Treatment of Hydrothermal-Liquefaction Wastewater with Crossflow UF for Oil and Particle Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feed Solution
2.2. Membranes
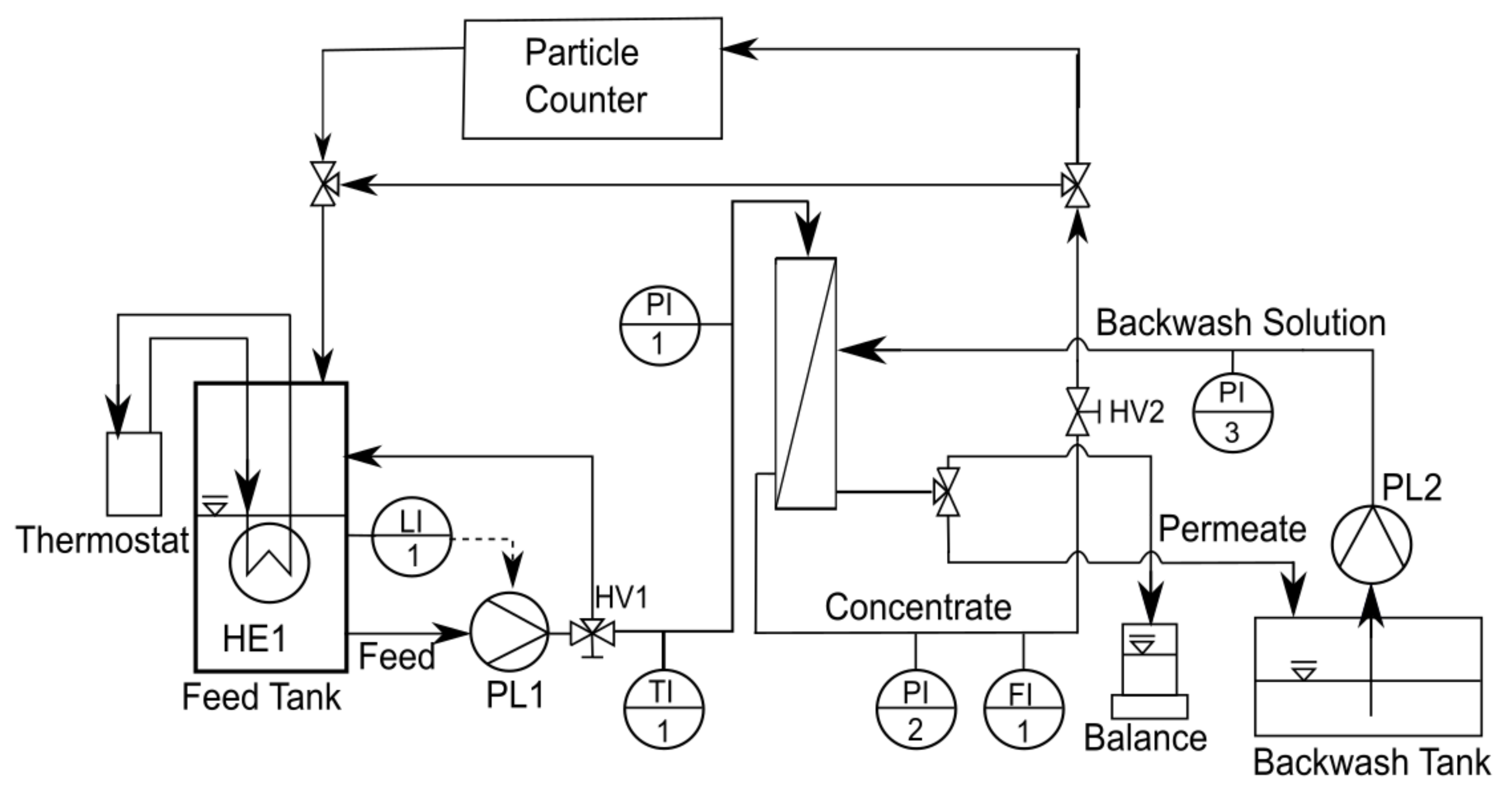
2.3. Filtration Setup
2.4. Cleaning Methods
2.5. Analytical Methods
2.6. Data Interpretation
3. Results and Discussion
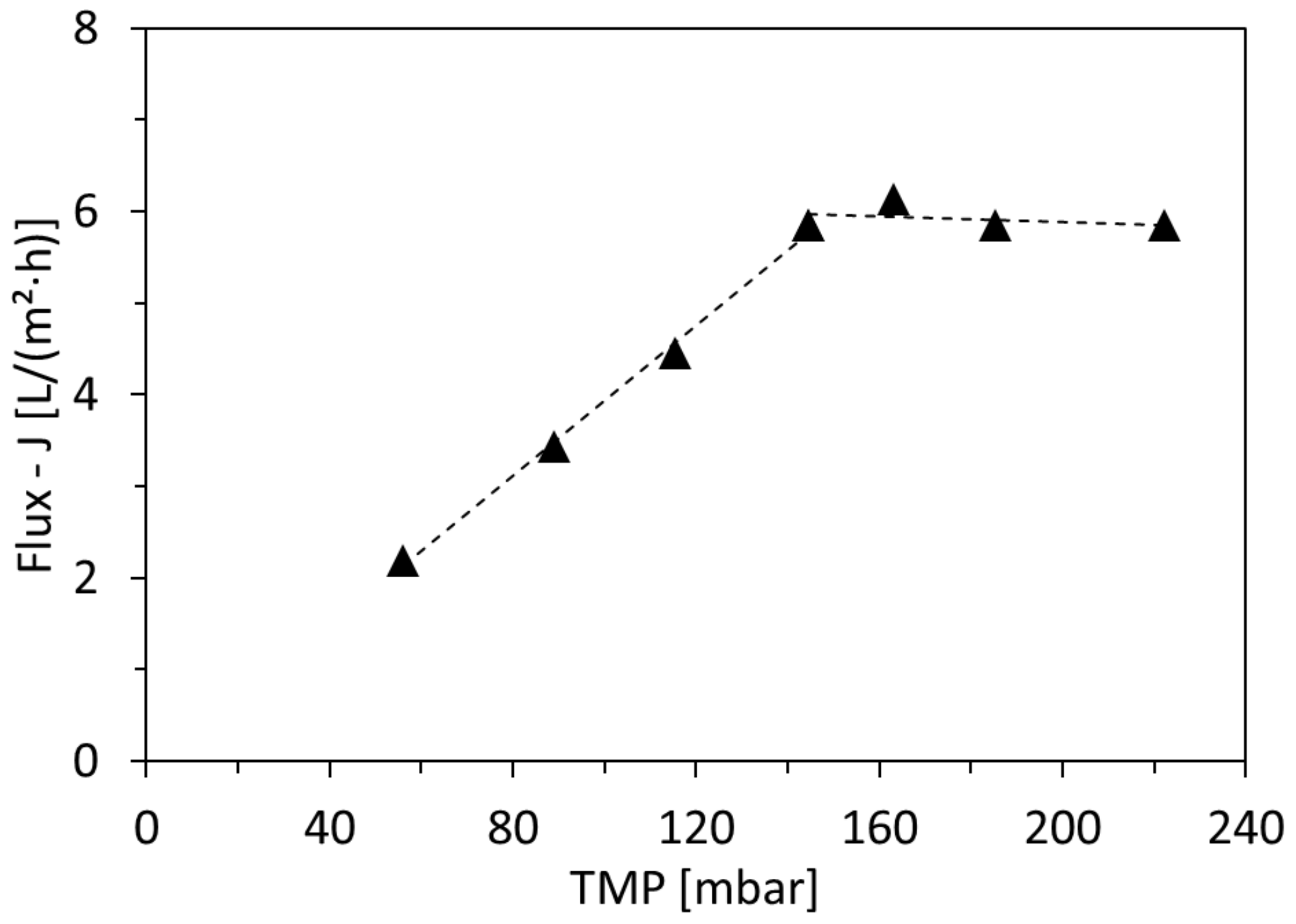
3.1. Critical-Flux Measurements
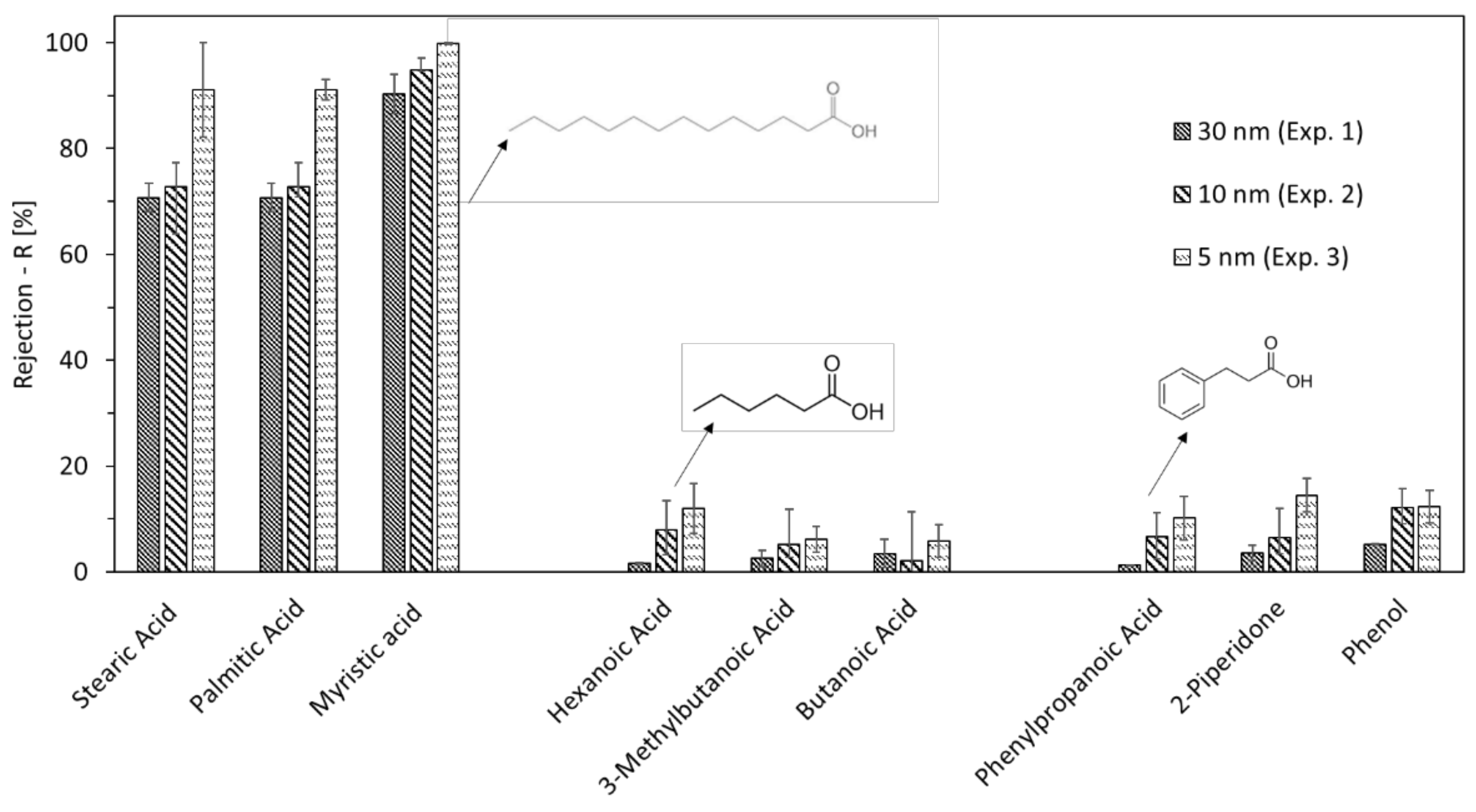
3.2. Permeate Quality
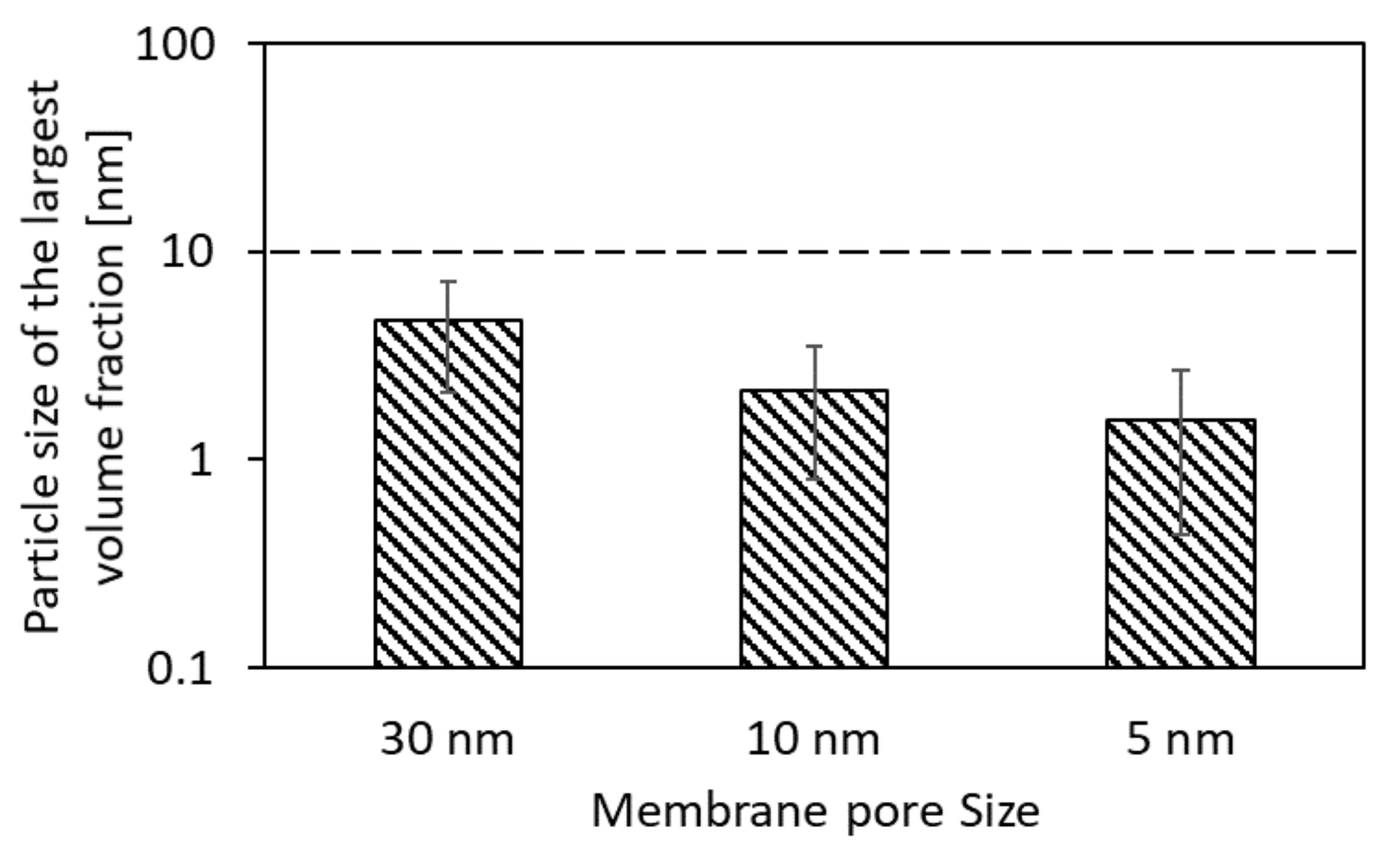
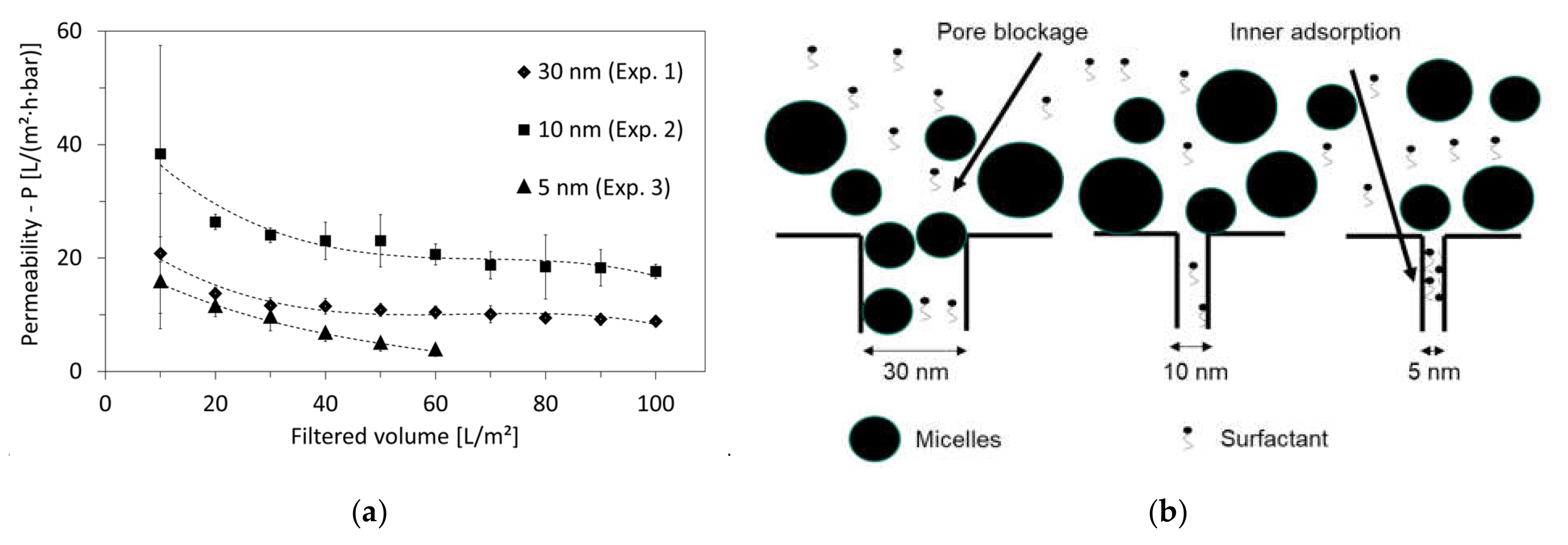
3.3. Optimal Membrane-Pore Size
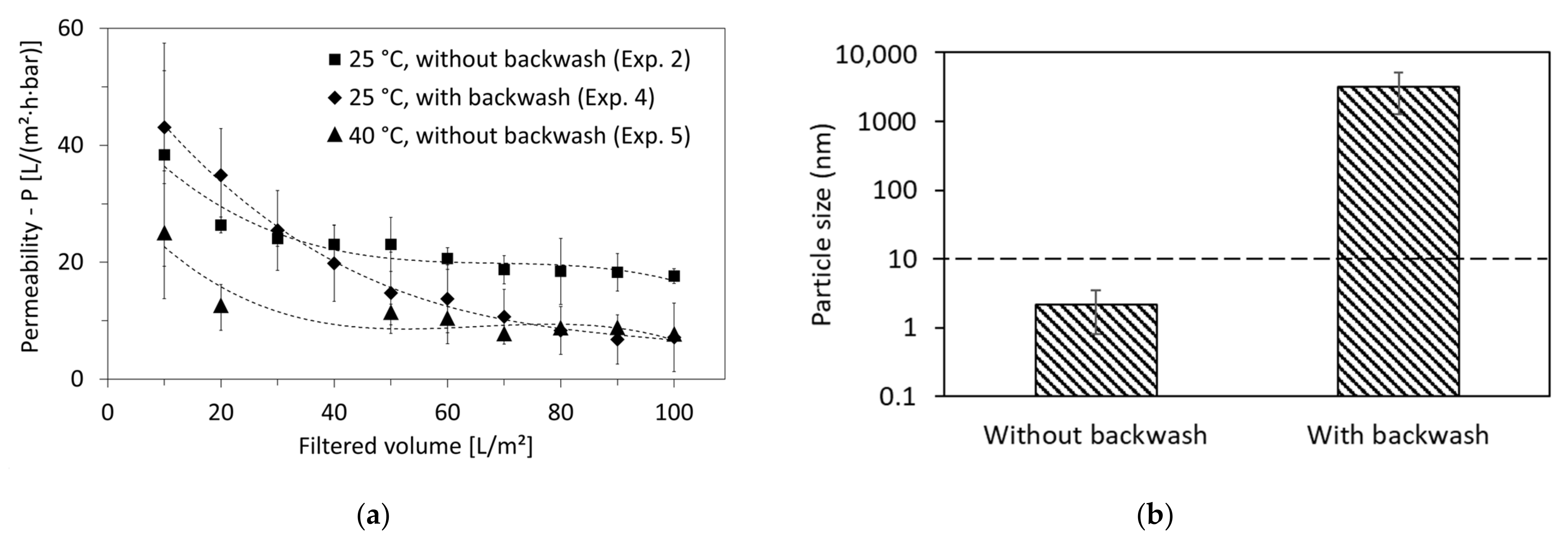
3.4. Optimal Operation Conditions
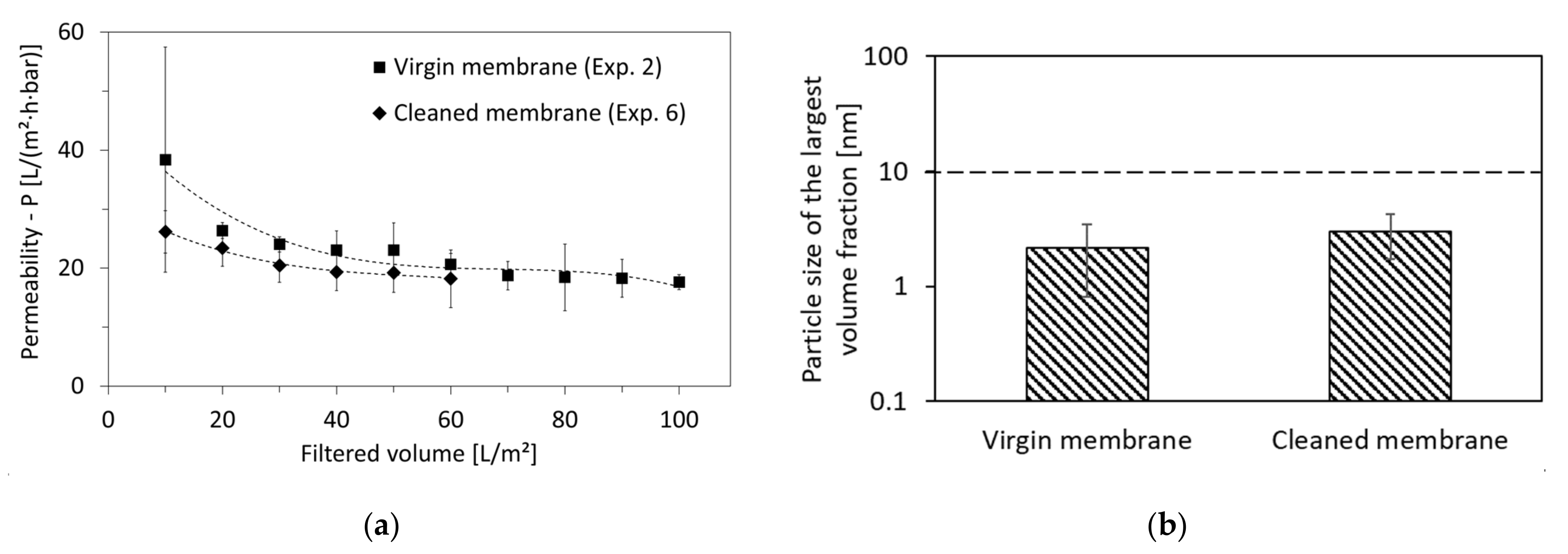
3.5. Optimal Cleaning Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maddi, B.; Panisko, E.; Wietsma, T.; Lemmon, T.; Swita, M.; Albrecht, K.; Howe, D. Quantitative characterization of aqueous byproducts from hydrothermal liquefaction of municipal wastes, food industry wastes, and biomass grown on waste. ACS Sustain. Chem. Eng. 2017, 5, 2205–2214. [Google Scholar] [CrossRef]
- Castello, D.; Pedersen, T.H.; Rosendahl, L.A. Continuous hydrothermal liquefaction of biomass: A critical review. Energies 2018, 11, 3165. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Scott, J.; Sharma, B.K.; Rajagopalan, N. Advanced treatment of hydrothermal liquefaction wastewater with nanofiltration to recover carboxylic acids. Environ. Sci. Water Res. Technol. 2018, 4, 520–528. [Google Scholar] [CrossRef]
