Foulant Identification and Performance Evaluation of Antiscalants in Increasing the Recovery of a Reverse Osmosis System Treating Anaerobic Groundwater
Abstract
:1. Introduction
- (a)
- To identify the foulant/scalants that would precipitate in the RO unit at 85% recovery.
- (b)
- To examine the role of antiscalants in increasing the RO recovery to at least 85%.
2. Materials and Methods
2.1. Feedwater (Anaerobic GW) Composition
Tested Antiscalants to Increase RO Recovery to at Least 85%
2.2. RO Pilot
2.3. Foulant Characterization
2.4. Lab-Scale RO Unit
3. Results and Discussion
3.1. Maximum Achievable Recovery Based on Antiscalant Suppliers’ Projection Programs
3.2. Foulant (Scalant) Characterization
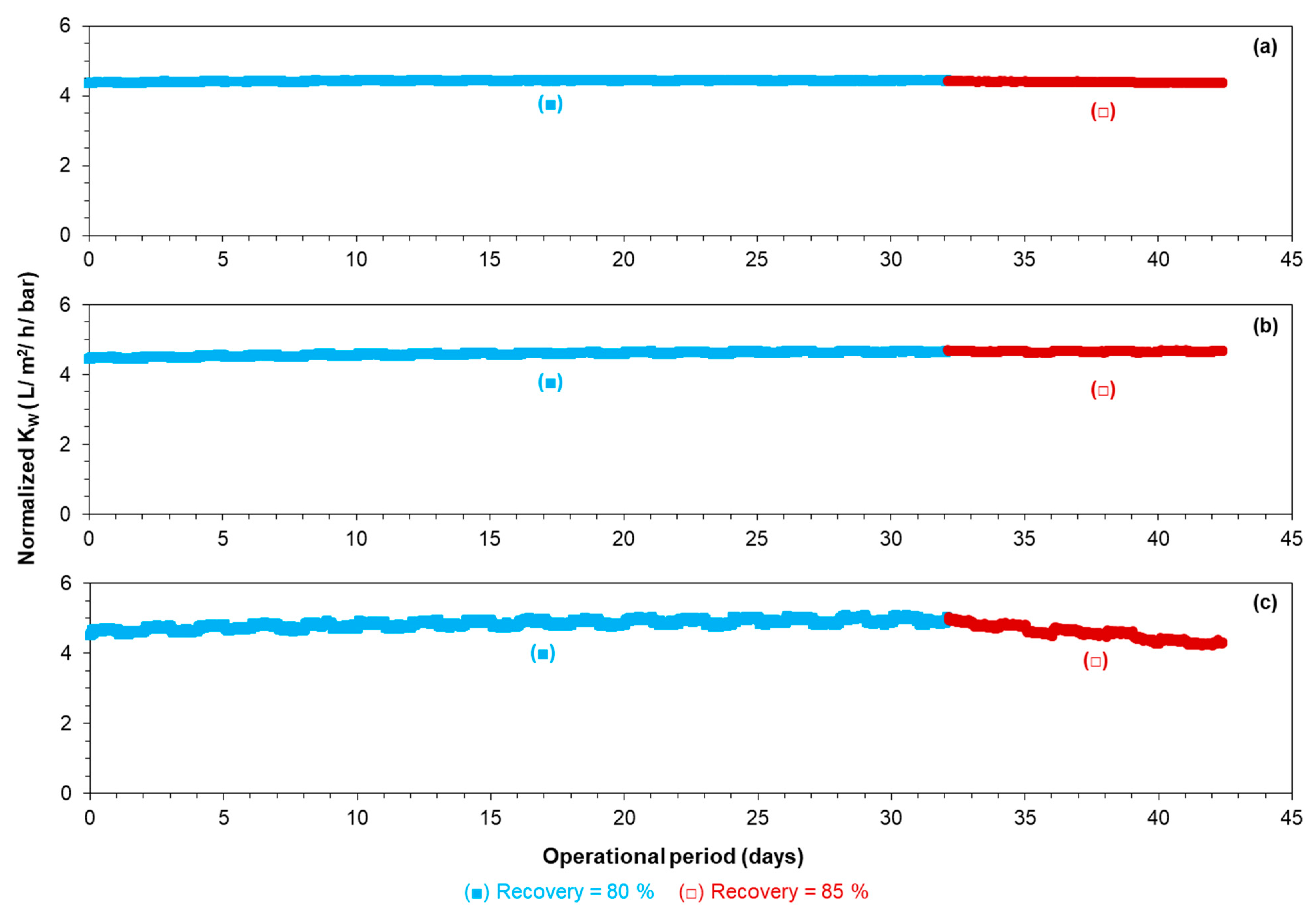
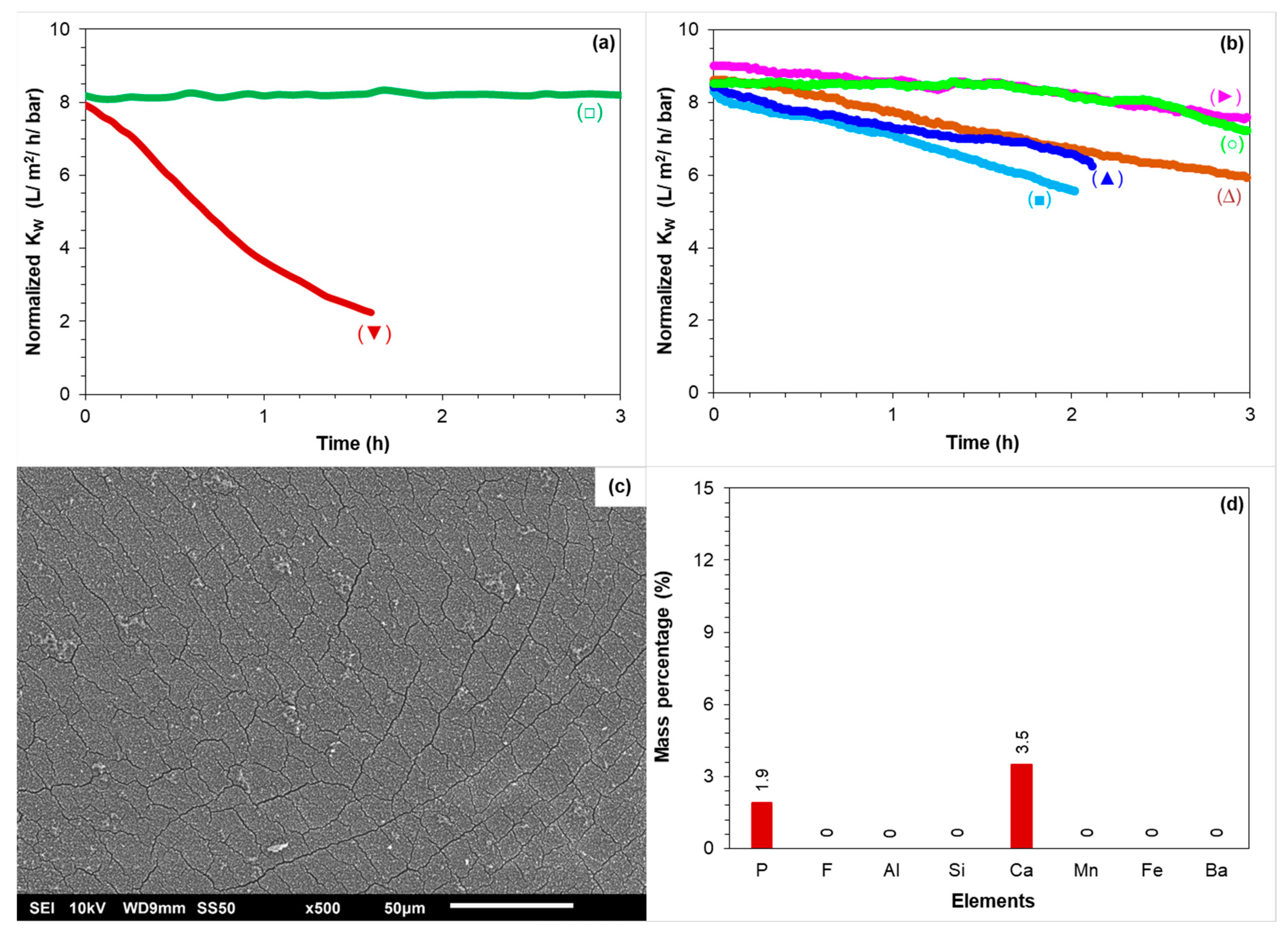
3.2.1. RO Operation at 80–85% Recoveries in the Absence of Antiscalants
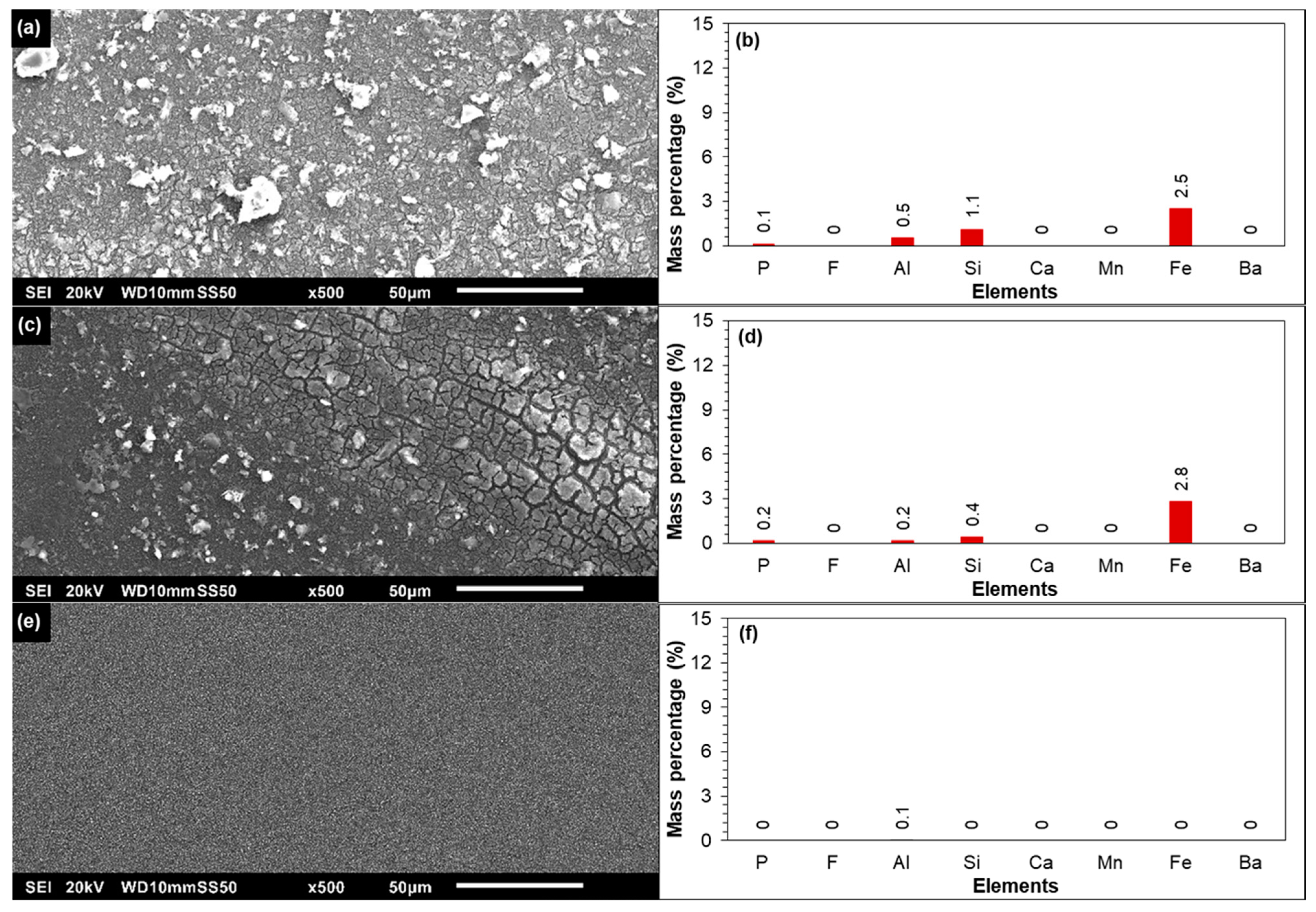
