Carbon Nanodots-Embedded Pullulan Nanofibers for Sulfathiazole Removal from Wastewater Streams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
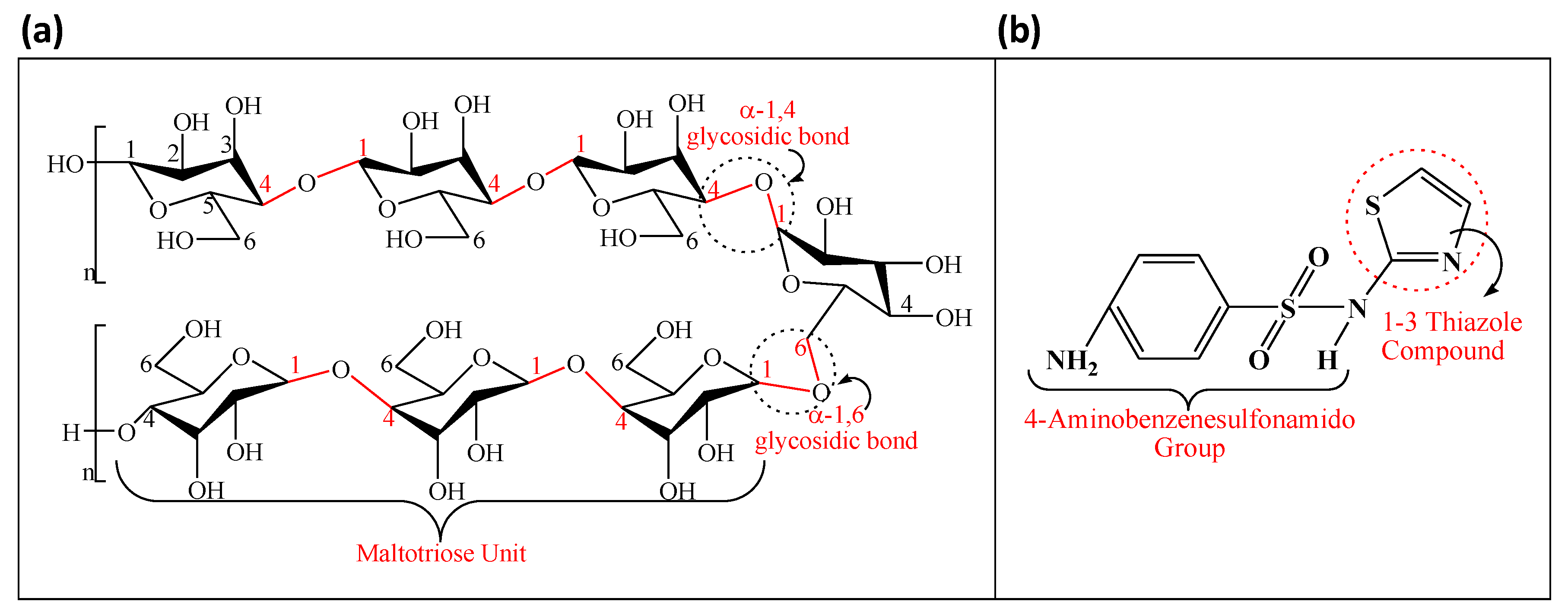
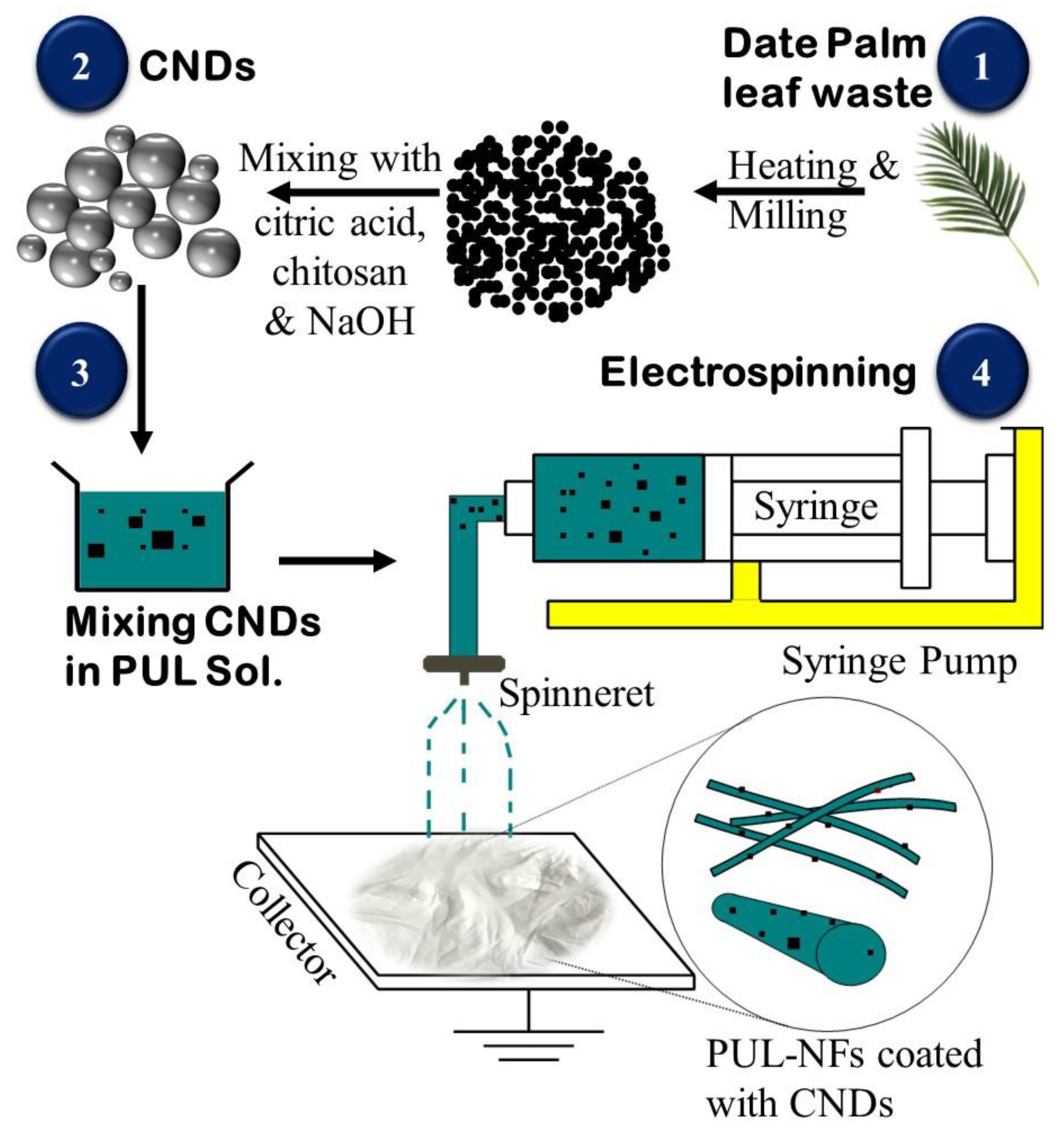
2.2. Synthesis of CNDs
2.3. Synthesis of CNDs-Embedded Pullulan NFs
2.4. Characterization
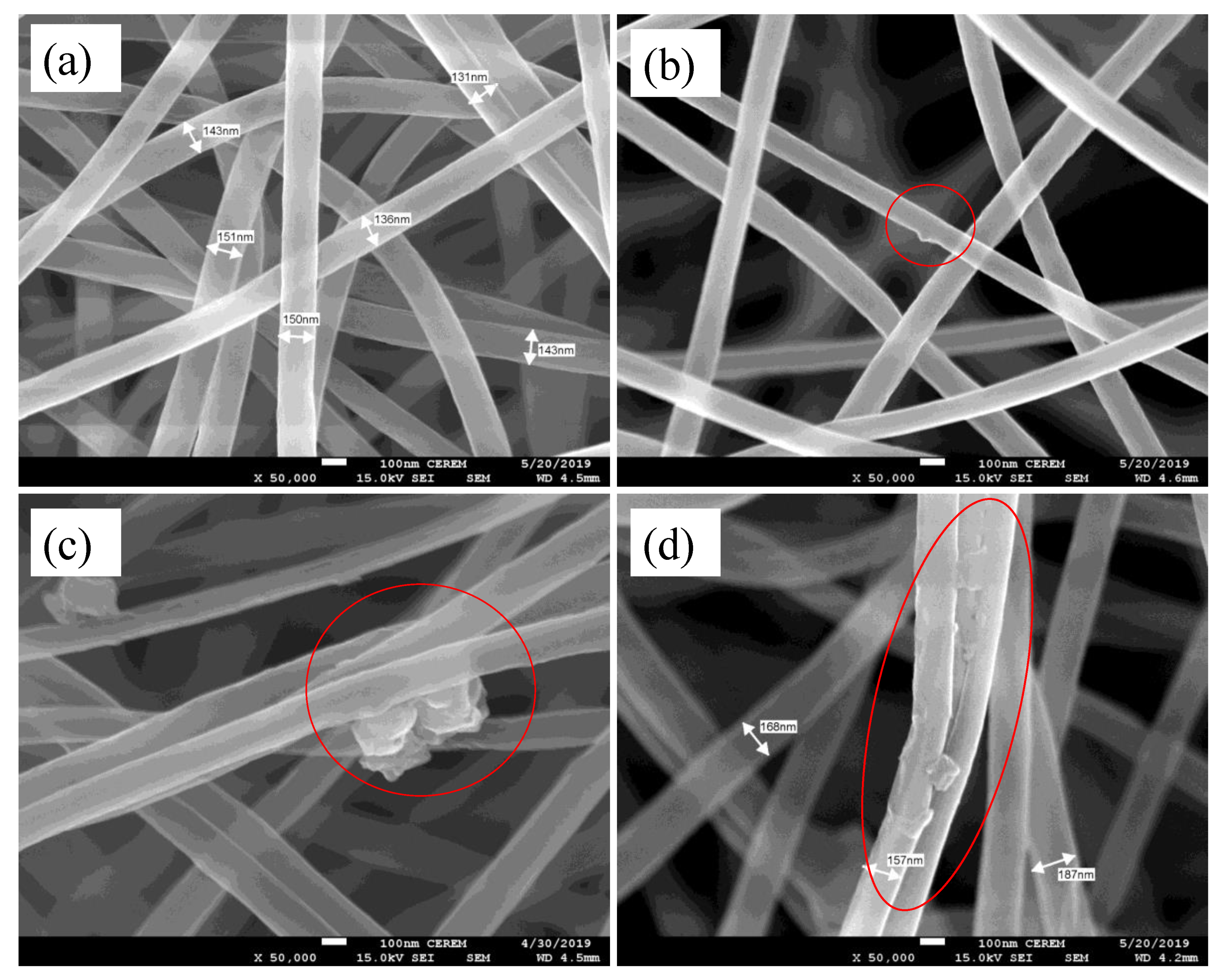
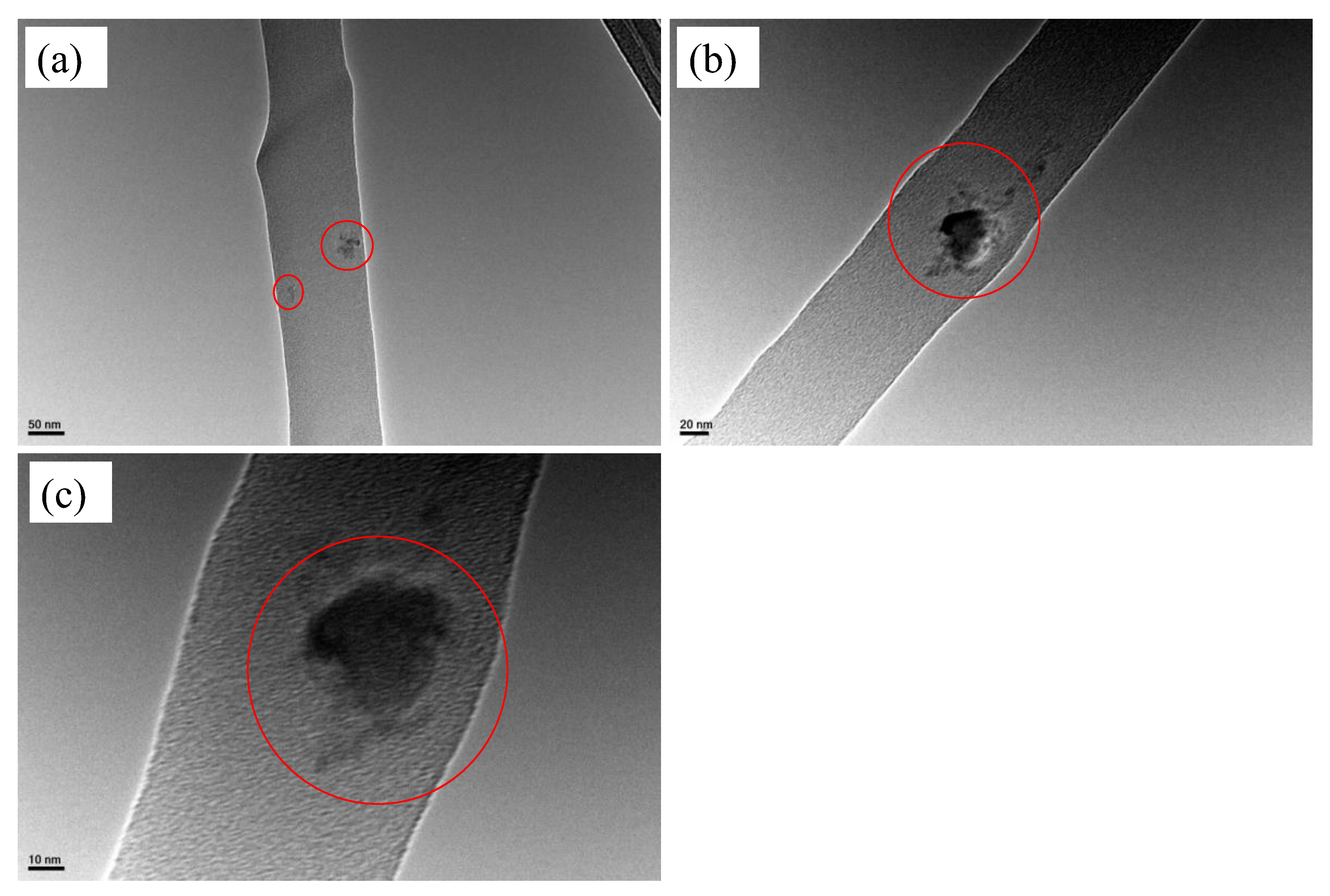
2.4.1. FE-SEM and TEM
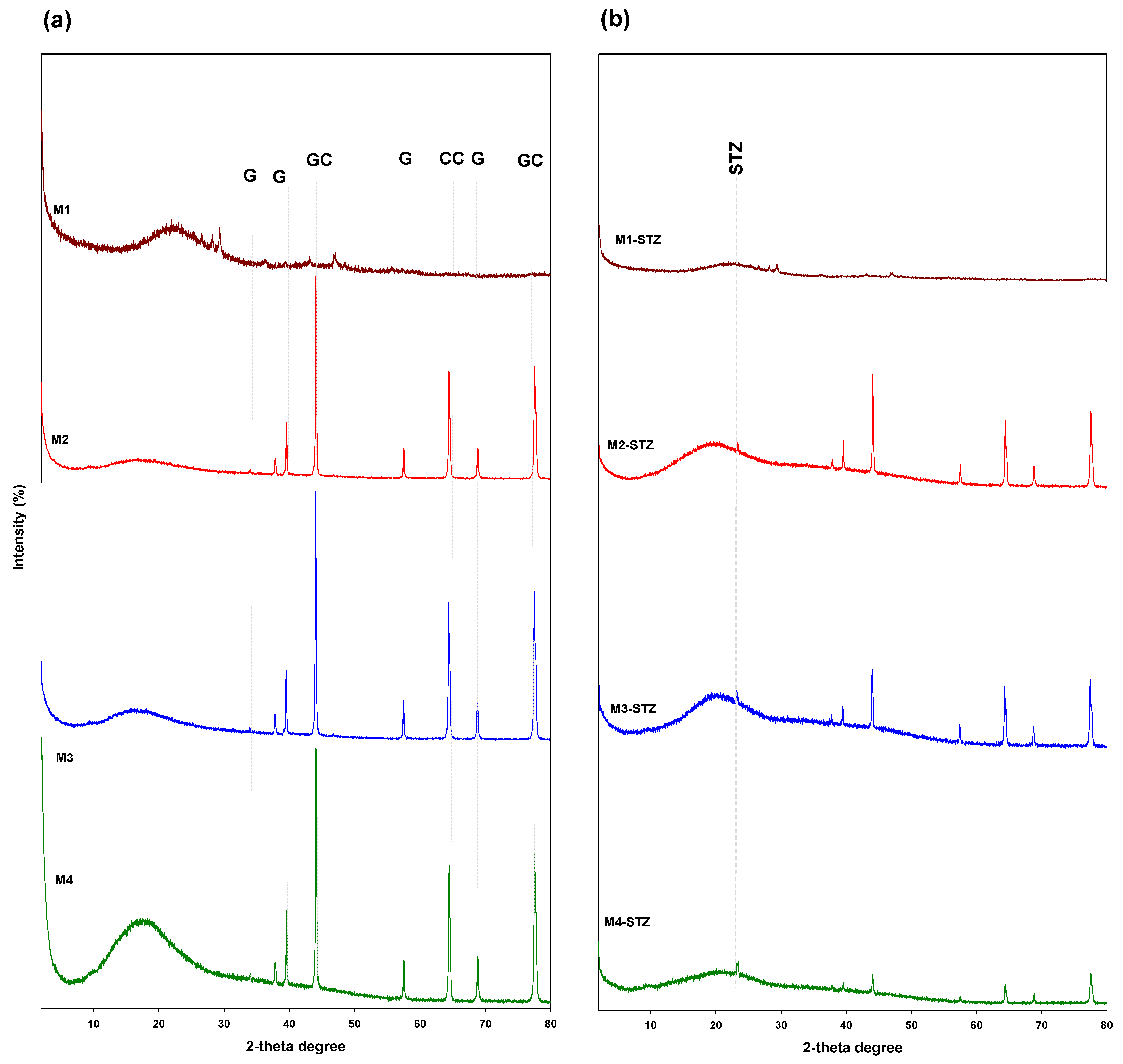
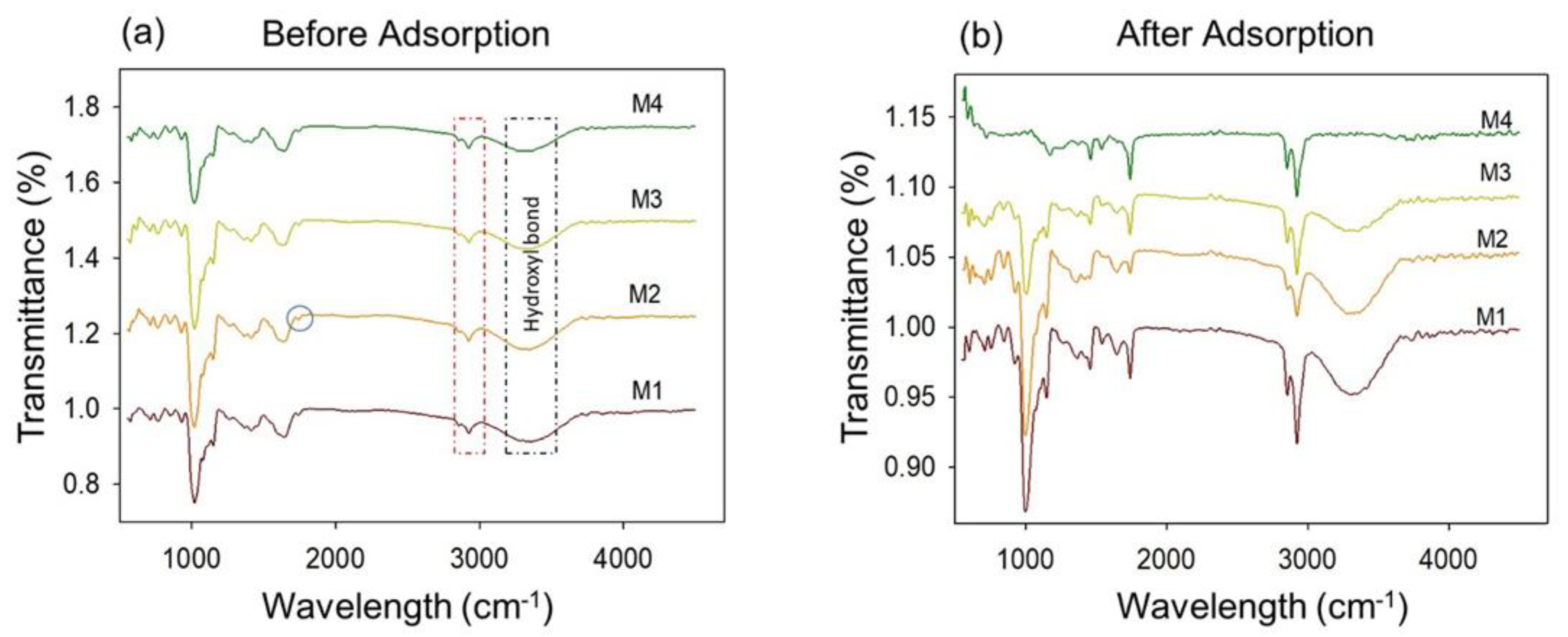
2.4.2. XRD, FTIR, and TGA
2.5. Adsorption Batch Studies
2.6. Kinetic Modelling
2.7. Isotherm Modelling
3. Results and Discussion
3.1. Characterization
3.1.1. SEM and TEM Analyses
3.1.2. XRD, FTIR, and TGA Analyses
3.2. STZ Adsorption Experiments
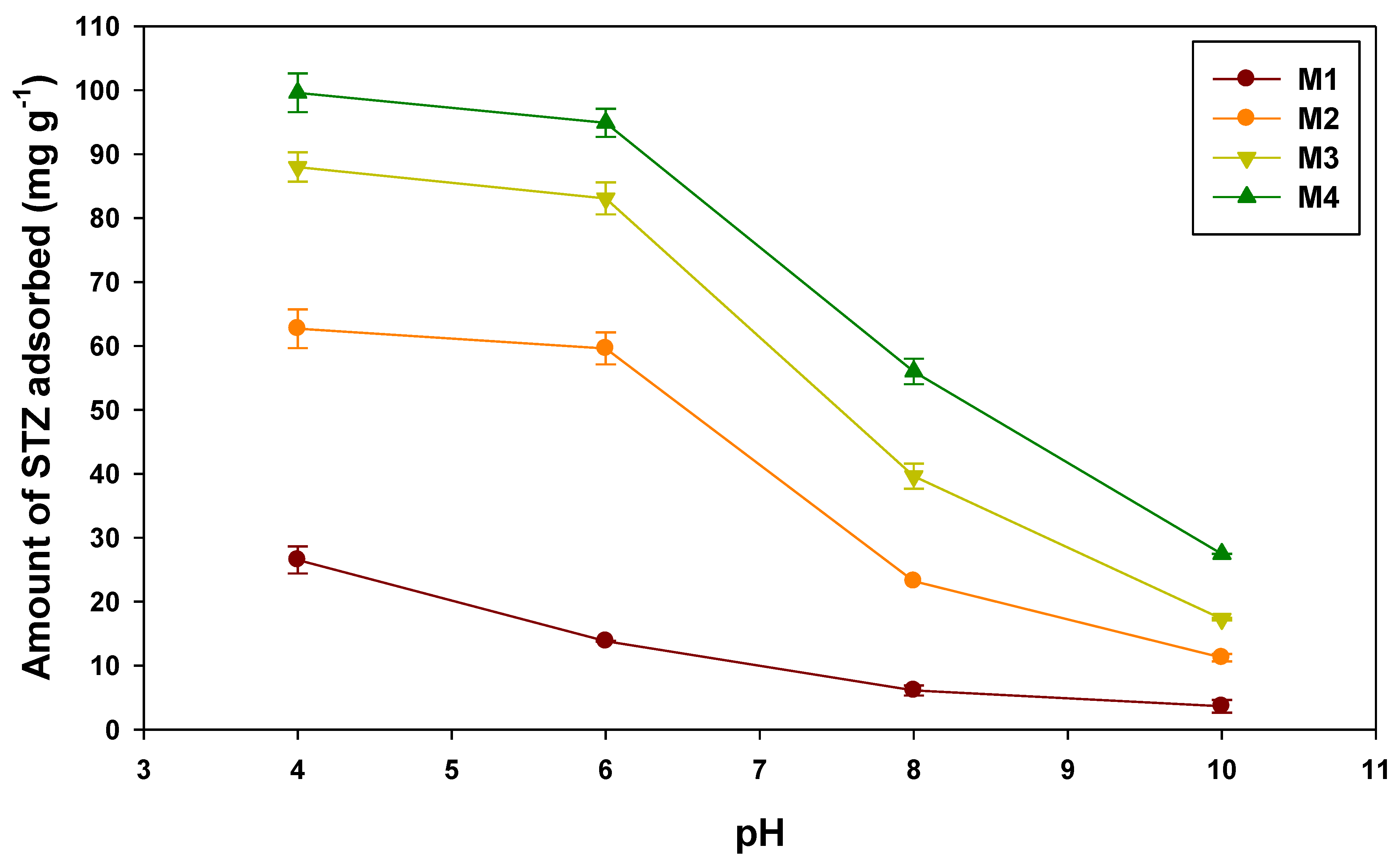
3.2.1. Effect of pH on STZ Adsorption
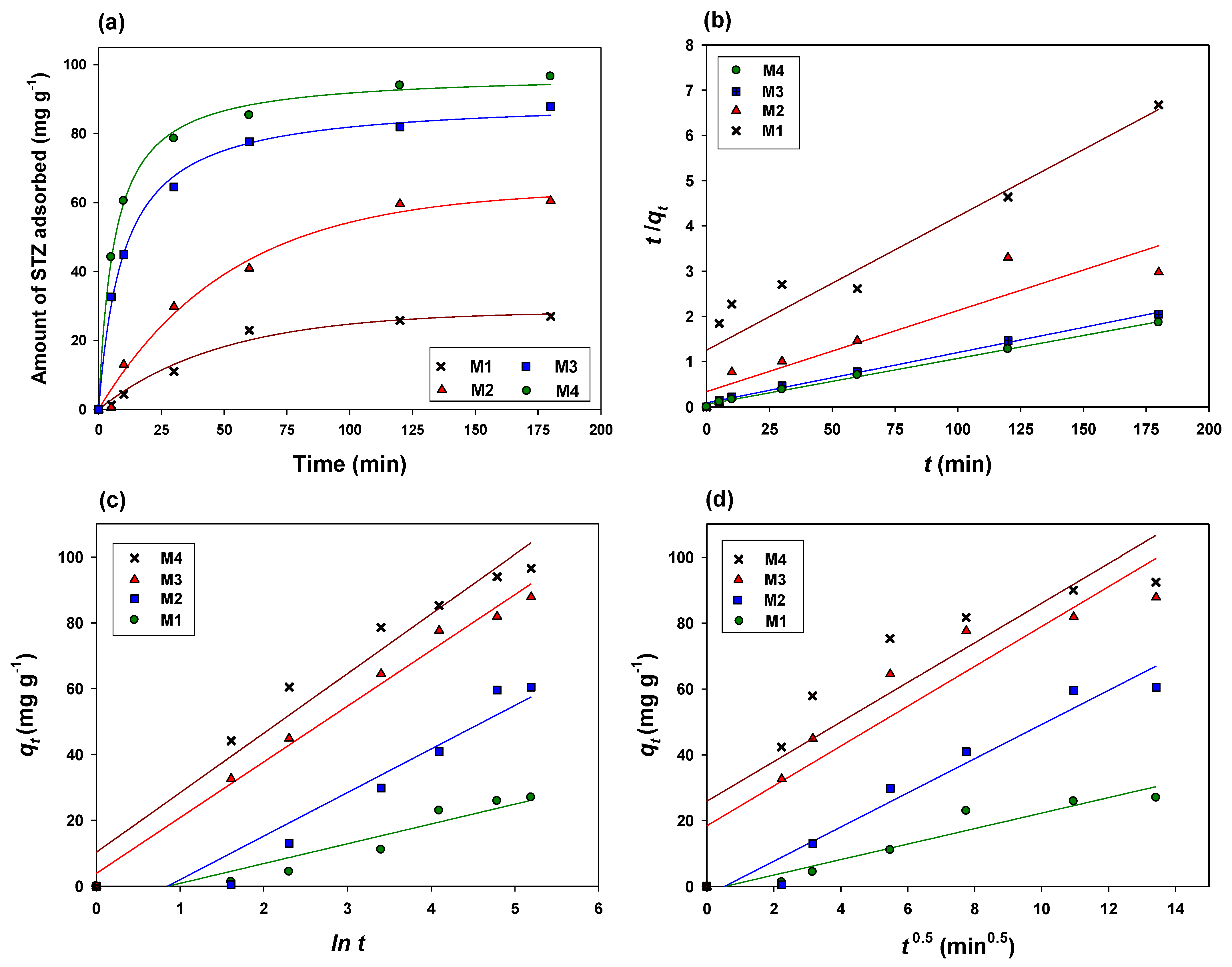
3.2.2. Adsorption Kinetics
3.2.3. Equilibrium Adsorption
3.3. STZ Removal Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashfaq, M.; Khan, K.N.; Rasool, S.; Mustafa, G.; Saif-Ur-Rehman, M.; Nazar, M.F.; Sun, Q.; Yu, C.-P. Occurrence and ecological risk assessment of fluoroquinolone antibiotics in hospital waste of Lahore, Pakistan. Environ. Toxicol. Pharmacol. 2016, 42, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Usman, A.R.A.; Rafique, M.I.; Al-Wabel, M.I. Engineered biochar composites with zeolite, silica, and nano-zerovalent iron for the efficient scavenging of chlortetracycline from aqueous solutions. Environ. Sci. Pollut. Res. 2019, 26, 15136–15152. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, R.H.; Fick, J.; Tysklind, M. Screening of antimycotics in Swedish sewage treatment plants—Waters and sludge. Water Res. 2010, 44, 649–657. [Google Scholar] [CrossRef]
- Makowska, N.; Koczura, R.; Mokracka, J. Class 1 integrase, sulfonamide and tetracycline resistance genes in wastewater treatment plant and surface water. Chemosphere 2016, 144, 1665–1673. [Google Scholar] [CrossRef]
