ121GAB | ΔG121SWS | ΔG123LW | ΔG123AB | ΔG123SWS |
| F1 | −3.47 ± 0.04 | −66.97 ± 1.93 | −70.43 ± 1.90 | −3.31 ± 0.02 | −45.43 ± 0.84 | −48.75 ± 0.83 |
| F2 | −3.27 ± 0.01 | −64.53 ± 0.48 | −67.80 ± 0.49 | −3.22 ± 0.01 | −44.21 ± 0.34 | −47.43 ± 0.34 |
| F3 | −4.72 ± 0.05 | −59.57 ± 0.82 | −64.28 ± 0.77 | −3.87 ± 0.02 | −43.25 ± 0.26 | −47.12 ± 0.24 |
| Membrane No. | Free Energy of Cohesion (mJ/m2) | Free Energy of Adhesion (mJ/m2) |
|---|---|---|
| F1 | −70.43 ± 1.90 | −48.75 ± 0.83 |
| F2 | −67.80 ± 0.49 | −47.43 ± 0.34 |
| F3 | −64.28 ± 0.77 | −47.12 ± 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zheng, M.; Zhou, Y.; Yang, L.; Zhang, Y.; Wu, Z.; Liu, G.; Zheng, J. Preparation of Nano-TiO2-Modified PVDF Membranes with Enhanced Antifouling Behaviors via Phase Inversion: Implications of Nanoparticle Dispersion Status in Casting Solutions. Membranes 2022, 12, 386. https://doi.org/10.3390/membranes12040386
Zhang J, Zheng M, Zhou Y, Yang L, Zhang Y, Wu Z, Liu G, Zheng J. Preparation of Nano-TiO2-Modified PVDF Membranes with Enhanced Antifouling Behaviors via Phase Inversion: Implications of Nanoparticle Dispersion Status in Casting Solutions. Membranes. 2022; 12(4):386. https://doi.org/10.3390/membranes12040386
Chicago/Turabian StyleZhang, Jie, Ming Zheng, Yun Zhou, Linlin Yang, Yuanyuan Zhang, Zhichao Wu, Guocong Liu, and Junjian Zheng. 2022. "Preparation of Nano-TiO2-Modified PVDF Membranes with Enhanced Antifouling Behaviors via Phase Inversion: Implications of Nanoparticle Dispersion Status in Casting Solutions" Membranes 12, no. 4: 386. https://doi.org/10.3390/membranes12040386
APA StyleZhang, J., Zheng, M., Zhou, Y., Yang, L., Zhang, Y., Wu, Z., Liu, G., & Zheng, J. (2022). Preparation of Nano-TiO2-Modified PVDF Membranes with Enhanced Antifouling Behaviors via Phase Inversion: Implications of Nanoparticle Dispersion Status in Casting Solutions. Membranes, 12(4), 386. https://doi.org/10.3390/membranes12040386