3.2.2. Membrane Autopsy
SEM-EDX of the Tail Element of the First Stage
SEM-EDX of the Tail Element of the Third Stage
XPS Analysis of the Tail Element of the Third Stage
FEEM Analysis of the 0.05 M HCl and 0.05 M NaOH Cleaning Solutions
3.3. Role of Antiscalants in Increasing RO Recovery to 85%
3.3.1. RO Pilot Operation at 85% Recovery with Various Antiscalants
3.3.2. Lab-Scale RO Tests with the Synthetic Concentrate of 85% Recovery with Various Antiscalants
4. Conclusions
- The maximum achievable recovery and the scalant limiting the RO recovery varied according to the projection programs of the different antiscalant suppliers, with some pointing to calcium carbonate and others to calcium phosphate as the limiting scaling compound. The maximum achievable recovery according to antiscalant suppliers was ranging between 77% and 89%.
- Operation of the RO at 80–85% recoveries without antiscalant:
- The normalized permeability of the third stage remained constant during a 1 month experimental period when the RO pilot was operated at 80% recovery without antiscalant, whereas the normalized permeability of the third stage decreased when the RO pilot was operated at 85% recovery without antiscalant.
- Membrane autopsy of the tail element of the third stage:
- Calcium phosphate was the main scalant causing permeability decline at 85% recovery and limiting the RO recovery.
- Calcium carbonate was not responsible for the permeability decline of the third stage at 85% recovery.
- Role of antiscalants in increasing the RO recovery to 85% (and higher):
- In the RO pilot measurements, the tested antiscalants were found to be ineffective in increasing the RO recovery to 85% as the permeability of the third stage decreased with each of the tested antiscalants.