- Simazaki, D.; Kubota, R.; Suzuki, T.; Akiba, M.; Nishimura, T.; Kunikane, S. Occurrence of selected pharmaceuticals at drinking water purification plants in Japan and implications for human health. Water Res. 2015, 76, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Kurt, A.; Mert, B.K.; Özengin, N.; Sivrioğlu, Ö.; Yonar, T. Treatment of Antibiotics in Wastewater Using Advanced Oxidation Processes (AOPs). In Physico-Chemical Wastewater Treatment and Resource Recovery; Farooq, R., Ahmad, Z., Eds.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Pan, X.; Ben, W.; Wang, J.; Hou, P.; Qiang, Z. Adsorptive removal of antibiotics from water using magnetic ion exchange resin. J. Environ. Sci. 2017, 52, 111–117. [Google Scholar] [CrossRef]
- Yi, Q.; Zhang, Y.; Gao, Y.; Tian, Z.; Yang, M. Anaerobic treatment of antibiotic production wastewater pretreated with enhanced hydrolysis: Simultaneous reduction of COD and ARGs. Water Res. 2017, 110, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-T.; Bui, X.-T.; Luu, V.-P.; Nguyen, P.-D.; Guo, W.; Ngo, H.-H. Removal of antibiotics in sponge membrane bioreactors treating hospital wastewater: Comparison between hollow fiber and flat sheet membrane systems. Bioresour. Technol. 2017, 240, 42–49. [Google Scholar] [CrossRef]
- Jojoa-Sierra, S.D.; Silva-Agredo, J.; Herrera-Calderon, E.; Torres-Palma, R.A. Elimination of the antibiotic norfloxacin in municipal wastewater, urine and seawater by electrochemical oxidation on IrO2 anodes. Sci. Total Environ. 2017, 575, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.R.; Aijaz, M.O.; Alharth, N.H.; Alharbi, H.F.; Al-Mubaddel, F.S.; Awual, M.R. Composite nanofibers membranes of poly(vinyl alcohol)/chitosan for selective lead(II) and cadmium(II) ions removal from wastewater. Ecotoxicol. Environ. Saf. 2019, 169, 479–486. [Google Scholar] [CrossRef]
