Roles of Sulfites in Reverse Osmosis (RO) Plants and Adverse Effects in RO Operation
Abstract
1. Introduction
2. Chemical Properties and Handling Precautions of Sodium Bisulfite (SBS)
3. Removal of Oxidative Disinfectants: Chlorine, Chloramine, Chlorine Dioxide, and DBNP
3.1. Dechlorination
3.2. Dechlorination Point Considerations
3.3. Monitoring Dechlorination
3.4. Precautions for Integrated Membrane System (IMS)
- Backwash: Backwash conducts to clean the fibers and, consequently, reduce the transmembrane pressure (TMP) accumulated during filtration. NaOCl has been the most widely used, and its typical range is 3–20 mg/L with a median of 10 mg/L [87].
- Chemical-enhanced backwash (CEB): the CEB occurs once or twice per day, is characterized by taking longer than the backwash, and is conducted by the use of chemicals. For example, NaOCl concentration is at 20–500 mg/L with a median of 150 mg/L.
- Cleaning in place (CIP): CIP occurs once every couple of months and is characterized by its longer duration (a few hours typically) and higher chemical concentrations used compared with CEB. NaOCl is used at elevated concentrations (up to 4000 mg/L with PVDF fibers) for oxidative cleaning.
3.5. Other Disinfectants Removal
3.5.1. Chloramine
- Free chlorine (pH < 11.0): 13 ms;
- Free chlorine (pH > 11.0): 4.3 s;
- Monochloramine (pH 4.0): 1.8 s;
- Monochloramine (pH 8.0): 2.0 min.
3.5.2. Chlorine Dioxide (ClO2)
3.5.3. 2,2-Dibromo-3-nitrilopropionamide (DBNPA)
(DBNPA) (Cyanoacetamide)
4. Preservative for New RO Elements and Storage in Plant Shutdown
- Clean membrane before applying SBS.
- Immerse membranes in the preservation solution directly in the pressure vessels.
- Vent the air from the system and isolate the system.
- Check pH during preservation to monitor the degradation of the preservation solution.
- Change preservation solution if pH is below 3.0.
- Change preservation solution every 30 days if the temperature is below 27 °C and 15 days if the temperature is above 27 °C.
5. Deoxygenation
6. Shock Treatment and Sanitization
6.1. Shock Treatment
6.2. Disinfection and Sanitization
7. Other Applications: Cleaning and pH Control
8. Adverse Effects of Sulfites on RO Membranes
- RO membrane oxidation;
- Trigger of biofouling.
8.1. RO Membrane Degradation/Oxidation by Reducing Agents
8.2. RO Membrane Degradation/Oxidation by Sulfites
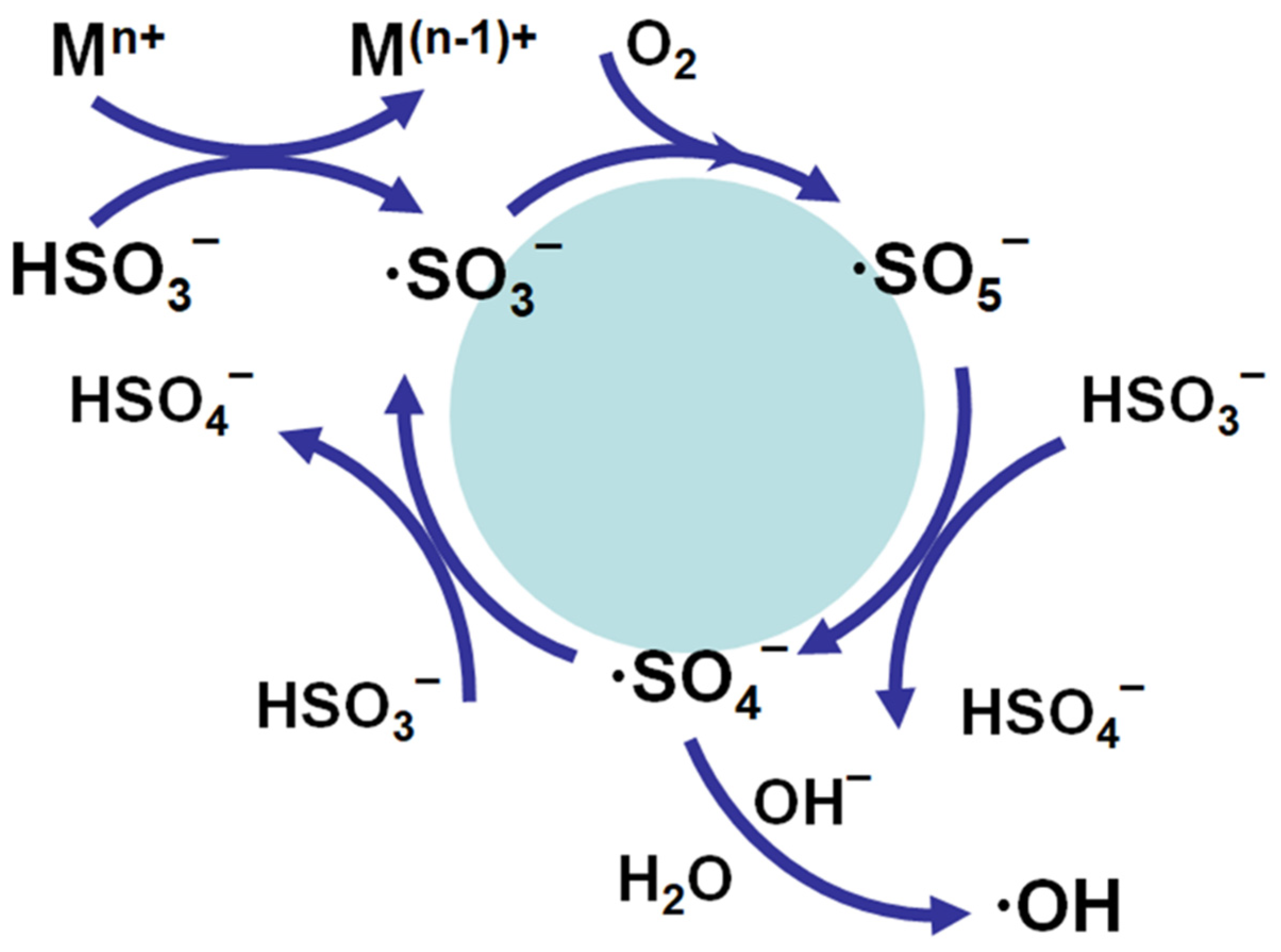
8.3. Mechanism Membrane Degradation with SBS
- ∙SO4− is the predominant radical at pH < 7.0;
- Both ∙SO4− and ∙OH are present at pH 9.0;
- ∙OH is the predominant radical at a more basic pH (i.e., pH 12.0).
8.4. Factors of Membrane Degradation with SBS
8.4.1. Effect of Heavy Metals
| Heavy Metal | Concentration | pH | Positive or Negative | Reference | ||
|---|---|---|---|---|---|---|
| Cupper | Cu | (+) | [157,248,251,252,253,309,310] | |||
| <2.5 ppb | 6.5 | (−) | Colorimetry | [248] | ||
| >5 ppb | (+) | ORP | [309] | |||
| 1 ppb | (−) | ORP | [309] | |||
| 30–50 ppb | (+) | Membrane | [253] | |||
| 0.1 ppm | 10 | (+) | Membrane | [255] | ||
| Cobalt | Co | (+) | [31,258,309,310] | |||
| <2.5 ppb | 6.5 | (−) | Colorimetry | [248] | ||
| Tin | Sn | (+) | ORP | [309] | ||
| Iron | Fe | 10 ppm | 10 | (−) | ORP | [255] |
| Precipitated | 9.5 9.6 | (+) | Membrane | [311,312] | ||
| 1.5 mg/L | 6.7 | (+) | Membrane | [313] | ||
| Manganese | Mn | 100 ppb | 6.5 | (−) | Colorimetry | [248] |
| Fe/Mn mix | 30/30 ppb | 10 | (+) | Membrane | [314] | |
| Zinc | Zn | 100 ppb | 6.5 | (−) | Colorimetry | [248] |
| Lead | Pb | 100 ppb | 6.5 | (−) | Colorimetry | [248] |
8.4.2. Effect of SBS Concentration and DO Concentration
8.4.3. Effect of Feed pH and Bicarbonate Concentration
8.4.4. Effect of Salinity and Any Other Ions (Chloride)
8.5. Countermeasures of Membrane Degradation Originated from SBS
| Preventive Countermeasures | Reference |
|---|---|
| Addition of chelating agents (e.g., EDTA, SHMP) | [249,252,309,315] |
| A scale inhibitor having a reducing function Phosphorous acid-based or phosphonate compounds | [316] |
| Addition of chelating agents to SBS preservative | [184] |
Addition of radical or oxidant scavengers
| [306] |
| Remove oxygen (e.g., vacuum degasification) | [257] |
| Preventive cleaning with acids to remove heavy metals | |
| [310] |
| [257,317] |
| Operate under lower pH (e.g., <pH 5.2) | [114] |
| Operate under lower pH (e.g., <pH 4.0, relates to HCO3+) | [315] |
| Operate and preserve under pH < pH 6.5 and/or <30 °C | [256] |
| Maintain feed or concentrate Cu or Co concentration < 2 µg/L | [310] |
| Alternative reducing agents for dechlorination | |
| [318] |
| [114] |
| High pH second-pass RO | |
| [314,319] |
| [311] |
| [312] |
| [15,255,320] |
9. SBS Acts as a Trigger of Biofouling
- Chlorine oxidizes NOM/humic substances, and assimilable organic carbon (AOC) is formed that can be considered as nutrients for surviving bacteria [104].
- Creating an anaerobic environment to enhance anaerobic bacterial growth, such as sulfate-reducing bacteria (SRB);
- SBS enhance some types of bacteria as food, such as sulfur-oxidizing bacteria;
- SBS increase AOC due to organic oxidation.
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2B3T | Two-bed, three-tower pure water system |
| AC | Activated carbon |
| AOC | Assimilable organic carbon |
| AOP | Advanced oxidation process |
| ATP | Adenosine triphosphate |
| BOM | Biodegradable organic material |
| BR | Bureau of Reclamation |
| BWRO | Brakish water reverse osmosis |
| CA | Cellulose acetate |
| CEB | Chemical-enhanced backwash |
| CF | Cartridge filter |
| CIP | Cleaning in place |
| CMIT | 5-Chloro-2-methyl-4-isothiazolin-3-one |
| COD | Chemical oxygen demand |
| CTA | Cellulose triacetate |
| DBNPA | 2,2-Dibromo-3-nitrilopropionamide |
| DBP | Disinfection by-product |
| DNA | Deoxyribonucleic Acid |
| DO | Dissolved oxygen |
| DOC | Dissolved organic carbon |
| DP | Differential pressure |
| DPD | N,N-diethyl-p-phenylenediamine |
| DTPMP | Diethylenetriamine pentamethylphosphonic acid |
| ED | Electrodialysis |
| EDTA | Ethylenediaminetetraacetic acid |
| EDX | Energy dispersive X-ray spectroscopy |
| EPR | Electron paramagnetic resonance |
| EPS | Extracellular polymeric substances |
| ESCA | Electron spectroscopy for chemical analysis |
| ESR | Electron spin resonance |
| FAC | Free available chlorine |
| HAA | Haloacetic acid |
| HPC | Heterotrophic plate count |
| ICI | Intermittent chlorine injection |
| IEX | Ion exchange |
| IMS | Integrated membrane system |
| LC-OCD | Liquid chromatography—organic carbon detection |
| LP | Low pressure |
| mBFR | Modified Biofilm Formation Rate |
| MC | Maintenance Cleaning |
| MC1 | Maintenance Cleaning with 200 ppm NaOCl solution |
| MF | Microfiltration |
| MIT | 2-Methyl-4-isothiazolin-3-one |
| MSF | Multi-stage flash evaporation |
| NF | Nanofiltration |
| NOM | Natural organic matter |
| O&M | Operation and maintenance |
| OIT | 2-Octyl-2H-isothiazol-3-one |
| ORP | Oxidation–reduction potential |
| PVDF | Polyvinylidene fluoride |
| RO | Reverse osmosis |
| SBS | Sodium bisulfite |
| SDGs | Sustainable development goals |
| SDI | Silt density index |
| SEM | Scanning electron microscopy |
| SHMP | Sodium hexametaphosphate |
| SMBS | Sodium metabisulfite |
| SOB | Sulfur-oxidizing bacteria |
| SRB | Sulfate-reducing bacteria |
| SWCC | Saline Water Conversion Corporation |
| SWRO | Seawater reverse osmosis |
| TDS | Total dissolved solids |
| TFC | Thin-film composite |
| TFN | Thin-film nanocomposite |
| THM | Trihalomethane |
| TOC | Total organic carbon |
| UF | Ultrafiltration |
| UPW | Ultra-pure water |
| WAC | Weak acid cation |
| XPS | X-ray photoelectron spectroscopy |
References
- Koros, W.J.; Ma, Y.H.; Shimidzu, T. Terminology for membranes and membrane processes (IUPAC Recommendations 1996). Pure Appl. Chem. 1996, 68, 1479–1489. [Google Scholar] [CrossRef]
- United Nations Sustainable Development. Water and Sanitation–United Nations Sustainable Development. 2016. Available online: https://www.un.org/sustainabledevelopment/water-and-sanitation/ (accessed on 8 July 2021).
- Van der Bruggen, B. Sustainable implementation of innovative technologies for water purification. Nat. Rev. Chem. 2021, 5, 217–218. [Google Scholar] [CrossRef]
- Lange, K.E. The Big Idea Get the Salt Out. In National Geographic. A Special issue. Water–Our Thirsty World; National Geographic Society: Washington, DC, USA, 2010; pp. 32–36. [Google Scholar]
- Zhang, Z.; Li, S.; Mi, B.; Wang, J.; Ding, J. Surface slip on rotating graphene membrane enables the temporal selectivity that breaks the permeability-selectivity trade-off. Sci. Adv. 2020, 6, eaba9471. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.; Hoek, E.M.; Yan, Y.; Subramani, A.K.; Huang, X.; Hurwitz, G.; Ghosh, A.K.; Jawor, A. Interfacial polymerization of thin film nanocomposites: A new concept for reverse osmosis membranes. J. Membr. Sci. 2007, 294, 1–7. [Google Scholar] [CrossRef]
- Bassyouni, M.; Abdel-Aziz, M.H.; Zoromba, M.S.; Abdel-Hamid, S.M.S.; Drioli, E. A review of polymeric nanocomposite membranes for water purification. J. Ind. Eng. Chem. 2019, 73, 19–46. [Google Scholar] [CrossRef]
- Wen, Y.; Yuan, J.; Ma, X.; Wang, S.; Liu, Y. Polymeric nanocomposite membranes for water treatment: A review. Environ. Chem. Lett. 2019, 17, 1539–1551. [Google Scholar] [CrossRef]
- Bergman, R.A. Cost of Membrane Softening in Florida. J.-Am. Water Work. Assoc. 1996, 88, 32–43. [Google Scholar] [CrossRef]
- Filteau, G.; Reilly, J.F. Nitrate Removal from Contaminated Groundwater Through Reverse Osmosis. In Proceedings of the AWWA Membrane Technology Conference, Baltimore, MD, USA, 1–4 August 1993; pp. 397–412. [Google Scholar]
- Zhai, Y.; Liu, G.; van der Meer, W.G. One-step reverse osmosis based on riverbank filtration for future drinking water purification. Engineering 2021, in press. [Google Scholar] [CrossRef]
- Ruiz-García, A.; Ruiz-Saavedra, E.; Pérez-Báez, S.O.; González-González, J.E. Evaluation of the first nine years operating data of a RO brackish water desalination plant in Las Palmas, Canary Islands, Spain. Desalinat. Water Treat. 2015, 55, 2555–2561. [Google Scholar] [CrossRef]
- Lagartos, A.; Rozenbaoum, E.; Oruc, M.; Hyung, H.; Armas JCd, S.D. Long-term boron rejection of thin-film nanocomposite membrane at Pembroke Desalination Plant in Malta: A case study. Desalinat. Water Treat. 2019, 157, 274–280. [Google Scholar] [CrossRef]
- Global Water Intelligence (GWI). Market profile: Membrane chemicals. Glob. Water Intell. Mag. 2012, 13, 37–40. [Google Scholar]
- Nada, N.; Attenborough, T.; Ito, Y.; Maeda, Y.; Tokunaga, K.; Iwahashi, H. SWRO drinking water project in Shuqaiq: Advanced BWRO, membrane oxidation, and scaling. IDA J. 2011, 3, 30–39. [Google Scholar] [CrossRef]
- Ito, Y.; Hanada, S.; Kitade, T.; Tanaka, Y.; Kurihara, M. Clarification of impact of biofouling triggered by chemical addition for designing of Mega-ton SWRO plant. In Proceedings of the IDA World Congress on Desalination and Water Reuse, Tianjin, China, 20–25 October 2013. [Google Scholar]
- Environex. Sodium Metabisulfite (SMBS) Solutions: Bisulfite Solutions for Sanitising, Dechlorination and RO Membranes. Technical Bulletin. 2016. Available online: https://environex.net.au/wp-content/uploads/2016/04/SodiumMetabisulfiteSolution.pdf (accessed on 9 July 2021).
- Southern Ionics Inc. Sulfur Products Handbook. 2004. Available online: http://www.southernionics.com/pdf/pb/sulfur_handbook.pdf (accessed on 9 July 2021).