- Van Doren, L.G.; Posmanik, R.; Bicalho, F.A.; Tester, J.W.; Sills, D.L. Prospects for energy recovery during hydrothermal and biological processing of waste biomass. Bioresour. Technol. 2017, 225, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Erkelens, M.; Ball, A.S.; Lewis, D.M. The application of activated carbon for the treatment and reuse of the aqueous phase derived from the hydrothermal liquefaction of a halophytic Tetraselmis sp. Bioresour. Technol. 2015, 182, 378–382. [Google Scholar] [CrossRef]
- Cherad, R.; Onwudili, J.; Biller, P.; Williams, P.; Ross, A. Hydrogen production from the catalytic supercritical water gasification of process water generated from hydrothermal liquefaction of microalgae. Fuel 2016, 166, 24–28. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Schideman, L.C.; Tommaso, G.; Chen, W.-T.; Zhou, Y.; Nair, K.; Qian, W.; Zhang, Y.; Wang, K. Anaerobic digestion of wastewater generated from the hydrothermal liquefaction of Spirulina: Toxicity assessment and minimization. Energy Convers. Manag. 2017, 141, 420–428. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Zhang, X.; Deal, B.; Han, L.; Zheng, J.; Ben, H. Advances in energy systems for valorization of aqueous byproducts generated from hydrothermal processing of biomass and systems thinking. Green Chem. 2019, 21, 2518–2543. [Google Scholar] [CrossRef]
- Shanmugam, S.R.; Adhikari, S.; Wang, Z.; Shakya, R. Treatment of aqueous phase of bio-oil by granular activated carbon and evaluation of biogas production. Bioresour. Technol. 2017, 223, 115–120. [Google Scholar] [CrossRef]
- Ravanchi, M.T.; Kaghazchi, T.; Kargari, A. Application of membrane separation processes in petrochemical industry: A review. Desalination 2009, 235, 199–244. [Google Scholar] [CrossRef]