- In once-through lab-scale RO tests, none of the tested antiscalants could prevent calcium phosphate scaling when the RO element was fed with the synthetic concentrate of 85% recovery.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beyer, F.; Rietman, B.M.; Zwijnenburg, A.; van den Brink, P.; Vrouwenvelder, J.S.; Jarzembowska, M.; Laurinonyte, J.; Stams, A.J.M.; Plugge, C.M. Long-term performance and fouling analysis of full-scale direct nanofiltration (NF) installations treating anoxic groundwater. J. Membr. Sci. 2014, 468, 339–348. [Google Scholar] [CrossRef]
- Hiemstra, P.; Kolpa, R.; Eekhout, J.M.J.M.; Kessel, T.; Adamse, E.; Paassen, J. ’Natural’ recharge of groundwater: Bank infiltration in the Netherlands. J. Water Supply Res. Technol.-AQUA 2003, 52, 37–47. [Google Scholar] [CrossRef]
- de Vet, W.; Genuchten, C.; van Loosdrecht, M.; Dijk, J. Water quality and treatment of river bank filtrate. Drink. Water Eng. Sci. Discuss. 2009, 3, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Kucera, J. Reverse Osmosis Membrane Fouling Control. In The Science and Technology of Industrial Water Treatment; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- Tang, C.Y.; Chong, T.H.; Fane, A.G. Colloidal interactions and fouling of NF and RO membranes: A review. Adv. Coll. Interface Sci. 2011, 164, 126–143. [Google Scholar] [CrossRef] [PubMed]
- Fridjonsson, E.O.; Vogt, S.J.; Vrouwenvelder, J.S.; Johns, M.L. Early non-destructive biofouling detection in spiral wound RO membranes using a mobile earth׳s field NMR. J. Membr. Sci. 2015, 489, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, Y.; Lienhard, J.H. How RO membrane permeability and other performance factors affect process cost and energy use: A review. Desalination 2019, 470, 114064. [Google Scholar] [CrossRef]
- Flemming, H.C. Mechanistic aspects of reverse osmosis membrane biofouling and prevention. Reverse Osm. 1993, 163–209. [Google Scholar]
- Potts, D.E.; Ahlert, R.C.; Wang, S.S. A critical review of fouling of reverse osmosis membranes. Desalination 1981, 36, 235–264. [Google Scholar] [CrossRef]
- Pandey, S.R.; Jegatheesan, V.; Baskaran, K.; Shu, L. Fouling in reverse osmosis (RO) membrane in water recovery from secondary effluent: A review. Rev. Environ. Sci. Biol./Technol. 2012, 11, 125–145. [Google Scholar] [CrossRef]
- Rodriguez, S.G.S. Particulate and Organic Matter Fouling of Seawater Reverse Osmosis Systems: Characterization, Modelling and Applications. UNESCO-IHE PhD Thesis; CRC Press/Balkema: Delft, The Netherlands, 2011. [Google Scholar]
- Martínez, C.; Gómez, V.; Pocurull, E.; Borrull, F. Characterization of organic fouling in reverse osmosis membranes by headspace solid phase microextraction and gas chromatography–mass spectrometry. Water Sc. Technol. 2014, 71, 117–125. [Google Scholar] [CrossRef]
- Xu, P.; Drewes, J.E.; Kim, T.-U.; Bellona, C.; Amy, G. Effect of membrane fouling on transport of organic contaminants in NF/RO membrane applications. J. Membr. Sci. 2006, 279, 165–175. [Google Scholar] [CrossRef]
- Vrouwenvelder, J.S.; Graf von der Schulenburg, D.A.; Kruithof, J.C.; Johns, M.L.; van Loosdrecht, M.C.M. Biofouling of spiral-wound nanofiltration and reverse osmosis membranes: A feed spacer problem. Water Res. 2009, 43, 583–594. [Google Scholar] [CrossRef]
- Vrouwenvelder, H.S.; van Paassen, J.A.M.; Folmer, H.C.; Hofman, J.A.M.H.; Nederlof, M.M.; van der Kooij, D. Biofouling of membranes for drinking water production. Desalination 1998, 118, 157–166. [Google Scholar] [CrossRef]
- Flemming, H.-C. Reverse osmosis membrane biofouling. Exp. Therm. Fluid Sci. 1997, 14, 382–391. [Google Scholar] [CrossRef]
- Hiemstra, P.; Van Paassen, J.; Rietman, B.; Verdouw, J. Aerobic versus anaerobic nanofiltration: Fouling of membranes. In Proceedings of the AWWA Membrane Conference, Long Beach, CA, USA, 28 February–3 March 1999. [Google Scholar]
- Ruiz-García, A.; Feo-García, J. Estimation of maximum water recovery in RO desalination for different feedwater inorganic compositions. Desalin. Water Treat. 2017, 70, 34–45. [Google Scholar] [CrossRef]
- Ruiz-García, A.; Feo-García, J. Antiscalant cost and maximum water recovery in reverse osmosis for different inorganic composition of groundwater. Desalin. Water Treat. 2017, 73, 46–53. [Google Scholar] [CrossRef]
- Antony, A.; Low, J.H.; Gray, S.; Childress, A.E.; Le-Clech, P.; Leslie, G. Scale formation and control in high pressure membrane water treatment systems: A review. J. Membr. Sci. 2011, 383, 1–16. [Google Scholar] [CrossRef]
- Tzotzi, C.; Pahiadaki, T.; Yiantsios, S.G.; Karabelas, A.J.; Andritsos, N. A study of CaCO3 scale formation and inhibition in RO and NF membrane processes. J. Membr. Sci. 2007, 296, 171–184. [Google Scholar] [CrossRef]