- Yu, F.; Li, Y.; Han, S.; Ma, J. Adsorptive removal of antibiotics from aqueous solution using carbon materials. Chemosphere 2016, 153, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-L.; Liu, L.-C.; Chen, W.-R. Adsorption of sulfamethoxazole and sulfapyridine antibiotics in high organic content soils. Environ. Pollut. 2017, 231 Pt 1, 1163–1171. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Yang, L.-M.; Paul Chen, J.; Zheng, Y.-M. Electrospun spongy zero-valent iron as excellent electro-Fenton catalyst for enhanced sulfathiazole removal by a combination of adsorption and electro-catalytic oxidation. J. Hazard. Mater. 2019, 371, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Akduman, Ç.; Morsümbül, S.; Kumbasar, E.P.A. The Removal of Reactive Red 141 from Wastewater: A Study of Dye Adsorption Capability of Water-Stable Electrospun Polyvinyl Alcohol Nanofibers. Autex Res. J. 2021, 21, 20–31. [Google Scholar] [CrossRef]
- Aijaz, M.O.; Karim, M.R.; Alharbi, H.F.; Alharthi, N.H.; Al-Mubaddel, F.S.; Abdo, H.S. Magnetic/Polyetherimide-Acrylonitrile Composite Nanofibers for Nickel Ion Removal from Aqueous Solution. Membranes 2021, 11, 50. [Google Scholar] [CrossRef]
- Aijaz, M.O.; Karim, M.R.; Alharbi, H.F.; Alharthi, N.H. Novel optimised highly aligned electrospun PEI-PAN nanofibre mats with excellent wettability. Polymer 2019, 180, 121665. [Google Scholar] [CrossRef]
- Liu, Q.; Zheng, Y.; Zhong, L.; Cheng, X. Removal of tetracycline from aqueous solution by a Fe3O4 incorporated PAN electrospun nanofiber mat. J. Environ. Sci. 2015, 28, 29–36. [Google Scholar] [CrossRef]
- Mogolodi Dimpe, K.; Nomngongo, P.N. Application of activated carbon-decorated polyacrylonitrile nanofibers as an adsorbent in dispersive solid-phase extraction of fluoroquinolones from wastewater. J. Pharm. Anal. 2019, 9, 117–126. [Google Scholar] [CrossRef]