- Padaki, M.; Murali, R.S.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.; Hilal, N.; Ismail, A. Membrane technology enhancement in oil–water separation. A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Lyu, H.; Fang, Y.; Ren, S.; Chen, K.; Luo, G.; Zhang, S.; Chen, J. Monophenols separation from monosaccharides and acids by two-stage nanofiltration and reverse osmosis in hydrothermal liquefaction hydrolysates. J. Membr. Sci. 2016, 504, 141–152. [Google Scholar] [CrossRef]
- Zhang, X.; Scott, J.; Sharma, B.K.; Rajagopalan, N. Fouling mitigation and carbon recovery in nanofiltration processing of hydrothermal liquefaction aqueous waste stream. J. Membr. Sci. 2020, 614, 118558. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; Comite, A.; Costa, C.; Firpo, R.; Jezowska, A.; Pagliero, M. Treatment of olive mill wastewater through integrated pressure-driven membrane processes. Membranes 2020, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-J.; Cao, Y.-M.; Qin, J.-J.; Jie, X.-M.; Wang, T.-H.; Liu, J.-H.; Yuan, Q. Development and characterization of anti-fouling cellulose hollow fiber UF membranes for oil–water separation. J. Membr. Sci. 2006, 279, 328–335. [Google Scholar] [CrossRef]
- Kennedy, M.; Siriphannon, S.; Van Hoof, S.; Schippers, J. Improving the performance of dead-end ultrafiltration systems: Comparing air and water flushing. Water Sci. Technol. Water Supply 2001, 1, 97–106. [Google Scholar] [CrossRef]
- Zondervan, E.; Roffel, B. Dynamic optimization of chemical cleaning in dead-end ultra filtration. J. Membr. Sci. 2008, 307, 309–313. [Google Scholar] [CrossRef] [Green Version]