- Koutsoukos, P. Calcium carbonate scale control in industrial water systems. In The Science and Technology of Industrial Water Treatment; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- Chesters, S.P. Innovations in the inhibition and cleaning of reverse osmosis membrane scaling and fouling. Desalination 2009, 238, 22–29. [Google Scholar] [CrossRef]
- Greenberg, G.; Hasson, D.; Semiat, R. Limits of RO recovery imposed by calcium phosphate precipitation. Desalination 2005, 183, 273–288. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates (CaPO4): Occurrence and properties. Prog. Biomater. 2016, 5, 9–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangal, M.N.; Salinas-Rodriguez, S.G.; Dusseldorp, J.; Kemperman, A.J.B.; Schippers, J.C.; Kennedy, M.D.; van der Meer, W.G.J. Effectiveness of antiscalants in preventing calcium phosphate scaling in reverse osmosis applications. J. Membr. Sci. 2021, 623, 119090. [Google Scholar] [CrossRef]
- Neofotistou, E.; Demadis, K.D. Use of antiscalants for mitigation of silica (SiO2) fouling and deposition: Fundamentals and applications in desalination systems. Desalination 2004, 167, 257–272. [Google Scholar] [CrossRef]
- Luo, M.; Wang, Z. Complex fouling and cleaning-in-place of a reverse osmosis desalination system. Desalination 2001, 141, 15–22. [Google Scholar] [CrossRef]
- Gallego, S.; Vigo, F.d.; Chesters, S.; Buñuel, P.L. Practical experience with high silica concentration in RO waters. In Proceedings of the WIM International Congress, Santiago, Chile, 9–11 July 2008. [Google Scholar]
- Lee, S.; Kim, J.; Lee, C.-H. Analysis of CaSO4 scale formation mechanism in various nanofiltration modules. J. Membr. Sci. 1999, 163, 63–74. [Google Scholar] [CrossRef]
- Pervov, A.G. Scale formation prognosis and cleaning procedure schedules in reverse osmosis systems operation. Desalination 1991, 83, 77–118. [Google Scholar] [CrossRef]
- Greenlee, L.F.; Testa, F.; Lawler, D.F.; Freeman, B.D.; Moulin, P. The effect of antiscalant addition on calcium carbonate precipitation for a simplified synthetic brackish water reverse osmosis concentrate. Water Res. 2010, 44, 2957–2969. [Google Scholar] [CrossRef]
- Chen, T.; Chen, P.; Montgomerie, H.; Hagen, T.; Leschied, C. Calcium Carbonate: Polymorph Stabilization in the Presence of Inhibitors. In The Science and Technology of Industrial Water Treatment; IWA Publishing: London, UK, 2009; pp. 61–79. [Google Scholar]
- Drak, A.; Glucina, K.; Busch, M.; Hasson, D.; Laîne, J.-M.; Semiat, R. Laboratory technique for predicting the scaling propensity of RO feed waters. Desalination 2000, 132, 233–242. [Google Scholar] [CrossRef]
- van Engelen, G.; Nolles, R. A sustainable antiscalant for RO processes. Desalin. Water Treat. 2013, 51, 921–923. [Google Scholar] [CrossRef]
- Mangal, M.N.; Salinas-Rodriguez, S.G.; Blankert, B.; Yangali-Quintanilla, V.A.; Schippers, J.C.; van der Meer, W.G.J.; Kennedy, M.D. Role of phosphate and humic substances in controlling calcium carbonate scaling in a groundwater reverse osmosis system. J. Environ. Chem. Eng. 2021, 9, 105651. [Google Scholar] [CrossRef]
- Boerlage, Ś.F.E.; Kennedy, M.D.; Bremere, I.; Witkamp, G.J.; van der Hoek, J.P.; Schippers, J.C. Stable barium sulphate supersaturation in reverse osmosis. J. Membr. Sci. 2000, 179, 53–68. [Google Scholar] [CrossRef]
- Hoch, A.R.; Reddy, M.M.; Aiken, G.R. Calcite crystal growth inhibition by humic substances with emphasis on hydrophobic acids from the Florida Everglades. Geochim. Et Cosmochim. Acta 2000, 64, 61–72. [Google Scholar] [CrossRef]
- Zuddas, P.; Pachana, K.; Faivre, D. The influence of dissolved humic acids on the kinetics of calcite precipitation from seawater solutions. Chem. Geol. 2003, 201, 91–101. [Google Scholar] [CrossRef]
- Klepetsanis, P.G.; Kladi, A.; Ostvold, T.; Kontoyiannis, C.G.; Koutsoukos, P.G.; Amjad, Z.; Reddy, M.M. The inhibition of calcium carbonate formation in aqueous supersaturated solutions, spontaneous precipitation and seeded crystal growth. In Advances in Crystal Growth Inhibition Technologies; Amjad, Z., Ed.; Springer: Boston, MA, USA, 2002; pp. 123–137. [Google Scholar]
- Amjad, Z.; Pugh, J.; Reddy, M. Kinetic inhibition of calcium carbonate crystal growth in the presence of natural and synthetic organic inhibitors. In Water Soluble Polymers: Solution Properties and Application; Plenum Press: New York, NY, USA, 1998; pp. 131–147. [Google Scholar]
- Inskeep, W.P.; Bloom, P.R. Kinetics of calcite precipitation in the presence of water-soluble organic ligands. Soil Sci. Soc. Am. J. 1986, 50, 1167–1172. [Google Scholar] [CrossRef]
- NIST Standard Reference Database Number 20; NIST X-ray Photoelectron Spectroscopy Database. National Institute of Standards and Technology: Gaithersburg, MD, USA, 2000.