- Singh, R.S.; Saini, G.K. Biosynthesis of Pullulan and Its Applications in Food and Pharmaceutical Industry. In Microorganisms in Sustainable Agriculture and Biotechnology; Satyanarayana, T., Johri, B.N., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 509–553. [Google Scholar] [CrossRef]
- Culica, M.E.; Kasperczyk, K.; Baron, R.I.; Biliuta, G.; Macsim, A.M.; Lazea-Stoyanova, A.; Orlinska, B.; Coseri, S. Recyclable Polymer-Supported N-Hydroxyphthalimide Catalysts for Selective Oxidation of Pullulan. Materials 2019, 12, 3585. [Google Scholar] [CrossRef] [Green Version]
- Karim, M.R.; Lee, H.W.; Kim, R.; Ji, B.C.; Cho, J.W.; Son, T.W.; Oh, W.; Yeum, J.H. Preparation and characterization of electrospun pullulan/montmorillonite nanofiber mats in aqueous solution. Carbohydr. Polym. 2009, 78, 336–342. [Google Scholar] [CrossRef]
- Semeniuk, M.; Yi, Z.; Poursorkhabi, V.; Tjong, J.; Jaffer, S.; Lu, Z.-H.; Sain, M. Future Perspectives and Review on Organic Carbon Dots in Electronic Applications. ACS Nano 2019, 13, 6224–6255. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Bai, X.; Cui, H.; Zhu, J.; Liu, W.; Zhang, T.; Dong, B.; Pan, G.; Xu, L.; Zhang, S.; et al. Carbon dot/polyvinylpyrrolidone hybrid nanofibers with efficient solid-state photoluminescence constructed using an electrospinning technique. Nanotechnology 2018, 29, 025706. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Wu, S.; Mensah, A.; Lu, K.; Wei, Q. Carbon quantum dots embedded electrospun nanofibers for efficient antibacterial photodynamic inactivation. Mater. Sci. Eng. C 2020, 108, 110377. [Google Scholar] [CrossRef] [PubMed]
- Safaei, B.; Youssefi, M.; Rezaei, B.; Irannejad, N. Synthesis and Properties of Photoluminescent Carbon Quantum Dot/Polyacrylonitrile Composite Nanofibers. Smart Sci. 2018, 6, 117–124. [Google Scholar] [CrossRef]