- Tanaka, S.; Hashimoto, Y. Preparation of Sulfur Dioxide with Constant Low Concentration from the pH Controlled Sodium Hydrogensulfite Solution. J. Chem. Soc. Jpn. 1977, 1977, 427–430. [Google Scholar] [CrossRef]
- Olabarria, P.M.G. Constructive Engineering of Large Reverse Osmosis Desalination Plants, 1st ed.; Chemical Publishing Company Inc.: Palm Springs, CA, USA, 2015. [Google Scholar]
- Byrne, W. Reverse Osmosis: A Practical Guide for Industrial Users, 2nd ed.; Tall Oaks Publishing: Littleton, CO, USA, 2002. [Google Scholar]
- Applegate, L.E.; Erkenbrecher, C.W. Monitoring and control of biological activity in Permasep® seawater RO plants. Desalination 1987, 65, 331–359. [Google Scholar] [CrossRef]
- FilmTec. In Dechlorinating Feedwater; FILMTEC Technical Bulletin Form FILMTEC-TB 4B; FilmTec: Minneaplois, MN, USA, 1988.
- Kucera, J. Reverse Osmosis: Design, Process, and Applications for Engineers, 2nd ed.; Scrivener Publishing/John Wiley & Sons: Beverly, MA, USA, 2015. [Google Scholar]
- Brandt, M.J.; Johnson, K.M.; Elphinston, A.J.; Ratnayaka, D.D. Chemical Storage, Dosing and Control. In Twort’s Water Supply, 7th ed.; Brandt, M.J., Johnson, K.M., Elphinston, A.J., Ratnayaka, D.D., Eds.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 513–552. [Google Scholar]
- Kunisada, Y.; Okada, K.; Sonoda, T.; Setogawa, S.; Ishiwatari, T. Energy-saving seawater desalination technology development: Part 5. J. Water Re-Use Technol. 1983, 9, 13–25. (In Japanese) [Google Scholar]
- Ishihara, T. Make-up water production equipment for a power generation boiler. J. Water Re-Use Technol. 1989, 15, 39–44. [Google Scholar]
- Furuichi, M.; Nakahara, R.; Iwahori, H.; Kuniyoshi, C.; Yamazato, T. Over-Eight-year Operation and Maintenance of 40,000 m3/day Seawater RO Plant in Japan. In Proceedings of the IDA World Congress on Desalination and Water Reuse, Singapore, 11–16 September 2005. [Google Scholar]
- Koizumi, M. Treatment of Water. Japanese Unexamined Patent Application Publication No. JPS59213495A, 20 May 1983. [Google Scholar]
- Sato, N.; Fujiwara, Y. Method for Treating Aqueous Solution of Sodium Hydrogensulfite. Japanese Unexamined Patent Application Publication No. JPS6458396A, 31 August 1987. [Google Scholar]
- Burns and Roe Industrial Services Corp. Reverse Osmosis Technical Manual, Prepared for OWRT; NTIS No. PBBO-186950; U.S. Department Inter.: Springfield, VA, USA, 1979; pp. 3.11, 4.24. [Google Scholar]
- Channabasappa, K.C.; Strobel, J.J. Status of Sea Water Reverse Osmosis Membrane Process Technology. In Proceedings of the 5th International Symposium on Fresh Water from the Sea, Sardinia, Italy, 16–20 May 1976; Volume 4, pp. 267–291. [Google Scholar]
- Khedr, M.G. Membrane fouling problems in reverse osmosis desalination applications. Desalinat.Water Reuse 2000, 10, 8–17. [Google Scholar]
- Chesters, S.P.; Pena, N.; Gallego, S.; Fazel, M.; Armstrong, M.W.; del Vigo, F. Results from 99 Seawater RO Membrane Autopsies. In Proceedings of the IDA World Congress on Desalination and Water Reuse, Perth, Australia, 4–9 September 2011. [Google Scholar]
- American Water Works Association. Reverse Osmosis and Nanofiltration (M46), 2nd ed.; American Water Works Association: Denver, CO, USA, 2007. [Google Scholar]
- Kucera, J. Biofouling of Polyamide Membranes: Fouling Mechanisms, Current Mitigation and Cleaning Strategies, and Future Prospects. Membranes 2019, 9, 111. [Google Scholar] [CrossRef]
- Massart, N.S. Dechlorination. In White’s Handbook of Chlorination and Alternative Disinfectants, 5th ed.; Black & Veatch Corp/John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 572–593. [Google Scholar]
- Basu, O.D.; Weerasinghe, B.N. Comparison of Reaction Kinetics under Varying Conditions for Dechlorination Chemicals. In Proceedings of the Water Environment Federation, Chicago, IL, USA, 18–22 October 2008; pp. 1922–1931. [Google Scholar]
- Basu, O.; Souza, N. Comparison of dechlorination rates and water quality impacts for sodium bisulfite, sodium thiosulfate and ascorbic acid. Aqua 2011, 60, 167–177. [Google Scholar] [CrossRef]
- Hermant, B.M.; Basu, O.D. Comparison of Reaction Rates and Relative Efficiencies for Various Dechlorination Chemicals. J. Environ. Eng. 2013, 139, 522–529. [Google Scholar] [CrossRef]
- Redondo, J.A.; Lomax, I. Y2K generation FILMTEC RO membranes combined with new pretreatment techniques to treat raw water with high fouling potential: Summary of experience. Desalination 2001, 136, 287–306. [Google Scholar] [CrossRef]
- Fritzmann, C.; Löwenberg, J.; Wintgens, T.; Melin, T. State-of-the-art of reverse osmosis desalination. Desalination 2007, 216, 1–76. [Google Scholar] [CrossRef]
- Moch, I.; Hamida, A.B.; Pohland, H. New Technology to Control Biofouling. In Proceedings of the IDA World Congress on Desalination, Abu Dhabi, United Arab Emirates, 18–24 November 1995; Volume 4, pp. 59–72. [Google Scholar]
- Bradley, R. Design Considerations for Reverse Osmosis Systems. In Reverse Osmosis: Membrane Technology, Water Chemistry and Industrial Applications; Amjad, Z., Ed.; Van Nostrand Reinhold: New York, NY, USA, 1993; pp. 104–138. [Google Scholar]
- El-Manharawy, S.; Hafez, A. Technical management of RO system. Desalination 2000, 131, 173–188. [Google Scholar] [CrossRef]
- Jefferies, M.; Freeman, D.; Comstock, D. Odourless Dechlorination for Reverse Osmosis. In Proceedings of the Desalination for the Environment: Clean Water and Energy, Limassol, Cyprus, 11–15 May 2014. [Google Scholar]
- Osta, T.K.; Bakheet, L.M. Pretreatment System in Reverse Osmosis Plants. Desalination 1987, 63, 71–80. [Google Scholar] [CrossRef]
- Light, W.G.; Chu, H.C.; Tran, C.N. Reverse osmosis TFC magnum elements for chlorinated/dechlorinated feedwater processing. Desalination 1987, 64, 411–421. [Google Scholar] [CrossRef]
- Nada, N.A.; Zahrani, A.; Ericsson, B. Experience on pre- and posttreatment from sea water desalination plants in Saudi Arabia. Desalination 1987, 66, 303–318. [Google Scholar] [CrossRef]
- Ayyash, Y.; Imai, H.; Yamada, T.; Fukuda, T.; Yanaga, Y.; Taniyama, T. Performance of reverse osmosis membrane in Jeddah Phase I plant. Desalination 1994, 96, 215–224. [Google Scholar] [CrossRef]
- Voutchkov, N. Desalination Engineering—Operation and Maintenance; McGraw Hill: New York, NY, USA, 2014; pp. 93–95. [Google Scholar]
- Byrne, W. Design and Care of Reverse Osmosis Systems. POWER 2018, 162, 53–59. [Google Scholar]
- Wilf, M. Alternative Dechlorination Methods in Reverse Osmosis (RO.) Applications. Pharm. Eng. 2013, 33, 66–74. [Google Scholar]
- Dow. FILMTEC Membranes: Technical Manual; Chap. 4 Water Chemistry and Pretreatment, 4.6 p. 3; FilmTec: Minneaplois, MN, USA, 1993. [Google Scholar]
- Echaniz, J.; Rodero, A.; Sallangos, O.; Santamaria, F.J. Dhekelia (Cyprus) Seawater Desalination Plant Design, Construction and Commissioning of the 20,000 m3/day R.O. Plant. In Proceedings of the IDA World Congress on Desalination and Water Reuse, Madrid, Spain, 6–9 October 1997; Volume II, pp. 371–392. [Google Scholar]
- Hafsi, M.; Khaoua, A.; Ben Abdellah, S.; El Mghari Tabib, M. Effects of the chemical injection points in pretreatment on reverse osmosis (RO) plant performance. Desalination 2004, 167, 209–216. [Google Scholar] [CrossRef]
- Saeed, M.O.; Jamaluddin, A.T.; Tisan, I.A.; Lawrence, D.A.; Al-Amri, M.M.; Chida, K. Biofouling in a Seawater Reverse Osmosis Plant on the Red Sea Coast, Saudi Arabia. In Proceedings of the IDA World Congress on Desalination and Water Reuse, San Diego, CA, USA, 29 August–3 September 1999; Volume II, pp. 207–221. [Google Scholar]
- Saeed, M.O. Effect of dechlorination point location and residual chlorine on biofouling in a seawater reverse osmosis plant. Desalination 2002, 167, 229–235. [Google Scholar] [CrossRef]
- Hassan, A.M.; Al-Jarrah, S.; Al-Lohibi, T.; Al-Hamdan, A.; Bakheet, L.M.; Al-Amri, M.M.I. Performance evaluation of SWCC SWRO plants. Desalination 1989, 74, 37–50. [Google Scholar] [CrossRef]
- Fethi, K. The RO 22500m3/D Gabès Plant: 10 Years of Operation without Membrane Replacement. In Proceedings of the IDA World Congress on Desalination and Water Reuse, Singapore, 11–16 September 2005; pp. SP05–SP032. [Google Scholar]
- Walker, T.; Cardenas, M.; Richinick, G. De-Bugging the Plant: Managing Reverse Osmosis Biofouling at a Groundwater Treatment Plant. J. AWWA 2016, 108, E137–E144. [Google Scholar] [CrossRef]
- Dorival, M.; Maduro, J.; Pereira, M.; Hallsby, A.; Lopez, D. Long-Term Operation of the Santa Barbara—Curacao Seawater Desalination Plant. In Proceedings of the IDA World Congress, Dubai, United Arab Emirates, 7–12 November 2009. IDAWC/DB09-301. [Google Scholar]
- Mindler, A.B.; Epstein, A.C. Chapter 2.6 Measurements and control in reverse osmosis desalination. Desalination 1986, 59, 343–379. [Google Scholar] [CrossRef]
- Sommariva, C.; Attenborough, A.; Poggi, G.F.; Al Mahdi, A.M.; Hori, T.; Tokunaga, K.; Ito, Y.; Maeda, Y. Matching Hollow-Fiber with Spiral-Wound Membranes: Process Compatibility and Optimization. In Proceedings of the IDA World Congress, Perth, Australia, 4–9 September 2011. IDAWC/PER11-225. [Google Scholar]
- Al-Mahdi, A.A.; Ki, Y.K.; Muneen, F.M. Process Design and Boron Removal in Saudi Arabia’s Largest SWRO Plant. In Proceedings of the IDA World Congress, Dubai, United Arab Emirates, 7–12 November 2009. IDAWC/DB09-001. [Google Scholar]
- Hayakawa, K.; Shimpo, C. Reverse Osmosis Treatment Method and Water Treatment Device. WO Publication Number WO2019031430A1, 14 February 2019. [Google Scholar]
- Singh, M.; Liang, L.; Basu, A.; Belsan, M.A.; Hallsby, G.A.; Morris, W.H.T. 3D TRASAR™ Technologies for Reliable Wastewater Recycling and Reuse. In Industrial Wastewater Treatment, Recycling, and Reuse; Ranade, V.V., Bhandari, V.M., Eds.; Butterworth-Heinemann: Waltham, MA, USA, 2014; pp. 435–462. [Google Scholar]
- DuPont, FilmTec™ Reverse Osmosis Membranes Technical Manual, Form No. 45-D01504-en, Rev. 7. Available online: https://www.dupont.com/content/dam/dupont/amer/us/en/water-solutions/public/documents/en/45-D01504-en.pdf (accessed on 5 August 2021).
- Hydranautics. Chemical Pretreatment for RO and NF; Technical Application Bulletin TAB111: Oceanside, CA, USA, 2020. Available online: https://membranes.com/wp-content/uploads/Documents/application-report/TAB111.pdf (accessed on 5 August 2021).
- LG Chem. Feed Water Quality Guidelines; Technical Application Bulletin TAB106: Seoul, Korea, 2020. Available online: https://www.lgwatersolutions.com/en/technical-document/technical-bulletins-tab (accessed on 5 August 2021).
- Toray, Operation, Maintenance, and Handling Manual, TMM-230 Operation Monitoring, 06-G-MB1-210401. 2021, pp. 26–37. Available online: http://s3-ap-northeast-1.amazonaws.com/water.toray/knowledge/manual/pdf/RO_HandlingManual.pdf (accessed on 5 August 2021).
- Gray, D.M. Instruments; Advances in Instrumentation used to monitor High-Purity Water Treatment Systems. Ultrapure Water 2003, 20, 24–28. [Google Scholar]
- Ito, Y.; Iwahashi, H.; Kakiue, H.; Matsui, K.; Nagai, M.; Takeuchi, K. Desalination Apparatus and Desalination Method. Japanese Unexamined Patent Application Publication No. JP2012120970A, 28 June 2012. [Google Scholar]
- Shulder, S.J. Experience and Use of Oxidation Reduction Potential (ORP) Measurements in Power Plant Applications. In Proceedings of the International Water Conference, Pittsburgh, PA, USA, 22–26 October 2006. IWC-06-032. [Google Scholar]
- Byrne, W. Common Mistakes in Design, Use of Reverse Osmosis Systems. Ethanol Prod. Mag. 2009, 15, 120–123. [Google Scholar]
- Xie, R.J.; Tan, E.K.; Puah, A.N. Oxidation-reduction potential in saline water reverse osmosis membrane desalination and its potential use for system control. Desalinat. Water Treat. 2009, 3, 193–203. [Google Scholar] [CrossRef]
- Tate, J. Industrial Reverse Osmosis System Design, WC&P. 21 July 2008. Available online: https://wcponline.com/2008/07/21/industrial-reverse-osmosis-system-design/ (accessed on 5 August 2021).
- Lindgren, M.; Casey, W. Multiple Applications of Membrane Technology for Water Treatment at a Semiconductor Manufacturing Facility. In Proceedings of the AMTA 2002 Biennial Conference and Exposition, Tampa, FL, USA, 6–9 August 2002. [Google Scholar]
- Martorell, A.; Miranda, P.; Beneyto, I.; Fernandez, R.; Palacios, E.; Ferrero, E. Tampa Bay Desalination Plant—7 Years of O&M. In Proceedings of the IDA World Congress on Desalination and Water Reuse, San Diego, CA, USA, 30 August–4 September 2015. IDAWC15 – Martorell_51563. [Google Scholar]
- 4500-SO32−SULFITE. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; Rice, E.W., Baird, R.B., Eaton, A.D., Eds.; American Public Health Association; American Water Works Association, Water Environment Federation: Washington, DC, USA, 2017. [Google Scholar]
- Ray, R.J.; Kucera-Gienger, J.; Retzlaff, S. Membrane-based hybrid processes for energy-efficient waste-water treatment. J. Membr. Sci. 1986, 28, 87–106. [Google Scholar] [CrossRef]
- Schmoldt, H.; Strathmann, H.; Kaschemekat, J. Desalination of sea water by an electrodialysis-reverse osmosis hybrid system. Desalination 1981, 38, 567–582. [Google Scholar] [CrossRef]
- Maeda, Y. High Performance Nanofiltration Membranes and Hybrid Processes. Membrane 1998, 23, 235–244. [Google Scholar] [CrossRef][Green Version]
- Nederlof, M.M.; Kruithof, J.C.; Taylor, J.S.; van der Kooij, D.; Schippers, J.C. Comparison of NF/RO membrane performance in integrated membrane systems. Desalination 2000, 131, 257–269. [Google Scholar] [CrossRef]
- Lozier, J.C.; Jones, G.; Bellamy, W. Integrated membrane treatment in Alaska. J. AWWA 1997, 89, 50–64. [Google Scholar] [CrossRef]
- Gilabert-Oriol, G.; Hassan, M.; Dewisme, J.; Garcia-Molina, V.; Busch, M. Backwashing pressurized ultrafiltration using reverse osmosis brine in seawater desalination and its potential costs savings. Desalination Water Treat. 2015, 55, 2800–2812. [Google Scholar] [CrossRef]
- Vial, D.; Doussau, G.; Galindo, R. Comparison of three pilot studies using Microza® membranes for Mediterranean seawater pre-treatment. Desalination 2003, 156, 43–50. [Google Scholar] [CrossRef]
- Busch, M.; García-Molina, V.; Rosenberg, S.; Gasia-Bruch, E. Performance Review of Reverse Osmosis Seawater Desalination Using Membrane Filtration Pretreatment. In Proceedings of the IDA World Congress, Perth, Australia, 4–9 September 2011. IDAWC/PER11-026. [Google Scholar]
- Henthorne, L.; Quigley, R. Evaluation of MF, UF and Conventional Pretreatment for Seawater RO Applications. In Proceedings of the IDA World Congress, Bahamas, 28 September–3 October 2003. BAH03-152. [Google Scholar]
- Brownsville Public Utilities Board. Final Pilot Study Report—Texas Seawater Desalination Demonstration Project. October 2008. Available online: https://www.twdb.texas.gov/innovativewater/desal/projects/brownsville/doc/BPUBPilot_Final_Report.pdf (accessed on 9 August 2021).