- Al-Malack, M.H.; Anderson, G. Formation of dynamic membranes with crossflow microfiltration. J. Membr. Sci. 1996, 112, 287–296. [Google Scholar] [CrossRef]
- Fan, G.; Su, Z.; Lin, R.; Lin, X.; Xu, R.; Chen, W. Influence of membrane materials and operational modes on the performance of ultrafiltration modules for drinking water treatment. Int. J. Polym. Sci. 2016, 2016, 6895235. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Tao, Z.; Chen, L.; Han, M.; Zhao, B.; Tian, X.; Wang, L.; Meng, F. An antifouling catechol/chitosan-modified polyvinylidene fluoride membrane for sustainable oil-in-water emulsions separation. Front. Environ. Sci. Eng. 2021, 15, 63. [Google Scholar] [CrossRef]
- Issaoui, M.; Limousy, L. Low-cost ceramic membranes: Synthesis, classifications, and applications. Comptes Rendus Chim. 2019, 22, 175–187. [Google Scholar] [CrossRef]
- Benito, J.; Conesa, A.; Rubio, F.; Rodriguez, M. Preparation and characterization of tubular ceramic membranes for treatment of oil emulsions. J. Eur. Ceram. Soc. 2005, 25, 1895–1903. [Google Scholar] [CrossRef]
- Hofs, B.; Ogier, J.; Vries, D.; Beerendonk, E.F.; Cornelissen, E.R. Comparison of ceramic and polymeric membrane permeability and fouling using surface water. Sep. Purif. Technol. 2011, 79, 365–374. [Google Scholar] [CrossRef]
- Fan, L.; Harris, J.L.; Roddick, F.A.; Booker, N.A. Influence of the characteristics of natural organic matter on the fouling of microfiltration membranes. Water Res. 2001, 35, 4455–4463. [Google Scholar] [CrossRef]
- Gupta, B.; Howell, J.; Wu, D.; Field, R. A helical baffle for cross-flow microfiltration. J. Membr. Sci. 1995, 102, 31–42. [Google Scholar] [CrossRef]
