Effects of Expanded Hemodialysis with Medium Cut-Off Membranes on Maintenance Hemodialysis Patients: A Review
Abstract
:1. Introduction
2. Unique Characteristics of Medium Cut-Off Membranes
2.1. Medium-Size Pore Radius and Tight Distribution of Pores
2.2. Steep Sieving Curve
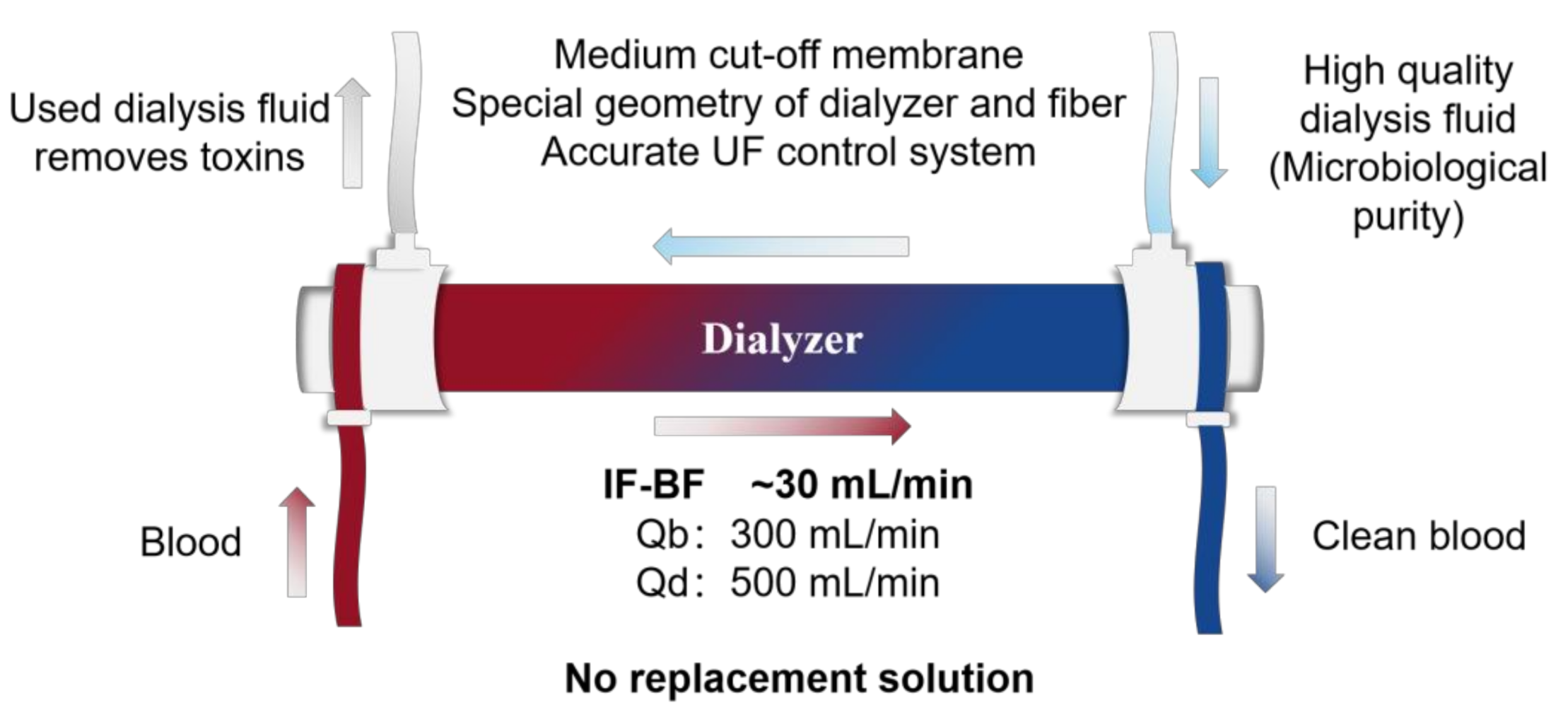
2.3. Internal Filtration–Backfiltration Mechanism (IF-BF)
3. The Effect of Expanded Hemodialysis on Uremic Toxins Removal
3.1. Dialysis Adequacy
3.2. Removal of β2 Microglobulin (β2-M)
3.3. Removal of Free Light Chains (FLCs) and Other Middle Molecules
3.4. Removal of Protein-Bound Uremic Toxins (PBUTs)
4. The Effect of Expanded Hemodialysis on Inflammation and Cardiovascular Risk
4.1. Effect of Expended Hemodialysis on Inflammation and Oxidative Stress
4.2. Effect of Expanded Hemodialysis on Cardiovascular Parameters
5. Effect of Expended Hemodialysis on Quality of Life (QoL)
6. Health Economics
6.1. Medication Costs for Erythropoietin-Stimulating Agents
6.2. Hospitalization Rates and Costs
7. Safety Concerns
7.1. Retention of Serum Albumin
7.2. Effects on Medication Clearance
7.3. Adverse Events
8. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Levey, A.S.; Eckardt, K.-U.; Dorman, N.M.; Christiansen, S.L.; Hoorn, E.J.; Ingelfinger, J.R.; Inker, L.A.; Levin, A.; Mehrotra, R.; Palevsky, P.M.; et al. Nomenclature for kidney function and disease: Report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2020, 97, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [Green Version]
- Liyanage, T.; Ninomiya, T.; Jha, V.; Neal, B.; Patrice, H.M.; Okpechi, I.; Zhao, M.-H.; Lv, J.; Garg, A.X.; Knight, J.; et al. World-wide access to treatment for end-stage kidney disease: A systematic review. Lancet 2015, 385, 1975–1982. [Google Scholar] [CrossRef]
- Himmelfarb, J.; Vanholder, R.; Mehrotra, R.; Tonelli, M. The current and future landscape of dialysis. Nat. Rev. Nephrol. 2020, 16, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Merrill, J.P. Dialysis versus transplantation in the treatment of end-stage renal Disease. Annu. Rev. Med. 1978, 29, 343–358. [Google Scholar] [CrossRef]
- Himmelfarb, J.; Ikizler, T.A. Hemodialysis. N. Engl. J. Med. 2010, 363, 1833–1845. [Google Scholar] [CrossRef]
- Rosner, M.H.; Reis, T.; Husain-Syed, F.; Vanholder, R.; Hutchison, C.; Stenvinkel, P.; Blankestijn, P.J.; Cozzolino, M.; Juillard, L.; Kashani, K.; et al. Classification of uremic toxins and their role in kidney failure. Clin. J. Am. Soc. Nephrol. 2021, 16, 1918–1928. [Google Scholar] [CrossRef]
- Van Biesen, W.; De Bacquer, D.; Verbeke, F.; Delanghe, J.; Lameire, N.; Vanholder, R. The glomerular filtration rate in an apparently healthy population and its relation with cardiovascular mortality during 10 years. Eur. Heart J. 2007, 28, 478–483. [Google Scholar] [CrossRef] [Green Version]
- Moe, S.; Drüeke, T.; Cunningham, J.; Goodman, W.; Martin, K.; Olgaard, K.; Ott, S.; Sprague, S.; Lameire, N.; Eknoyan, G. Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006, 69, 1945–1953. [Google Scholar] [CrossRef] [Green Version]
- Viggiano, D.; Wagner, C.A.; Martino, G.; Nedergaard, M.; Zoccali, C.; Unwin, R.; Capasso, G. Mechanisms of cognitive dysfunction in CKD. Nat. Rev. Nephrol. 2020, 16, 452–469. [Google Scholar] [CrossRef]
- Wang, H.E.; Gamboa, C.; Warnock, D.G.; Muntner, P. Chronic kidney disease and risk of death from infection. Am. J. Nephrol. 2011, 34, 330–336. [Google Scholar] [CrossRef] [Green Version]
- Ciceri, P.; Cozzolino, M. Expanded haemodialysis as a current strategy to remove uremic toxins. Toxins 2021, 13, 380. [Google Scholar] [CrossRef]
- Boschetti-De-Fierro, A.; Voigt, M.; Storr, M.; Krause, B. MCO Membranes: Enhanced selectivity in high-flux class. Sci. Rep. 2015, 5, 18448. [Google Scholar] [CrossRef] [Green Version]
- Humes, H.; Fissell, W.; Tiranathanagul, K. The future of hemodialysis membranes. Kidney Int. 2006, 69, 1115–1119. [Google Scholar] [CrossRef] [Green Version]
- Boschetti-De-Fierro, A.; Voigt, M.; Storr, M.; Krause, B. Extended characterization of a new class of membranes for blood purification: The high cut-off membranes. Int. J. Artif. Organs 2013, 36, 455–463. [Google Scholar] [CrossRef]