- Alam, A.-M.; Liu, Y.; Park, M.; Park, S.-J.; Kim, H.-Y. Preparation and characterization of optically transparent and photoluminescent electrospun nanofiber composed of carbon quantum dots and polyacrylonitrile blend with polyacrylic acid. Polymer 2015, 59, 35–41. [Google Scholar] [CrossRef]
- Lee, C.; Pant, B.; Alam, A.-M.; An, T.; Chung, H.-J.; Hong, S.-T.; Park, S.-J.; Park, M.; Kim, H.-Y. Biocompatible and photoluminescent keratin/poly(vinyl alcohol)/carbon quantum dot nanofiber: A novel multipurpose electrospun mat. Macromol. Res. 2016, 24, 924–930. [Google Scholar] [CrossRef]
- Li, J.-Y.; Liu, Y.; Shu, Q.-W.; Liang, J.-M.; Zhang, F.; Chen, X.-P.; Deng, X.-Y.; Swihart, M.T.; Tan, K.-J. One-Pot Hydrothermal Synthesis of Carbon Dots with Efficient Up- and Down-Converted Photoluminescence for the Sensitive Detection of Morin in a Dual-Readout Assay. Langmuir 2017, 33, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Usman, A.R.A.; Hussain, Q.; Al-Farraj, A.S.F.; Tsang, Y.F.; Bundschuh, J.; Al-Wabel, M.I. Fabrication and evaluation of silica embedded and zerovalent iron composited biochars for arsenate removal from water. Environ. Pollut. 2020, 266, 115256. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmad, M.; Usman, A.R.A.; Al-Faraj, A.S.; Ok, Y.S.; Hussain, Q.; Abduljabbar, A.S.; Al-Wabel, M.I. An efficient phosphorus scavenging from aqueous solution using magnesiothermally modified bio-calcite. Environ. Technol. 2018, 39, 1638–1649. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Chao, H.-P. Effect of pyrolysis temperatures and times on the adsorption of cadmium onto orange peel derived biochar. Waste Manag. Res. 2015, 34, 129–138. [Google Scholar] [CrossRef]
- Teixidó, M.; Pignatello, J.J.; Beltrán, J.L.; Granados, M.; Peccia, J. Speciation of the Ionizable Antibiotic Sulfamethazine on Black Carbon (Biochar). Environ. Sci. Technol. 2011, 45, 10020–10027. [Google Scholar] [CrossRef]
