Modification of Thin Film Composite Pressure Retarded Osmosis Membrane by Polyethylene Glycol with Different Molecular Weights
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of the Membranes
2.3. Membrane Characterizations
2.3.1. Morphology, Surface Roughness and Functional Group
2.3.2. Equilibrium Water Content, Porosity, Average Pore Size and Contact Angle
2.3.3. RO Test for Intrinsic Parameters
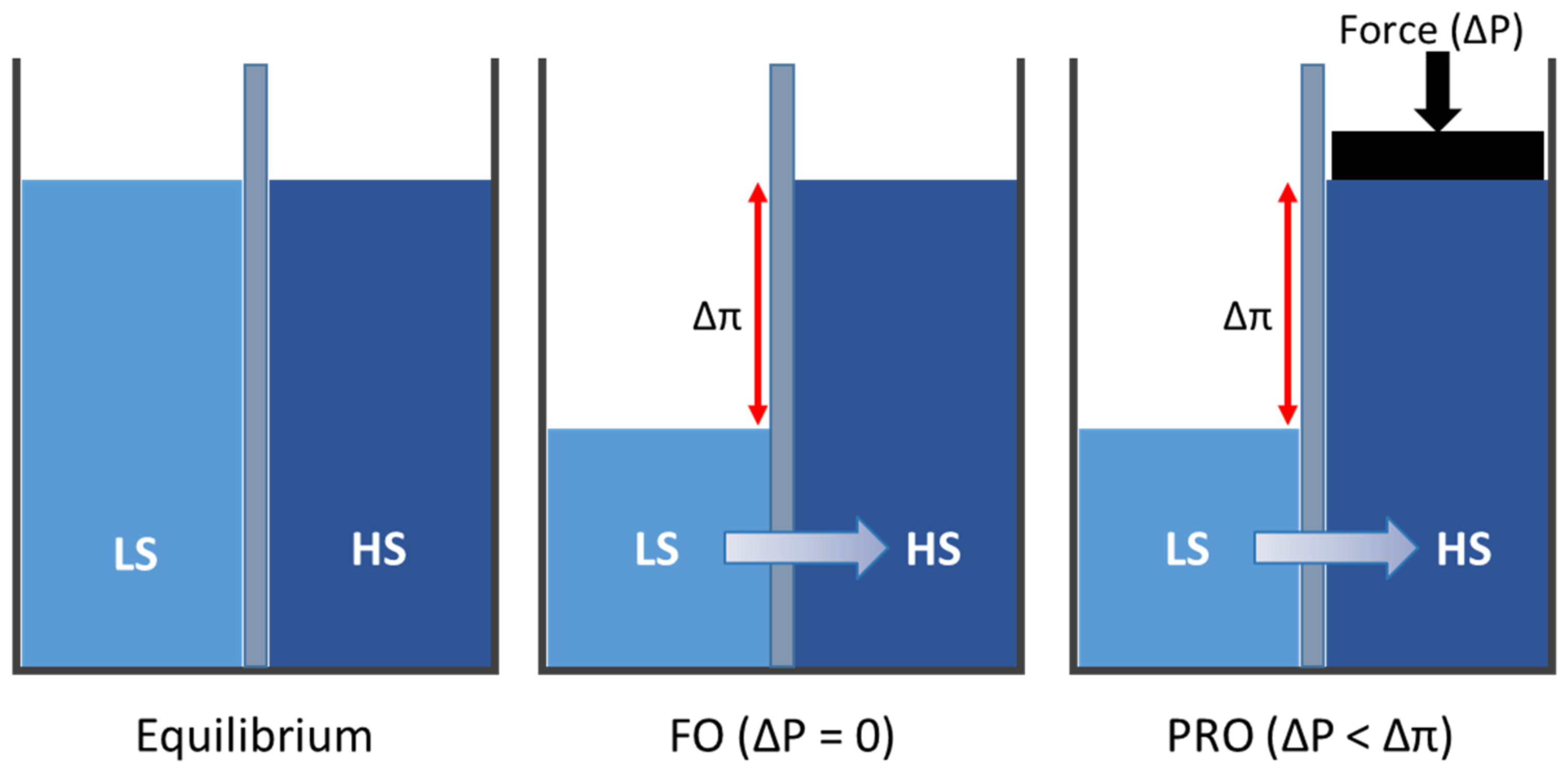
2.4. FO and PRO Membrane Performances
3. Results and Discussions
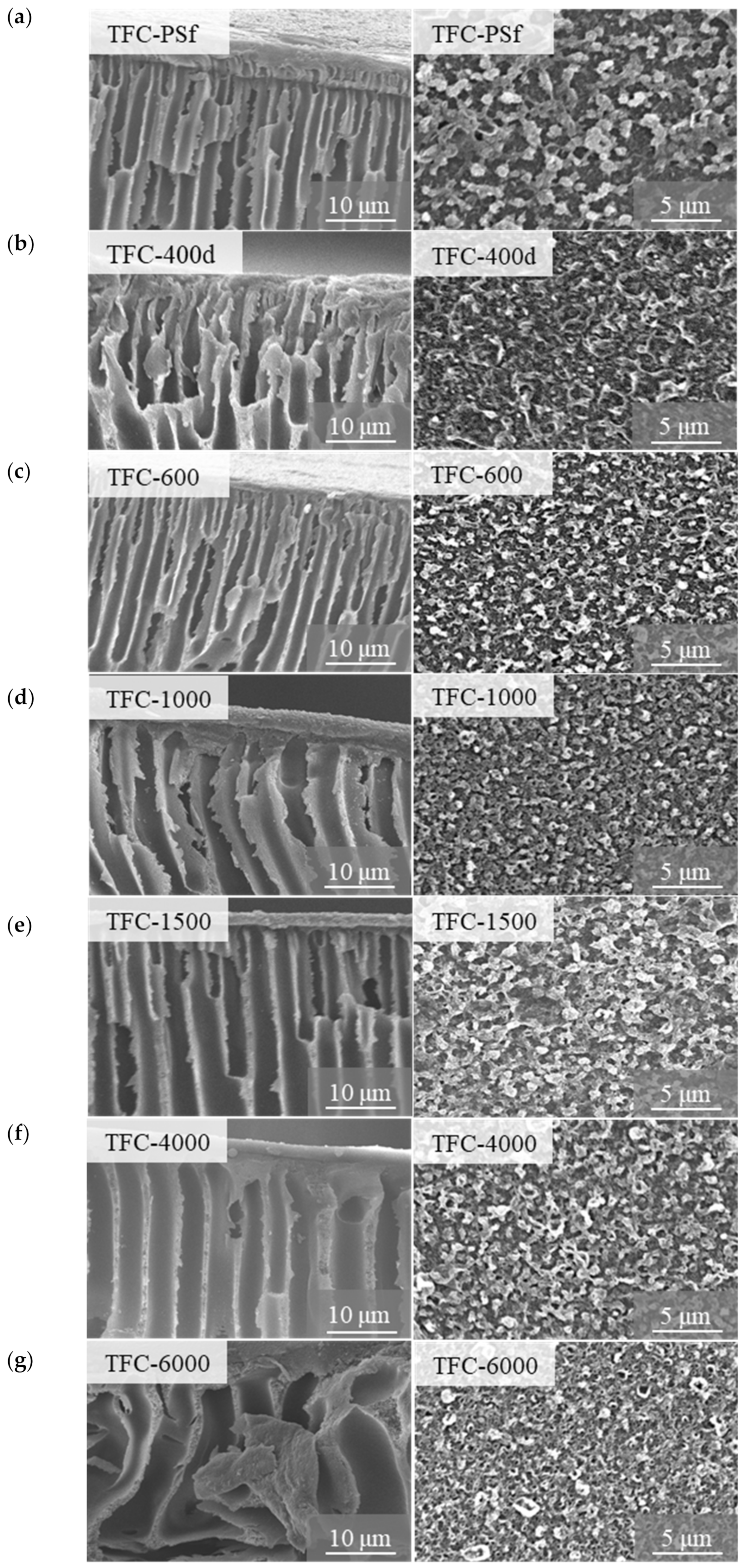
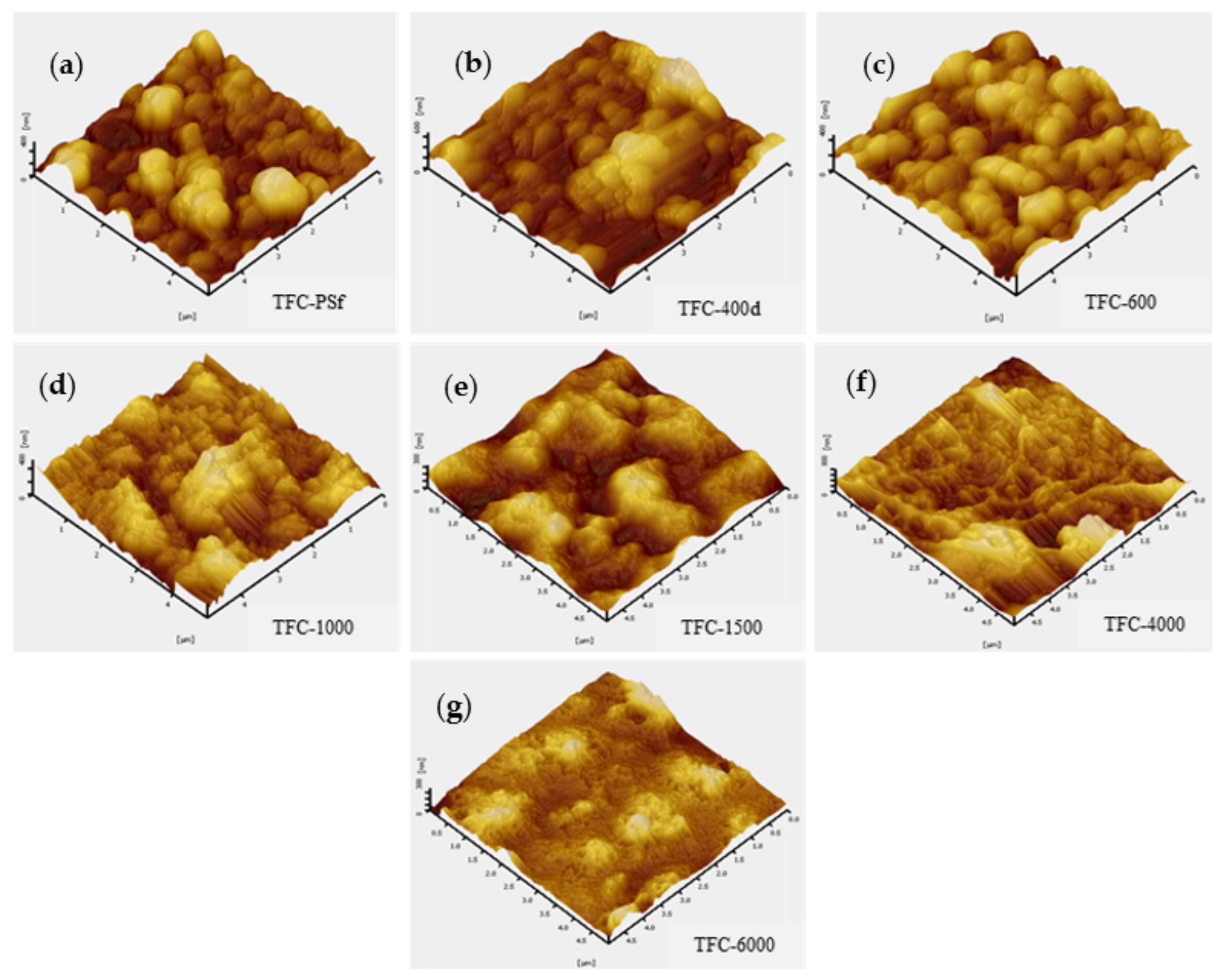
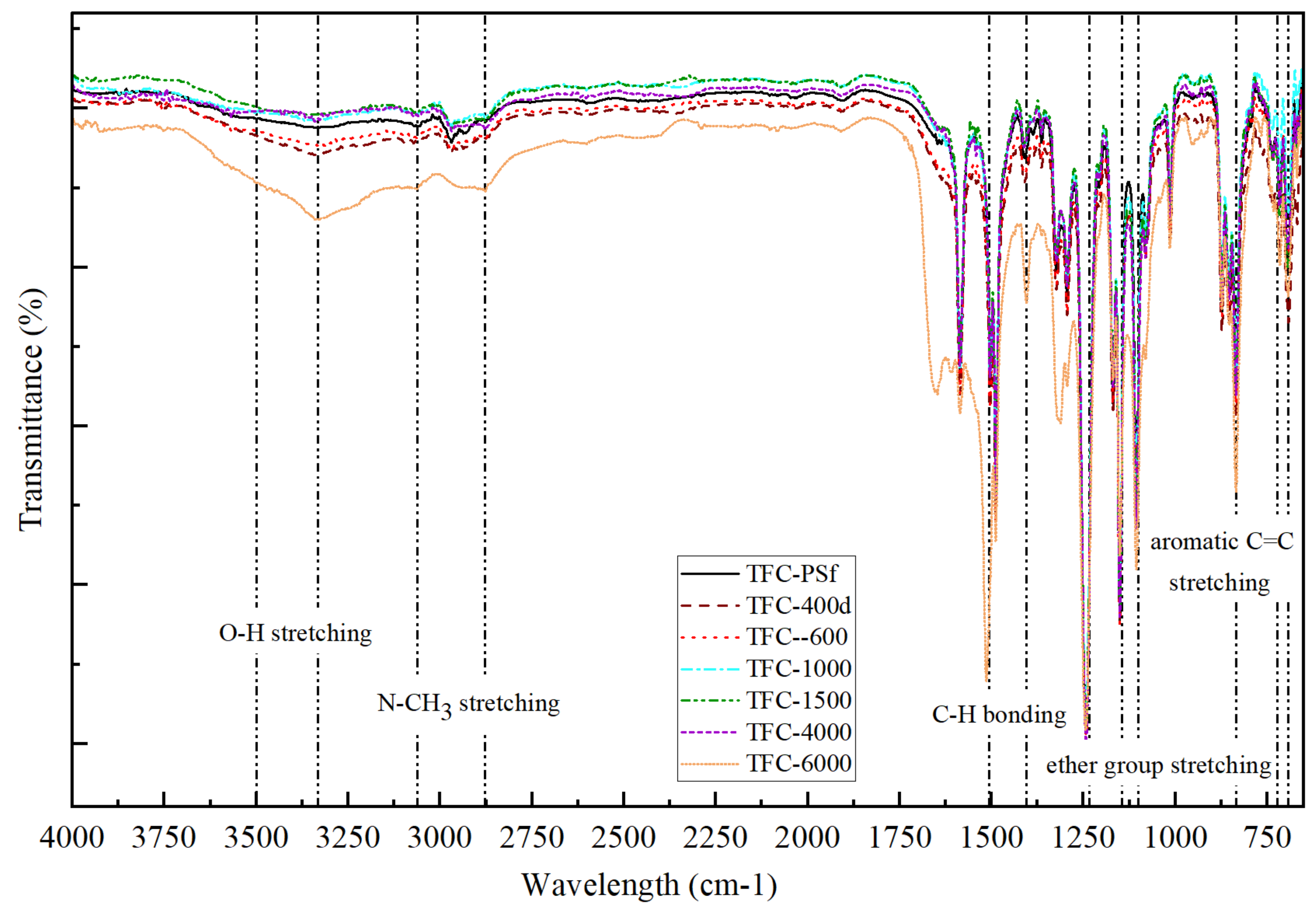
3.1. Membrane Morphology, Surface Roughness and Functional Group
3.2. Effects of PEG on the Intrinsic Properties of the TFC PA/PSf Membranes
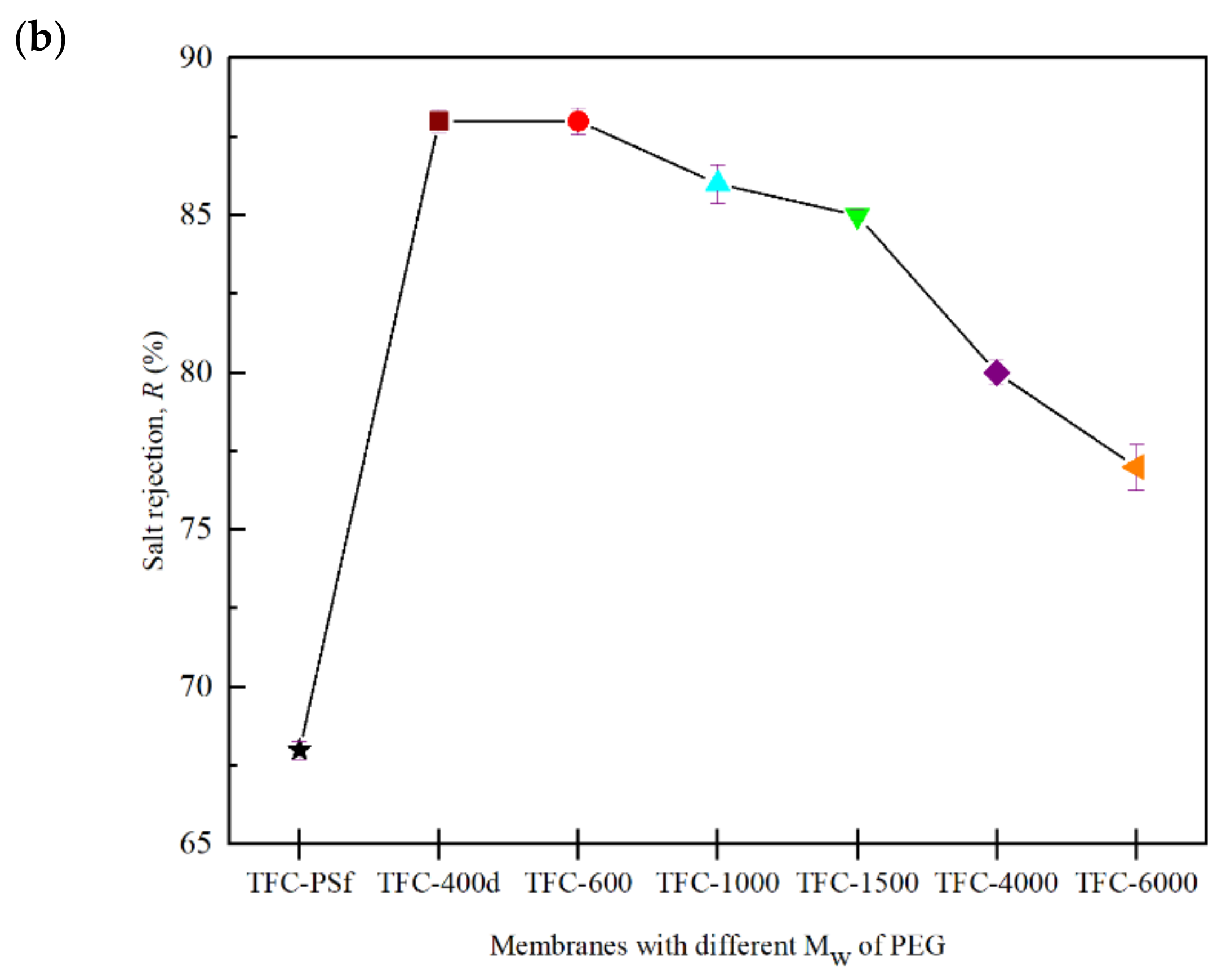
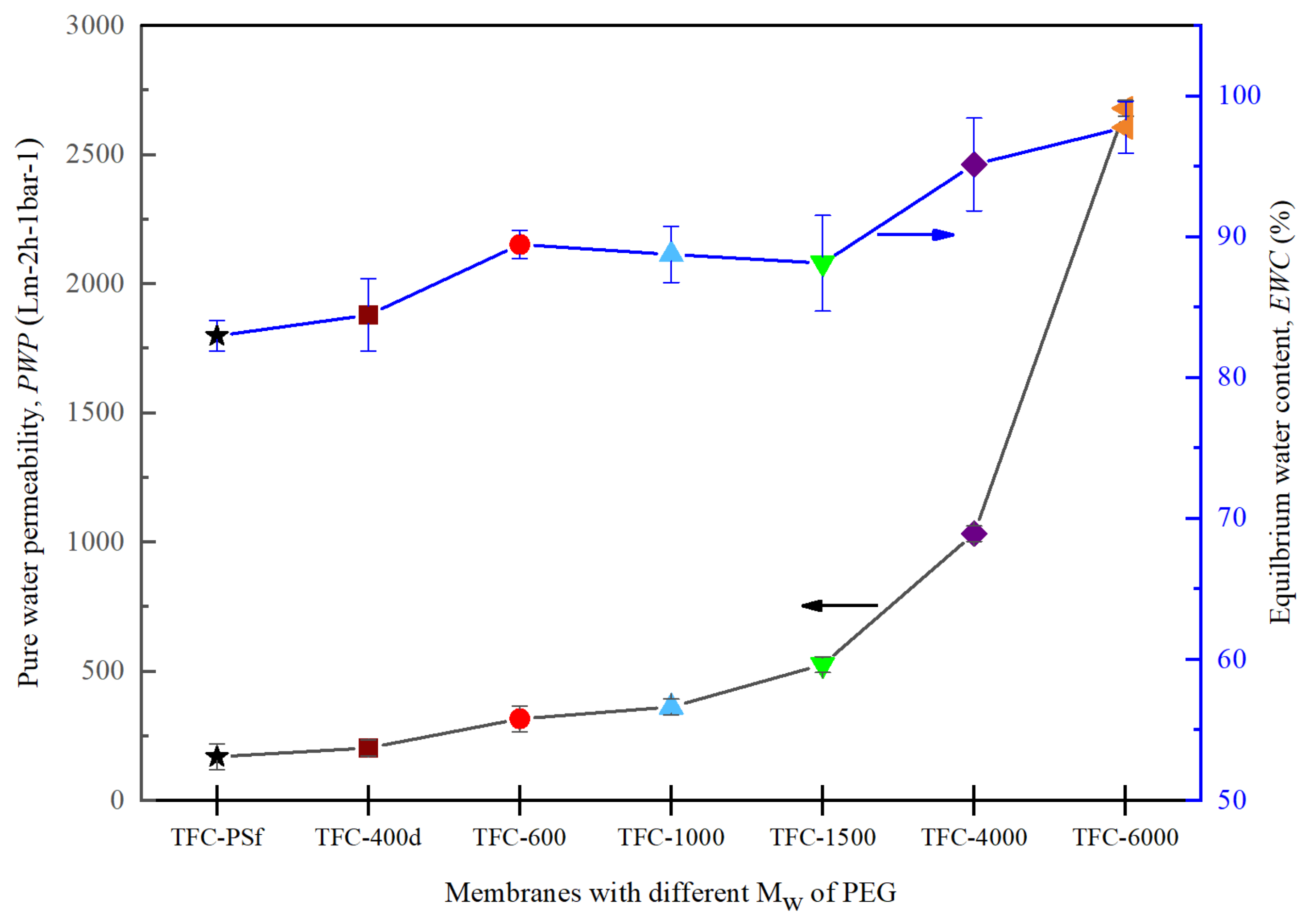
3.3. Effects of the Mw of PEG on PWP and EWC
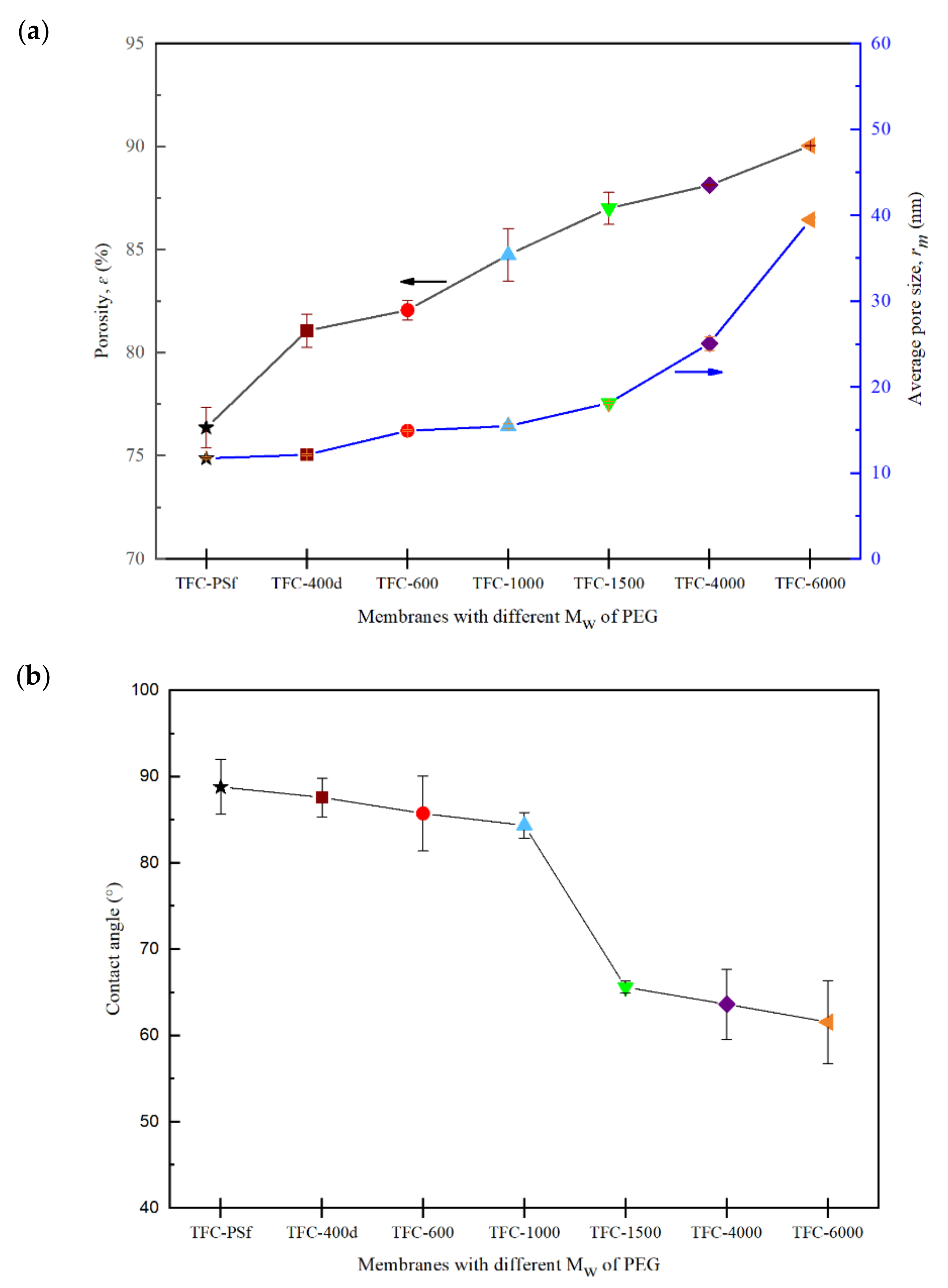
3.4. Effects of the Mw of PEG on Membrane Porosity and Hydrophilicity
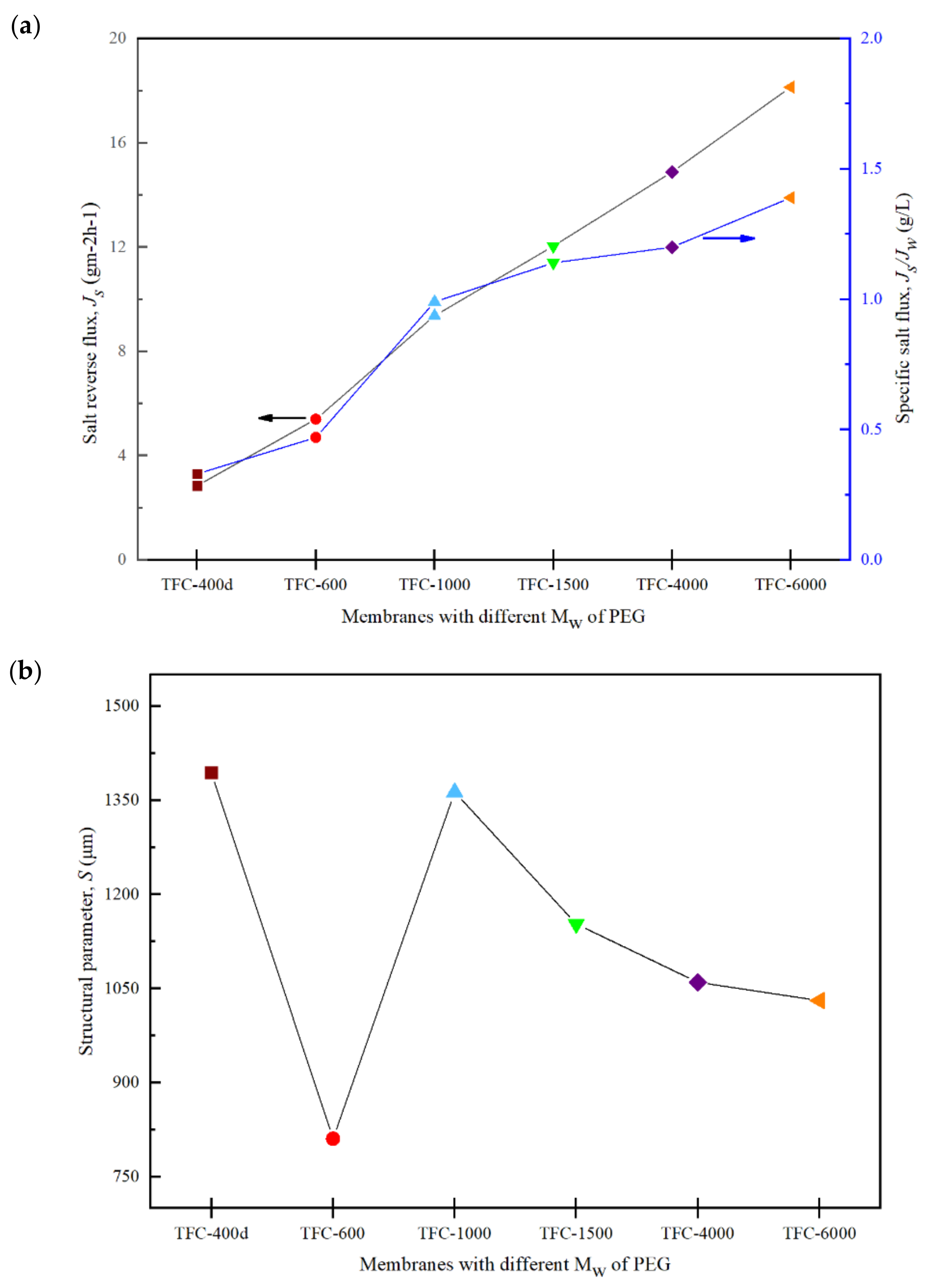
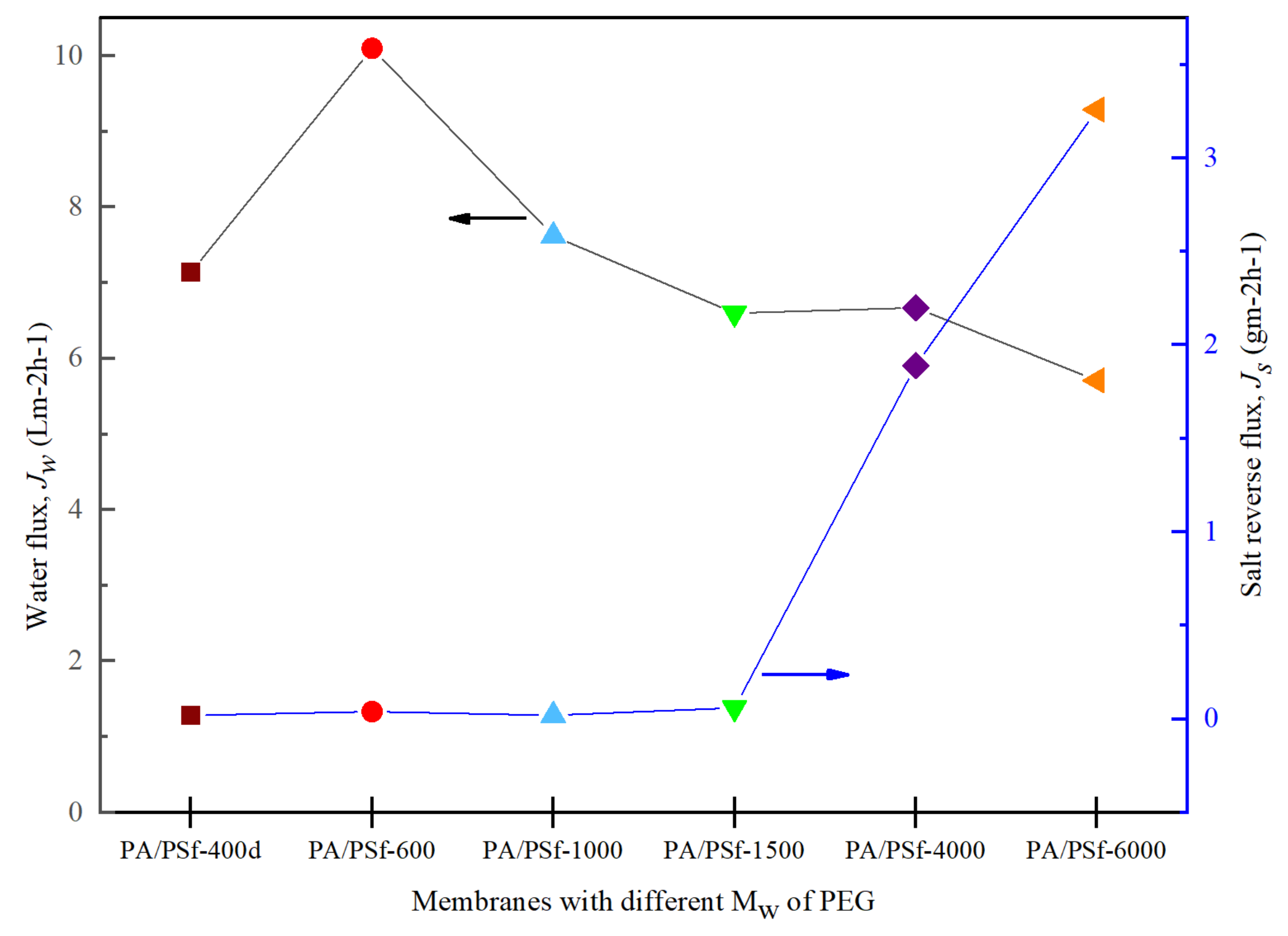
3.5. FO Membrane Performance and Structural Parameter
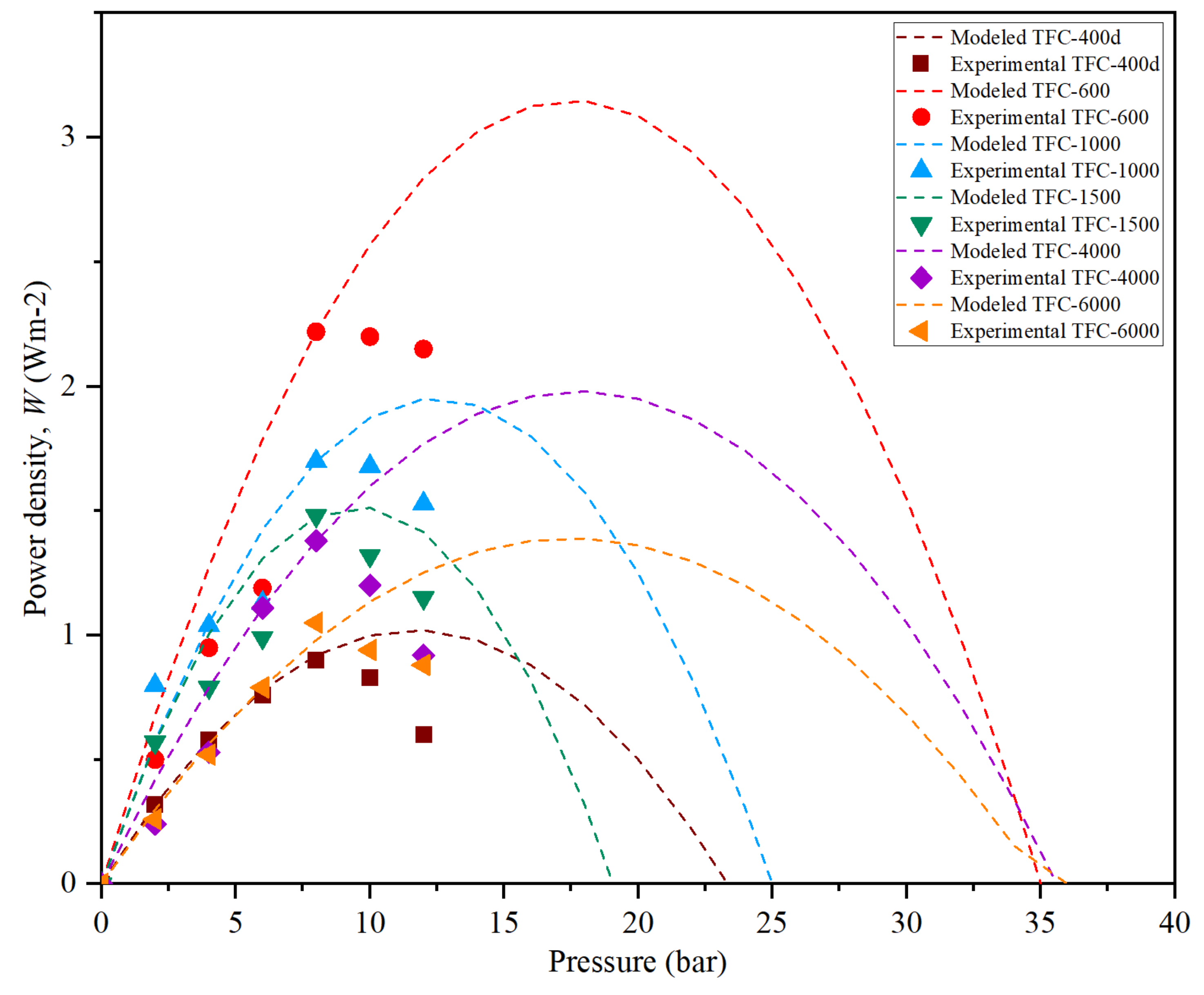
3.6. PRO Membrane Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ahmad, A.; Waheed, S.; Khan, S.M.; Gul, S.-E.; Shafiq, M.; Farooq, M.; Sanaullah, K.; Jamil, T. Effect of silica on the properties of cellulose acetate/polyethylene glycol membranes for reverse osmosis. Desalination 2015, 355, 1–10. [Google Scholar] [CrossRef]
- Ali, A.; Tufa, R.A.; Macedonio, F.; Curcio, E.; Drioli, E. Membrane technology in renewable-energy-driven desalination. Renew. Sustain. Energy Rev. 2018, 81, 1–21. [Google Scholar] [CrossRef]