- Maduell, F.; Rodas, L.; Broseta, J.J.; Gomez, M.; Xipell, M.; Guillen, E.; Montagud-Marrahi, E.; Arias-Guillén, M.; Fontseré, N.; Vera, M.; et al. medium cut-off dialyzer versus eight hemodiafiltration dialyzers: Comparison using a global removal score. Blood Purif. 2019, 48, 167–174. [Google Scholar] [CrossRef]
- Canaud, B.; Bragg-Gresham, J.; Marshall, M.; Desmeules, S.; Gillespie, B.; Depner, T.; Klassen, P.; Port, F. Mortality risk for patients receiving hemodiafiltration versus hemodialysis: European results from the DOPPS. Kidney Int. 2006, 69, 2087–2093. [Google Scholar] [CrossRef] [Green Version]
- Maduell, F.; Moreso, F.; Pons, M.; Ramos, R.; Mora-Macià, J.; Carreras, J.; Soler, J.; Torres, F.; Campistol, J.M.; Martinez-Castelao, A.; et al. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J. Am. Soc. Nephrol. 2013, 24, 487–497. [Google Scholar] [CrossRef] [Green Version]
- Ronco, C. Hemodiafiltration: Evolution of a technique towards better dialysis care. Contrib. Nephrol. 2011, 168, 19–27. [Google Scholar] [CrossRef]
- Hulko, M.; Dietrich, V.; Koch, I.; Gekeler, A.; Gebert, M.; Beck, W.; Krause, B. Pyrogen retention: Comparison of the novel medium cut-off (MCO) membrane with other dialyser membranes. Sci. Rep. 2019, 9, 6791. [Google Scholar] [CrossRef]
- Uchino, S.; Bellomo, R.; Goldsmith, D.; Davenport, P.; Cole, L.; Baldwin, I.; Panagiotopoulos, S.; Tipping, P. Super high flux hemofiltration: A new technique for cytokine removal. Intensive Care Med. 2002, 28, 651–655. [Google Scholar] [CrossRef]
- Haase, M.; Bellomo, R.; Morger, S.; Baldwin, I.; Boyce, N.; Haase, M.; Bellomo, R.; Morger, S.; Baldwin, I.; Boyce, N. High cut-off point membranes in septic acute renal failure: A systematic review. Int. J. Artif. Organs 2007, 30, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Naka, T.; Haase, M.; Bellomo, R. ‘Super high-flux’ or ‘high cut-off’ hemofiltration and hemodialysis. Contrib. Nephrol. 2010, 166, 181–189. [Google Scholar] [PubMed]
- Gondouin, B.; Hutchison, C.A. High cut-off dialysis membranes: Current uses and future potential. Adv. Chronic Kidney Dis. 2011, 18, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, C.A.; Heyne, N.; Airia, P.; Schindler, R.; Zickler, D.; Cook, M.; Cockwell, P.; Grima, D. Immunoglobulin free light chain levels and recovery from myeloma kidney on treatment with chemotherapy and high cut-off haemodialysis. Nephrol. Dial. Transplant. 2012, 27, 3823–3828. [Google Scholar] [CrossRef]
- Hutchison, C.A.; Bradwell, A.; Cook, M.; Basnayake, K.; Basu, S.; Harding, S.; Hattersley, J.; Evans, N.; Chappel, M.J.; Sampson, P.; et al. Treatment of acute renal failure secondary to multiple myeloma with chemotherapy and extended high cut-off hemodialysis. Clin. J. Am. Soc. Nephrol. 2009, 4, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Rimmelé, T.; Kellum, J.A. Clinical review: Blood purification for sepsis. Crit. Care 2011, 15, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girndt, M.; Fiedler, R.; Martus, P.; Pawlak, M.; Storr, M.; Bohler, T.; Glomb, M.A.; Liehr, K.; Henning, C.; Templin, M.; et al. High cut-off dialysis in chronic haemodialysis patients. Eur. J. Clin. Investig. 2015, 45, 1333–1340. [Google Scholar] [CrossRef]
- Ronco, C.; Clark, W.R. Haemodialysis membranes. Nat. Rev. Nephrol. 2018, 14, 394–410. [Google Scholar] [CrossRef]
- Ronco, C.; La Manna, G. Expanded hemodialysis: A new therapy for a new class of membranes. Contrib. Nephrol. 2017, 190, 124–133. [Google Scholar] [CrossRef]
- Ronco, C. The Rise of Expanded Hemodialysis. Blood Purif. 2017, 44, I–VIII. [Google Scholar] [CrossRef]
- Boschetti-De-Fierro, A.; Beck, W.; Hildwein, H.; Krause, B.; Storr, M.; Zweigart, C. Membrane Innovation in Dialysis. Contrib. Nephrol. 2017, 191, 100–114. [Google Scholar] [CrossRef]
- Kirsch, A.H.; Lyko, R.; Nilsson, L.-G.; Beck, W.; Amdahl, M.; Lechner, P.; Schneider, A.; Wanner, C.; Rosenkranz, A.R.; Krieter, D.H. Performance of hemodialysis with novel medium cut-off dialyzers. Nephrol. Dial. Transplant. 2017, 32, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Schepers, E.; Glorieux, G.; Eloot, S.; Hulko, M.; Boschetti-De-Fierro, A.; Beck, W.; Krause, B.; Van Biesen, W. Assessment of the association between increasing membrane pore size and endotoxin permeability using a novel experimental dialysis simulation set-up. BMC Nephrol. 2018, 19, 1. [Google Scholar] [CrossRef] [Green Version]