- Baars, E.; Heijman, S.; Bosklopper, T.G. Red alert on transmembrane pressure (TMP). Desalination 2005, 179, 125–130. [Google Scholar] [CrossRef]
- Jones, K.L.; O’Melia, C.R. Protein and humic acid adsorption onto hydrophilic membrane surfaces: Effects of pH and ionic strength. J. Membr. Sci. 2000, 165, 31–46. [Google Scholar] [CrossRef]
- Kumar, R.; Ismail, A. Fouling control on microfiltration/ultrafiltration membranes: Effects of morphology, hydrophilicity, and charge. J. Appl. Polym. Sci. 2015, 132, 132. [Google Scholar] [CrossRef]
- Meng, S.; Wang, R.; Zhang, K.; Meng, X.; Xue, W.; Liu, H.; Liang, D.; Zhao, Q.; Liu, Y. Transparent exopolymer particles (TEPs)-associated protobiofilm: A neglected contributor to biofouling during membrane filtration. Front. Environ. Sci. Eng. 2021, 15, 64. [Google Scholar] [CrossRef]
- Shi, X.; Tal, G.; Hankins, N.P.; Gitis, V. Fouling and cleaning of ultrafiltration membranes: A review. J. Water Process Eng. 2014, 1, 121–138. [Google Scholar] [CrossRef]
- Sayegh, A.; Prakash, N.S.; Pedersen, T.H.; Horn, H.; Saravia, F. Treatment of hydrothermal liquefaction wastewater with ultrafiltration and air stripping for oil and particle removal and ammonia recovery. J. Water Process Eng. 2021, 44, 102427. [Google Scholar] [CrossRef]
- Burguera, J.L.; Burguera, M. Analytical applications of emulsions and microemulsions. Talanta 2012, 96, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Madani, S.; Amirfazli, A. Oil drop shedding from solid substrates by a shearing liquid. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 796–806. [Google Scholar] [CrossRef]
- Nachtigall, S.; Zedel, D.; Kraume, M. Analysis of drop deformation dynamics in turbulent flow. Chin. J. Chem. Eng. 2016, 24, 264–277. [Google Scholar] [CrossRef]