- Cath, T.Y.; Elimelech, M.; McCutcheon, J.R.; McGinnis, R.L.; Achilli, A.; Anastasio, D.; Brady, A.R.; Childress, A.E.; Farr, I.V.; Hancock, N.T.; et al. Standard Methodology for Evaluating Membrane Performance in Osmotically Driven Membrane Processes. Desalination 2013, 312, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Loh, C.H.; Zhang, L.; Setiawan, L.; She, Q.; Fang, W.; Hu, X.; Wang, R. Module scale-up and performance evaluation of thin film composite hollow fiber membranes for pressure retarded osmosis. J. Membr. Sci. 2018, 548, 398–407. [Google Scholar] [CrossRef]
- Chiao, Y.-H.; Sengupta, A.; Chen, S.-T.; Hung, W.-S.; Lai, J.-Y.; Upadhyaya, L.; Qian, X.; Wickramasinghe, S.R. Novel thin-film composite forward osmosis membrane using polyethylenimine and its impact on membrane performance. Sep. Sci. Technol. 2019, 55, 590–600. [Google Scholar] [CrossRef]
- Gai, J.-G.; Gong, X.-L.; Kang, W.-L.; Zhang, X.; Wang, W.-W. Key factors influencing water diffusion in aromatic PA membrane: Hydrates, nanochannels and functional groups. Desalination 2014, 333, 52–58. [Google Scholar] [CrossRef]
- Han, G.; Zhang, S.; Li, X.; Chung, N.T.-S. High performance thin film composite pressure retarded osmosis (PRO) membranes for renewable salinity-gradient energy generation. J. Membr. Sci. 2013, 440, 108–121. [Google Scholar] [CrossRef]
- Hong, S.S.; Ryoo, W.; Chun, M.-S.; Chung, G.-Y. Effects of membrane characteristics on performances of pressure retarded osmosis power system. Korean J. Chem. Eng. 2015, 32, 1249–1257. [Google Scholar] [CrossRef]
- Hou, J.; Cao, M.; Liu, P. Development and utilization of geothermal energy in China: Current practices and future strategies. Renew. Energy 2018, 125, 401–412. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, J.; Kim, J.M.; Kang, S.W. Water treatment by polysulfone membrane modified with tetrahydrofuran and water pressure. Macromol. Res. 2016, 24, 1020–1023. [Google Scholar] [CrossRef]
- Idarraga-Mora, J.A.; Ladner, D.A.; Husson, S.M. Thin-film composite membranes on polyester woven mesh with variable opening size for pressure-retarded osmosis. J. Membr. Sci. 2018, 549, 251–259. [Google Scholar] [CrossRef]