- Wolley, M.; Jardine, M.; Hutchison, C.A. Exploring the clinical relevance of providing increased removal of large middle molecules. Clin. J. Am. Soc. Nephrol. 2018, 13, 805–814. [Google Scholar] [CrossRef]
- Ronco, C.; Marchionna, N.; Brendolan, A.; Neri, M.; Lorenzin, A.; Rueda, A.J.M. Expanded haemodialysis: From operational mechanism to clinical results. Nephrol. Dial. Transplant. 2018, 33 (Suppl. 3), iii41–iii47. [Google Scholar] [CrossRef] [Green Version]
- Axelsson, J.; Mahmutovic, I.; Rippe, A.; Rippe, B. Loss of size selectivity of the glomerular filtration barrier in rats following laparotomy and muscle trauma. Am. J. Physiol. Physiol. 2009, 297, F577–F582. [Google Scholar] [CrossRef]
- Storr, M.; Ward, R.A. Membrane innovation: Closer to native kidneys. Nephrol. Dial. Transplant. 2018, 33 (Suppl. 3), iii22–iii27. [Google Scholar] [CrossRef]
- Zweigart, C.; Boschetti-De-Fierro, A.; Hulko, M.; Nilsson, L.-G.; Beck, W.; Storr, M.; Krause, B. Medium Cut-Off Membranes—Closer to the Natural Kidney Removal Function. Int. J. Artif. Organs 2017, 40, 328–334. [Google Scholar] [CrossRef]
- Ronco, C.; Brendolan, A.; Lupi, A.; Metry, G.; Levin, N.W. Effects of a reduced inner diameter of hollow fibers in hemodialyzers. Kidney Int. 2000, 58, 809–817. [Google Scholar] [CrossRef] [Green Version]
- Lorenzin, A.; Golino, G.; de Cal, M.; Pajarin, G.; Savastano, S.; Lupi, A.; Sandini, A.; Fiorin, F.; Ronco, C. Flow dynamic analysis by contrast-enhanced imaging techniques of medium cutoff membrane hemodialyzer. Blood Purif. 2021, 51, 138–146. [Google Scholar] [CrossRef]
- Macias, N.; Vega, A.; Abad, S.; Aragoncillo, I.; Maria Garcia-Prieto, A.; Santos, A.; Torres, E.; Luno, J. Middle molecule elimination in expanded haemodialysis: Only convective transport? Clin. Kidney J. 2019, 12, 447–455. [Google Scholar] [CrossRef]
- Lorenzin, A.; Neri, M.; Lupi, A.; Todesco, M.; Santimaria, M.; Alghisi, A.; Brendolan, A.; Ronco, C. Quantification of internal filtration in hollow fiber hemodialyzers with medium cut-off membrane. Blood Purif. 2018, 46, 196–204. [Google Scholar] [CrossRef]
- Clark, W.R.; Gao, D.; Neri, M.; Ronco, C. Solute transport in hemodialysis: Advances and limitations of current membrane technology. Contrib. Nephrol. 2017, 191, 84–99. [Google Scholar] [CrossRef]
- Lorenzin, A.; Neri, M.; Clark, W.R.; Garzotto, F.; Brendolan, A.; Nalesso, F.; Marchionna, N.; Zanella, M.; Sartori, M.; Fiore, G.B.; et al. Modeling of Internal Filtration in Theranova Hemodialyzers. Contrib. Nephrol. 2017, 191, 127–141. [Google Scholar] [CrossRef]
- Hong, W.-P.; Lee, Y.-J. The association of dialysis adequacy, body mass index, and mortality among hemodialysis patients. BMC Nephrol. 2019, 20, 382–388. [Google Scholar] [CrossRef]
- National Kidney Foundation. KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 update. Am. J. Kidney Dis. 2015, 66, 884–930. [Google Scholar] [CrossRef] [Green Version]
- Kimata, N.; Karaboyas, A.; Bieber, B.A.; Pisoni, R.L.; Morgenstern, H.; Gillespie, B.W.; Saito, A.; Akizawa, T.; Fukuhara, S.; Robinson, B.M.; et al. Gender, low Kt/V, and mortality in Japanese hemodialysis patients: Opportunities for improvement through modifiable practices. Hemodial. Int. 2014, 18, 596–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duong, T.V.; Wu, P.-Y.; Wong, T.-C.; Chen, H.-H.; Chen, T.-H.; Hsu, Y.-H.; Peng, S.-J.; Kuo, K.-L.; Liu, H.-C.; Lin, E.-T.; et al. Mid-arm circumference, body fat, nutritional and inflammatory biomarkers, blood glucose, dialysis adequacy influence all-cause mortality in hemodialysis patients: A prospective cohort study. Medicine 2019, 98, e14930. [Google Scholar] [CrossRef] [PubMed]
- Béguin, L.; Krummel, T.; Longlune, N.; Galland, R.; Couchoud, C.; Hannedouche, T. Dialysis dose and mortality in hemodialysis: Is higher better? Nephrol. Dial. Transplant. 2021, 36, 2300–2307. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-H.; Park, Y.; Yook, J.-M.; Choi, S.-Y.; Jung, H.-Y.; Choi, J.-Y.; Park, S.-H.; Kim, C.-D.; Kim, Y.-L.; Cho, J.-H. Randomized controlled trial of medium cut-off versus high-flux dialyzers on quality of life outcomes in maintenance hemodialysis patients. Sci. Rep. 2020, 10, 7780. [Google Scholar] [CrossRef]
