Poly(ethylene-co-vinyl alcohol) Electrospun Nanofiber Membranes for Gravity-Driven Oil/Water Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
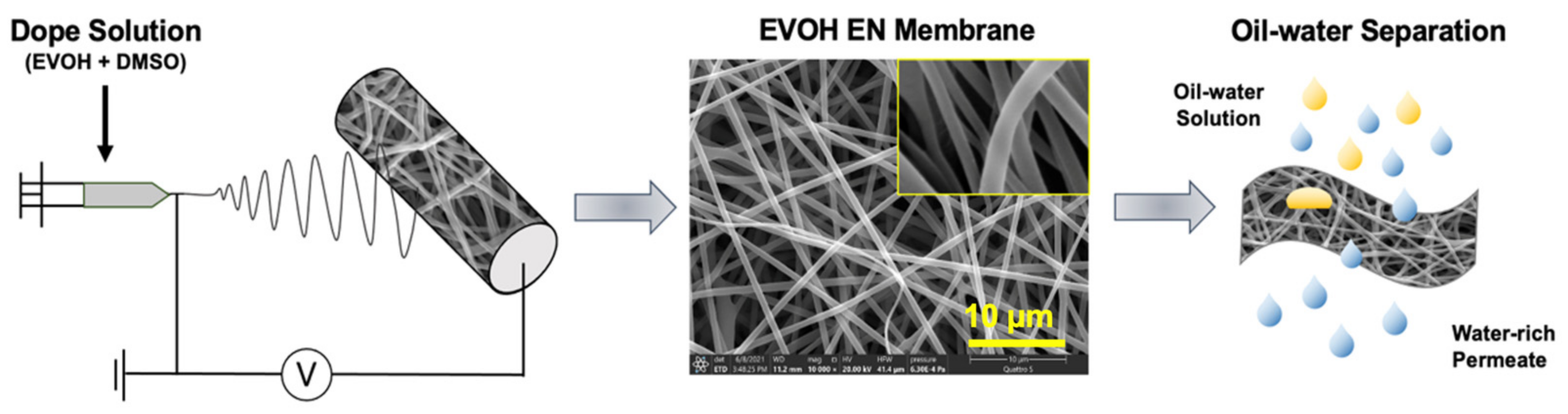
2.2. Preparation of EVOH Electrospun Nanofiber Membranes
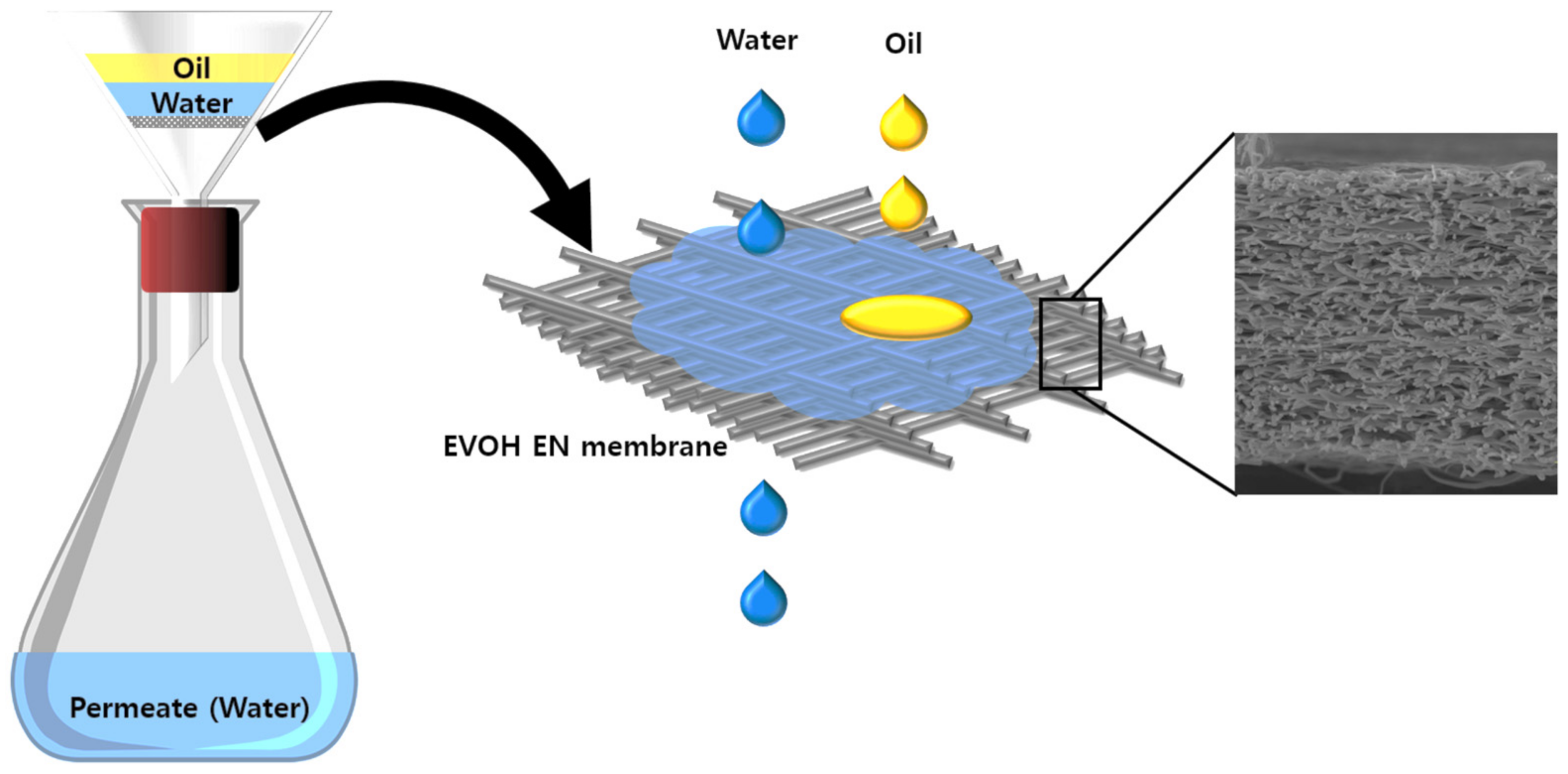
2.3. Separation Setup
2.4. Characterization
3. Results and Discussion
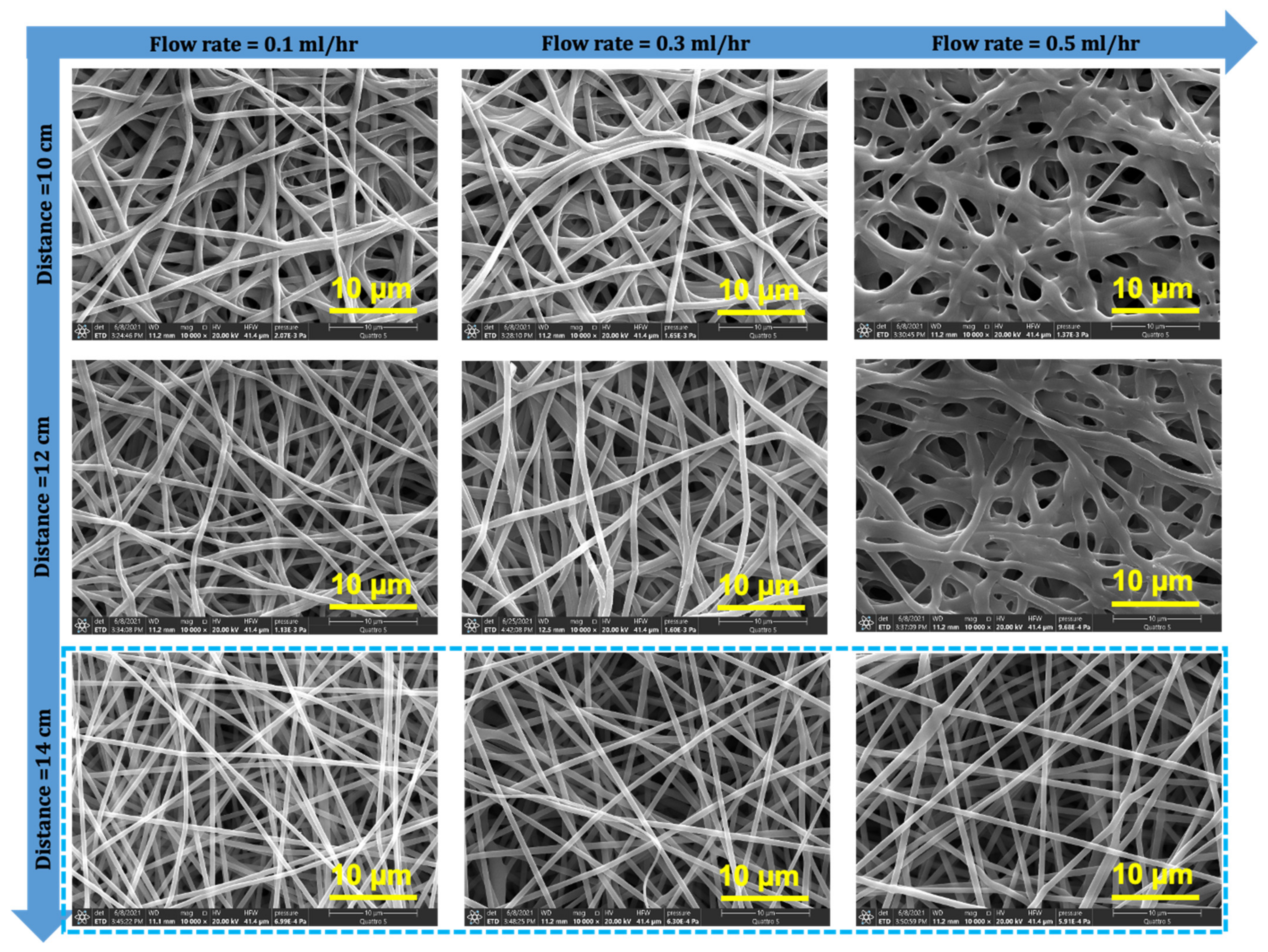
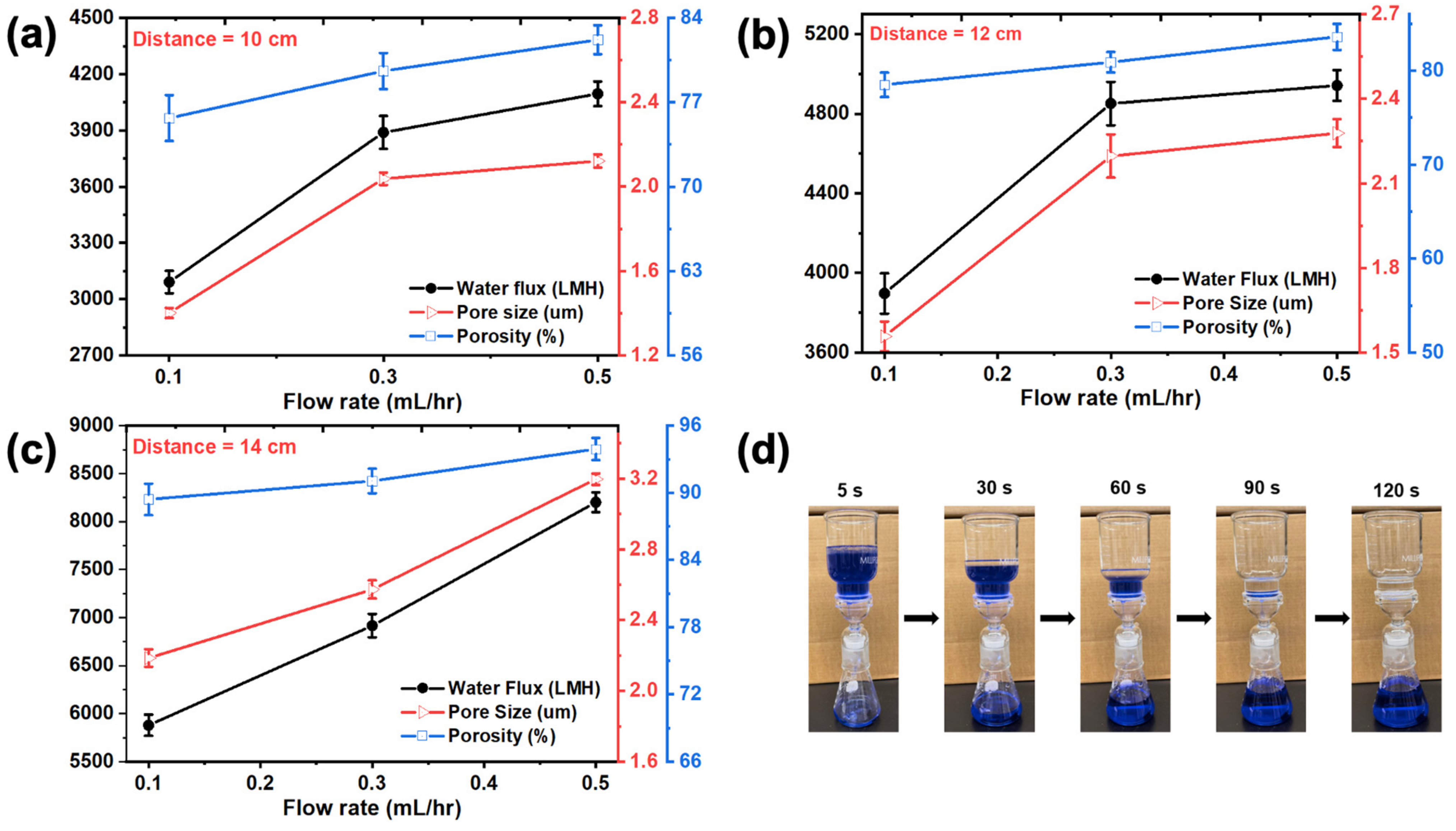
3.1. Optimization of the Electrospinning Conditions
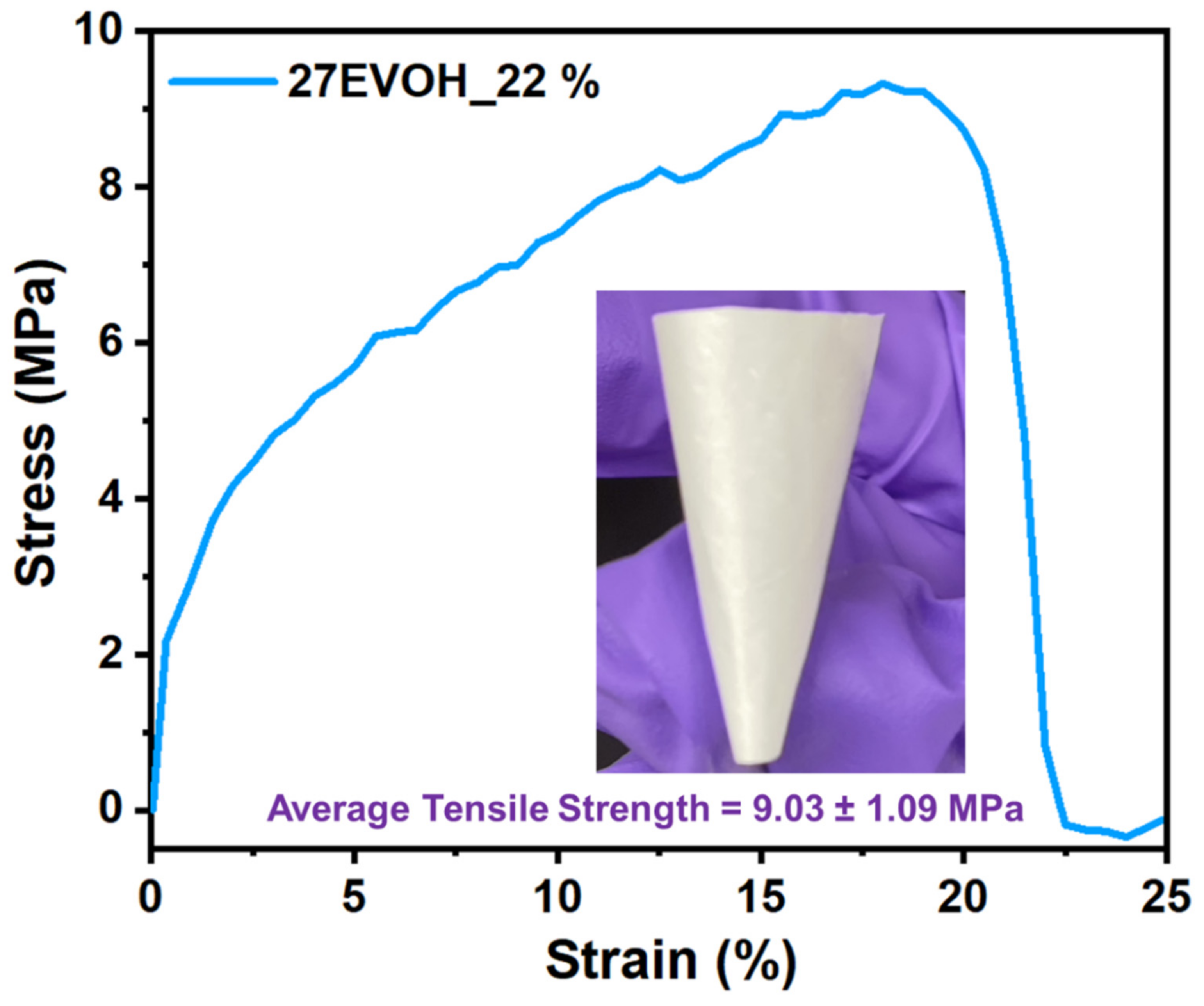
3.2. Mechanical Property Analysis
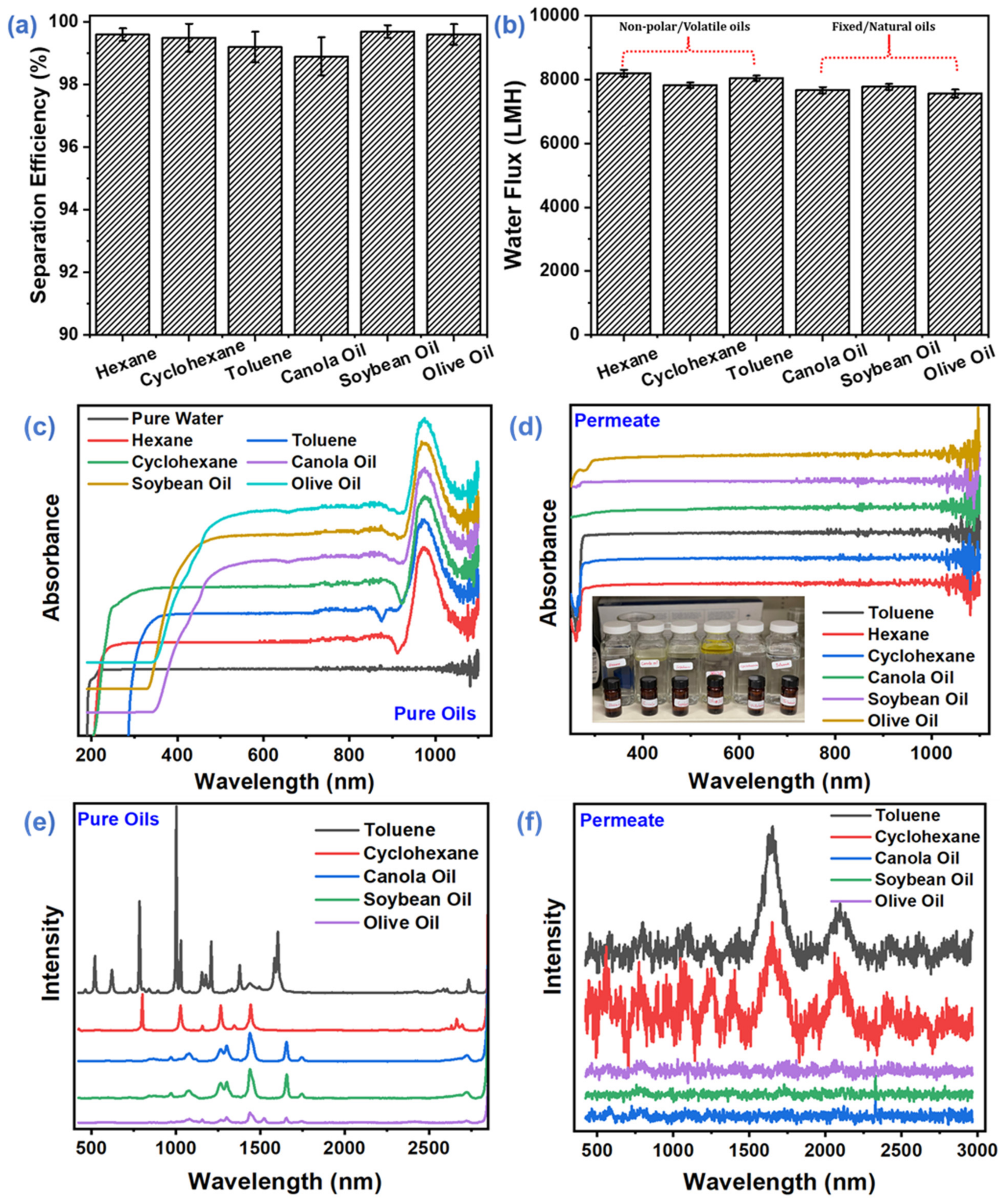
3.3. Membrane Performance Evaluation
3.3.1. Separation Efficiency of Different Oil-Water Systems
3.3.2. Oil-Water Emulsion Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, R.K.; Dunderdale, G.J.; England, M.W.; Hozumi, A. Oil/water separation techniques: A review of recent progresses and future directions. J. Mater. Chem. A 2017, 5, 16025–16058. [Google Scholar] [CrossRef]
- Padaki, M.; Murali, R.; Abdullah, M.; Misdan, N.; Moslehyani, A.; Kassim, M.; Hilal, N.; Ismail, A. Membrane technology enhancement in oil–water separation. A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Chen, P.-C.; Xu, Z.-K. Mineral-Coated Polymer Membranes with Superhydrophilicity and Underwater Superoleophobicity for Effective Oil/Water Separation. Sci. Rep. 2013, 3, 2776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nanosci. Technol. 2010, 452, 337–346. [Google Scholar]
- Elhemmali, A.; Anwar, S.; Zhang, Y.; Shirokoff, J. A comparison of oil-water separation by gravity and electrolysis separation process. Sep. Sci. Technol. 2021, 56, 359–373. [Google Scholar] [CrossRef]
- Barnaby, W. Do nations go to war over water? Nature 2009, 458, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Qi, Y.; Zhang, Y.; Luo, J.; Cui, P.; Jiang, W. A Review on Oil/Water Mixture Separation Material. Ind. Eng. Chem. Res. 2020, 59, 14546–14568. [Google Scholar] [CrossRef]
- Ventikos, N.P.; Vergetis, E.; Psaraftis, H.N.; Triantafyllou, G. A high-level synthesis of oil spill response equipment and countermeasures. J. Hazard. Mater. 2004, 107, 51–58. [Google Scholar] [CrossRef]