- Leal, J.; White, J.M.; Dietrich, J.A. Seawater Desalination in Brownsville, Texas. In Proceedings of the IDA World Congress, Dubai, United Arab Emirates, 7–12 November 2009. IDAWC/DB09-148. [Google Scholar]
- Henthorne, L. Evaluation of Membrane Pretreatment for Seawater RO Desalination. United States Bureau of Reclamation, Desalination and Water Purification Research and Development Program, Report No. 106. October 2007. Available online: https://www.usbr.gov/research/dwpr/reportpdfs/Report106.pdf (accessed on 9 August 2021).
- Alfred Arias, D.A.; Vila Cremer, S.; Suarez, J.; Gilabert, G. Pressurized Ultrafiltration: Use of Reverse Osmosis Brine for UF Backwashes. In Proceedings of the IDA World Congress on Desalination and Water reuse, San Diego, CA, USA, 30 August–4 September 2015. IDA15WC-Arias_51486. [Google Scholar]
- Nordham, D.; Varnava, B.; Miller, M.; Hoffard, T. Desalination: Coastal Seawater Testing of a Full-Scale MF System for Shipboard RO Pretreatment. Ultrapure Water 2010, 27, 18–26. [Google Scholar]
- Esteban Martin, M.; Craig Bartels, C.; Franks, R.; Garrote, R.; Rodriguez, M.A.; Neculau, M.; Vartolomei, F. Versatility of UF+RO Plant in Marbella for Testing Hollow Fiber and Spiral Wound Membranes Performance and CIP. In Proceedings of the IDA World Congress 2017 on Water Reuse & Desalination, São Paulo, Brazil, 15–20 October 2017. DA17WC-57945_Garrote. [Google Scholar]
- Guibert, D.; Laverty, P. Ultrafiltration Pretreatment for Reverse Osmosis Plants: Design Perspectives and Considerations. In Proceedings of the IDA World Congress on Desalination and Water Reuse, Tianjin, China, 20–25 October 2013. IDAWC/TIAN13-189. [Google Scholar]
- Suárez, J.; Salgado, B.; Casañas, A.; González, J.C.; Pordomingo, J. One-year operational experience with ultrafiltration as pretreatment of seawater reverse osmosis desalination system (Maspalomas-I Plant). Desalinat. Water Treat. 2015, 55, 2813–2821. [Google Scholar] [CrossRef]
- Suárez, J.; Barberà, L.; Gilabert-Oriol, G.; González, J.C.; Pordomingo, J. Two Years Operational Experience with Ultrafiltration as Pre-treatment of Seawater Desalination System. In Proceedings of the IDA World Congress on Desalination and Water Reuse, San Diego, CA, USA, 30 August–4 September 2015. IDAWC15-51405_Suarez. [Google Scholar]
- Matsumoto, K.; Aoki, T.; Hirao, T.; Yoda, K.; Morita, S.; Nagai, N.; Ikuno, N.; Uemura, K. New Combined Chlorine Disinfection for Bio-fouling Control. In Proceedings of the IDA World Congress, Dubai, United Arab Emirates, 7–12 November 2009. IDAWC/DB09-069. [Google Scholar]
- Endou, Y.; Kawakatsu, T. The Influence to Polyamide Reverse Osmosis (RO) Membranes of Slime Control Agent for RO Membranes, “KURIVERTER® IK-110”. Membrane 2016, 41, 44–47. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Tsuji, M.; Araki, C.; Someya, S.; Eguchi, M. A new stabilized-inorganic-oxidative biocide for biofouling control in RO Systems. In Proceedings of the Desalination for Clean Water and Energy Cooperation Among Mediterranean Countries of Europe and the MENA Region, Palermo, Italy, 10–14 May 2015. [Google Scholar]
- Bertheas, U.; Majamaa, K.; Arzu, A.; Pahnke, R. Use of DBNPA to control biofouling in RO systems. Desalinat. Water Treat. 2009, 3, 175–178. [Google Scholar] [CrossRef]
- Wood, J.; Arba, J.; Zoccolante, G. Membrane Technologies for purified water that meets USP 23. Ultrapure Water 1999, 16, 45–50. [Google Scholar]
- Applegate, L.E.; Erkenbrecher, C.W.; Winters, H. New chloramine process to control aftergrowth and biofouling in permasepR B-10 RO surface seawater plants. Desalination 1989, 74, 51–67. [Google Scholar] [CrossRef]
- Chua, I.; Moch, I. Potable Water for a Remote Fishing Port. Desalination Water Reuse 1994, 4, 20–24. [Google Scholar]
- Chang, N.; Singer, P. Formation and Fate of Bromoform During Desalination. J. Environ. Eng. 1984, 110, 1189–1193. [Google Scholar] [CrossRef]
- Kurihara, M.; Fusaoka, Y.; Sasaki, T.; Bairinji, R.; Tadahiro Uemura, T. Development of crosslinked fully aromatic polyamide ultra-thin composite membranes for seawater desalination. Desalination 1994, 96, 133–143. [Google Scholar] [CrossRef]
- Tanaka, S.; Numata, K.; Kuzumoto, H.; Sekino, M. New disinfection method in RO seawater desalination systems. Desalination 1994, 96, 191–199. [Google Scholar] [CrossRef]
- Ammerlaan, A.; Franklin, J.; Moody, C. Yuma desalting plant. Membrane degradation during test operations. Desalination 1992, 88, 33–49. [Google Scholar] [CrossRef]
- Kremen, S.S.; Knappe, P. Chloramination for RO system disinfection while preventing head-end loss of cellulosic membrane performance. Ultrapure Water 1994, 11, 38–42. [Google Scholar]
- The DOW Chemical Brochure, DOW Antimicrobial 7287 and DOW Antimicrobial 8536: The Fast-Acting, Broad-Spectrum Biocides with Low Environmental Impact. Form No. 253-01464-06/18/02. 2002. Available online: https://aniq.org.mx/pqta/pdf/ANTIMICROBIANO%20%207287%20(HT).pdf (accessed on 26 December 2021).
- Durham, L. Biological growth control problem in RO systems. Ultrapure Water 1989, 6, 30–33. [Google Scholar]
- Auerswald, D.C. Optimizing the Performance of a Reverse Osmosis/Electrodeionization System. In Proceedings of the International Water Conference, Pittsburgh, PA, USA, 30 October–1 November 1995. IWC-95-40. [Google Scholar]
- Tanaka, S.; Kuzumoto, H.; Sekino, M. Using Chlorine Dioxide for Trihalomethane Control on RO Seawater Desalination System. In Proceedings of the IDA World Congress on Desalination, Abu Dhabi, United Arab Emirates, 18–24 November 1995; Volume III, pp. 79–89. [Google Scholar]
- Redondo, J.A.; Lomax, I. Experiences with the pretreatment of raw water with high fouling potential for reverse osmosis plant using FILMTEC membranes. Desalination 1997, 110, 167–182. [Google Scholar] [CrossRef]
- Johnson, C.; Alvarez, M.; Salsano, J. Treatment of a Seasonally Brackish Surface Water Using Integrated Membrane Systems. In Proceedings of the AWWA Water Quality Technology Conference, Philadelphia, PA, USA, 2–6 November 2003. 03wqtc_wed9-2. [Google Scholar]
- Lozier, J.C. Evaluating Chloramines for Control of RO Membrane Biofouling with Ground and Surface Water Supplies. In Proceedings of the AWWA Membrane Tech. Conference, Phoenix, AZ, USA, 6–9 March 2005. [Google Scholar]
- Marshall, T.; Don, L. Delivery and Performance of the Luggage Point Wastewater Treatment Plant Water Reclamation Project. In Proceedings of the AWA 19th Convention—A Water Odyssey, Canberra, ACT, Australia, 1–4 April 2001. [Google Scholar]
- Thompson, M.; Powell, D. Case Study—Kranji High Grade Water Reclamation Plant, Singapore. In Proceedings of the IMSTEC ’03, Sydney, Australia, 10–14 November 2003. imstec215. [Google Scholar]
- Andes, K.; Bartels, C.R.; Iong, J.; Wilf, M. Design Considerations for Wastewater Treatment by Reverse Osmosis. In Proceedings of the IDA World Congress on Desalination and Water Reuse, Paradise Island, Bahamas, 28 September–3 October 2003. BAH03-060. [Google Scholar]
- Franks, R.; Bartels, C.R.; Andes, K.; Patel, M.; Yong, T. Implementing Energy Saving RO Technology in Large Scale Wastewater Treatment Plants. In Proceedings of the IDA World Congress, Maspalomas, Gran Canaria, Spain, 21–26 October 2007. IDAWC/MP07-148. [Google Scholar]
- Sugawara, Y.; Takahata, H.; Nakatsuji, H. Fresh Water Generation Method and Fresh Water Generator. Japanese Unexamined Patent Application Publication No. JP2015186773A, 29 October 2015. [Google Scholar]
- Jew, V.; Shoenberger, P.; Filteau, G.; Alt, S. Desalination of Pacific Ocean Water via Microfiltration and Reverse Osmosis. In Proceedings of the AWWA Annual Conference and Exhibition, Anaheim, CA, USA, 15–19 June 2003. [Google Scholar]
- Sharma, R.; Trussell, S.; Lauri, P.; Shoenberger, P.; Filteau, G.; Trussell, R. Bench-Scale studies on the Use of Pre-Formed Chloramines in Seawater Desalination. In Proceedings of the AWWA Annual Conference and Exhibition (ACE09), San Diego, CA, USA, 14–18 June 2009. [Google Scholar]
- West Basin Municipal Water District Ocean Water Desalination Pilot Program Final Comprehensive Report 2002–2009. 2 September 2010. Available online: https://www.westbasin.org/wp-content/uploads/2020/07/OWD-Pilot-Final-comprehensive-Report-2002-2009.pdf (accessed on 1 September 2021).
- West Basin Municipal Water District Ocean Water Desalination Demonstration Project: Final Report. 29 February 2016. Available online: https://www.westbasin.org/wp-content/uploads/2020/07/OWDDF_Final_Report_032818.pdf (accessed on 1 September 2021).
- Valentine, R.L. Bromochloramine oxidation of N,N-diethyl-p-phenylenediamine in the presence of monochloramine. Environ. Sci. Technol. 1986, 20, 166–170. [Google Scholar] [CrossRef]
- Gabelich, C.J.; Yun, T.; Coffey, B.; Suffet, I. Effects of aluminum sulfate and ferric chloride coagulant residuals on polyamide membrane performance. Desalination 2002, 150, 15–30. [Google Scholar] [CrossRef]
- Gabelich, C.J.; Frankin, J.C.; Gerringer, F.; Ishida, K.P.; Suffet, I. Enhanced oxidation of polyamide membranes using monochloramine and ferrous iron. J. Membr. Sci. 2005, 258, 64–70. [Google Scholar] [CrossRef]
- Knoell, T.; Martin, E.; Ishida, K.; Phipps, D. The Effects of Chlorine Exposure on the Performance and Properties of Polyamide Reverse Osmosis Membranes. In Proceedings of the AWWA Membrane Tech. Conference, Phoenix, AZ, USA, 6–9 March 2005. [Google Scholar]
- Silva, M.K.; Tessaro, I.C.; Wada, K. Investigation of oxidative degradation of polyamide reverse osmosis membranes by monochloramine solutions. J. Membr. Sci. 2006, 282, 375–382. [Google Scholar]
- Cran, M.; Bigger, S.; Gray, S. Degradation of polyamide reverse osmosis membranes in the presence of chloramine. Desalination 2011, 283, 58–63. [Google Scholar] [CrossRef]
- Fu, J.; Qu, J.; Liu, R.; Qiang, Z.; Zhao, X.; Liu, H. Mechanism of Cu(II)-catalyzed monochloramine decomposition in aqueous solution. Sci. Total Environ. 2009, 407, 4105–4109. [Google Scholar] [CrossRef] [PubMed]
- Gabelich, C.J.; Ishida, K.P.; Gerringer, F.; Evangelista, R.; Kalyan, M.; Suffet, I. Control of residual aluminum from conventional treatment to improve reverse osmosis performance. Desalination 2006, 190, 147–160. [Google Scholar] [CrossRef]
- Martínez, A.; Vargas, R.; Galano, A. Citric acid: A promising copper scavenger. Comput. Theor. Chem. 2018, 1133, 47–50. [Google Scholar] [CrossRef]
- Lozier, J.C. Chloramination for Control of RO Membrane Biofouling on Ground and Surface Water. In Proceedings of the IDA World Congress on Desalination and Water Reuse, Singapore, 11–16 September 2005; pp. SP05–SP147. [Google Scholar]
- Vargas, C.; Roux, A.; Zavlanos, V. Treatment of the Reverse Osmosis Concentrate from the Western Corridor Recycled Water Project: Nutrient Load Reduction and Operational Challenges. In Proceedings of the Membranes and Desalination Specialty Conference III, Double Bay, NSW, Australia, 11–13 February 2009. [Google Scholar]
- Susarla, P.; Brammer, T.; Staib, C.; Kinser, K.; Rohe, D. The Murrumba Downs Advanced Water Treatment Plant in Queensland, Australia Produces High Quality Industrial Water. In Proceedings of the AWWA Annual Conference and Exposition (ACE09), San Diego, CA, USA, 14–18 June 2009. Poster Session WED17, Water Reuse. [Google Scholar]
- Ekkad, N.; Huber, C.O. Residual sulfite after dechlorination of water. Water Air Soil Pollut. 1996, 90, 295–300. [Google Scholar] [CrossRef]
- Comb, L. Chloramines: Their Chemistry and Their Role in Water Treatment. In Proceedings of the Membranes in Drinking and Industrial Water Production MDIW 2002, Mülheim an der Ruhr, Germany, 22–26 September 2002. [Google Scholar]
- Comb, L. Chloramines: Their Chemistry and Role in Water Treatment. Ultrapure Water 2003, 20, 31–50. [Google Scholar]
- Sandín, R.; Ferrero, E.; Repollés, C.; Navea, S.; Bacardit, J.; Espinós, J.P.; Malfeito, J. Reverse osmosis membranes oxidation by hypochlorite and chlorine dioxide: Spectroscopic techniques vs. Fujiwara test. Desalinat. Water Treat. 2013, 51, 318–327. [Google Scholar] [CrossRef]
- Mizuta, K. Effect of the Water Disinfectant Chlorine Dioxide on the Integrity of a Reverse Osmosis Membrane. Master’s Thesis, University of Illinois at Urbana-Champaign, Champaign, IL, USA, 2014. [Google Scholar]
- Alayemieka, E.; Lee, S. Modification of polyamide membrane surface with chlorine dioxide solutions of differing pH. Desalinat. Water Treat. 2012, 45, 84–90. [Google Scholar] [CrossRef]
- Kim, Y.-K. Effect of Chlorine Dioxide on Polyamide Membranes at Various pH Values. Master’s Thesis, Graduate School of UNIST, Ulsan, Korea, 2016. Available online: http://unist.dcollection.net/public_resource/pdf/000002237166_20210825180018.pdf (accessed on 2 September 2021).