- Idris, S.N.A.; Jullok, N.; Lau, W.J.; Ong, H.L.; Dong, C.-D. Graphene Oxide Incorporated Polysulfone Substrate for Flat Sheet Thin Film Nanocomposite Pressure Retarded Osmosis Membrane. Membranes 2020, 10, 416. [Google Scholar] [CrossRef] [PubMed]
- Kabir, E.; Kumar, P.; Kumar, S.; Adelodun, A.A.; Kim, K.-H. Solar energy: Potential and future prospects. Renew. Sustain. Energy Rev. 2018, 82, 894–900. [Google Scholar] [CrossRef]
- Hickenbottom, K.L.; Miller-Robbie, L.; Vanneste, J.; Marr, J.M.; Heeley, M.B.; Cath, T.Y. Comparative life-cycle assessment of a novel osmotic heat engine and an organic Rankine cycle for energy production from low-grade heat. J. Clean. Prod. 2018, 191, 490–501. [Google Scholar] [CrossRef]
- Kim, B.; Gwak, G.; Hong, S. Review on methodology for determining forward osmosis (FO) membrane characteristics: Water permeability (A), solute permeability (B), and structural parameter (S). Desalination 2017, 422, 5–16. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, K.-H. Effect of PEG additive on membrane formation by phase inversion. J. Membr. Sci. 1998, 138, 153–163. [Google Scholar] [CrossRef]
- Kwak, S.Y.; Jung, S.G.; Yoon, Y.S.; Ihm, D.W. Details of surface features in aromatic polyamide reverse osmosis mem-branes characterized by scanning electron and atomic force microscopy. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 1429–1440. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Fu, F.; Chung, N.T.-S. Deformation and reinforcement of thin-film composite (TFC) polyamide-imide (PAI) membranes for osmotic power generation. J. Membr. Sci. 2013, 434, 204–217. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, S.; Setiawan, L.; Zhang, L.; Wang, R. Integral hollow fiber membrane with chemical cross-linking for pressure retarded osmosis operated in the orientation of active layer facing feed solution. J. Membr. Sci. 2018, 550, 163–172. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Li, C.; Hou, L.-A. Comparison of performance and biofouling resistance of thin-film composite forward osmosis membranes with substrate/active layer modified by graphene oxide. RSC Adv. 2019, 9, 6502–6509. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Chen, C.; Zhao, P.; Li, T.; Liu, C.; Wang, Q.; Chen, Y.; Crittenden, J. Thin-film composite forward osmosis membranes with substrate layer composed of polysulfone blended with PEG or polysulfone grafted PEG methyl ether methacrylate. Front. Chem. Sci. Eng. 2016, 10, 562–574. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, F.; Ma, J.; Wu, M.; Zhang, J.; Gao, C. Effect of PEG additive on the morphology and performance of polysulfone ultrafiltration membranes. Desalination 2011, 272, 51–58. [Google Scholar] [CrossRef]
- Mansourpanah, Y.; Ostadchinigar, A. Preparation of chemically attached polyamide thin film membrane using different diamines: Separation and computational investigation. J. Polym. Res. 2017, 24, 1–6. [Google Scholar] [CrossRef]
- Mayer, F.D.; Brondani, M.; Carrillo, M.C.V.; Hoffmann, R.; Lora, E.E.S. Revisiting energy efficiency, renewability, and sustainability indicators in biofuels life cycle: Analysis and standardization proposal. J. Clean. Prod. 2020, 252, 119850. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; Elimelech, M. Influence of membrane support layer hydrophobicity on water flux in osmotically driven membrane processes. J. Membr. Sci. 2008, 318, 458–466. [Google Scholar] [CrossRef]
- Misdan, N.; Lau, W.; Ismail, A.; Matsuura, T. Formation of thin film composite nanofiltration membrane: Effect of polysulfone substrate characteristics. Desalination 2013, 329, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Park, H.M.; Jee, K.Y.; Lee, Y.T. Preparation and characterization of a thin-film composite reverse osmosis membrane using a polysulfone membrane including metal-organic frameworks. J. Membr. Sci. 2017, 541, 510–518. [Google Scholar] [CrossRef]
- Raouf, M.; Abedini, R.; Omidkhah, M.; Nezhadmoghadam, E. A favored CO2 separation over light gases using mixed matrix membrane comprising polysulfone/polyethylene glycol and graphene hydroxyl nanoparticles. Process Saf. Environ. Prot. 2020, 133, 394–407. [Google Scholar] [CrossRef]