- Yu, L.; Han, M.; He, F. A review of treating oily wastewater. Arab. J. Chem. 2017, 10, S1913–S1922. [Google Scholar] [CrossRef] [Green Version]
- Adebajo, M.O.; Frost, R.L.; Kloprogge, J.T.; Carmody, O.; Kokot, S. Porous Materials for Oil Spill Cleanup: A Review of Synthesis and Absorbing Properties. J. Porous Mater. 2003, 10, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Yuying, D. Recent development of super-wettable materials and their applications in oil-water separation. J. Clean. Prod. 2020, 266, 121624–122020. [Google Scholar] [CrossRef]
- Yong, J.; Huo, J.; Chen, F.; Yang, Q.; Hou, X. Oil/water separation based on natural materials with super-wettability: Recent advances. Phys. Chem. Chem. Phys. 2018, 20, 25140–25163. [Google Scholar] [CrossRef]
- Lee, A.; Elam, J.W.; Darling, S.B. Membrane materials for water purification: Design, development, and application. Environ. Sci. Water Res. Technol. 2016, 2, 17–42. [Google Scholar] [CrossRef]
- Abadi, S.R.H.; Sebzari, M.R.; Hemati, M.; Rekabdar, F.; Mohammadi, T. Ceramic membrane performance in microfiltration of oily wastewater. Desalination 2011, 265, 222–228. [Google Scholar] [CrossRef]
- Singh, V.; Purkait, M.K.; Das, C. Cross-Flow Microfiltration of Industrial Oily Wastewater: Experimental and Theoretical Consideration. Sep. Sci. Technol. 2011, 46, 1213–1223. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Willershausen, D.; Ashaghi, K.S.; Engel, L.; Placido, L.; Mund, P.; Bolduan, P.; Czermak, P. Investigations on the use of different ceramic membranes for efficient oil-field produced water treatment. Desalination 2010, 250, 991–996. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Wang, J.-N.; Wu, S.-Z.; Chen, Q.-D.; Zhao, S.; Zhang, H.; Sun, H.-B.; Jiang, L. Three-Level Biomimetic Rice-Leaf Surfaces with Controllable Anisotropic Sliding. Adv. Funct. Mater. 2011, 21, 2927–2932. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, X.; Akbulut, O.; Hu, J.; Suib, S.L.; Kong, J.; Stellacci, F. Superwetting nanowire membranes for selective absorption. Nat. Nanotechnol. 2008, 3, 332–336. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Z.; Mai, Z.; Ma, Y.; Liu, B.; Jiang, L.; Zhu, D. A Super-Hydrophobic and Super-Oleophilic Coating Mesh Film for the Separation of Oil and Water. Angew. Chem. 2004, 43, 2012–2014. [Google Scholar] [CrossRef]