- Marcon, J.; Mortha, G.; Marlin, N.; Molton, F.; Duboc, C.; Burnet, A. New insights into the decomposition mechanism of chlorine dioxide at alkaline pH. Holzforschung 2017, 71, 599–610. [Google Scholar] [CrossRef]
- Hydranautics. Potential Use of ClO2 and a Disinfectant for Polyamide RO/NF Membranes; Technical Applications Bulletin 115; Hydranautics: Oceanside, CA, USA, 2020. [Google Scholar]
- Hupperich, K.; Mutke, X.; Abdighahroudi, M.S.; Jütte, M.; Schmidt, T.; Lutze, H. Reaction of chlorine dioxide with organic matter—Formation of inorganic products. Environ. Sci. Water Res. Technol. 2020, 6, 2597–2606. [Google Scholar] [CrossRef]
- Cooper, W.J.; Zika, R.; Steinhauer, M. Bromide-oxidant interactions and THM formation: A literature review. J. Am. Water Work. Assoc. 1985, 77, 116–121. [Google Scholar] [CrossRef]
- Hijós, G.; Miranda, P.; Palacios, E.; López, N.; Jiménez, A.; Shea, A. Tampa Bay Desalination Plant: Pre-Treatment and RO Unit Main Operation Parameters. In Proceedings of the AMTA/SEDA Joint Conference and Exposition, Naples, FL, USA, 14 July 2008. [Google Scholar]
- Gordon, G.; Slootmaekers, B.; Tachiyashiki, S.; Wood, D.W. Minimizing Chlorite Ion and Chlorate Ion in Water Treated with Chlorine Dioxide. J. Am. Water Work. Assoc. 1990, 82, 160–165. [Google Scholar] [CrossRef]
- Yonkos, L.; Fisher, D.; Burton, D.; Whitekettle, W.K.; Peterille, J.C. Effectiveness of the sulfur (IV) compound, sodium bisulfite, in reducing chlorine, chlorine dioxide, and chlorite toxicity to Daphnia magna in well water and pond water. Environ. Toxicol. Chem. 2001, 20, 530–536. [Google Scholar] [CrossRef]
- Ferrero, E.; Llansana, A.; Ayala, V.; Malfeito, J.J. Chlorite and Chlorate Effect on the Reverse Osmosis Membranes Performance. In Proceedings of the IDA World Congress-Maspalomas, Gran Canaria, Spain, 21–26 October 2007. IDAWC/MP07-127. [Google Scholar]
- Suzuki, K.; Gordon, G. Stoichiometry and kinetics of the reaction between chlorine dioxide and sulfur (IV) in basic solutions. Inorg. Chem. 1978, 10, 3115–3118. [Google Scholar] [CrossRef]
- Kuzumoto, E.; Sekino, M.; Tanaka, S. Reduction of Disinfected Byproduct in Membrane Separation Process. Japanese Unexamined Patent Application Publication No. JPH0929075A, 4 February 1997. [Google Scholar]
- Hoehn, R.C.; Darby, H.S.; Neemann, J. Chlorine Dioxide. In White’s Handbook of Chlorination and Alternative Disinfectants, 5th ed.; Black & Veatch Corp/JohnWiley & Sons: Hoboken, NJ, USA, 2010; pp. 700–766. [Google Scholar]
- Doñaque, E.P.; Cebrian, A.M.; Luján, P.J.M. Chlorine Dioxide as Disinfectant for Pretreatment in Seawater Desalination Plants. In Proceedings of the AMTA/AWWA Membrane Technology Conference, Orlando, FL, USA, 2–6 March 2015. [Google Scholar]
- Griese, M.; Hauser, K.; Berkemeier, M.; Gordon, G. Using Reducing Agents to Eliminate Chlorine Dioxide and Chlorite Ion Residuals in Drinking Water. J. Am. Water Work. Assoc. 1991, 83, 56–61. [Google Scholar] [CrossRef]
- Katz, A.; Narkis, N. Removal of chlorine dioxide disinfection by-products by ferrous salts. Water Res. 2001, 35, 101–108. [Google Scholar] [CrossRef]
- Blanchard, F.; Gonsior, S.; Hopkins, D. 2,2-Dibromo-3-nitrilopropionamide (DBNPA) chemical degradation in natural waters: Experimental evaluation and modeling of competitive pathways. Water Res. 1987, 21, 801–807. [Google Scholar] [CrossRef]
- Zeiher, E.H.K.; Yu, F.P. Biocides used for Industrial Membrane System Sanitization. Ultrapure Water 2000, 17, 55–61. [Google Scholar]
- Exner, J.; Burk, G.A.; Kyriacou, D. Rates and products of decomposition of 2,2-dibromo-3-nitrilopropionamide. J. Agric. Food Chem. 1973, 21, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Boorsma, M.; Dost, S.; Klinkhamer, S.; Schippers, J. Monitoring and controlling biofouling in an integrated membrane system. Desalinat. Water Treat. 2011, 31, 347–353. [Google Scholar] [CrossRef]
- Dow Chemical Company. Recommendations for Proper Installation and Operation of Dowex* Permeators; Dow Chemical: Midland, MI, USA, 1979. [Google Scholar]
- Boegli, J.W.; Murphy, A.P.; Price, M.K. Oxidation of Formaldehyde Solutions Used for the Preservation of Reverse Osmosis Membranes; Applied Sciences Branch, Division of Research and Laboratory Services, Engineering and Research Center, US Department of the Interior, Bureau of Reclamation: Denver, CO, USA, 1984. [Google Scholar]
- Rowley, L.H. A screening study of 12 biocides for potential use with cellulose acetate reverse osmosis membranes. Desalination 1992, 88, 71–83. [Google Scholar] [CrossRef]
- Du Pont Company. Sterilizing (Sanitizing) Procedure, Permasep Engineering Manual, #509; Du Pont Company: Willmington, DE, USA, 1982. [Google Scholar]
- FilmTec. FT30 Reverse Osmosis Membrane Biological Protection and Disinfection; FILMTEC Technical Bulletin; Form FILMTEC-4002B; FilmTec: Minneapolis MN, USA, 1988. [Google Scholar]
- FilmTec. FT30 Reverse Osmosis Membrane Biological Protection and Disinfection; Technical Bulletin; FILMTEC Membranes; Form No. 609-24010-790 AMS; FilmTec: Minneapolis MN, USA, 1990. [Google Scholar]
- Nakagawa, Y.; Fujino, H.; Inoue, T. Preservation Method for Semipermeable Composite Membrane. Japanese Unexamined Patent Application Publication No. JPS57207503A, 20 December 1982. [Google Scholar]
- Toray Operation, Maintenance, and Handling Manual, TMM-500 Storage of RO Element Outside of Pressure Vessel, 06-G-MB1-210401. 2021, pp. 68–69. Available online: http://s3-ap-northeast-1.amazonaws.com/water.toray/knowledge/manual/pdf/RO_HandlingManual.pdf (accessed on 5 August 2021).
- LG Chem. Receipt of Elements, Short-Term Storage, and Disposal of Used Elements; Technical Service Bulletin. TSB101: 2021. Available online: https://www.lgwatersolutions.com/en/technical-document/technical-bulletins-tsb (accessed on 10 September 2021).
- Wilbert, M.C. The Desalting and Water Treatment Membrane Manual: A Guide to Membranes for Municipal Water Treatment. Water Treatment Technology Program Report No. 1; US Bureau of Reclamation: Denver, CO, USA, 1993. [Google Scholar]
- LG Chem. Membrane Storage Inside Pressure Vessel; Technical Service Bulletin. TSB105: 2021. Available online: https://www.lgwatersolutions.com/en/technical-document/technical-bulletins-tab (accessed on 10 September 2021).
- William Varnava, W.; Silbernagel, M.; Kuepper, T.; Miller, M. Reverse Osmosis (RO) Element Preservation Study. In Proceedings of the 1996 Biennial Conference and Exposition of the ADA, Monterey, CA, USA, 4–8 August 1996; pp. 308–327. [Google Scholar]
- Toray Operation, Maintenance, and Handling Manual, TMM-240 Shutdown Considerations for RO Systems, 06-G-MB1-210401. 2021, pp. 41–43. Available online: http://s3-ap-northeast-1.amazonaws.com/water.toray/knowledge/manual/pdf/RO_HandlingManual.pdf (accessed on 5 August 2021).
- Larson, R.; Cadotte, J.E.; Petersen, R.J. The FT-30 seawater reverse osmosis membrane—Element test results. Desalination 1981, 38, 473–483. [Google Scholar] [CrossRef]
- Petersen, R.J.; Cadotte, J.E.; Buettner, J.M. Development of FT-30 Membranes in Spiral Wound Modules; FilmTec Corp.: Minneapolis, MN, USA, 1982. [Google Scholar]
- Henthorne, L.; Hassan, A.; Jamaluddin, A.T.M. Storage of Thin Film Reverse Osmosis Membranes in Three Biocides. In Proceedings of the AWWA Membrane Technology Conference, New Orleans, LA, USA, 23–26 February 1997; pp. 1059–1073. [Google Scholar]
- Rowley, L.H. Eleven biocides are investigated for microbial efficacy and membrane compatibility to use at the Yuma Desalting Plant. Desalination 1994, 97, 35–43. [Google Scholar] [CrossRef]
- Ventresque, C.; Habarou, H.; Gaid, A. New Applications of Nanofiltration. In Proceedings of the International Desalination Association World Congress on Desalination and Water Reuse, Tianjin, China, 20–25 October 2013. IDAWC/TIAN13-078. [Google Scholar]
- Tu, K.L.; Chivas, A.; Nghiem, L. Effects of chemical preservation on flux and solute rejection by reverse osmosis membranes. J. Membr. Sci. 2014, 472, 202–209. [Google Scholar] [CrossRef][Green Version]
- Ventresque, C.; Cohen, D.; Camenzuli, J.; Chauvin, S.; Barbe, C. Preservation of RO Membranes in Drinking Water Plants: Operational Experience and Case Studies. In Proceedings of the IDA World Congress on Desalination and Water Reuse, San Diego, CA, USA, 30 August–4 September 2015. IDAWC15- Ventresque_51765. [Google Scholar]
- Fusaoka, Y.; Okada, K.; Takeuchi, H. Treatment of Reverse Osmosis Membrane Separation Apparatus. Japanese Unexamined Patent Application Publication No. JPH07328391A, 19 December 1995. [Google Scholar]
- Farooque, A.; Al-amoudi, A.; O’Hara, J.; Munshi, H.A. RO membrane failure: Investigation of failure during preservation. Filtr. Sep. 2007, 44, 22–24. [Google Scholar] [CrossRef]
- Majamaa, K.; Bertheas, U.; Finlayson, F.; Levy, R.B. Preservation of reverse osmosis membranes with non oxidizing biocides—Comparison with SMBS. Water Sci. Technol. Water Supply 2011, 11, 342–351. [Google Scholar] [CrossRef]
- Salgado, B.; Majamaa, K.; Sanz, J.; Molist, J. Design and start-up experiences of 19,000 m3/d Camp de Tarragona-Vilaseca Water Reclamation Plant. Desalinat. Water Treat. 2013, 51, 1519–1526. [Google Scholar] [CrossRef]
- Sanz, J.; Suescún, J.; Molist, J.; Rubio, F.; Mujeriego, R.; Salgado, B. Reclaimed water for the Tarragona petrochemical park. Water Sci. Technol. Water Supply 2015, 15, 308–316. [Google Scholar] [CrossRef]
- Sanz, J.; Salgado, B.; Taberna, E.; Busch, M.; Mas, J.; Levy, R.; Vigués, N.; Molist, J. 2014 Operation and long-term preservation experience with FILMTEC™ Reverse Osmosis elements at the Camp de Tarragona Wastewater Reclamation Plant. In Proceedings of the Abstract of the Desalination for the Environment, Clean Water and Energy Conference, European Desalination Society, Limassol, Cyprus, 11–15 May 2014. [Google Scholar]
- Miguel, C.; Hernandez, M. Management of Barcelona SWRO Plant During Low and Discontinuous Operation. In Proceedings of the IDA World Congress on Desalination and Water Reuse, San Diego, CA, USA, 30 August–4 September 2015. IDAWC15-Miguel_51447. [Google Scholar]
- Malik, A.; Kutty, P.; Siddiqi, N.; Andijani, I.; Thankachan, T. Effect of Deaeration and Sodium Sulfite Addition to MSF Make-up Water on Corrosion of Evaporator and Heat Exchanger Materials. J. King Saud Univ. Eng. Sci. 1996, 8, 21–35. [Google Scholar] [CrossRef]
- Inoue, T.; Kurihara, M.; Watanabe, T. Semipermeable Membrane. Japanese Unexamined Patent Application Publication No. JPS54107882A, 24 August 1979. [Google Scholar]
- Kurihara, M.; Kanamaru, N.; Harumiya, N.; Yoshimura, K.; Hagiwara, S. Spiral-wound new thin film composite membrane for a single-stage seawater desalination by reverse osmosis. Desalination 1980, 32, 13–23. [Google Scholar] [CrossRef]
- Kurihara, M.; Harumiya, N.; Kanamaru, N.; Tonomura, T.; Nakasatomi, M. Development of the PEC-1000 composite membrane for single-stage seawater desalination and the concentration of dilute aqueous solutions containing valuable materials. Desalination 1981, 38, 449–460. [Google Scholar] [CrossRef]
- Petersen, R.J. Composite reverse osmosis and nanofiltration membranes. J. Membr. Sci. 1993, 83, 81–150. [Google Scholar] [CrossRef]
- Kurihara, M.; Nakagawa, Y.; Takeuchi, H.; Kanamaru, N.; Tonomura, T. Single-stage seawater desalination at high temperature and salinity as present in the Middle East using PEC-1000 membrane modules. Desalination 1983, 46, 101–110. [Google Scholar] [CrossRef]
- Matsuka, N.; Nakagawa, Y.; Kurihara, M.; Tonomura, T. Reaction kinetics of sodium bisulfite and dissolved oxygen in seawater and their applications to seawater reverse osmosis. Desalination 1984, 51, 163–171. [Google Scholar] [CrossRef]
- Eriksson, P. The effect of dissolved oxygen in water on the performance of a high rejection polyamide reverse osmosis membrane. Desalination 1991, 83, 249–260. [Google Scholar] [CrossRef]
- Hiemstra, P.; Van Paassen, J.; Rietman, B.; Verdouw, J. Aerobic Versus Anaerobic Nanofiltration: Fouling of Membranes. In Proceedings of the AWWA Membrane Conference, Long Beach, CA, USA, 28 February–3 March 1999. [Google Scholar]
- Castle, R.J.; Harn, J.N.P.E. Case Studies: Aerobic vs. Anaerobic Pretreatment of Groundwater. In Proceedings of the AMTA Biennial Conference and Exposition, Anaheim, CA, USA, 30 July–2 August 2006. [Google Scholar]
- Harn, J.N.; Troyer, S. Direct Membrane Treatment of Anaerobic High Iron and Manganese Groundwaters. In Proceedings of the AMTA/AWWA Membrane Technology Conference & Exposition, Long Beach, CA, USA, 13–17 February 2017. [Google Scholar]
- Beyer, F.; Rietman, B.; Zwijnenburg, A.; Brink, P.V.; Vrouwenvelder, J.; Jarzembowska, M.; Laurinonyte, J.; Stams, A.; Plugge, C. Long-term performance and fouling analysis of full-scale direct nanofiltration (NF) installations treating anoxic groundwater. J. Membr. Sci. 2014, 468, 339–348. [Google Scholar] [CrossRef]
- Hart, G.K.; Messner, S.M. Iron Removal at the Corkscrew Water Treatment Plant Using Nanofiltration Membranes. In Proceedings of the AWWA Membrane Technologies in the Water Industry, Orlando, FL, USA, 10–13 March 1991; pp. 139–147. [Google Scholar]
- Loeb, S.; Selover, E. Sixteen months of field experience on the Coalinga pilot plant. Desalination 1967, 2, 75–80. [Google Scholar] [CrossRef]
- Sieveka, E.H. Reverse-Osmosis Pilot Plants. In Desalination by Reverse Osmosis; Merten, U., Ed.; The MIT Press: Cambridge, MA, USA, 1967. [Google Scholar]
- Mccutchan, J.W.; Johnson, J.S. Reverse Osmosis at Coalinga, California. J. Am. Water Work. Assoc. 1970, 62, 346–353. [Google Scholar] [CrossRef]
- Yallaly, B.; Seacord, T.; Kalkman, T. Meeting Criteria for Water and Wastewater Systems Simultaneously Using Reverse Osmosis and Zero Liquid Discharge Technology. In Proceedings of the AMTA Annual Conference & Exposition, San Diego, CA, USA, 12–15 July 2010. [Google Scholar]
- Keller, M. Iron Removal by Ion Exchange: Standing on Solid Ground. Water Cond. Purif. 2004, 46, 20–23. [Google Scholar]
- Martin, C.J.; Kartinen, E.O. Ion Exchange Softening as Pretreatment for Reverse Osmosis Feed Water for Iron Contaminated Water. In Proceedings of the ADA North American Biennial Conference & Exposition, Williamsburg, VA, USA, 2–6 August 1998. [Google Scholar]
- Mitchenko, T.; Stender, P.; Kozlov, P. Ion-Exchange Pretreatment of Well Water with the High Level of Iron for Reverse Osmosis Plant. J. Ion Exch. 2007, 18, 616–619. [Google Scholar] [CrossRef]
- Bornak, W.E. A Difficult Iron RO Pretreatment Problem; Paper IWC-98-12. In Proceedings of the 59th International Water Conference, Pittsburgh, PA, USA, 19–21 October 1998. [Google Scholar]
- UNEP. Desalination Resource and Guidance Manual for Environmental Impact Assessments. United Nations Environment Programme. 2008. Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/30011/DesalRG.pdf?sequence=1&isAllowed=y (accessed on 17 September 2021).