- Said, M.M.; El-Aassar, A.-H.; Kotp, Y.H.; Shawky, H.A.; Mottaleb, M.S.A. Performance assessment of prepared polyamide thin film composite membrane for desalination of saline groundwater at Mersa Alam-Ras Banas, Red Sea Coast, Egypt. Desalin. Water Treat. 2013, 51, 4927–4937. [Google Scholar] [CrossRef]
- Sark, J.; Jullok, N.; Lau, W. Improving the Structural Parameter of the Membrane Sublayer for Enhanced Forward Osmosis. Membranes 2021, 11, 448. [Google Scholar] [CrossRef]
- Sharma, M.; Mondal, P.; Chakraborty, A.; Kuttippurath, J.; Purkait, M. Effect of different molecular weight polyethylene glycol on flat sheet cellulose acetate membranes for evaluating power density performance in pressure retarded osmosis study. J. Water Process Eng. 2018, 30, 100632. [Google Scholar] [CrossRef]
- She, Q.; Wei, J.; Ma, N.; Sim, V.; Fane, A.G.; Wang, R.; Tang, C.Y. Fabrication and characterization of fabric-reinforced pressure retarded osmosis membranes for osmotic power harvesting. J. Membr. Sci. 2016, 504, 75–88. [Google Scholar] [CrossRef]
- She, Q.; Zhang, L.; Wang, R.; Krantz, W.B.; Fane, A.G. Pressure-retarded osmosis with wastewater concentrate feed: Fouling process considerations. J. Membr. Sci. 2017, 542, 233–244. [Google Scholar] [CrossRef]
- Sinha, M.K.; Purkait, M.K. Increase in hydrophilicity of polysulfone membrane using polyethylene glycol methyl ether. J. Membr. Sci. 2013, 437, 7–16. [Google Scholar] [CrossRef]
- Vogel, E.; Saravia, G.; Kobe, S.; Schumann, R.; Schuster, R. A novel method to optimize electricity generation from wind energy. Renew. Energy 2018, 126, 724–735. [Google Scholar] [CrossRef]
- Wan, C.F.; Yang, T.; Gai, W.; De Lee, Y.; Chung, T.-S. Thin-film composite hollow fiber membrane with inorganic salt additives for high mechanical strength and high power density for pressure-retarded osmosis. J. Membr. Sci. 2018, 555, 388–397. [Google Scholar] [CrossRef]