- Weiner, D.E.; Falzon, L.; Skoufos, L.; Bernardo, A.; Beck, W.; Xiao, M.; Tran, H. Efficacy and safety of expanded hemodialysis with the theranova 400 dialyzer: A randomized controlled trial. Clin. J. Am. Soc. Nephrol. CJASN 2020, 15, 1310–1319. [Google Scholar] [CrossRef]
- Sevinc, M.; Hasbal, N.B.; Yilmaz, V.; Basturk, T.; Ahbap, E.; Sakaci, T.; Ozcafer, P.N.; Unsal, A. Comparison of circulating levels of uremic toxins in hemodialysis patients treated with medium cut-off membranes and high-flux membranes: Theranova in Sisli Hamidiye Etfal (THE SHE) Randomized Control Study. Blood Purif. 2020, 49, 733–742. [Google Scholar] [CrossRef]
- Belmouaz, M.; Bauwens, M.; Hauet, T.; Bossard, V.; Jamet, P.; Joly, F.; Chikhi, E.; Joffrion, S.; Gand, E.; Bridoux, F. Comparison of the removal of uraemic toxins with medium cut-off and high-flux dialysers: A randomized clinical trial. Nephrol. Dial. Transplant. 2020, 35, 328–335. [Google Scholar] [CrossRef]
- Bunch, A.; Sanchez, R.; Nilsson, L.; Bernardo, A.A.; Vesga, J.I.; Ardila, F.; Guerrero, I.M.; Sanabria, R.M.; Rivera, A.S. The colombian registry of expanded hemodialysis investigators medium cut-off dialyzers in a large population of hemodialysis patients in colombia: Corexh registry. Ther. Apher. Dial. 2021, 25, 33–43. [Google Scholar] [CrossRef]
- Gejyo, F.; Yamada, T.; Odani, S.; Nakagawa, Y.; Arakawa, M.; Kunitomo, T.; Kataoka, H.; Suzuki, M.; Hirasawa, Y.; Shirahama, T. A new form of amyloid protein associated with chronic hemodialysis was identified as beta 2-microglobulin. Biochem. Biophys. Res. Commun. 1985, 129, 701–706. [Google Scholar] [CrossRef]
- Winchester, J.F.; Salsberg, J.A.; Levin, N.W. Beta-2 microglobulin in ESRD: An in-depth review. Adv. Ren. Replace. Ther. 2003, 10, 279–309. [Google Scholar] [CrossRef]
- Cho, N.-J.; Park, S.; Islam, I.; Song, H.-Y.; Lee, E.Y.; Gil, H.-W. Long-term effect of medium cut-off dialyzer on middle uremic toxins and cell-free hemoglobin. PLoS ONE 2019, 14, e0220448. [Google Scholar] [CrossRef] [Green Version]
- Kirsch, A.H.; Rosenkranz, A.R.; Lyko, R.; Krieter, D.H. Effects of Hemodialysis Therapy Using Dialyzers with Medium Cut-Off Membranes on Middle Molecules. Contrib. Nephrol. 2017, 191, 158–167. [Google Scholar] [CrossRef]
- Ward, R.A.; Greene, T.; Hartmann, B.; Samtleben, W. Resistance to intercompartmental mass transfer limits beta2-microglobulin removal by post-dilution hemodiafiltration. Kidney Int. 2006, 69, 1431–1437. [Google Scholar] [CrossRef] [Green Version]
- Fraser, S.D.; Fenton, A.; Harris, S.; Shardlow, A.; Liabeuf, S.; Massy, Z.A.; Burmeister, A.; Hutchison, C.A.; Landray, M.; Emberson, J.; et al. The Association of Serum Free Light Chains with Mortality and Progression to End-Stage Renal Disease in Chronic Kidney Disease: Systematic Review and Individual Patient Data Meta-analysis. Mayo Clin. Proc. 2017, 92, 1671–1681. [Google Scholar] [CrossRef] [Green Version]
- Krishnasamy, R.; Hawley, C.M.; Jardine, M.J.; Roberts, M.A.; Cho, Y.; Wong, M.; Heath, A.; Nelson, C.L.; Sen, S.; Mount, P.F.; et al. A trial evaluating mid cut-off value membrane clearance of albumin and light chains in hemodialysis patients: A safety device study. Blood Purif. 2020, 49, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Jourde-Chiche, N.; Dou, L.; Cerini, C.; Dignat-George, F.; Brunet, P. Vascular incompetence in dialysis patients-protein-bound uremic toxins and endothelial dysfunction. Semin. Dial. 2011, 24, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Lekawanvijit, S.; Kompa, A.R.; Wang, B.H.; Kelly, D.J.; Krum, H. Cardiorenal syndrome: The emerging role of protein-bound uremic toxins. Circ. Res. 2012, 111, 1470–1483. [Google Scholar] [CrossRef] [Green Version]
- Vanholder, R.; Schepers, E.; Pletinck, A.; Nagler, E.V.; Glorieux, G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: A systematic review. J. Am. Soc. Nephrol. 2014, 25, 1897–1907. [Google Scholar] [CrossRef] [PubMed]
- Tiong, M.K.; Krishnasamy, R.; Smith, E.R.; Hutchison, C.A.; Ryan, E.G.; Pascoe, E.M.; Hawley, C.M.; Hewitson, T.D.; Jardine, M.J.; Roberts, M.A.; et al. Effect of a medium cut-off dialyzer on protein-bound uremic toxins and mineral metabolism markers in patients on hemodialysis. Hemodial. Int. 2021, 25, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Reque, J.; Pérez Alba, A.; Panizo, N.; Sánchez-Canel, J.J.; Pascual, M.J.; Pons Prades, R. Is expanded hemodialysis an option to online hemodiafiltration for small- and middle-sized molecules clearance? Blood Purif. 2019, 47, 126–131. [Google Scholar] [CrossRef]