- Christensen, I.L.; Sun, Y.-P.; Juzenas, P. Carbon Dots as Antioxidants and Prooxidants. J. Biomed. Nanotechnol. 2011, 7, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, J.; Kim, M.; Hyun, S.; Moon, D.H. Sorption of sulfathiazole in the soil treated with giant Miscanthus-derived biochar: Effect of biochar pyrolysis temperature, soil pH, and aging period. Environ. Sci. Pollut. Res. 2018, 25, 25681–25689. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tian, D.; Hu, J.; Shen, F.; Long, L.; Zhang, Y.; Yang, G.; Zeng, Y.; Zhang, J.; He, J.; et al. Functionalizing bottom ash from biomass power plant for removing methylene blue from aqueous solution. Sci. Total Environ. 2018, 634, 760–768. [Google Scholar] [CrossRef]
- Ter Laak, T.L.; Gebbink, W.A.; Tolls, J. The effect of pH and ionic strength on the sorption of sulfachloropyridazine, tylosin, and oxytetracycline to soil. Environ. Toxicol. Chem. 2006, 25, 904–911. [Google Scholar] [CrossRef]
- Pei, Z.; Yang, S.; Li, L.; Li, C.; Zhang, S.; Shan, X.; Wen, B.; Guo, B. Effects of copper and aluminum on the adsorption of sulfathiazole and tylosin on peat and soil. Environ. Pollut. 2014, 184, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Xu, Z.; Wei, Y.; Zhou, Y.; Yang, X.; Yang, Y.; Yang, J.; Zhang, J.; Luo, L.; Zhou, Z. Carbon-based materials as adsorbent for antibiotics removal: Mechanisms and influencing factors. J. Environ. Manag. 2019, 237, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-F.; Zhao, J.; Wang, S.; Xing, B. Adsorption of sulfonamides on reduced graphene oxides as affected by pH and dissolved organic matter. Environ. Pollut. 2016, 210, 85–93. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Johir, M.A.H.; Sornalingam, K. Single and competitive sorption properties and mechanism of functionalized biochar for removing sulfonamide antibiotics from water. Chem. Eng. J. 2017, 311, 348–358. [Google Scholar] [CrossRef]