- Wei, J.; Li, Y.; Setiawan, L.; Wang, R. Influence of macromolecular additive on reinforced flat-sheet thin film composite pressure-retarded osmosis membranes. J. Membr. Sci. 2016, 511, 54–64. [Google Scholar] [CrossRef]
- Wei, J.; Qiu, C.; Tang, C.Y.; Wang, R.; Fane, A.G. Synthesis and characterization of flat-sheet thin film composite forward osmosis membranes. J. Membr. Sci. 2011, 372, 292–302. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, H.; Feng, L.; Zhang, L. Effects of polyethylene glycol on the structure and filtration performance of thin-film PA-Psf composite forward osmosis membranes. Sep. Sci. Technol. 2016, 51, 862–873. [Google Scholar] [CrossRef]
- Yi, Z.; Zhu, L.-P.; Zhao, Y.-F.; Zhu, B.-K.; Xu, Y.-Y. An extending of candidate for the hydrophilic modification of polysulfone membranes from the compatibility consideration: The polyethersulfone-based amphiphilic copolymer as an example. J. Membr. Sci. 2012, 390–391, 48–57. [Google Scholar] [CrossRef]
- Yunos, M.Z.; Harun, Z.; Basri, H.; Ismail, A.F. Effects of Water as Non-Solvent Additive on Performance of Polysulfone Ultrafiltration Membrane. Adv. Mater. Res. 2012, 488–489, 46–50. [Google Scholar] [CrossRef] [Green Version]
- Yunos, M.Z.; Harun, Z.; Basri, H.; Ismail, A.F. Studies on fouling by natural organic matter (NOM) on polysulfone membranes: Effect of polyethylene glycol (PEG). Desalination 2014, 333, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Zambare, R.S.; Dhopte, K.B.; Nemade, P.R.; Tang, C.Y. Separation and Purification Technology Effect of oxidation degree of GO nanosheets on microstructure and performance of polysulfone-GO mixed matrix membranes. Sep. Purif. Technol. 2020, 244, 116865. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, S.; Yang, B.; Huo, J.; Zhang, X.; Bao, J.; Ruan, X.; He, G. Effect of Hydrogen-Bonding Interaction on the Arrangement and Dynamics of Water Confined in a Poly-amide Membrane: A Molecular Dynamics Simulation. J. Phys. Chem. B 2018, 122, 4719–4728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tian, J.; Ren, Z.; Shi, W.; Zhang, Z.; Xu, Y.; Gao, S.; Cui, F. High performance thin-film composite (TFC) forward osmosis (FO) membrane fabricated on novel hydrophilic disulfonated poly(arylene ether sulfone) multiblock copolymer/polysulfone substrate. J. Membr. Sci. 2016, 520, 529–539. [Google Scholar] [CrossRef]
- Zheng, K.; Zhou, S.; Zhou, X. A low-cost and high-performance thin-film composite forward osmosis membrane based on an SPSU/PVC substrate. Sci. Rep. 2018, 8, 10022. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; He, H.L.; Sun, F.; Su, Y.; Yu, H.Y.; Gu, J.S. Structural parameters reduction in polyamide forward osmosis membranes via click modification of the polysulfone support. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124082. [Google Scholar] [CrossRef]




| Membrane | Mw of PEG (gmol−1) | PEG/NMP/PSf (wt%) |
|---|---|---|
| PSf | 0 | 0/82/18 |
| PSf-400a | 400 | 0.25/81.75/18 |
| PSf-400b | 400 | 0.5/81.50/18 |
| PSf-400c | 400 | 0.75/81.25/18 |
| PSf-400d | 400 | 10/82/18 |
| PSf-600 | 600 | 10/82/18 |
| PSf-1000 | 1000 | 10/82/18 |
| PSf-1500 | 1500 | 10/82/18 |
| PSf-4000 | 4000 | 10/82/18 |
| PSf-6000 | 6000 | 10/82/18 |
| Membrane | Maximum Peak-to-Valley Distance, Rp-v (μm) | Surface Roughness (μm) | |
|---|---|---|---|
| Average, Ravg | Root-Mean-Squared, Rrms | ||
| TFC-PSf | 54.63 | 84.83 | 10.75 |
| TFC-400d | 60.48 | 73.54 | 94.37 |
| TFC-600 | 62.43 | 68.11 | 86.49 |
| TFC-1000 | 53.89 | 62.26 | 78.32 |
| TFC-1500 | 32.64 | 43.44 | 53.42 |
| TFC-4000 | 41.05 | 38.89 | 51.93 |
| TFC-6000 | 37.17 | 31.42 | 41.64 |
| TFC Membrane | Water Permeability, A (Lm−2h−1bar−1) | Salt Permeability, B (Lm−2h−1) | Salt Rejection, R (%) | Structural Parameter, S (μm) |
|---|---|---|---|---|
| TFC-PSf | 0.49 ± 0.12 | 0.07 ± 0.26 | 68 ± 0.29 | 1803 |
| TFC-400a | 0.89 ± 0.02 | 0.11 ± 0.04 | 71 ± 1.01 | 1414 |
| TFC-400b | 1.01 ± 0.01 | 0.12 ± 0.05 | 79 ± 0.50 | 1407 |