- García-Prieto, A.; Vega, A.; Linares, T.; Abad, S.; Macías, N.; Aragoncillo, I.; Torres, E.; Hernández, A.; Barbieri, D.; Luño, J. Evaluation of the efficacy of a medium cut-off dialyser and comparison with other high-flux dialysers in conventional haemodialysis and online haemodiafiltration. Clin. Kidney J. 2018, 11, 742–746. [Google Scholar] [CrossRef] [Green Version]
- Lindgren, A.; Fjellstedt, E.; Christensson, A. Comparison of hemodialysis using a medium cutoff dialyzer versus hemodiafiltration: A controlled cross-over study. Int. J. Nephrol. Renov. Dis. 2020, 13, 273–280. [Google Scholar] [CrossRef]
- Belmouaz, M.; Diolez, J.; Bauwens, M.; Duthe, F.; Ecotiere, L.; Desport, E.; Bridoux, F. Comparison of hemodialysis with medium cut-off dialyzer and on-line hemodiafiltration on the removal of small and middle-sized molecules. Clin. Nephrol. 2018, 89, 50–56. [Google Scholar] [CrossRef]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and oxidative stress in chronic kidney disease-potential therapeutic role of minerals, vitamins and plant-derived metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef] [Green Version]
- Scholze, A.; Jankowski, J.; Pedraza-Chaverri, J.; Evenepoel, P. Oxidative stress in chronic kidney disease. Oxid. Med. Cell. Longev. 2016, 2016, 8375186. [Google Scholar] [CrossRef] [Green Version]
- Ali, B.H.; Al-Salam, S.; Al Suleimani, Y.; Al Kalbani, J.; Al Bahlani, S.; Ashique, M.; Manoj, P.; Al Dhahli, B.; Al Abri, N.; Naser, H.T.; et al. Curcumin ameliorates kidney function and oxidative stress in experimental chronic kidney disease. Basic Clin. Pharmacol. Toxicol. 2018, 122, 65–73. [Google Scholar] [CrossRef]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef] [Green Version]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.-M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. J. Immunol. Res. 2018, 2018, 2180373. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Mitra, N.; Kanetsky, P.A.; Devaney, J.; Wing, M.R.; Reilly, M.; Shah, V.O.; Balakrishnan, V.S.; Guzman, N.J.; Girndt, M.; et al. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin. J. Am. Soc. Nephrol. 2012, 7, 1938–1946. [Google Scholar] [CrossRef] [Green Version]
- Modaresi, A.; Nafar, M.; Sahraei, Z. Oxidative stress in chronic kidney disease. Iran. J. Kidney Dis. 2015, 9, 165–179. [Google Scholar]
- Lumlertgul, N.; Hall, A.; Camporota, L.; Crichton, S.; Ostermann, M. Clearance of inflammatory cytokines in patients with septic acute kidney injury during renal replacement therapy using the EMiC2 filter (Clic-AKI study). Crit. Care 2021, 25, 1–11. [Google Scholar] [CrossRef]
- Zickler, D.; Schindler, R.; Willy, K.; Martus, P.; Pawlak, M.; Storr, M.; Hulko, M.; Boehler, T.; Glomb, M.A.; Liehr, K.; et al. Medium Cut-off (mco) membranes reduce inflammation in chronic dialysis patients—A randomized controlled clinical trial. PLoS ONE 2017, 12, e0169024. [Google Scholar] [CrossRef]
- Lim, J.-H.; Jeon, Y.; Yook, J.-M.; Choi, S.-Y.; Jung, H.-Y.; Choi, J.-Y.; Park, S.-H.; Kim, C.-D.; Kim, Y.-L.; Cho, J.-H. Medium cut-off dialyzer improves erythropoiesis stimulating agent resistance in a hepcidin-independent manner in maintenance hemodialysis patients: Results from a randomized controlled trial. Sci. Rep. 2020, 10, 16062. [Google Scholar] [CrossRef]
- Cozzolino, M.; Magagnoli, L.; Ciceri, P.; Conte, F.; Galassi, A. Effects of a medium cut-off (Theranova(®)) dialyser on haemodialysis patients: A prospective, cross-over study. Clin. Kidney J. 2021, 14, 382–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeter, H.H.; Korucu, B.; Akcay, O.F.; Derici, K.; Derici, U.; Arinsoy, T. Effects of medium cut-off dialysis membranes on inflammation and oxidative stress in patients on maintenance hemodialysis. Int. Urol. Nephrol. 2020, 52, 1779–1789. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.N.; Parfrey, P.S.; Sarnak, M.J. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am. J. Kidney Dis. 1998, 32, S112–S119. [Google Scholar] [CrossRef] [PubMed]