- Tanaka, R.; Samu, Y.; Suzuki, T.; Niinae, M.; Lin, L.; Luh, J.; Coronell, O. The effects of humic acid fouling on the performane of polyamide composite reverse osmosis membranes. J. MMIJ 2016, 132, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Kwon, Y.-N.; Leckie, J. Characterization of humic acid fouled reverse osmosis and nanofiltration membranes by transmission electron microscopy and streaming potential measurements. Environ. Sci. Technol. 2007, 41, 942–949. [Google Scholar] [CrossRef]
- Tang, C.Y.; Kwon, Y.-N.; Leckie, J.O. Fouling of reverse osmosis and nanofiltration membranes by humic acid—effects of solution composition and hydrodynamic conditions. J. Membr. Sci. 2007, 290, 86–94. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, Y.; Zhang, X.; Tong, X.; Yu, T.; Wang, Y.; Ikuno, N.; Ishii, K.; Hu, H. Ozonation as an efficient pretreatment method to alleviate reverse osmosis membrane fouling caused by complexes of humic acid and calcium ion. Front. Environ. Sci. Eng. 2019, 13, 55. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence excitation−emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
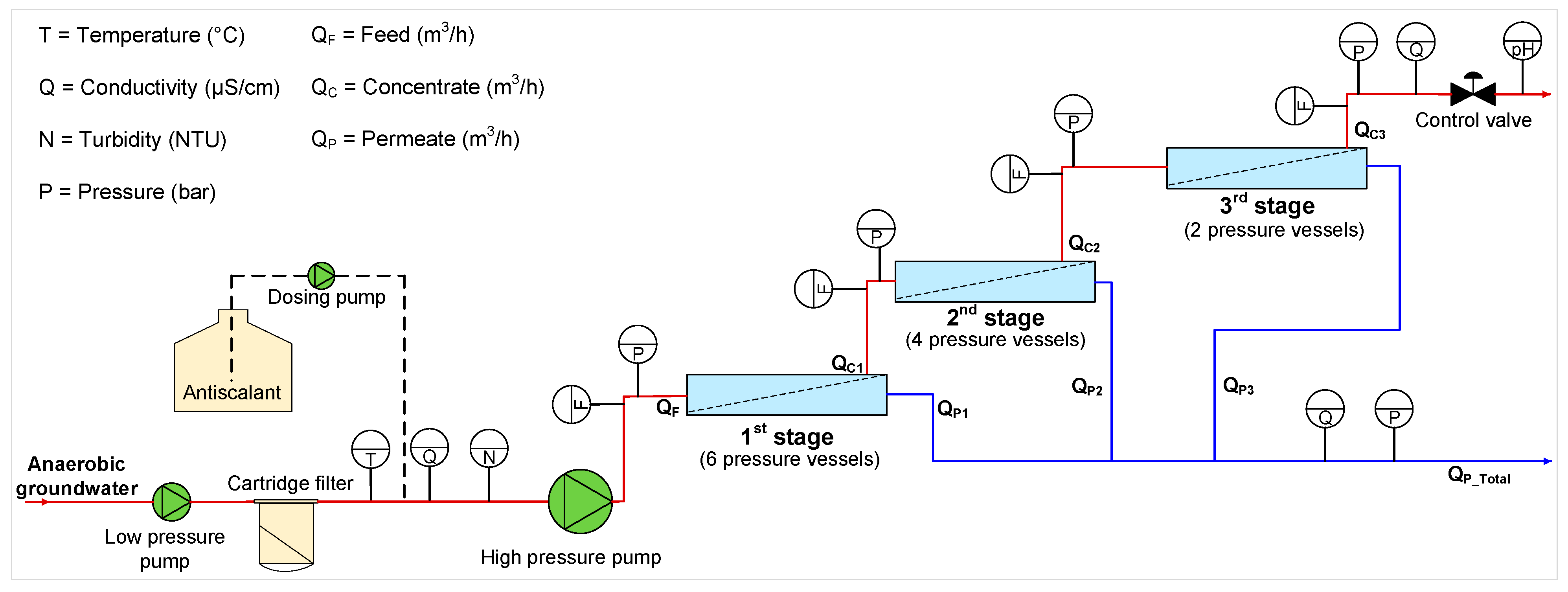
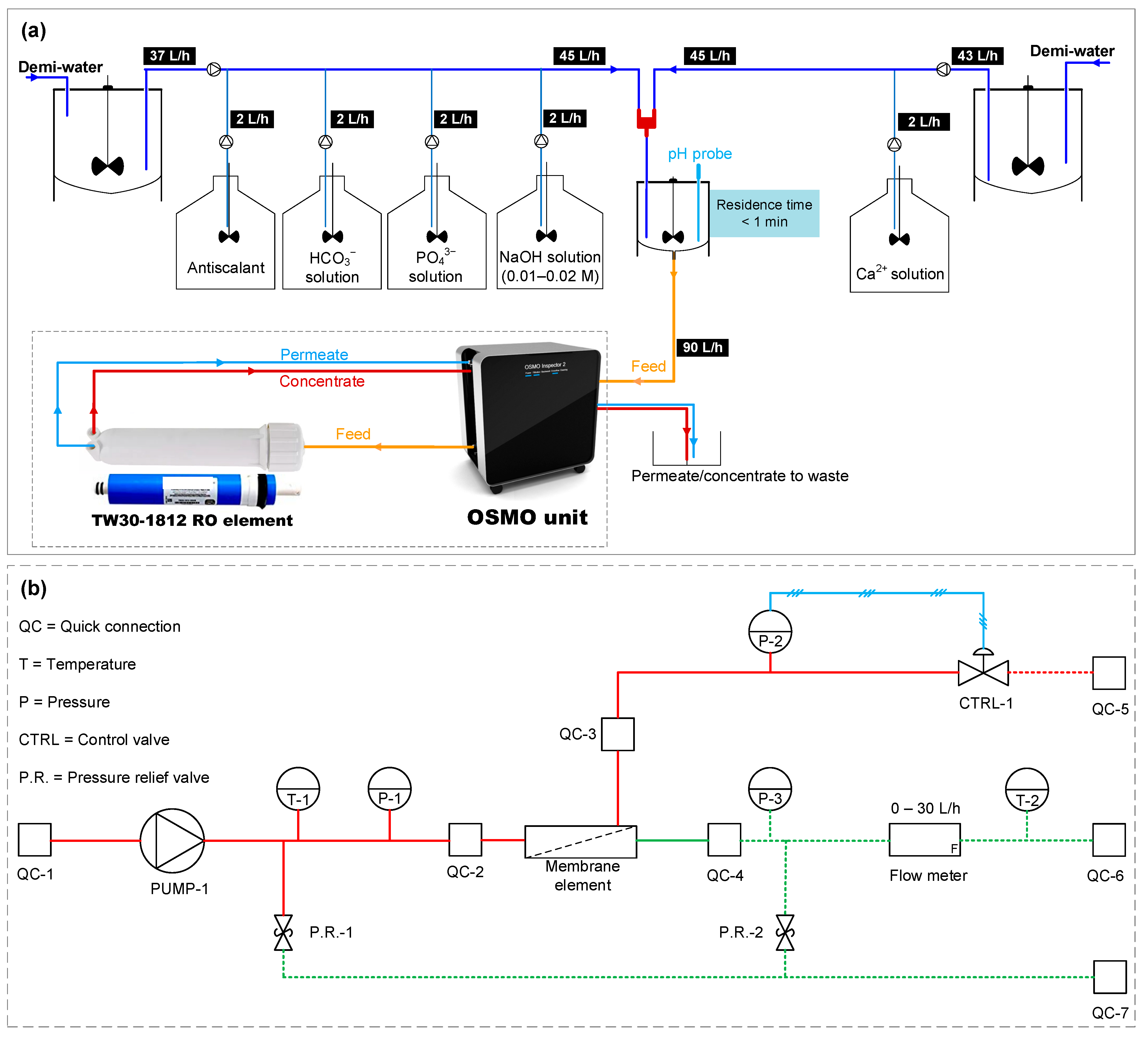





| Cations | Concentration (mg/L) | Anions | Concentration (mg/L) |
|---|---|---|---|
| Calcium | 115.2 | Sulphate | 43.4 |
| Magnesium | 17.4 | Chloride | 113.6 |
| Sodium | 55.2 | Fluoride | 0.1 |
| Potassium | 5.6 | Bicarbonate | 391.8 |
| Barium | 0.1 | Carbonate | - |
| Strontium | 0.5 | Nitrate | 0.2 |
| Iron (II) | 8.5 | Silica | 16.7 |
| Ammonium | 3.7 | Orthophosphate | 2.1 |
| Other properties of the RO feed: | |||
| pH | 7.0 | TDS (mg/L) | 750–800 |
| Temperature (°C) | 12 | DOC (HS) (mg/L) | 8.6 (5.3) |
| Turbidity (NTU) | <0.1 | ||
| Antiscalants ▲ | Chemical Nature | Target Scalants | |