- Le Clech, P.; Jefferson, B.; Chang, I.S.; Judd, S.J. Critical flux determination by the flux-step method in a submerged membrane bioreactor. J. Membr. Sci. 2003, 227, 81–93. [Google Scholar] [CrossRef]
- Defrance, L.; Jaffrin, M. Comparison between filtrations at fixed transmembrane pressure and fixed permeate flux: Application to a membrane bioreactor used for wastewater treatment. J. Membr. Sci. 1999, 152, 203–210. [Google Scholar] [CrossRef]
- Obotey Ezugbe, E.; Rathilal, S. Membrane technologies in wastewater treatment: A review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, T.; Ma, J. Ceramic membrane fouling during ultrafiltration of oil/water emulsions: Roles played by stabilization surfactants of oil droplets. Environ. Sci. Technol. 2015, 49, 4235–4244. [Google Scholar] [CrossRef]
- Sutzkover-Gutman, I.; Hasson, D.; Semiat, R. Humic substances fouling in ultrafiltration processes. Desalination 2010, 261, 218–231. [Google Scholar] [CrossRef]
- Salahi, A.; Abbasi, M.; Mohammadi, T. Permeate flux decline during UF of oily wastewater: Experimental and modeling. Desalination 2010, 251, 153–160. [Google Scholar] [CrossRef]
- Crozes, G.; Jacangelo, J.; Anselme, C.; Laine, J. Impact of ultrafiltration operating conditions on membrane irreversible fouling. J. Membr. Sci. 1997, 124, 63–76. [Google Scholar] [CrossRef]
- Ye, Y.; Sim, L.N.; Herulah, B.; Chen, V.; Fane, A. Effects of operating conditions on submerged hollow fibre membrane systems used as pre-treatment for seawater reverse osmosis. J. Membr. Sci. 2010, 365, 78–88. [Google Scholar] [CrossRef]