- Hu, B.; Scott, K. Influence of membrane material and corrugation and process conditions on emulsion microfiltration. J. Membr. Sci. 2007, 294, 30–39. [Google Scholar] [CrossRef]
- Tian, D.; Zhang, X.; Wang, X.; Zhai, J.; Jiang, L. Micro/nanoscale hierarchical structured ZnO mesh film for separation of water and oil. Phys. Chem. Chem. Phys. 2011, 13, 14606–14610. [Google Scholar] [CrossRef]
- Cheryan, M.; Rajagopalan, N. Membrane processing of oily streams. Wastewater treatment and waste reduction. J. Membr. Sci. 1998, 151, 13–28. [Google Scholar] [CrossRef]
- Yue, X.; Li, Z.; Zhang, T.; Yang, D.; Qiu, F. Design and fabrication of superwetting fiber-based membranes for oil/water separation applications. Chem. Eng. J. 2019, 364, 292–309. [Google Scholar] [CrossRef]
- Wei, Y.; Qi, H.; Gong, X.; Zhao, S. Specially Wettable Membranes for Oil–Water Separation. Adv. Mater. Interfaces 2018, 5, 1800576. [Google Scholar] [CrossRef]
- Mosadegh, S.; Rodrigue, D.; Brisson, J.; Iliuta, M. Wetting phenomenon in membrane contactors—Causes and prevention. J. Membr. Sci. 2014, 452, 332–353. [Google Scholar] [CrossRef]
- Barredo-Damas, S.; Alcaina-Miranda, M.I.; Bes-Piá, A.; Iborra-Clar, M.I.; Iborra-Clar, A.; Mendoza-Roca, J.A. Ceramic membrane behavior in textile wastewater ultrafiltration. Desalination 2010, 250, 623–628. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Ashaghi, K.S.; Engel, L.; Willershausen, D.; Mund, P.; Bolduan, P.; Czermak, P. Characterization and application of different ceramic membranes for the oil-field produced water treatment. Desalination 2009, 245, 533–540. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Z.; Wei, X.; Zhao, B.; Wang, J.; Yang, S.; Wang, S. Performance improvement of polysulfone ultrafiltration membrane using PANiEB as both pore forming agent and hydrophilic modifier. J. Membr. Sci. 2011, 385, 251–262. [Google Scholar] [CrossRef]