- Cannesson, N.; Johnstone, P.; Mitchell, M.; Boerlage, S. Community, environmental and marine impact minimisation at the Gold Coast desalination plant. In Proceedings of the IDA World Congress on Desalination and Water Reuse, Dubai, United Arab Emirates, 7–12 November 2009. [Google Scholar]
- Ozair, G.; Feda, M.O.A. Brine Disposal Implications on Nearshore Physico-Chemical Quality of Marine Environment. In Proceedings of the IDA World Congress on Desalination and Water Reuse, Tianjin, China, 20–25 October 2013. IDAWC/TIAN13-214. [Google Scholar]
- Patel, K.G.; Harris, F.L.; Channabasappa, K. Operational experience of reverse osmosis plants at Wrightsville Beach, North Carolina, USA. Desalination 1976, 19, 381–388. [Google Scholar] [CrossRef]
- Baker, J.S.; Dudley, L. Biofouling in membrane systems—A review. Desalination 1998, 118, 81–89. [Google Scholar] [CrossRef]
- Matani, K.; Kimura, T. Method and Apparatus for Treating Fresh Water or Salt Water. Japanese Unexamined Patent Application Publication No. JP2005040661A, 17 February 2005. [Google Scholar]
- Obaid, M.; Hamida, A.B. Practical solutions to problems experienced in open seawater RO plants operating on the Arabian Gulf. Desalination 1998, 120, 137–142. [Google Scholar] [CrossRef]
- Arrayedhy, M.A. Pre- and post-treatment at the RO plant at RA’s Abu Jarjur, Bahrain. Desalination 1987, 63, 81–94. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Konishi, K.; Edogawa, K. Method for Sterilizing Reverse Osmosis Equipment. Japanese Unexamined Patent Application Publication No. JPS62110705A, 21 May 1987. [Google Scholar]
- Kimura, S.; Ohya, H.; Murayama, Y.; Kikuchi, K.; Hirai, M.; Toyoda, M.; Sonoda, T.; Setogawa, S. Five years operating experience of a 800 cubic meters per day R.O. seawater desalination plant. Desalination 1985, 54, 45–54. [Google Scholar] [CrossRef]
- Taniguchi, Y. An overview of pretreatment technology for reverse osmosis desalination plants in Japan. Desalination 1997, 110, 21–35. [Google Scholar] [CrossRef]
- Heyden, W. Seawater desalination by reverse osmosis: Plant design, performance data, operation and maintenance (Tanajib, Arabian Gulf Coast). Desalination 1985, 52, 187–199. [Google Scholar] [CrossRef]
- Arrayedh, M.A.; Ericsson, B.; Saad, M.A.; Yoshioka, H. Reverse osmosis desalination plant, RA’s Abu Jarjur, State of Bahrain—Two years operational experience for the 46,000 m3/day RO plant. Desalination 1987, 65, 197–230. [Google Scholar] [CrossRef]
- Alawadhi, A.A.; Hussain, A.R. Design Optimisation and Production Enhancement at Ras Abu Jarjur R.O. Desalination Plant. In Proceedings of the IDA World Congress, Maspalomas, Gran Canaria, Spain 21–26 October 2007. IDAWC/MP07-253. [Google Scholar]
- Hafsi, M. Analysis of Boujdour desalination plant performance. Desalination 2001, 134, 93–104. [Google Scholar] [CrossRef]
- Kurihara, M.; Kuniyoshi, C.; Yamazato, T.; Takeuchi, H. Japan: 10 Years of Experience in Okinawa Seawater Desalination Plant. In Proceedings of the 2006 AWWA Desalination symposium, Honolulu, HI, USA, 7–9 May 2006. [Google Scholar]
- Kimura, T.; Ito, Y.; Nakaoki, Y. Innovative Biofouling Prevention on Seawater Desalination Reverse Osmosis Membrane. In Proceedings of the IDA World Congress on Desalination and Water Reuse, Manama, Bahrain, 8–13 March 2002. [Google Scholar]
- Sunil, W.; Liu, Y.; Chai, S.; Han, L. Biofouling Control for Reverse Osmosis and Nanofiltration Membranes. In Proceedings of the IDA World Congress, Dubai, United Arab Emirates, October 20–24 2019. IDAWC19-Liu. [Google Scholar]
- Lu, Y.; Komori, H.; Hayakawa, K.; Ho, J.; Lee, J.; Sim, L.; Chong, T. New Dosing Strategies of the Stabilized Chlorine Biofouling Control Agent in Seawater Reverse Osmosis (SWRO). In Proceedings of the IDA World Congress, Dubai, United Arab Emirates, October 20–24 2019. IDAWC19- Lu. [Google Scholar]
- Fujioka, T.; Ngo, M.T.; Boivin, S.; Kawahara, K.; Takada, A.; Nakamura, Y.; Yoshikawa, H. Controlling biofouling and disinfection by-product formation during reverse osmosis treatment for seawater desalination. Desalination 2020, 488, 114507. [Google Scholar] [CrossRef]
- Parise, P.L.; Parekh, B.S.; Smith, R. Development of Sanitary Design Reverse-Osmosis Systems for the Pharmaceutical Industry. In Reverse Osmosis and Ultrafiltration; Sourirajan, S., Matsuura, T., Eds.; American Chemical Society: Washington, DC, USA, 1985; pp. 297–312. [Google Scholar]
- McDonough, F.E.; Hargrove, R.E. Sanitation of Reverse Osmosis/Ultrafiltration Equipment. J. Milk Food Technol. 1972, 35, 102–106. [Google Scholar] [CrossRef]
- Ahmed, S.; Alansari, M.; Kannari, T. Biological fouling and control at Ras Abu Jarbur RO plant—A new approach. Desalination 1989, 74, 69–84. [Google Scholar] [CrossRef]
- Alawadhi, A.A.; Ericsson, B. Improving the Long-term Reliability of a 10 MIGD R.O. Desalination Plant, Ras Abu Jarjur, Bahrain, by installing an Additional RO Train—Ten Years Operational Experience. In Proceedings of the IDA World Congress on Desalination and Water Reuse, Bahrain, Madrid, Spain, 6–9 October 1997; Volume II, pp. 197–214. [Google Scholar]
- Byrne, W. Reverse Osmosis: A Practical Guide for Industrial Users, 1st ed.; Tall Oaks Publishing, Inc.: Littleton, CO, USA, 1995; p. 130. [Google Scholar]
- Hayashi, Y.; Furusawa, T. Sulfur Scale Cleaning Method. Japanese Unexamined Patent Application Publication No. JPH04141299A, 14 May 1992. [Google Scholar]
- Smith, B.M.; Whipple, S.S. Reducing Boiler Feedwater Demands with Reverse Osmosis. In Proceedings of the TAPPI Engineering Conference, Boston, MA, USA, 17 September 1992. [Google Scholar]
- Reiss, C.R.; Talton, E.; Schuck, R. Ultrafiltration-nanofiltration for removal of hydrogen sulfide, elemental sulfur, and sulfate from a municipal groundwater. Water Sci. Technol. Water Supply 2003, 3, 329–335. [Google Scholar] [CrossRef]
- Hydranautics. Foulants and Cleaning Procedures for Composite Polyamide RO/NF Membrane Elements; Technical Service Bulletin 107; Hydranautics: Oceanside, CA, USA, 2020. [Google Scholar]
- Shimizu, K. Membrane Washing Method. Japanese Unexamined Patent Application Publication No. JP2006305444A, 9 November 2006. [Google Scholar]
- Beyer, F.; Laurinonyte, J.; Zwijnenburg, A.; Stams, A.J.; Plugge, C.M. Membrane Fouling and Chemical Cleaning in Three Full-Scale Reverse Osmosis Plants Producing Demineralized Water. J. Eng. 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Yamashiro, Y.; Goto, T. Experience of ten years running the Okinawa plant. Desalination Water Reuse 2009, 18, 14–20. [Google Scholar]
- Yamasato, T. Operation status at the Okinawa Prefecture Seawater Desalination Center. The 21st New Membrane Technology Symposium. In Proceedings of the Membrane Society of Japan and Japan Management Association, Tokyo, Japan, 26–29 October 2009; pp. 3–11. (In Japanese). [Google Scholar]
- Ebrahim, S. Cleaning and regeneration of membranes in desalination and wastewater applications: State-of-the-art. Desalination 1994, 96, 225–238. [Google Scholar] [CrossRef]
- Tsuruguchi, H.; Hitotsuyanagi, N. Water Treatment Method Using Reverse Osmosis Membrane. Japanese Unexamined Patent Application Publication No. JP2004025027A, 29 January 2004. [Google Scholar]
- Yamamoto, K.; Kawanishi, S. Site-specific DNA damage induced by hydrazine in the presence of manganese and copper ions. The role of hydroxyl radical and hydrogen atom. J. Biol. Chem. 1991, 266, 1509–1515. [Google Scholar] [CrossRef]
- Nagai, M.; Iwahashi, H.; Hayashi, Y.; Ogino, Y. The behavior of an oxidizing/reducing agent in seawater. Desalination 1994, 96, 291–301. [Google Scholar] [CrossRef]
- Harumiya, N.; Kurihara, M.; Uemura, T. Separation of Liquid by Semipermeable Composite Membrane. Japanese Unexamined Patent Application Publication No. JPS5621604A, 28 February 1981. [Google Scholar]
- Light, W.G.; Perlman, J.L.; Riedinger, A.B.; Needham, D.F. Desalination of non-chlorinated surface seawater using TFCR membrane elements. Desalination 1988, 70, 47–64. [Google Scholar] [CrossRef]
- Tsuchida, H.; Inoue, S. RO desalination facilities in solitary islands of Okinawa. J. Water Re-Use Technol. 1994, 20, 58–63. (In Japanese) [Google Scholar]
- Kawada, I.; Nagura, K. Operation of the reverse osmosis seawater desalination demonstration plant in Okinawa. J. Water Re-Use Technol. 1995, 21, 53–58. (In Japanese) [Google Scholar]
- Kojima, Y.; Kusakabe, Y.; Hatakeyama, S. Development of a Reverse-osmosis Seawater Desalination System. Ebara Eng. Rev. 1996, 173, 33–42. (In Japanese) [Google Scholar]
- Talavera, J.L.P. Recent Operating Methods of Reverse Osmosis Plants: A New Approach. In Proceedings of the JDA Forum 2011, Tokyo, Japan, 16 February 2011. [Google Scholar]
- Nada, N.; Ito, Y.; Maeda, Y.; Tokunaga, K.; Iwahashi, H. Large SWRO Project for Drinking Water in Shuqaiq. In Proceedings of the IDA World Congress, Perth, Australia, 4–9 September 2011. IDAWC/PER11-189. [Google Scholar]
- Fusaoka, Y.; Okada, K.; Takeuchi, H. Treatment of Reverse Osmosis Membrane Separation Apparatus. Japanese Unexamined Patent Application Publication No. JPH07328392A, 19 December 1995. [Google Scholar]
- Kajio, S.; Someya, Y.; Okada, T.; Murakami, Y.; Shimozaka, K. Desalting Treatment Using Reverse Osmotic Membrane, Device Therefor and Operation of the Device. Japanese Unexamined Patent Application Publication No. JPH09290259A, 11 November 1997. [Google Scholar]
- Hu, L.C.C.; Maeda, Y. Integrated Membrane-Based Reclamation System in a Major Petrochemical Plant—3 Years Experience in Taiwan. In Proceedings of the IDA World Congress, Bahamas, 28 September–3 October 2003; pp. BAH3–BAH83. [Google Scholar]
- Iwahori, H.; Ando, M.; Ishihara, S.; Tada, N.; Nishida, Y. Risk Management of RO Seawater Desalination Projects by Membrane Supplier. In Proceedings of the IDA World Congress, Maspalomas, Gran Canaria, Spain 21–26 October 2007. IDAWC/MP07-062. [Google Scholar]
- Brandt, C.; Eldik, R. Transition Metal-Catalyzed Oxidation of Sulfur (IV) Oxides. Atmospheric-Relevant Processes and Mechanisms. Chem. Rev. 1995, 95, 119–190. [Google Scholar] [CrossRef]
- Neta, P.; Huie, R.E. Free-radical chemistry of sulfite. Environ. Health Perspect. 1985, 64, 209–217. [Google Scholar] [CrossRef]
- Kuo, D.T.; Kirk, D.W.; Jia, C. The chemistry of aqueous S(IV)-Fe-O2 system: State of the art. J. Sulfur Chem. 2006, 27, 461–530. [Google Scholar] [CrossRef]
- Inoue, M.; Hayatsu, H.; Tanooka, H. Concentration effect of bisulfite on the inactivation of transforming activity of DNA. Chem. -Biol. Interact. 1972, 5, 85–95. [Google Scholar] [CrossRef]
- Hayatsu, H.; Miller, R. The cleavage of DNA by the oxygen-dependent reaction of bisulfite. Biochem. Biophys. Res. Commun. 1972, 46, 120–124. [Google Scholar] [CrossRef]
- Hayatsu, H. Bisulfite modification of nucleic acids and their constituents. Prog. Nucleic Acid Res. Mol. Biol. 1976, 16, 75–124. [Google Scholar]
- Shapiro, R.H. Genetic effects of bisulfite (sulfur dioxide). Mutat. Res. 1977, 39, 149–175. [Google Scholar] [CrossRef]
- Gunnison, A.F. Sulphite toxicity: A critical review of in vitro and in vivo data. Food Cosmet. Toxicol. 1981, 19, 667–682. [Google Scholar] [CrossRef]
- Kawanishi, S.; Yamamoto, K.; Inoue, S. Site-specific DNA damage induced by sulfite in the presence of cobalt (II) ion. Role of sulfate radical. Biochem. Pharmacol. 1989, 38, 3491–3496. [Google Scholar] [PubMed]
- Shi, X.; Mao, Y. 8-Hydroxy-2′-deoxyguanosine formation and DNA damage induced by sulfur trioxide anion radicals. Biochem. Biophys. Res. Commun. 1994, 205, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.G.; Hickerson, R.P.; Perez, R.J.; Burrows, C.J. DNA Damage from Sulfite Autoxidation Catalyzed by a Nickel (II) Peptide. J. Am. Chem. Soc. 1997, 119, 1501–1506. [Google Scholar] [CrossRef]
- Muller, J.G.; Burrows, C.J. Metallodrug complexes that mediate DNA and lipid damage via sulfite autoxidation: Copper (II) famotidine and iron (III) bis (salicyglycine). Inorg. Chim. Acta 1998, 275–276, 314–319. [Google Scholar] [CrossRef]
- Lepentsiotis, V.; Domagała, J.; Grgić, I.; van Eldik, R.; Muller, J.G.; Burrows, C.J. Mechanistic Information on the Redox Cycling of Nickel(II/III) Complexes in the Presence of Sulfur Oxides and Oxygen. Correlation with DNA Damage Experiments. Inorg. Chem. 1999, 38, 3500–3505. [Google Scholar] [CrossRef]
- Jameton, R.A.; Muller, J.G.; Burrows, C.J. Oxidative DNA damage from sulfite autoxidation catalyzed by manganese (III). Comptes Rendus Chim. 2002, 5, 461–466. [Google Scholar] [CrossRef]
- Moreno, R.; Alipázaga, M.V.; Medeiros, M.H.; Coichev, N. DNA damage induced by sulfite autoxidation catalyzed by copper (II) tetraglycine complexes. Dalton Trans. 2005, 6, 1101–1107. [Google Scholar] [CrossRef]
- Guo, D.; Yuan, X.; Liang, J. Influence of Cu (II) on the interaction of sulfite with DNA. Spectrochimica acta. Part A Mol. Biomol. Spectrosc. 2006, 65, 459–462. [Google Scholar] [CrossRef]
- Alipázaga, M.V.; Moreno, R.; Linares, E.; Medeiros, M.H.; Coichev, N. DNA damage by sulfite autoxidation catalyzed by cobalt complexes. Dalton Trans. 2008, 41, 5636–5644. [Google Scholar] [CrossRef] [PubMed]
- Alipázaga, M.V.; Cerchiaro, G.; Moyac, H.D.; Coichev, N. Oxidative DNA Damage Induced by S (IV) in the Presence of Cu (II) and Cu(I) Complexes. J. Braz. Chem. Soc. 2009, 20, 1302–1312. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Heydari-Bafrooei, E.; Rezaei, B. DNA-based biosensor for comparative study of catalytic effect of transition metals on autoxidation of sulfite. Anal. Chem. 2013, 85, 991–997. [Google Scholar] [CrossRef]
- Cheh, A.M.; Skochdopole, J.; Koski, P.; Cole, L.D. Nonvolatile mutagens in drinking water: Production by chlorination and destruction by sulfite. Science 1980, 207, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Rochelle, G.T. Oxidative degradation of organic acid conjugated with sulfite oxidation in flue gas desulfurization: Products, kinetics, and mechanism. Environ. Sci. Technol. 1987, 21, 266–272. [Google Scholar] [CrossRef]
- Croué, J.P.; Reckhow, D.A. Destruction of chlorination byproducts with sulfite. Environ. Sci. Technol. 1989, 23, 1412–1419. [Google Scholar] [CrossRef]
- Kulkarni, U.; Dixit, S.G. Destruction of phenol from wastewater by oxidation with sulfite-oxygen. Ind. Eng. Chem. Res. 1991, 30, 1916–1920. [Google Scholar] [CrossRef]
- Pasiuk-Bronikowska, W.; Bronikowski, T.; Ulejczyk, M. Solubilization of organics in water coupled with sulphite autoxidation. Water Res. 1997, 31, 1767–1775. [Google Scholar] [CrossRef]
- Liang, C.; Wang, Z.; Bruell, C.J. Influence of pH on persulfate oxidation of TCE at ambient temperatures. Chemosphere 2007, 66, 106–113. [Google Scholar] [CrossRef]
- Chen, L.; Peng, X.; Liu, J.; Li, J.; Wu, F. Decolorization of Orange II in Aqueous Solution by an Fe (II)/sulfite System: Replacement of Persulfate. Ind. Eng. Chem. Res. 2012, 51, 13632–13638. [Google Scholar] [CrossRef]
- Zhou, D.; Yuan, Y.; Yang, S.; Gao, H.; Chen, L. Roles of oxysulfur radicals in the oxidation of acid orange 7 in the Fe (III)–sulfite system. J. Sulfur Chem. 2015, 36, 373–384. [Google Scholar] [CrossRef]
- Sun, B.; Guan, X.; Fang, J.; Tratnyek, P.G. Activation of Manganese Oxidants with Bisulfite for Enhanced Oxidation of Organic Contaminants: The Involvement of Mn (III). Environ. Sci. Technol. 2015, 49, 12414–12421. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Liu, Y.; Zheng, J.; Tan, M.; Wang, Z.; Wu, M. Synergetic Transformations of Multiple Pollutants Driven by Cr(VI)-Sulfite Reactions. Environ. Sci. Technol. 2015, 49, 12363–12371. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Yang, S.; Zhou, D.; Wu, F. A simple Cr(VI)-S(IV)-O2 system for rapid and simultaneous reduction of Cr(VI) and oxidative degradation of organic pollutants. J. Hazard. Mater. 2016, 307, 294–301. [Google Scholar] [CrossRef]