- Jing, X.-R.; Wang, Y.-Y.; Liu, W.-J.; Wang, Y.-K.; Jiang, H. Enhanced adsorption performance of tetracycline in aqueous solutions by methanol-modified biochar. Chem. Eng. J. 2014, 248, 168–174. [Google Scholar] [CrossRef]


| Analyses | M1 | M2 | M3 | M4 |
|---|---|---|---|---|
| Tonset (°C) | 288.0 | 291.0 | 290.5 | 289.3 |
| Residue (%) | 14.60 | 18.51 | 18.51 | 17.76 |
| Tpeak (°C) | 303.1 | 309.5 | 309.7 | 307.0 |
| Tm (°C) | 308.9 | 316.2 | 316.0 | 312.5 |
| Tg (°C) | 262.6 | 264.3 | 265.1 | 263.9 |
| Specific heat capacity (J/(g °C)) | 0.022 | 0.284 | 0.255 | 0.270 |
| Heat flow (W/g) | −0.0037 | −0.047 | −0.042 | −0.044 |
| Adsorbent | First Order | Second Order | Pseudo-First Order | Pseudo-Second Order | Elovich | Intraparticle Diffusion | Power Function | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | SEE | R2 | SEE | R2 | SEE | R2 | SEE | R2 | SEE | R2 | SEE | R2 | SEE | |
| M1 | 0.81 | 0.007 | 0.18 | 0.004 | 0.75 | 0.007 | 0.88 | 0.004 | 0.89 | 0.109 | 0.93 | 0.001 | 0.84 | 0.000 |
| M2 | 0.84 | 0.005 | 0.12 | 0.002 | 0.76 | 0.005 | 0.87 | 0.036 | 0.91 | 0.002 | 0.96 | 0.001 | 0.81 | 0.000 |
| M3 | 0.63 | 0.006 | 0.06 | 0.000 | 0.66 | 0.006 | 0.99 | 0.003 | 0.99 | 0.003 | 0.86 | 0.002 | 0.73 | 0.000 |
| M4 | 0.54 | 0.004 | 0.02 | 0.000 | 0.73 | 0.004 | 0.99 | 0.008 | 0.96 | 0.001 | 0.82 | 0.000 | 0.68 | 0.000 |
| Adsorbent | First Order | Second Order | Pseudo-First Order | Pseudo-Second Order | Elovich | Intraparticle Diffusion | Power Function | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k1 | k2 | k1′ | qe | k2′ | qe | h | α | β | kid | c | Kf | B | |
| M1 | 0.016 | −0.002 | −0.022 | 3.13 | 4.1 × 10−4 | 38.79 | 0.56 | 6.018 | −5.16 | 2.35 | −1.24 | 0.73 | −0.27 |
| M2 | 0.021 | −0.004 | −0.028 | 4.12 | 9.0 × 10−4 | 55.82 | 2.96 | 13.24 | −11.26 | 5.19 | −2.71 | 0.96 | −0.48 |
| M3 | 0.012 | −0.000 | −0.023 | 3.95 | 1.4 × 10−3 | 89.94 | 11.21 | 16.93 | 3.89 | 6.05 | 18.44 | 0.73 | 1.28 |