- Obaid, M.; Barakat, N.A.M.; Fadali, O.A.; Motlak, M.; Almajid, A.A.; Khalil, K.A. Effective and reusable oil/water separation membranes based on modified polysulfone electrospun nanofiber mats. Chem. Eng. J. 2015, 259, 449–456. [Google Scholar] [CrossRef]
- Teow, Y.H.; Ahmad, A.L.; Lim, J.K.; Ooi, B.S. Preparation and characterization of PVDF/TiO2 mixed matrix membrane via in situ colloidal precipitation method. Desalination 2012, 295, 61–69. [Google Scholar] [CrossRef]
- Yu, S.; Zuo, X.; Bao, R.; Xu, X.; Wang, J.; Xu, J. Effect of SiO2 nanoparticle addition on the characteristics of a new organic–inorganic hybrid membrane. Polymer 2009, 50, 553–559. [Google Scholar] [CrossRef]
- Gunawan, P.; Guan, C.; Song, X.; Zhang, Q.; Leong, S.S.J.; Tang, C.; Chen, Y.; Chan-Park, M.B.; Chang, M.W.; Wang, K.; et al. Hollow Fiber Membrane Decorated with Ag/MWNTs: Toward Effective Water Disinfection and Biofouling Control. ACS Nano 2011, 5, 10033–10040. [Google Scholar] [CrossRef] [PubMed]
- Medina-Gonzalez, Y.; Remigy, J.-C. Sonication-assisted preparation of pristine MWCNT–polysulfone conductive microporous membranes. Mater. Lett. 2011, 65, 229–232. [Google Scholar] [CrossRef]
- Khayet, M.; Villaluenga, J.; Valentin, J.; López-Manchado, M.; Mengual, J.; Seoane, B.J.P. Filled poly(2,6-dimethyl-1,4-phenylene oxide) dense membranes by silica and silane modified silica nanoparticles: Characterization and application in pervaporation. Polymer 2005, 46, 9881–9891. [Google Scholar] [CrossRef]
- Pan, Z.; Cao, S.; Li, J.; Du, Z.; Cheng, F. Anti-fouling TiO2 nanowires membrane for oil/water separation: Synergetic effects of wettability and pore size. J. Membr. Sci. 2019, 572, 596–606. [Google Scholar] [CrossRef]
- Qian, D.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. TiO2/sulfonated graphene oxide/Ag nanoparticle membrane: In situ separation and photodegradation of oil/water emulsions. J. Membr. Sci. 2018, 554, 16–25. [Google Scholar] [CrossRef]
- Lin, Q.; Zeng, G.; Yan, G.; Luo, J.; Cheng, X.; Zhao, Z.; Li, H. Self-cleaning photocatalytic MXene composite membrane for synergistically enhanced water treatment: Oil/water separation and dyes removal. Chem. Eng. J. 2022, 427, 131668. [Google Scholar] [CrossRef]