| TFC-400c | 1.14 ± 0.01 | 0.13 ± 0.09 | 82 ± 0.87 | 1399 |
| TFC-400d | 1.19 ± 0.04 | 0.16 ± 0.05 | 88 ± 0.37 | 1394 |
| Membranes | Water Permeability, A (Lm−2h−1bar−1) | Salt Permeability, B (Lm−2h−1) | Salt Rejection, R (%) | Ref. |
|---|---|---|---|---|
| TFC-600 | 1.24 ± 0.02 | 0.13 ± 0.02 | 88 ± 0.43 | This study |
| MS3(PSf/PEG-600/NMP) a | 1.37 ± 0.21 | 1.89 ± 0.29 | 82 ± 1.52 | [38] |
| TFN-0.1GO (PSf/PEG-600/GO/NMP) b | 1.04 ± 0.33 | 0.37 ± 0.02 | 96.2 ± 0.08 | [39] |
| PSf/PEG-600/Tween80/ * DMF | n/a | n/a | 83.2 | [40] |
| ** CA/PEG-600 (10 g) c | 0.27 | n/a | 52 | [41] |
| CA/PEG-600 (8 g) c | 0.25 | n/a | 60 | |
| CA/PEG-600 (6 g) c | 0.20 | n/a | 69.2 | |
| CA/PEG-600 (4 g) c | 1.68 | n/a | 81.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idris, S.N.A.; Jullok, N.; Lau, W.J.; Ma’Radzi, A.H.; Ong, H.L.; Ramli, M.M.; Dong, C.-D. Modification of Thin Film Composite Pressure Retarded Osmosis Membrane by Polyethylene Glycol with Different Molecular Weights. Membranes 2022, 12, 282. https://doi.org/10.3390/membranes12030282
Idris SNA, Jullok N, Lau WJ, Ma’Radzi AH, Ong HL, Ramli MM, Dong C-D. Modification of Thin Film Composite Pressure Retarded Osmosis Membrane by Polyethylene Glycol with Different Molecular Weights. Membranes. 2022; 12(3):282. https://doi.org/10.3390/membranes12030282
Chicago/Turabian StyleIdris, Siti Nur Amirah, Nora Jullok, Woei Jye Lau, Akmal Hadi Ma’Radzi, Hui Lin Ong, Muhammad Mahyidin Ramli, and Cheng-Di Dong. 2022. "Modification of Thin Film Composite Pressure Retarded Osmosis Membrane by Polyethylene Glycol with Different Molecular Weights" Membranes 12, no. 3: 282. https://doi.org/10.3390/membranes12030282
APA StyleIdris, S. N. A., Jullok, N., Lau, W. J., Ma’Radzi, A. H., Ong, H. L., Ramli, M. M., & Dong, C.-D. (2022). Modification of Thin Film Composite Pressure Retarded Osmosis Membrane by Polyethylene Glycol with Different Molecular Weights. Membranes, 12(3), 282. https://doi.org/10.3390/membranes12030282