- Di Lullo, L.; House, A.; Gorini, A.; Santoboni, A.; Russo, D.; Ronco, C. Chronic kidney disease and cardiovascular complications. Hear. Fail. Rev. 2015, 20, 259–272. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Shaper, A.G.; Lowe, G.D.; Lennon, L.; Rumley, A.; Whincup, P.H. Renal function and cardiovascular mortality in elderly men: The role of inflammatory, procoagulant, and endothelial biomarkers. Eur. Heart J. 2006, 27, 2975–2981. [Google Scholar] [CrossRef] [Green Version]
- Himmelfarb, J.; Stenvinkel, P.; Ikizler, T.; Hakim, R.M. The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002, 62, 1524–1538. [Google Scholar] [CrossRef] [Green Version]
- Cheung, A.K.; Sarnak, M.J.; Yan, G.; Dwyer, J.; Heyka, R.J.; Rocco, M.V.; Teehan, B.P.; Levey, A.S. Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int. 2000, 58, 353–362. [Google Scholar] [CrossRef] [Green Version]
- Zoccali, C.; Mallamaci, F.; Tripepi, G. Novel cardiovascular risk factors in end-stage renal disease. J. Am. Soc. Nephrol. 2004, 15, 77–80. [Google Scholar] [CrossRef] [Green Version]
- Zoccali, C.; Mallamaci, F.; Tripepi, G. Traditional and emerging cardiovascular risk factors in end-stage renal disease. Kidney Int. 2003, 63, S105–S110. [Google Scholar] [CrossRef] [Green Version]
- Lekawanvijit, S. Cardiotoxicity of uremic toxins: A driver of cardiorenal syndrome. Toxins 2018, 10, 352. [Google Scholar] [CrossRef] [Green Version]
- Meléndez, G.C.; McLarty, J.L.; Levick, S.P.; Du, Y.; Janicki, J.S.; Brower, G.L. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension 2010, 56, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Libby, P. Interleukin-1 Beta as a target for atherosclerosis therapy: Biological basis of CANTOS and beyond. J. Am. Coll. Cardiol. 2017, 70, 2278–2289. [Google Scholar] [CrossRef]
- Willy, K.; Hulko, M.; Storr, M.; Speidel, R.; Gauss, J.; Schindler, R.; Zickler, D. In vitro dialysis of cytokine-rich plasma with high and medium cut-off membranes reduces its procalcific activity. Artif. Organs 2017, 41, 803–809. [Google Scholar] [CrossRef]
- Willy, K.; Girndt, M.; Voelkl, J.; Fiedler, R.; Martus, P.; Storr, M.; Schindler, R.; Zickler, D. Expanded haemodialysis therapy of chronic haemodialysis patients prevents calcification and apoptosis of vascular smooth muscle cells in vitro. Blood Purif. 2018, 45, 131–138. [Google Scholar] [CrossRef]
- Ahn, S.-H.; Ko, M.M.; Song, J.H.; Jung, J.H. Changes in plasma sclerostin level associated with use of a medium cut-off dialyzer in end-stage renal disease. Kidney Res. Clin. Pr. 2021, 40, 120–134. [Google Scholar] [CrossRef]
- Lee, Y.; Jang, M.-J.; Jeon, J.; Lee, J.E.; Huh, W.; Choi, B.S.; Park, C.W.; Chin, H.J.; Kang, C.L.; Kim, D.K.; et al. Cardiovascular risk comparison between expanded hemodialysis using theranova and online hemodiafiltration (CARTOON): A multicenter randomized controlled trial. Sci. Rep. 2021, 11, 10807. [Google Scholar] [CrossRef]
- Abdel-Kader, K.; Unruh, M.L.; Weisbord, S.D. Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin. J. Am. Soc. Nephrol. 2009, 4, 1057–1064. [Google Scholar] [CrossRef]
- Gorodetskaya, I.; Zenios, S.; Mcculloch, C.E.; Bostrom, A.; Hsu, C.-Y.; Bindman, A.B.; Go, A.S.; Chertow, G.M. Health-related quality of life and estimates of utility in chronic kidney disease. Kidney Int. 2005, 68, 2801–2808. [Google Scholar] [CrossRef] [Green Version]
- Tsai, Y.-C.; Hung, C.-C.; Hwang, S.-J.; Wang, S.-L.; Hsiao, S.-M.; Lin, M.-Y.; Kung, L.-F.; Hsiao, P.-N.; Chen, H.-C. Quality of life predicts risks of end-stage renal disease and mortality in patients with chronic kidney disease. Nephrol. Dial. Transplant. 2010, 25, 1621–1626. [Google Scholar] [CrossRef] [Green Version]
- Alarcon, J.C.; Bunch, A.; Ardila, F.; Zuñiga, E.; Vesga, J.I.; Rivera, A.; Sánchez, R.; Sanabria, R.M. On behalf of the colombian registry of expanded hemodialysis investigators impact of medium cut-off dialyzers on patient-reported outcomes: COREXH registry. Blood Purif. 2021, 50, 110–118. [Google Scholar] [CrossRef]
- Reis, T.; Martino, F.; Dias, P.; de Freitas, G.R.R.; Filho, E.R.D.S.; de Azevedo, M.L.C.; Reis, F.; Cozzolino, M.; Rizo-Topete, L.; Ronco, C. Removal of middle molecules with medium cutoff dialyzer in patients on short frequent hemodialysis. Hemodial. Int. 2021, 25, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; on behalf of the European Kidney Health Alliance; Annemans, L.; Brown, E.; Gansevoort, R.; Gout-Zwart, J.J.; Lameire, N.; Morton, R.L.; Oberbauer, R.; Postma, M.J.; et al. Reducing the costs of chronic kidney disease while delivering quality health care: A call to action. Nat. Rev. Nephrol. 2017, 13, 393–409. [Google Scholar] [CrossRef] [PubMed]