- Nasreen, S.A.A.N.; Sundarrajan, S.; Nizar, S.A.S.; Balamurugan, R.; Ramakrishna, S. Advancement in electrospun nanofibrous membranes modification and their application in water treatment. Membranes 2013, 3, 266–284. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Barhate, R.S.; Loong, C.K.; Ramakrishna, S. Preparation and characterization of nanofibrous filtering media. J. Membr. Sci. 2006, 283, 209–218. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, Q.; Hua, D.; Xiong, R.; Zhao, J.; Rao, W.; Huang, S.; Zhan, X.; Chen, F.; Huang, C.J.R.A. Electrospun fibers for oil–water separation. RSC Adv. 2016, 6, 12868–12884. [Google Scholar] [CrossRef]
- Tarus, B.; Fadel, N.; Al-Oufy, A.; El-Messiry, M. Effect of polymer concentration on the morphology and mechanical characteristics of electrospun cellulose acetate and poly (vinyl chloride) nanofiber mats. Alex. Eng. J. 2016, 55, 2975–2984. [Google Scholar] [CrossRef] [Green Version]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Tan, N.C.B. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Jacobs, V.; Anandjiwala, R.D.; Maaza, M. The influence of electrospinning parameters on the structural morphology and diameter of electrospun nanofibers. J. Appl. Polym. Sci. 2010, 115, 3130–3136. [Google Scholar] [CrossRef]
- Mokwena, K.; Tang, J. Ethylene Vinyl Alcohol: A Review of Barrier Properties for Packaging Shelf Stable Foods. Crit. Rev. Food Sci. Nutr. 2012, 52, 640–650. [Google Scholar] [CrossRef]
- Robertson, G.L. Food Packaging: Principles and Practice; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Jabur, A.; Najim, M.; Rahman, S. Study the effect of flow rate on some physical properties of different polymeric solutions. J. Phys. Conf. Ser. 2018, 1003, 012069. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C. Effects of Working Parameters on Electrospinning. In One-Dimensional Nanostructures: Electrospinning Technique and Unique Nanofibers; Li, Z., Wang, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 15–28. [Google Scholar]