|---|---|---|---|
| Primary Scalants Targeted | Additional Scalants Targeted | ||
| AS–1 | Blend of phosphonates and carboxylic acids | Calcium phosphate/carbonate | Silica, iron/clay fouling, etc. |
| AS–2 | Proprietary acrylic polymer with chelate agent | Silica, calcium phosphate | Calcium carbonate, etc. |
| AS–3 | Information not available | Calcium phosphate/carbonate | Silica, clay, metal oxides, etc. |
| AS–4 | A modified polycarboxylate | Calcium phosphate/carbonate | Silica, etc. |
| AS–5 | Sulfonated polycarboxylate | Calcium phosphate | Silica, calcium carbonate, etc. |
| Run | Pressure Vessel Configuration | Recovery (%) | Antiscalant | Antiscalant Dose ● (mg/L) | Run Period (Days) |
|---|---|---|---|---|---|
| A | 3-2-1 (6 elements) | 80 | - | 0 | 10 |
| 85 | - | 0 | 32 | ||
| B | 6-2-1 (3 elements) | 85 | - | 0 | 5 |
| AS–1 | 2.5 | 3 | |||
| AS–2 | 2.5 | 5 | |||
| AS–3 | 2.5 | 4 | |||
| AS–4 | 5.0 | 5 | |||
| AS–5 | 5.0 | 4 |
| Feed Solution | Antiscalant | Antiscalant Dose ● (mg/L) | Ca2+ (mg/L) | PO43− (mg/L) | HCO3− (mg/L) | pH (−) |
|---|---|---|---|---|---|---|
| Synthetic concentrate of 80% recovery | - | 0 | 575 | 10.5 | 200 | 7.4 |
| Synthetic concentrate of 85% recovery | - | 0 | 767 | 14 | 200 | 7.6 |
| AS–1 | 33.3 | 767 | 14 | 200 | 7.6 | |
| AS–2 | ||||||
| AS–3 | ||||||
| AS–4 | ||||||
| AS–5 |
| Elements | C | N | O | P | S | Ca | Mn | Fe |
|---|---|---|---|---|---|---|---|---|
| Average atomic concentration (%) | 46.35 | 1.9 | 38.47 | 3.45 | 1.18 | 6.19 | 0.47 | 1.98 |
| Standard deviations | 0.9 | 0.29 | 0.48 | 0.42 | 0.06 | 0.32 | 0.17 | 0.26 |
| Element | Binding Energy (eV) | Identified Compound(s) According to the NIST Database |
|---|---|---|
| Carbon (C1s) | 284.8 | Reference value |
| Calcium (Ca2p3/2) | 347.2 | Ca3(PO4)2, Ca8H2(PO4)6·5H2O, Ca10(PO4)6(OH)2 |