- Mohajeri, E.; Noudeh, G.D. Effect of temperature on the critical micelle concentration and micellization thermodynamic of nonionic surfactants: Polyoxyethylene sorbitan fatty acid esters. E-J. Chem. 2012, 9, 2268–2274. [Google Scholar] [CrossRef]
- Schork, N.; Schuhmann, S.; Arndt, F.; Schütz, S.; Guthausen, G.; Nirschl, H. MRI investigations of filtration: Fouling and cleaning processes. Microporous Mesoporous Mater. 2018, 269, 60–64. [Google Scholar] [CrossRef]
- Li, F.; An, X.; Feng, C.; Kang, J.; Wang, J.; Yu, H. Research on Operation Efficiency and Membrane Fouling of A2/O-MBR in Reclaimed Water Treatment. Membranes 2019, 9, 172. [Google Scholar] [CrossRef] [Green Version]
- Dudziak, M.; Wyczarska-Kokot, J.; Łaskawiec, E.; Stolarczyk, A. Application of ultrafiltration in a swimming pool water treatment system. Membranes 2019, 9, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Parameter | Value (g/L) | Parameter | Value (g/L) |
|---|---|---|---|
| TSS | 0.8 | Stearic Acid | 0.3 |
| TOC | 35 | Benzenpropanoic Acid | 1.0 |
| Non-ionic surfactants | 1.3 | 2-Piperidone | 4.1 |
| Cationic surfactants | 0.4 | Butanoic Acid | 0.7 |
| Anionic surfactants | 0.3 | 3-Methylbutanoic Acid | 1.3 |
| Mystiric Acid | 0.1 | Hexanoic Acid | 0.4 |
| Palmitic Acid | 0.5 | Phenol | 0.3 |
| Experiment No. | Time of Operation [d] | Membrane-Pore Size [nm] | Temperature [°C] | Backwash Cycles [yes/no] |
|---|---|---|---|---|
| 1 | 7 | 30 | 25 | no |
| 2 | 7 | 10 | 25 | no |
| 3 | 7 | 5 | 25 | no |
| 4 | 8 | 10 | 25 | yes |
| 5 | 8 | 10 | 40 | no |
| 6 * | 3 | 10 | 25 | no |
| Pore Size [nm] | Temperature [°C] | Critical TMP [mbar] | Critical Flux [L/(m2·h)] |