- Sanabria, R.M.; Hutchison, C.A.; Vesga, J.I.; Ariza, J.G.; Sanchez, R.; Suarez, A.M. Expanded Hemodialysis and Its Effects on Hospitalizations and Medication Usage: A Cohort Study. Nephron Exp. Nephrol. 2021, 145, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Ariza, J.G.; Walton, S.M.; Suarez, A.M.; Sanabria, M.; Vesga, J.I. An initial evaluation of expanded hemodialysis on hospitalizations, drug utilization, costs, and patient utility in Colombia. Ther. Apher. Dial. 2021, 25, 621–627. [Google Scholar] [CrossRef]
- Bae, M.N.; Kim, S.H.; Kim, Y.O.; Jin, D.C.; Song, H.C.; Choi, E.J.; Kim, Y.K.; Kim, Y.-S.; Kang, S.-W.; Kim, N.-H.; et al. Association of Erythropoietin-Stimulating Agent Responsiveness with Mortality in Hemodialysis and Peritoneal Dialysis Patients. PLoS ONE 2015, 10, e0143348. [Google Scholar] [CrossRef] [Green Version]
- Duong, U.; Kalantar-Zadeh, K.; Molnar, M.Z.; Zaritsky, J.J.; Teitelbaum, I.; Kovesdy, C.P.; Mehrotra, R. Mortality Associated with Dose Response of Erythropoiesis-Stimulating Agents in Hemodialysis versus Peritoneal Dialysis Patients. Am. J. Nephrol. 2012, 35, 198–208. [Google Scholar] [CrossRef] [Green Version]
- Suliman, M.E.; Stenvinkel, P.; Bárány, P.; Heimbürger, O.; Anderstam, B.; Lindholm, B. Hyperhomocysteinemia and its relationship to cardiovascular disease in ESRD: Influence of hypoalbuminemia, malnutrition, inflammation, and diabetes mellitus. Am. J. Kidney Dis. 2003, 41, S89–S95. [Google Scholar] [CrossRef]
- Cordeiro, I.S.; Cordeiro, L.; Wagner, C.S.; Araújo, L.K.R.; Pereira, B.J.; Abensur, H.; Elias, R.M.; Silva, B.C. High-Flux versus High-Retention-Onset Membranes: In vivo Small and Middle Molecules Kinetics in Convective Dialysis Modalities. Blood Purif. 2020, 49, 8–15. [Google Scholar] [CrossRef]
- Bushljetik, I.R.; Trajceska, L.; Biljali, S.; Balkanov, T.; Dejanov, P.; Spasovski, G. Efficacy of Medium Cut-Off Dialyzer and Comparison with Standard High-Flux Hemodialysis. Blood Purif. 2021, 50, 492–498. [Google Scholar] [CrossRef]
- Samtleben, W.; Dengler, C.; Reinhardt, B.; Nothdurft, A.; Lemke, H.-D. Comparison of the new polyethersulfone high-flux membrane DIAPES(R) HF800 with conventional high-flux membranes during on-line haemodiafiltration. Nephrol. Dial. Transplant. 2003, 18, 2382–2386. [Google Scholar] [CrossRef] [Green Version]
- Ward, R.A. Protein-leaking membranes for hemodialysis: A new class of membranes in search of an application? J. Am. Soc. Nephrol. 2005, 16, 2421–2430. [Google Scholar] [CrossRef] [Green Version]
- Fiedler, R.; Neugebauer, F.; Ulrich, C.; Wienke, A.; Gromann, C.; Storr, M.; Böhler, T.; Seibert, E.; Girndt, M. Randomized controlled pilot study of 2 weeks’ treatment with high cutoff membrane for hemodialysis patients with elevated c-reactive protein. Artif. Organs 2012, 36, 886–893. [Google Scholar] [CrossRef]
- Berg, A.H.; Drechsler, C.; Wenger, J.; Buccafusca, R.; Hod, T.; Kalim, S.; Ramma, W.; Parikh, S.M.; Steen, H.; Friedman, D.J.; et al. Carbamylation of serum albumin as a risk factor for mortality in patients with kidney failure. Sci. Transl. Med. 2013, 5, 175ra29. [Google Scholar] [CrossRef] [Green Version]
- Voigt, M.; Gebert, M.; Haug, U.; Hulko, M.; Storr, M.; Boschetti-De-Fierro, A.; Beck, W.; Krause, B. Retention of beneficial molecules and coagulation factors during haemodialysis and haemodiafiltration. Sci. Rep. 2019, 9, 6370. [Google Scholar] [CrossRef] [Green Version]
- Allawati, H.; Dallas, L.; Nair, S.; Palmer, J.; Thaikandy, S.; Hutchison, C. A Pharmacokinetic study comparing the clearance of vancomycin during haemodialysis using medium cut-off membrane (Theranova) and high-flux membranes (Revaclear). Toxins 2020, 12, 317. [Google Scholar] [CrossRef]



| Uremic Toxin Class | Molecular Weight (kDa) | Representative Biomarkers |
|---|---|---|
| Small water-soluble molecules | <0.5 | Urea (60 Da), creatinine (113 Da), uric acid (168 Da) |
| Small-middle molecules | 0.5–15 | PTH (9.5 kDa), β2-MG (11.8 kDa), cystatin C(13.3 kDa) |