- Yu, Y.; Li, S.; Peng, X.; Yang, S.; Zhu, Y.; Chen, L.; Wu, F.; Mailhot, G. Efficient oxidation of bisphenol A with oxysulfur radicals generated by iron-catalyzed autoxidation of sulfite at circumneutral pH under UV irradiation. Environ. Chem. Lett. 2016, 14, 527–532. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, S.; Yuan, Y.; Xu, J.; Zhu, Y.; Li, J.; Wu, F. A novel heterogeneous system for sulfate radical generation through sulfite activation on a CoFe2O4 nanocatalyst surface. J. Hazard. Mater. 2017, 324, 583–592. [Google Scholar] [CrossRef]
- Xie, P.; Guo, Y.; Chen, Y.; Wang, Z.; Shang, R.; Wang, S.; Ding, J.; Wan, Y.; Jiang, W.; Ma, J. Application of a novel advanced oxidation process using sulfite and zero-valent iron in treatment of organic pollutants. Chem. Eng. J. 2017, 314, 240–248. [Google Scholar] [CrossRef]
- Sun, S.; Pang, S.; Jiang, J.; Ma, J.; Huang, Z.; Zhang, J.; Liu, Y.; Xu, C.; Qingliang, L.; Yuan, Y. The combination of ferrate (VI) and sulfite as a novel advanced oxidation process for enhanced degradation of organic contaminants. Chem. Eng. J. 2018, 333, 11–19. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, D.; Li, J.; Wu, F.; Brigante, M.; Mailhot, G. Rapid oxidation of paracetamol by Cobalt (II) catalyzed sulfite at alkaline pH. Catal. Today 2018, 313, 155–160. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, J.; Song, H.; Sun, S.; Zhang, Z.; Yang, T. Organic contaminants degradation from the S (IV) autoxidation process catalyzed by ferrous-manganous ions: A noticeable Mn(III) oxidation process. Water Res. 2018, 133, 227–235. [Google Scholar] [CrossRef]
- Zhou, D.; Chen, L.; Chen, L.; Li, J.; Wu, F. Transition metal catalyzed sulfite auto-oxidation systems for oxidative decontamination in waters: A state-of-the-art minireview. Chem. Eng. J. 2018, 346, 726–738. [Google Scholar] [CrossRef]
- Wang, J.; Teng, Y.; Zhang, C.; Liao, X.; Zhai, Y.; Zuo, R. Activation of manganese dioxide with bisulfite for enhanced abiotic degradation of typical organophosphorus pesticides: Kinetics and transformation pathway. Chemosphere 2019, 226, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Luo, T.; Xu, J.; Li, J.; Wu, F.; Brigante, M.; Mailhot, G. Enhanced oxidation of aniline using Fe (III)-S(IV) system: Role of different oxysulfur radicals. Chem. Eng. J. 2019, 362, 183–189. [Google Scholar] [CrossRef]
- Zhang, H.; Guan, W.; Zhang, L.; Guan, X.; Wang, S. Degradation of an Organic Dye by Bisulfite Catalytically Activated with Iron Manganese Oxides: The Role of Superoxide Radicals. ACS Omega 2020, 5, 18007–18012. [Google Scholar] [CrossRef] [PubMed]
- Backstrom, H. The chain mechanism in the autooxidation of sodium sulfite solutions. Z. Phys. Chem. 1934, B25, 99–122. [Google Scholar]
- Erben-Russ, M.; Bors, W.; Winter, R.; Saran, M. The reaction of sulfite anion radical (SO-3) with polyunsaturated fatty acids. Int. J. Radiat. Appl. Instrumentation. Part C. Radiat. Phys. Chem. 1986, 27, 419–424. [Google Scholar] [CrossRef]
- Pagano, D.A.; Zeiger, E.; Stark, A.A. Autoxidation and mutagenicity of sodium bisulfite. Mutat. Res. 1990, 228, 89–96. [Google Scholar] [CrossRef]
- Das, T.N. Reactivity and Role of SO5•-Radical in Aqueous Medium Chain Oxidation of Sulfite to Sulfate and Atmospheric Sulfuric Acid Generation. J. Phys. Chem. A 2001, 105, 9142–9155. [Google Scholar] [CrossRef]
- Shi, X.; Dalal, N.S. ESR spin trapping evidence for SO-3 and OH radicals in sulfite oxidation. Res. Chem. Intermed. 1990, 13, 103–115. [Google Scholar] [CrossRef]
- Liang, C.; Su, H. Identification of Sulfate and Hydroxyl Radicals in Thermally Activated Persulfate. Ind. Eng. Chem. Res. 2009, 48, 5558–5562. [Google Scholar] [CrossRef]
- Sugawara, Y.; Kenji Saito, K.; Takahata, H. Preparing Method of Water Treatment Supply Water, and Fresh Water Producing Method. Japanese Unexamined Patent Application Publication No. JP2020049418A, 2 April 2020. [Google Scholar]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Huie, R.E.; Neta, P. Chemical behavior of SO3- and SO5- radicals in aqueous solutions. J. Phys. Chem. 1984, 88, 5665–5669. [Google Scholar] [CrossRef]
- Kawada, I.; Nagura, K.; lwahori, H.; Yoshiyasu Kamiyama, Y. Generation of Certain Oxidizing Agents by SBS and Their Inhibition With High Copper Concentrations in Sea Water Desalination Systems. In Proceedings of the IDA World Congress on Desalination and Water Sciences, Abu Dhabi, United Arab Emirates, 18–24 November 1995; Volume VI, pp. 381–390. [Google Scholar]
- Fusaoka, Y.; Okada, K.; Takeuchi, H. Separation with Reverse Osmotic Membrane and Apparatus Therefor. Japanese Unexamined Patent Application Publication No. JPH0957067A, 4 March 1997. [Google Scholar]
- Tsuge, H.; Noshita, M.; Takesaka., K. Seawater Desalination Method and Seawater Desalination Apparatus. Unexamined Patent Application Publication No. JP2005246282A, 15 September 2005. [Google Scholar]
- Hirokawa, M. Membrane Separation Process and Membrane Separation Apparatus. Unexamined Patent Application Publication No. JP2008029965A, 14 February 2008. [Google Scholar]
- Ferrer, O.; Gibert, O.; Cortina, J.L. Reverse osmosis membrane composition, structure and performance modification by bisulphite, iron(III), bromide and chlorite exposure. Water Res. 2016, 103, 256–263. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ando, M.; Ishihara, S. Method of Multi-Stage Reverse Osmosis Treatment. U.S. Patent Application Publication No. US20040050793A1, 18 March 2004. [Google Scholar]
- Nagura, K.; Kawada, I. Seawater Pretreatment Process for Seawater Desalination by Reverse Osmosis Membrane Module. Unexamined Patent Application Publication No. JPH07308671A, 28 November 1995. [Google Scholar]
- Taniguchi, M.; Lagref, J.J.; Kallenberg, K. Water Production Method. International Publication Patent No. WO2016084905A1, 2 June 2016. [Google Scholar]
- Takeuchi, H.; Okada, K.; Fusaoka, Y. Operation of Reverse Osmosis Membrane Device. Unexamined Patent Application Publication No. JPH0957076A, 4 March 1997. [Google Scholar]
- Sugita, K. Reverse Osmosis Membrane Separation Method and Reverse Osmosis Membrane Separation Apparatus. Unexamined Patent Application Publication No. JP2007090288A, 12 April 2007. [Google Scholar]
- Ando, M.; Ishihara, S. Multi-Stage Type Reverse Osmosis Treating Method. Unexamined Patent Application Publication No. JP2003071252A, 11 April 2003. [Google Scholar]
- Ito, Y.; Nagai, M.; Tokunaga, K.; Iwahashi, H. Reverse Osmosis Treatment Method and Reverse Osmosis Treatment Apparatus. Unexamined Patent Application Publication No. JP2013052333A, 21 March 2013. [Google Scholar]
- Fowles, E.H.; Gilbert, B.C.; Giles, M.R.; Whitwood, A.C. The effects of chelating agents on radical generation in alkaline peroxide systems, and the relevance to substrate damage. Free. Radic. Res. 2007, 41, 515–522. [Google Scholar] [CrossRef]
- Rochelle, G.T.; Owens, D.R.; Chang, J.C.; Bma, T.G. Thiosulfate as an Oxidation Inhibitor in Flue Gas Desulfurization Processes: A Review of R&D Results. J. Air Pollut. Control. Assoc. 1986, 36, 1138–1146. [Google Scholar]
- Flemming, H.C.; Schaule, G.; Griebe, T.; Schmitt, J.; Tamachkiarowa, A. Biofouling—The Achilles heel of membrane processes. Desalination 1997, 113, 215–225. [Google Scholar] [CrossRef]
- Dudley, L.Y.; Annunziata, U.A.; Robinson, J.S.; Latham, L.J. Practical Studies to Investigate Microbiological Fouling in RO Plant. In Proceedings of the IDA World Congress on Desalination and Water Science, Abu Dhabi, United Arab Emirates, 18–24 November 1995. [Google Scholar]
- Dudley, L.Y.; Christopher, N. Practical experiences of biofouling in reverse osmosis systems. Spec. Publ. R. Soc. Chem. 1999, 242, 101–108. [Google Scholar]
- Vrouwenvelder, J.S.; Kooij, D.V. Diagnosis, prediction and prevention of biofouling of NF and RO membranes. Desalination 2001, 139, 65–71. [Google Scholar] [CrossRef]
- Mansouri, J.; Harrisson, S.; Chen, V. Strategies for controlling biofouling in membrane filtration systems: Challenges and opportunities. J. Mater. Chem. 2010, 20, 4567–4586. [Google Scholar] [CrossRef]
- Vrouwenvelder, J.S.; Kruithof, J.C.; van Loosdrecht, M.C. Integrated approach for biofouling control. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2010, 62, 2477–2490. [Google Scholar] [CrossRef]
- Nguyen, T.; Roddick, F.A.; Fan, L. Biofouling of Water Treatment Membranes: A Review of the Underlying Causes, Monitoring Techniques and Control Measures. Membranes 2012, 2, 804–840. [Google Scholar]
- Arza, A.; Kucera, J. Minimize Biofouling of RO Membranes. Chem. Eng. Prog. 2016, 112, 60–67. [Google Scholar]
- Maddah, H.A.; Chogle, A.M. Biofouling in reverse osmosis: Phenomena, monitoring, controlling and remediation. Appl. Water Sci. 2016, 7, 2637–2651. [Google Scholar] [CrossRef]
- Bucs, S.S.; Farhat, N.M.; Kruithof, J.C.; Picioreanu, C.; Loosdrecht, M.C.; Vrouwenvelder, J.S. Review on strategies for biofouling mitigation in spiral wound membrane systems. Desalination 2018, 434, 189–197. [Google Scholar] [CrossRef]
- Idais, R.H.; Abuhabib, A.A.; Hamzah, S. Recent Advances in Measuring and Controlling Biofouling of Seawater Reverse Osmosis SWRO: A Review. Osmotically Driven Membr. Processes 2021. Available online: https://www.intechopen.com/online-first/75237 (accessed on 26 December 2021). [CrossRef]
- Pichardo-Romero, D.; Garcia-Arce, Z.P.; Zavala-Ramírez, A.; Castro-Muñoz, R. Current advances in biofouling mitigation in membranes for water treatment: An overview. Processes 2020, 8, 182. [Google Scholar] [CrossRef]
- Winters, H.; Isquith, I.R.; Arthur, W.; Mindler, A. Control of biological fouling in seawater reverse osmosis desalination. Desalination 1983, 47, 233–238. [Google Scholar] [CrossRef]
- Winters, H. Biofouling—Its History and How it Affects Today’s Desalination Industry. In Proceedings of the IDA World Congress on Desalination, Abu Dhabi, United Arab Emirates, 18–24 November 1995; Volume I, pp. 255–264. [Google Scholar]
- Winters, H.; Isquith, I. A Critical Evaluation of Pretreatment to Control Fouling in Open Seawater Reverse Osmosis, Has it been a Success? In Proceedings of the IDA World Congress on Desalination, Abu Dhabi, United Arab Emirates, 18–24 November 1995; Volume I, pp. 321–322. [Google Scholar]
- Khan, M.T. Fouling of Seawater Reverse Osmosis (SWRO) Membrane: Chemical and Microbiological Characterization. Ph.D. Dissertation, King Abdullah University of Science & Technology (KAUST), Thuwal, Saudi Arabia, December 2013. [Google Scholar]
- Ito, Y.; Takahashi, Y.; Hanada, S.; Chiura, H.X.; Ijichi, M.; Iwasaki, W.; Machiyama, A.; Kitade, T.; Tanaka, Y.; Kurihara, M.; et al. Impact of Chemical Addition on the Establishment of Mega-Ton Per Day Sized SWRO Desalination Plant. In Proceedings of the AWA Membranes and Desalination Conference 2013, Brisbane, Australia, 1–4 July 2013. [Google Scholar]
- Alawadhi, A.A. Pretreatment plant design—Key to a successful reverse osmosis desalination plant. Desalination 1997, 110, 1–10. [Google Scholar] [CrossRef]
- Flemming, H.C.; Ridgway, H.F. Biofouling: Open questions, insufficient solutions, innovative approaches. In Proceedings of the EDS Conference and Exhibition on Desalination for the Environment: Clean Water and Energy, Baden-Baden, Germany, 17–20 May 2009. [Google Scholar]
- Hamida, A.B.; Moch, I. Controlling Biological Fouling in Open Sea Intake RO Plants Without Continuous Chlorination. Desalination Water Reuse 1996, 6, 40–45. [Google Scholar]
- Moch, I. Controlling Biofouling in Brackish and Sea Water RO Plants Using Intermittent Chlorine Addition. In Proceedings of the American Desalting Association 1998 North American Biennial Conference & Exposition, Williamsburg, VA, USA, 2–8 August 1998. [Google Scholar]
- Ureña, C.C.; Morales, J.A. Management of Operation and Optimization of Desalination Plants: Ceuta and Melilla Experiences. In Proceedings of the IDA World Congress on Desalination and Water Reuse, Tianjin, China, 20–25 October 2013. IDAWC/TIAN13-084. [Google Scholar]
- Franks, R.N.; Neculau, M.; Bartels, C.C.; Dalmau, M.; Donaque, E.P.; El Filali, A.C.; Miranda, P.; Ferrero, E. Reducing Fouling at Fouka SWRO Plant by Optimizing Chemical and Physical Pretreatment. In Proceedings of the IDA World Congress on Desalination and Water reuse, San Diego, CA, USA, 30 August–4 September 2015. IDA15WC_Franks_51546. [Google Scholar]
- Jacangelo, J.G.; Voutchkov, N.; Badruzzaman, M.; Weinrich, L.A. Pretreatment for Seawater Reverse Osmosis: Existing Plant Performance and Selection Guidance; The Water Resolution Foundation: Denver, CO, USA, 2018. [Google Scholar]
- Badruzzaman, M.; Voutchkov, N.; Weinrich, L.A.; Jacangelo, J.G. Selection of pretreatment technologies for seawater reverse osmosis plants: A review. Desalination 2019, 449, 78–91. [Google Scholar] [CrossRef]
- Sadhwani, J. Operating Costs. Las Palmas III Desalinating Plant. In Proceedings of the IDA World Congress on Desalination and Water Reuse, Madrid, Spain, 6–9 October 1997; Volume 2, pp. 261–293. [Google Scholar]
- Irwin, K.; Ramroop, I. Pretreatment for Seawater Reverse Osmosis: Three Years Operation of the 27.6 MIGD (125,530 m³/Day) Seawater Desalination Plant. Point Lisas, Trinidad and Tobago. In Proceedings of the International Water Conference, Orland, FL, USA, 9–13 October 2005. IWC-05-037. [Google Scholar]
- Marshall, K.C.; Blainey, B.L. Role of Bacterial Adhesion in Biofilm Formation and Biocorrosion. In Biofouling and Biocorrosion in Industrial Water Systems; Springer: Berlin/Heidelberg, Germany, 1991; pp. 29–46. [Google Scholar]
- Lovins, W.A.; Duranceau, S.J.; Taylor, J.S. Aerobic Membrane Feedwater Conditions: Friend or Foe? In Proceedings of the AWWA Membrane Tech. Conference, Phoenix, AZ, USA, 6–9 March 2005. [Google Scholar]
- Bak, F.; Pfennig, N. Chemolithotrophic growth of Desulfovibrio sulfodismutans sp. nov. by disproportionation of inorganic sulfur compounds. Arch. Microbiol. 2004, 147, 184–189. [Google Scholar] [CrossRef]
- Park, H.S.; Chatterjee, I.; Dong, X.; Wang, S.; Sensen, C.W.; Caffrey, S.M.; Jack, T.R.; Boivin, J.; Voordouw, G. Effect of Sodium Bisulfite Injection on the Microbial Community Composition in a Brackish-Water-Transporting Pipeline. Appl. Environ. Microbiol. 2011, 77, 6908–6917. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Chiura, H.X.; Ijichi, M.; Ito, Y.; Hanada, S.; Kogure, K. Chemical Operation Shifts Microbial Community Structure in Seawater Desalination Process. In Proceedings of the IDA World Congress on Desalination and Water Reuse, Tianjin, China, 20–25 October 2013. IDAWC/TIAN13-298. [Google Scholar]
- Khan, M.T.; Busch, M.; Molina, V.; Emwas, A.M.; Aubry, C.; Croué, J.P. How different is the composition of the fouling layer of wastewater reuse and seawater desalination RO membranes? Water Res. 2014, 59, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Iwahashi, H.; Kishi, M.; Goto, T.; Hirai, M.; Al Mohannadi, F.H.; Jolo, K.M. A New Method of Bio-Fouling Prevention for SWRO. In Proceedings of the IDA World Congress, Maspalomas, Gran Canaria, Spain, 21–26 October 2007. IDAWC/MP07-178. [Google Scholar]
- Yamaki, Y. Ion Exchange Equipment and its Operation Examples in Ultrapure Water Systems. In Proceedings of the 20th Ion Exchange Seminar, Tokyo, Japan, 6 July 2007; Available online: http://www.jaie.gr.jp/linkfile/seminar/proceedings_20.pdf (accessed on 5 October 2021). (In Japanese).