- Mazoochi, T.; Hamadanian, M.; Ahmadi, M.; Jabbari, V. Investigation on the morphological characteristics of nanofiberous membrane as electrospun in the different processing parameters. Int. J. Ind. Chem. 2012, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Rnjak-Kovacina, J.; Wise, S.G.; Li, Z.; Maitz, P.K.; Young, C.J.; Wang, Y.; Weiss, A.S. Tailoring the porosity and pore size of electrospun synthetic human elastin scaffolds for dermal tissue engineering. Biomaterials 2011, 32, 6729–6736. [Google Scholar] [CrossRef]
- Garg, K.; Sell, S.A.; Madurantakam, P.; Bowlin, G.L. Angiogenic potential of human macrophages on electrospun bioresorbable vascular grafts. Biomed. Mater. 2009, 4, 031001. [Google Scholar] [CrossRef] [Green Version]
- Lifshutz, N. On the ‘Mean Flow’ Pore Size Distribution of Microfiber and Nanofiber Webs. Int. Non-Wovens J. 2005, 14, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, F.E.; Lalia, B.S.; Hilal, N.; Hashaikeh, R. Underwater superoleophobic cellulose/electrospun PVDF–HFP membranes for efficient oil/water separation. Desalination 2014, 344, 48–54. [Google Scholar] [CrossRef]
- Tai, M.H.; Gao, P.; Tan, B.Y.L.; Sun, D.D.; Leckie, J.O. Highly Efficient and Flexible Electrospun Carbon–Silica Nanofibrous Membrane for Ultrafast Gravity-Driven Oil–Water Separation. ACS Appl. Mater. Interfaces 2014, 6, 9393–9401. [Google Scholar] [CrossRef]
- Zhang, G.; Li, L.; Huang, Y.; Hozumi, A.; Sonoda, T.; Su, Z. Fouling-resistant membranes for separation of oil-in-water emulsions. RSC Adv. 2018, 8, 5306–5311. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Lim, Y.; Choi, W. One-Step Synthesis of Environmentally Friendly Superhydrophilic and Superhydrophobic Sponges for Oil/Water Separation. Materials 2019, 12, 1182. [Google Scholar] [CrossRef] [Green Version]
- Yue, X.; Li, J.; Zhang, T.; Qiu, F.; Yang, D.; Xue, M. In situ one-step fabrication of durable superhydrophobic-superoleophilic cellulose/LDH membrane with hierarchical structure for efficiency oil/water separation. Chem. Eng. J. 2017, 328, 117–123. [Google Scholar] [CrossRef]