|---|---|---|---|
| 30 | 25 | 90 | 8.3 |
| 10 | 25 | 75 | 6.6 |
| 5 | 25 | 150 | 6.1 |
| 10 | 40 | 60 | 5.2 |
| Surfactants’ Rejection—R [%] | |||
|---|---|---|---|
| Membrane-Pore Size | Anionic | Cationic | Non-Ionic |
| 30 nm | 85 | 20 | 3 |
| 10 nm | >90 | 31 | 5 |
| 5 nm | 87 | 30 | 18 |
| Cleaning Step | Time of Operation (h) | Cleaning Method | PWP (L/m2·h·Bar) | Recovery (%) |
|---|---|---|---|---|
| 1 | 1 | Rising CFV (1.5 m/s) | 89 | 42 |
| 2 | 1 | Rising temperature (50 °C) | 100 | 47 |
| 3 | 1 | Applying backwash | 93 | 44 |
| 4 | 24 | Alkaline cleaning (pH 12) | 149 | 70 |
| 5 | 24 | Acid cleaning (pH 2) | 157 | 75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayegh, A.; Merkert, S.; Zimmermann, J.; Horn, H.; Saravia, F. Treatment of Hydrothermal-Liquefaction Wastewater with Crossflow UF for Oil and Particle Removal. Membranes 2022, 12, 255. https://doi.org/10.3390/membranes12030255
Sayegh A, Merkert S, Zimmermann J, Horn H, Saravia F. Treatment of Hydrothermal-Liquefaction Wastewater with Crossflow UF for Oil and Particle Removal. Membranes. 2022; 12(3):255. https://doi.org/10.3390/membranes12030255
Chicago/Turabian StyleSayegh, Ali, Simon Merkert, Joscha Zimmermann, Harald Horn, and Florencia Saravia. 2022. "Treatment of Hydrothermal-Liquefaction Wastewater with Crossflow UF for Oil and Particle Removal" Membranes 12, no. 3: 255. https://doi.org/10.3390/membranes12030255
APA StyleSayegh, A., Merkert, S., Zimmermann, J., Horn, H., & Saravia, F. (2022). Treatment of Hydrothermal-Liquefaction Wastewater with Crossflow UF for Oil and Particle Removal. Membranes, 12(3), 255. https://doi.org/10.3390/membranes12030255