- Weinrich, L.A. The Impact of Assimilable Organic Carbon on Biological Fouling of Reverse Osmosis Membranes in Seawater Desalination. Ph.D. Thesis, Drexel University, Philadelphia, PA, USA, February 2015. [Google Scholar]
- Weinrich, L.A.; LeChevallier, M.W.; Haas, C.N. Application of the Bioluminescent Saltwater Assimilable Organic Carbon Test as a Tool for Identifying and Reducing Reverse Osmosis Membrane Fouling in Desalination; Water Reuse Research Foundation: Alexandria, VA, USA, 2015. [Google Scholar]
- Weinrich, L.A.; Lechevallier, M.W.; Haas, C.N. Contribution of assimilable organic carbon to biological fouling in seawater reverse osmosis membrane treatment. Water Res. 2016, 101, 203–213. [Google Scholar] [CrossRef]
- Khan, M.T.; Hong, P.; Nada, N.; Croué, J.P. Does chlorination of seawater reverse osmosis membranes control biofouling? Water Res. 2015, 78, 84–97. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, Y.; Ye, T.; Shi, N.; Tang, F.; Hu, H. Characterization of trihalomethane, haloacetic acid, and haloacetonitrile precursors in a seawater reverse osmosis system. Sci. Total Environ. 2017, 576, 391–397. [Google Scholar] [CrossRef]
- Hirai, M.; Kanno, T.; Goto, T.; Al-Mohannadi, F.H.; Al-Khalaf, J.; Nagai, M.; Iwahashi, H.; Kihara, M.; Kitade, T. SWRO Desalination for High Salinity. In Proceedings of the IDA World Congress, Dubai, United Arab Emirates, 7–12 November 2009. IDAWC/DB09–173. [Google Scholar]