| Phosphorous (P2p3/2) | 132.9 | Ca3(PO4)2 |
| Iron (Fe2p3/2) | 711.1 | Fe2O3 |
| Manganese (Mn2p3/2) | 642.4 | MnO2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangal, M.N.; Salinas-Rodriguez, S.G.; Dusseldorp, J.; Blankert, B.; Yangali-Quintanilla, V.A.; Kemperman, A.J.B.; Schippers, J.C.; van der Meer, W.G.J.; Kennedy, M.D. Foulant Identification and Performance Evaluation of Antiscalants in Increasing the Recovery of a Reverse Osmosis System Treating Anaerobic Groundwater. Membranes 2022, 12, 290. https://doi.org/10.3390/membranes12030290
Mangal MN, Salinas-Rodriguez SG, Dusseldorp J, Blankert B, Yangali-Quintanilla VA, Kemperman AJB, Schippers JC, van der Meer WGJ, Kennedy MD. Foulant Identification and Performance Evaluation of Antiscalants in Increasing the Recovery of a Reverse Osmosis System Treating Anaerobic Groundwater. Membranes. 2022; 12(3):290. https://doi.org/10.3390/membranes12030290
Chicago/Turabian StyleMangal, Muhammad Nasir, Sergio G. Salinas-Rodriguez, Jos Dusseldorp, Bastiaan Blankert, Victor A. Yangali-Quintanilla, Antoine J. B. Kemperman, Jan C. Schippers, Walter G. J. van der Meer, and Maria D. Kennedy. 2022. "Foulant Identification and Performance Evaluation of Antiscalants in Increasing the Recovery of a Reverse Osmosis System Treating Anaerobic Groundwater" Membranes 12, no. 3: 290. https://doi.org/10.3390/membranes12030290
APA StyleMangal, M. N., Salinas-Rodriguez, S. G., Dusseldorp, J., Blankert, B., Yangali-Quintanilla, V. A., Kemperman, A. J. B., Schippers, J. C., van der Meer, W. G. J., & Kennedy, M. D. (2022). Foulant Identification and Performance Evaluation of Antiscalants in Increasing the Recovery of a Reverse Osmosis System Treating Anaerobic Groundwater. Membranes, 12(3), 290. https://doi.org/10.3390/membranes12030290