| Medium-middle molecules | 15–25 | Myoglobin (17 kDa),TNF-α (17 kDa), sTNFR2 (17 kDa), IL-10 (18 kDa), FGF-2 (18 kDa), prolactin (22 kDa), κ-FLC (22.5 kDa), complement factor D (23.75 kDa), IL-18 (24 kDa), IL-6 (24.5 kDa) |
| Large-middle molecules | 25–58 | sTNFR1 (27 kDa), FGF-23 (32 kDa), VEGF (34.2 kDa), YKL-40 (40 kDa), λ-FLC (45 kDa) |
| Large molecules | >58 | AOPP (>60 kDa), modified albumin (65 kDa) |
| Protein-bound uremic toxins | mostly < 0.5 | Homocysteine, IS, pCS |
| Device | Membrane Type | Structural Characteristics | ||||
|---|---|---|---|---|---|---|
| Pore Radius * (nm) | Fiber Inner Diameter (μm) | Fiber Wall Thickness (μm) | Effective Surface Area (m2) | UF-Coefficient ** (mL/h/mmHg) | ||
| Pollyflux 17L | Low-flux | 3.1 ± 0.2 | 215 | 50 | 1.7 | 12.5 |
| Revaclear 400 | High-flux | 3.9 ± 0.1 | 190 | 35 | 1.8 | 54 |
| Theranova 400 | Medium cut-off | 5.0 ± 0.1 | 180 | 35 | 1.7 | 48 |
| Theranova 500 | Medium cut-off | 5.0 ± 0.1 | 180 | 35 | 2.0 | 59 |
| Theralite 2100 | High cut-off | 10.0 ± 2.0 | 215 | 50 | 2.1 | 52 |
| Year | First Author | Patients (N) | Dialysis Treatment | Time | Study Design | Cytokines Significantly Removed by MCO Pre-Post Dialysis | Cytokines Significantly Removed by MCO at End of Study Period | Cytokines Removed by MCO Pre-Post Dialysis but No Significance | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 2017 | Zickler | 48 | HD MCO vs. HF | 12 weeks | 4-week MCO 4-week HF pre-post dialysis 8-week extension | TNF-α mRNA IL-6 mRNA sTNFR1 | TNF-α mRNA IL-6 mRNA sTNFR1 | - | [79] |
| 2019 | Belmouaz | 40 | HD MCO vs. HF | 6 months | 3-month MCO 3-month HF pre-post dialysis | Homocysteine | Homocysteine | IL-1b, IL-6, TNF-a, Ox-LDL, 8-iso-Prostaglandin F2a, SOD activity | [54] |
| 2019 | Cozzolino | 20 | HD MCO vs. HF | 6 months | 3-month MCO 3-month HF pre-post dialysis | - | - | IL-1b, IL-6, TNF-α | [81] |
| 2020 | Lim | 49 | HD MCO vs. HF | 12 weeks | 12 weeks | TNF-α | TNF-α | - | [80] |
| 2020 | Sevinc | 52 | HD MCO vs. HF | 6 months | 3-month MCO 3-month HF pre-post dialysis | VEGF | VEGF | FGF-23, IFN-γ, IL-6, IL-10, IL-17A | [53] |
| 2020 | Weiner | 172 | HD MCO vs. HF | 24 weeks | 24 weeks | TNF-α | TNF-α | IL-6 | [52] |
| 2020 | Yeter | 42 | HD MCO vs. HF vs. LF | 6 months | 6 months | - | - | TOS, TAS, PON-1, CRP | [82] |
| Year | First Author | Sample Size | Intervention | Time | Study Design | Pre-Dialysis Albumin Level (g/dL, Baseline vs. End) | Percentage Reduction | Reference |
|---|---|---|---|---|---|---|---|---|
| 2017 | Zickler | 48 | HD MCO vs. HF | 12 weeks | 4-week MCO 4-week HF pre-post dialysis 8-week extension | 3.70 ± 0.36 3.53 ± 0.37 | 4.50% | [79] |
| 2019 | Belmouaz | 40 | HD MCO vs. HF | 6 months | 3-month MCO 3-month HF pre-post dialysis | 3.71 ± 0.31 3.69 ± 0.43 | - | [54] |
| 2019 | Cozzolino | 20 | HD MCO vs. HF | 6 months | 3-month MCO 3-month HF pre-post dialysis | 3.8 (3.30–4.20) 3.6 (2.98–3.90) | 5.20% | [81] |
| 2020 | Sevinc | 52 | HD MCO vs. HF | 6 months | 3-month MCO 3-month HF pre-post dialysis | 3.88 (3.71–4.04) 3.62 (3.45–3.88) | 6.70% | [53] |
| 2020 | Bunch | 638 | MCO | 12 months | 12 months | 4.05 (4.04–4.07) 3.98 (3.96–4.00) | 1.70% | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Yang, T.; Li, Y.; Li, J.; Yang, Q.; Wang, L.; Jiang, L.; Su, B. Effects of Expanded Hemodialysis with Medium Cut-Off Membranes on Maintenance Hemodialysis Patients: A Review. Membranes 2022, 12, 253. https://doi.org/10.3390/membranes12030253
Zhang Z, Yang T, Li Y, Li J, Yang Q, Wang L, Jiang L, Su B. Effects of Expanded Hemodialysis with Medium Cut-Off Membranes on Maintenance Hemodialysis Patients: A Review. Membranes. 2022; 12(3):253. https://doi.org/10.3390/membranes12030253
Chicago/Turabian StyleZhang, Zhuyun, Tinghang Yang, Yupei Li, Jiameng Li, Qinbo Yang, Liya Wang, Luojia Jiang, and Baihai Su. 2022. "Effects of Expanded Hemodialysis with Medium Cut-Off Membranes on Maintenance Hemodialysis Patients: A Review" Membranes 12, no. 3: 253. https://doi.org/10.3390/membranes12030253
APA StyleZhang, Z., Yang, T., Li, Y., Li, J., Yang, Q., Wang, L., Jiang, L., & Su, B. (2022). Effects of Expanded Hemodialysis with Medium Cut-Off Membranes on Maintenance Hemodialysis Patients: A Review. Membranes, 12(3), 253. https://doi.org/10.3390/membranes12030253