| M4 | 0.012 | −0.000 | −0.025 | 3.93 | 1.8 × 10−3 | 98.58 | 17.69 | 18.09 | 10.30 | 6.29 | 26.94 | 0.73 | 1.45 |
| Adsorbents | Langmuir | Freundlich | Temkin | Dubinin-Radushkevich | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| QL (mg g−1) | KL (L g−1) | R2 | KF L g−1 | 1/n | R2 | b (J mol−1) | A (L g−1) | R2 | QD (mg g−1) | E (kJ g−1) | R2 | |
| M1 | 35.04 | 0.045 | 0.97 | 4.06 | 0.42 | 0.91 | 6.65 | 0.70 | 0.93 | 29.20 | 0.083 | 0.98 |
| M2 | 93.09 | 0.039 | 0.98 | 8.03 | 0.49 | 0.92 | 14.89 | 0.92 | 0.88 | 69.12 | 0.050 | 0.99 |
| M3 | 124.27 | 0.128 | 0.97 | 11.77 | 0.49 | 0.91 | 16.76 | 1.92 | 0.77 | 97.05 | 0.046 | 0.99 |
| M4 | 133.68 | 0.041 | 0.97 | 26.48 | 0.35 | 0.88 | 17.57 | 5.14 | 0.88 | 106.45 | 0.012 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aijaz, M.O.; Ahmad, M.; Al-Wabel, M.I.; Karim, M.R.; Usman, A.R.A.; Assaifan, A.K. Carbon Nanodots-Embedded Pullulan Nanofibers for Sulfathiazole Removal from Wastewater Streams. Membranes 2022, 12, 228. https://doi.org/10.3390/membranes12020228
Aijaz MO, Ahmad M, Al-Wabel MI, Karim MR, Usman ARA, Assaifan AK. Carbon Nanodots-Embedded Pullulan Nanofibers for Sulfathiazole Removal from Wastewater Streams. Membranes. 2022; 12(2):228. https://doi.org/10.3390/membranes12020228
Chicago/Turabian StyleAijaz, Muhammad Omer, Munir Ahmad, Mohammad I. Al-Wabel, Mohammad Rezaul Karim, Adel R. A. Usman, and Abdulaziz K. Assaifan. 2022. "Carbon Nanodots-Embedded Pullulan Nanofibers for Sulfathiazole Removal from Wastewater Streams" Membranes 12, no. 2: 228. https://doi.org/10.3390/membranes12020228
APA StyleAijaz, M. O., Ahmad, M., Al-Wabel, M. I., Karim, M. R., Usman, A. R. A., & Assaifan, A. K. (2022). Carbon Nanodots-Embedded Pullulan Nanofibers for Sulfathiazole Removal from Wastewater Streams. Membranes, 12(2), 228. https://doi.org/10.3390/membranes12020228