- Song, J.; Li, S.; Zhao, C.; Lu, Y.; Zhao, D.; Sun, J.; Roy, T.; Carmalt, C.J.; Deng, X.; Parkin, I.P. A superhydrophilic cement-coated mesh: An acid, alkali, and organic reagent-free material for oil/water separation. Nanoscale 2018, 10, 1920–1929. [Google Scholar] [CrossRef]
- Shi, H.; He, Y.; Pan, Y.; Di, H.; Zeng, G.; Zhang, L.; Zhang, C. A modified mussel-inspired method to fabricate TiO2 decorated superhydrophilic PVDF membrane for oil/water separation. J. Membr. Sci. 2016, 506, 60–70. [Google Scholar] [CrossRef]
- Sadler, E.; Crick, C.R. Suction or gravity-fed oil-water separation using PDMS-coated glass filters. Sustain. Mater. Technol. 2021, 29, e00321. [Google Scholar] [CrossRef]
- Ren, C.; Chen, W.; Chen, C.; Winnubst, L.; Yan, L. Gravity-Driven Separation of Oil/Water Mixture by Porous Ceramic Membranes with Desired Surface Wettability. Materials 2021, 14, 457. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, W.; Wu, S.; Tang, G.; Cui, J.; Zhang, Q.; Chen, F.; Xiong, R.; Huang, C. Electrospun frogspawn structured membrane for gravity-driven oil-water separation. J. Colloid Interface Sci. 2019, 547, 136–144. [Google Scholar] [CrossRef]
- Wang, F.-p.; Zhao, X.-j.; Wahid, F.; Zhao, X.-q.; Qin, X.-t.; Bai, H.; Xie, Y.-y.; Zhong, C.; Jia, S.-r. Sustainable, superhydrophobic membranes based on bacterial cellulose for gravity-driven oil/water separation. Carbohydr. Polym. 2021, 253, 117220. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.; Wu, Y.; Liu, Y.; Xue, C.; Ding, G.; Cheng, G.; Cui, J.; Pan, J. Robust antifouling NH2-MIL-88B coated quartz fibrous membrane for efficient gravity-driven oil-water emulsion separation. J. Membr. Sci. 2022, 644, 120093. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, T.C.; Wei, B.; Chen, S.; Liang, Y.; Yuan, S. Durable CNTs Reinforced Porous Electrospun Superhydrophobic Membrane for Efficient Gravity Driven Oil/Water Separation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125342. [Google Scholar] [CrossRef]



| Dope Solution for Electrospinning | Electrospinning Conditions | |||
|---|---|---|---|---|
| ID | Polymer (wt%) | DMSO (wt%) | Parameter | Number |
| 44EVOH_20% | 20 | 80 | Voltage (kV) | 12 |
| 44EVOH_22% | 22 | 78 | Distance (cm) | 12 |
| 44EVOH_24% | 24 | 76 | Flow rate (mL/h) | 0.1 |
| Membrane Material | Method | Oil-Water System | Flux (L m−2 h−1) | Pore Size (µm) | Cycle Numbers | Reference |
|---|---|---|---|---|---|---|
| EVOH ENM | Gravity | Water/ n-hexane | 8200.3 ± 103.2 | 3.2 ± 0.032 | 20 | This work |
| MFS/CC-DKGM | Filtration | Water/ n-hexane | 4702 | - | 20 | [56] |
| Cellulose fiber/LDH | Gravity | Water/ Chloroform | 4968 | ~0.15 | 50 | [57] |
| CU mesh/ Cement | Gravity | Water/ n-hexane | 5000 | - | 1 | [58] |
| TiO2-PVDF | Vacuum F. | Water/ n-hexane | 785 | - | 3 | [59] |
| PDMS glass filter | Gravity | Water/ Chloroform | 6351 | 1.6 | 11 | [60] |
| Al2O3 membranes | Gravity | Water/Octane | 14.1 L m−2 s−1 kPa−1 | 15~30 | 1 | [61] |
| PDMS-PI membrane | Gravity | Water/DCM | 4443.2 ± 70 | - | 20 | [62] |
| BN-CuSA2 membrane | Gravity | Water/DCM | 1667.63 | 0.00306 | 10 | [63] |
| NM88B@QFM | Gravity | Water/ n-hexane | 489 | 1.148 | 15 | [64] |
| CNTs@PVDF | Gravity | Water/ Chloroform | 6600 | 0.0124 | 15 | [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, A.A.; Yoo, Y.; Park, A.; Cho, Y.H.; Park, Y.-I.; Park, H. Poly(ethylene-co-vinyl alcohol) Electrospun Nanofiber Membranes for Gravity-Driven Oil/Water Separation. Membranes 2022, 12, 382. https://doi.org/10.3390/membranes12040382
Shah AA, Yoo Y, Park A, Cho YH, Park Y-I, Park H. Poly(ethylene-co-vinyl alcohol) Electrospun Nanofiber Membranes for Gravity-Driven Oil/Water Separation. Membranes. 2022; 12(4):382. https://doi.org/10.3390/membranes12040382
Chicago/Turabian StyleShah, Aatif Ali, Youngmin Yoo, Ahrumi Park, Young Hoon Cho, You-In Park, and Hosik Park. 2022. "Poly(ethylene-co-vinyl alcohol) Electrospun Nanofiber Membranes for Gravity-Driven Oil/Water Separation" Membranes 12, no. 4: 382. https://doi.org/10.3390/membranes12040382
APA StyleShah, A. A., Yoo, Y., Park, A., Cho, Y. H., Park, Y.-I., & Park, H. (2022). Poly(ethylene-co-vinyl alcohol) Electrospun Nanofiber Membranes for Gravity-Driven Oil/Water Separation. Membranes, 12(4), 382. https://doi.org/10.3390/membranes12040382