- Kurihara, M.; Takeuchi, H.; Ito, Y. A reliable seawater desalination system based on membrane technology and biotechnology considering reduction of the environmental impact. Environments 2018, 5, 127. [Google Scholar] [CrossRef]
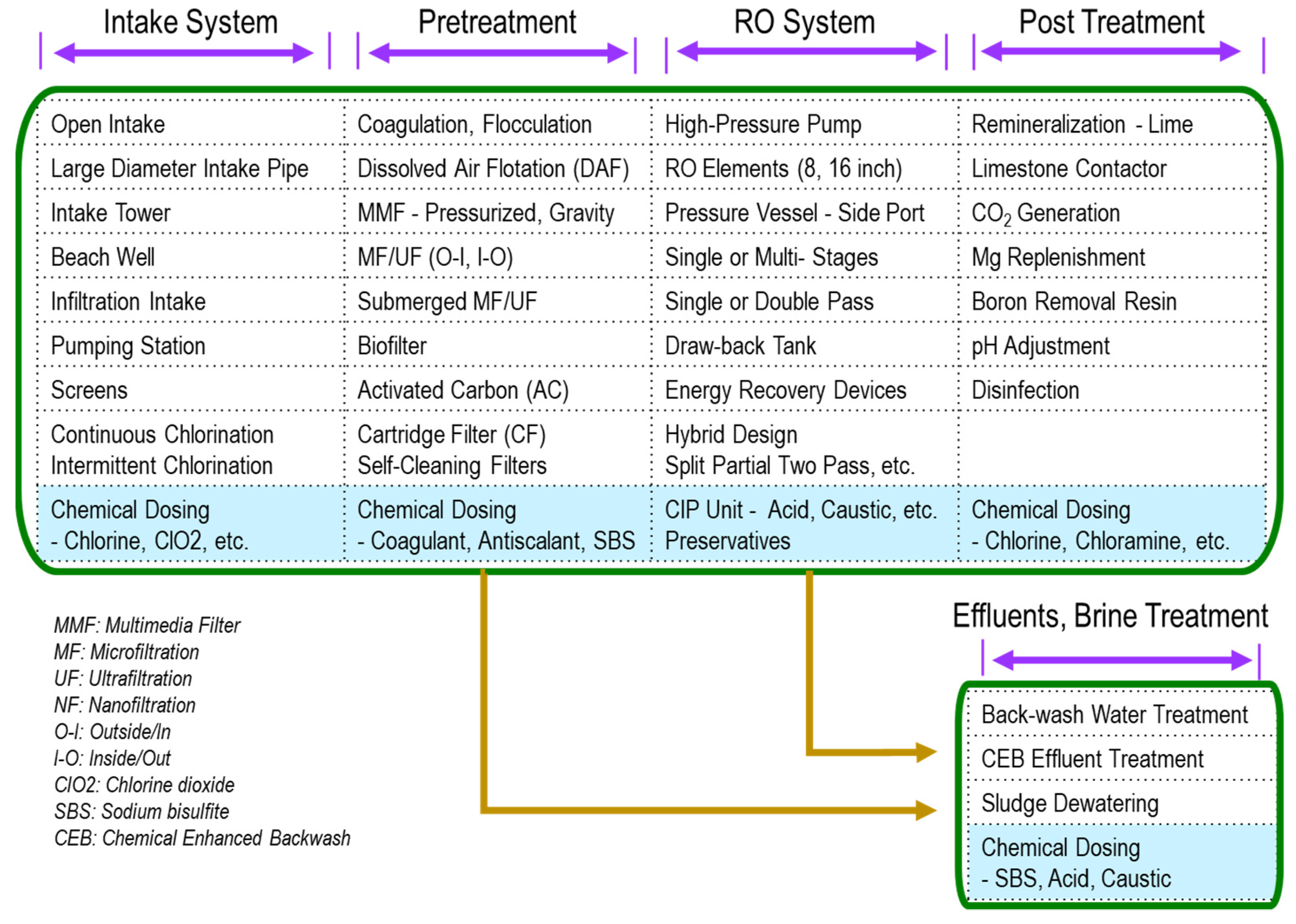
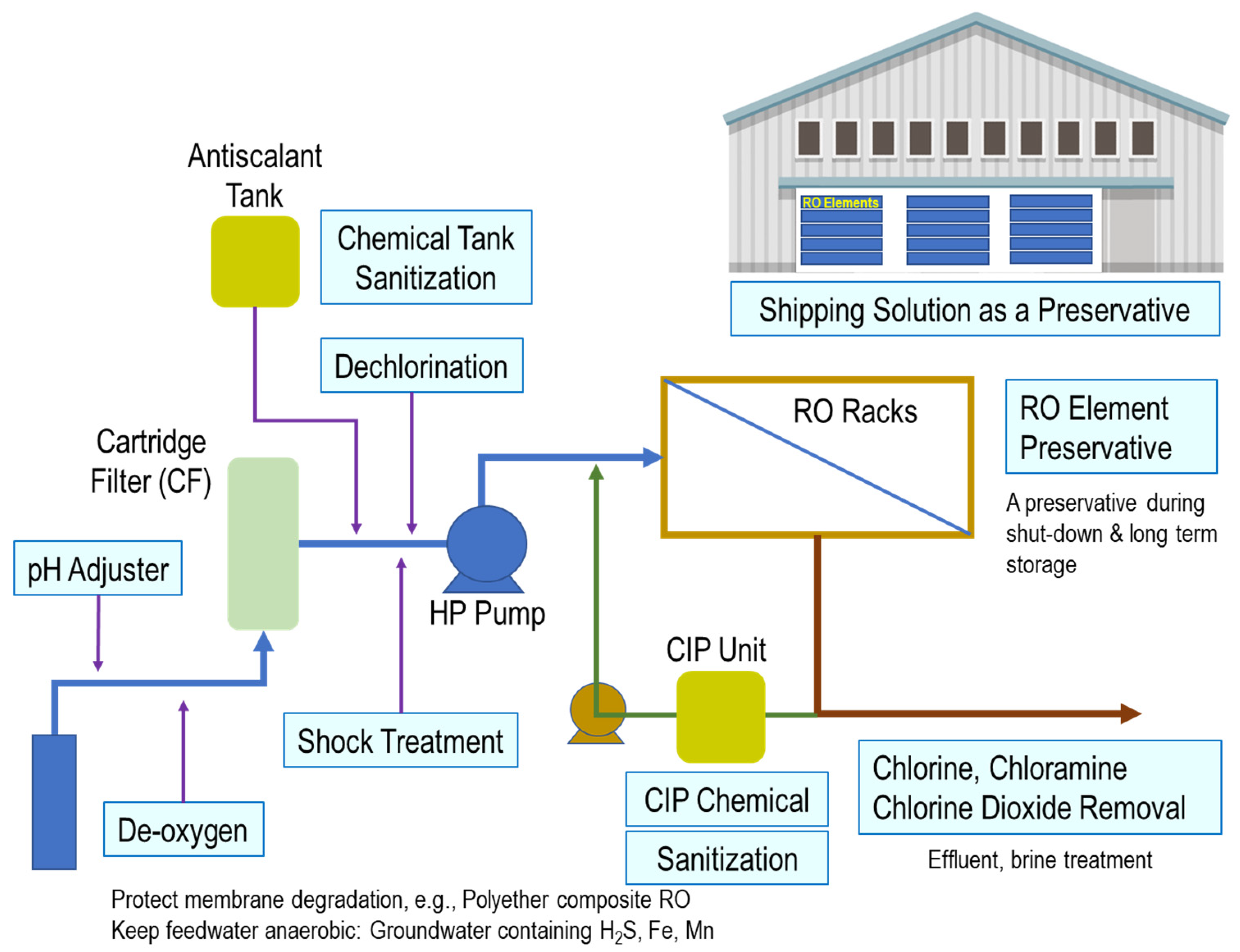
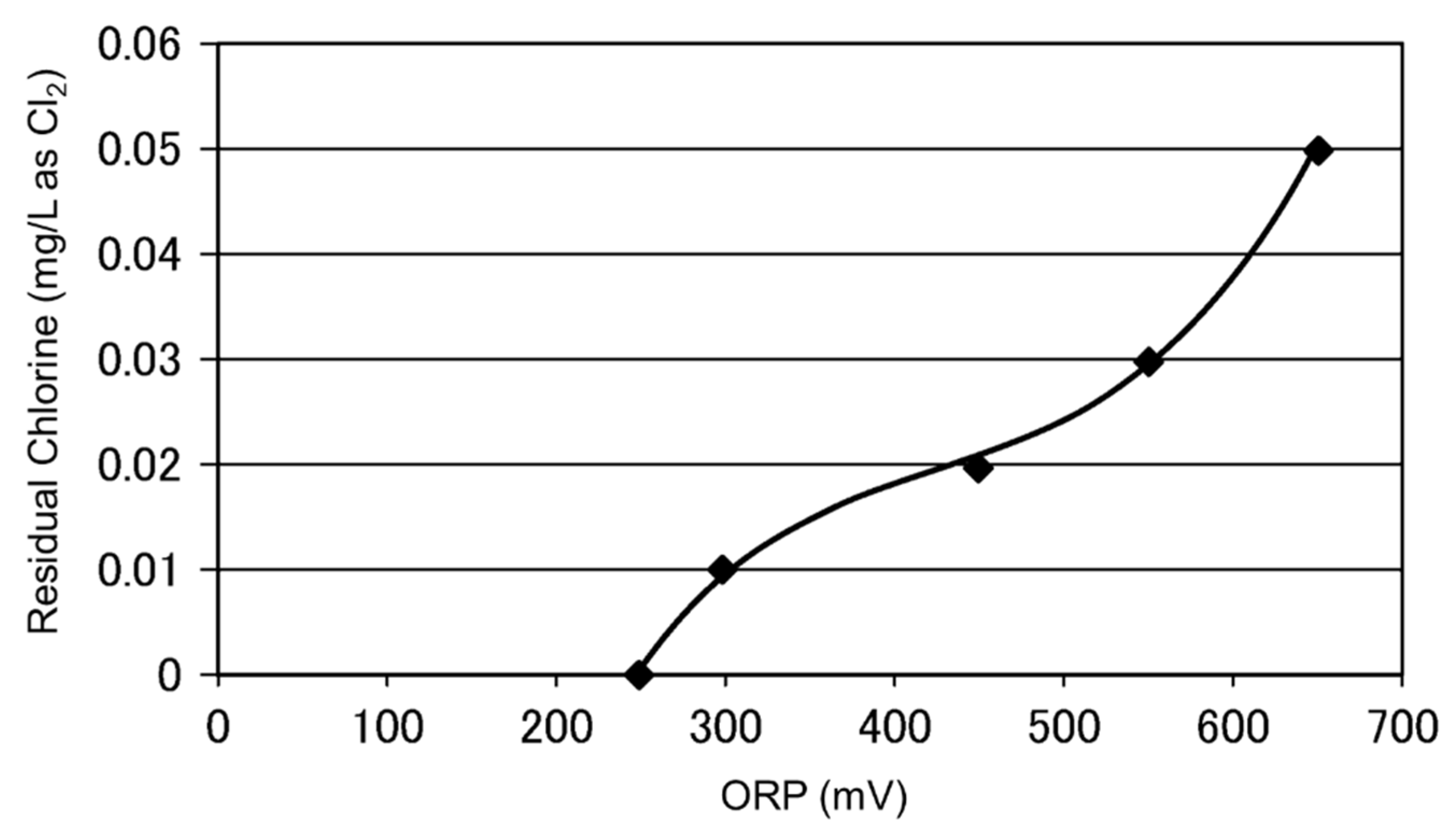
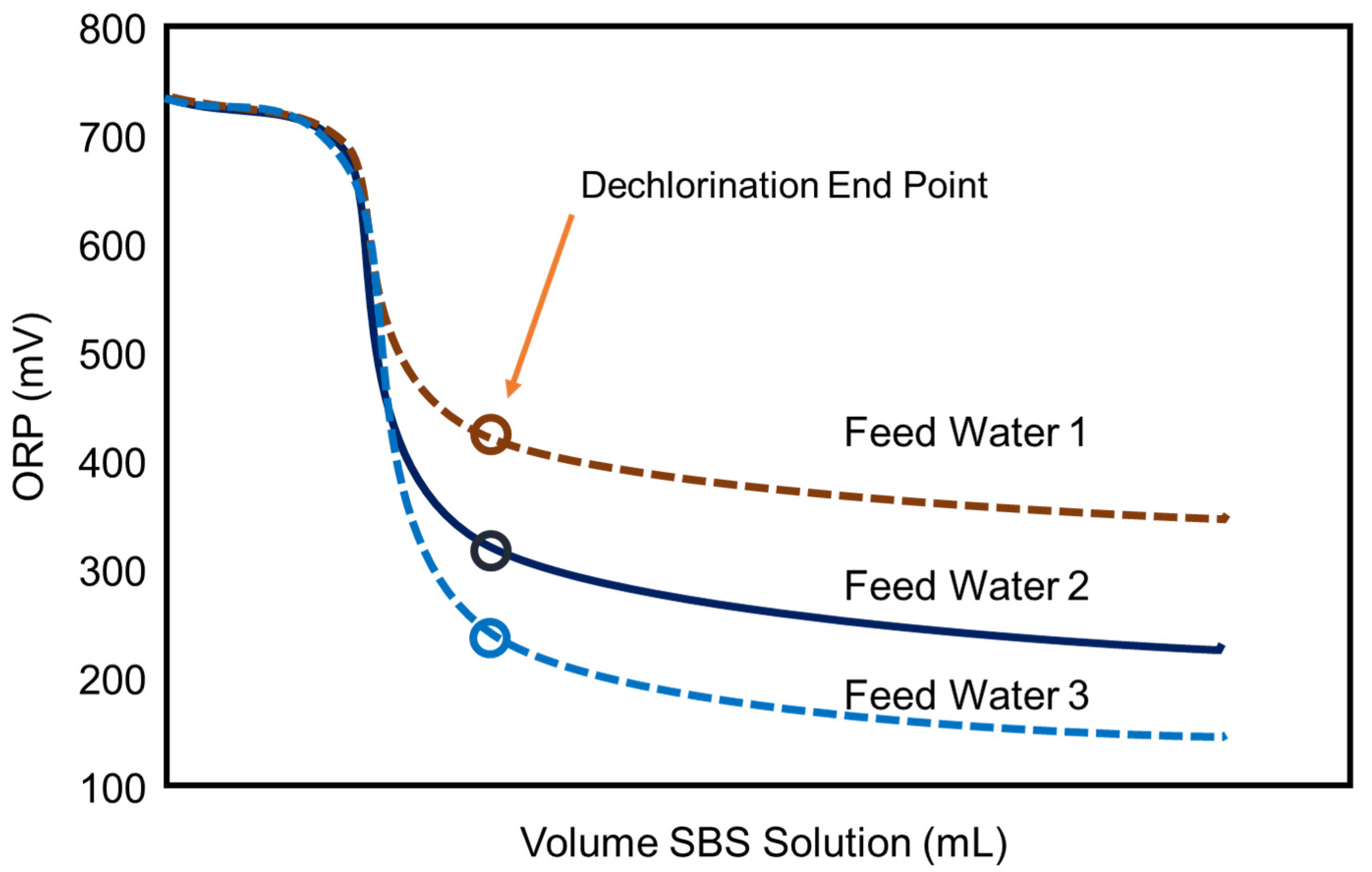

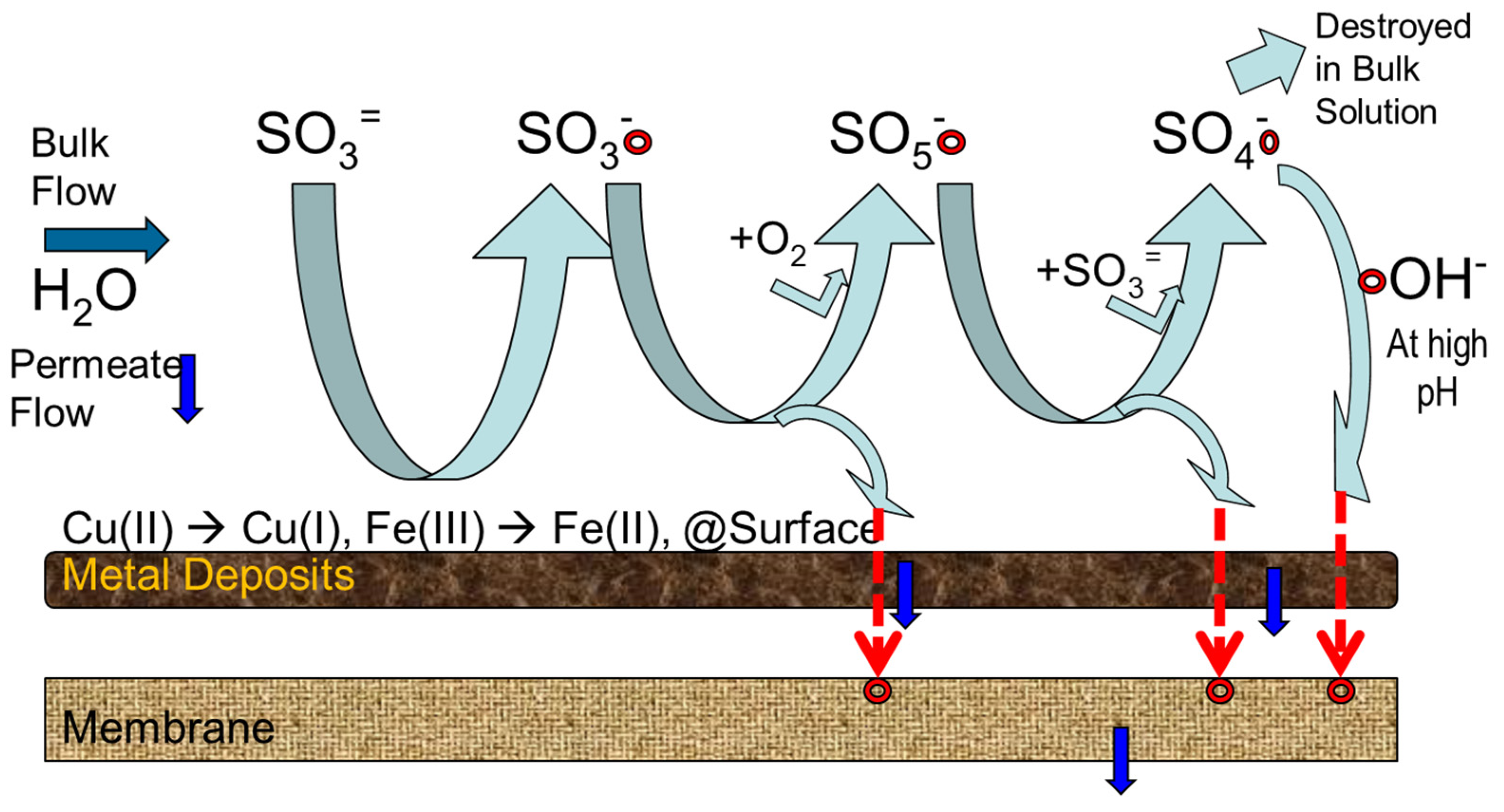
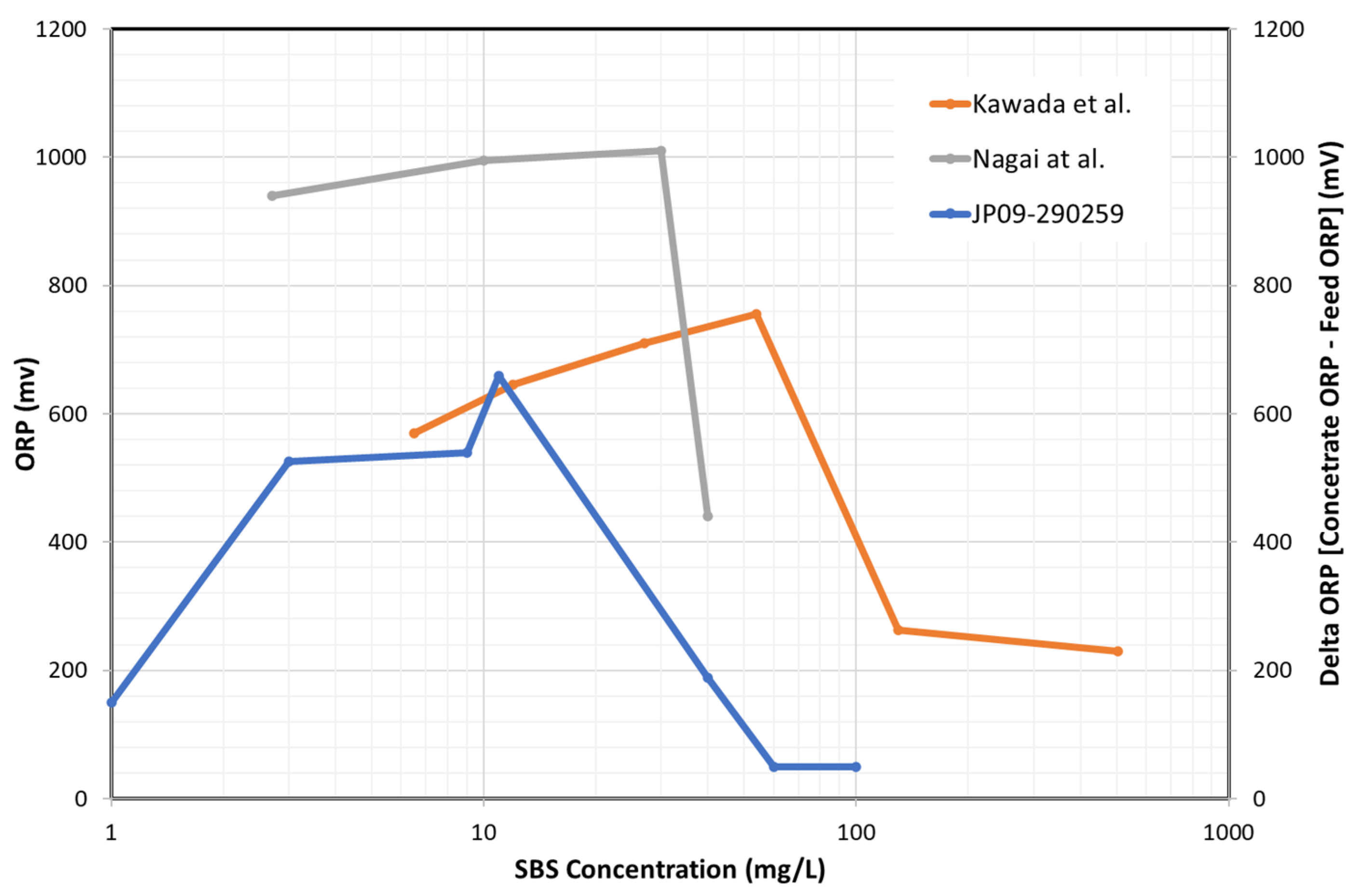
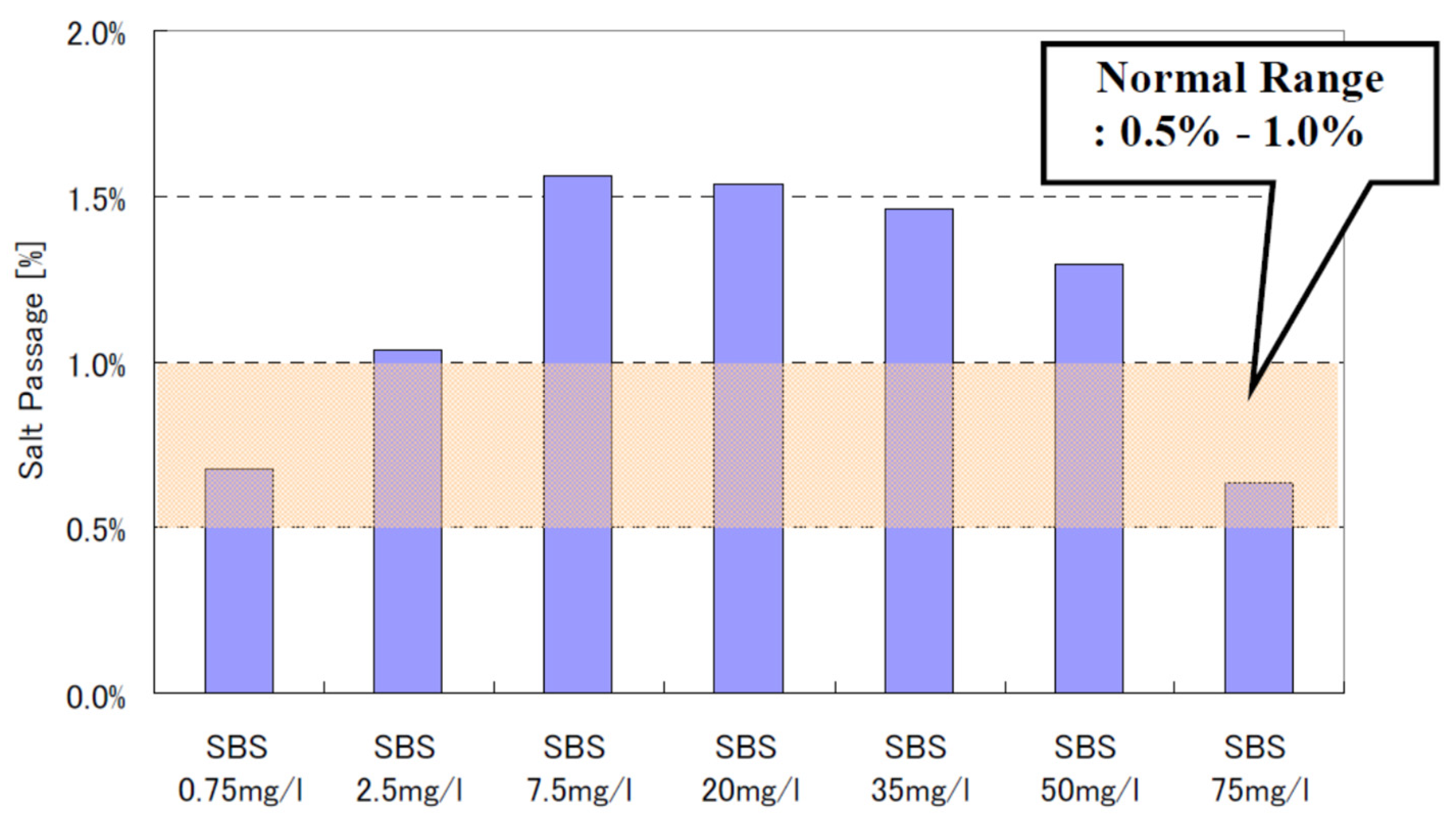
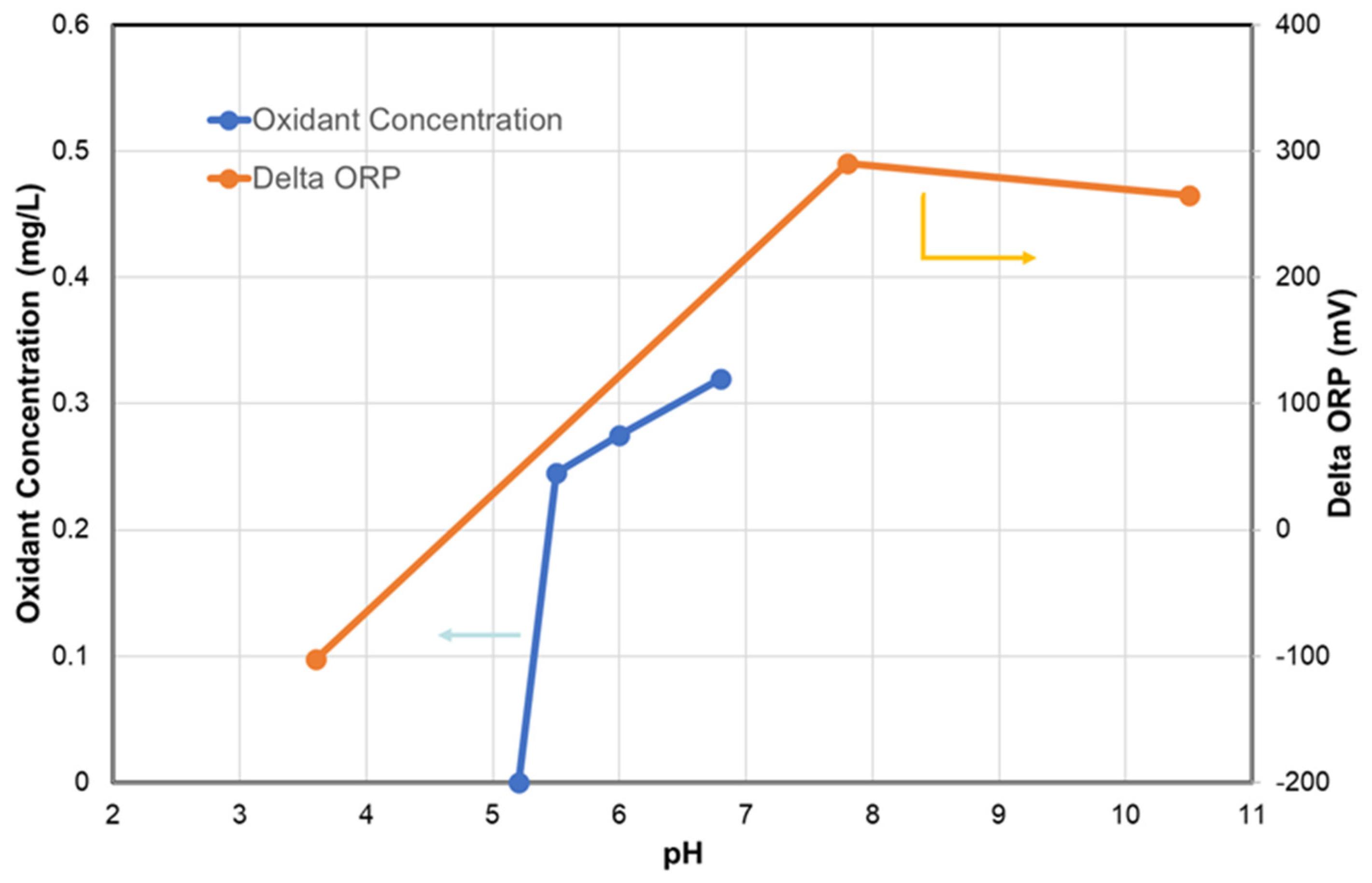
| IUPAC Name | Sodium Hydrogen Sulfite |
|---|---|
| CAS Number | 7631-90-5 |
| Molecular Formula | NaHSO3 |
| Molar Mass | 104.06 g/mol |
| Solubility in Water | 42 wt% in water 20 °C |
| Solution Density (20 °C) | 1.304 (37 wt% aq. Solution) 1.360 (42 wt% aq. Solution) |
| Odor | A slight odor of sulfur dioxide |
| Solution (wt%) | Shelf Life |
|---|---|
| 2 | Three (3) days |
| 10 | One (1) week |
| 20 | One (1) month |
| 30 | Six (6) months |
| Sulfites | Molecular Weight | Theoretical Dosage to Remove 1 mg Chlorine (mg) |
|---|---|---|
| Sodium sulfite | 126.1 | 1.78 |
| Sodium bisulfite (SBS) | 104.1 | 1.46 |
| Sodium metabisulfite (SMBS) | 190.2 | 1.34 |
| Sulfites | Dosage to Remove 1 mg Chlorine (mg) | Stochiometric Amount Ratio | Comments | Reference |
|---|---|---|---|---|
| SMBS | 3 >3 | 2.24 >2.24 | Brackish water Seawater | [23] |
| 3 | 2.24 | [41,42] | ||
| 2 | 1.49 | [24] | ||
| SBS | 3–5 | Aramid polyamide | [43] | |
| >2 | [44] | |||
| 2 | 1.37 | [45] |
| Membrane Manufacturer | High Alarm H (mV) | High-High Alarm HH (mV) | Reference |
|---|---|---|---|
| A | - | 175–200 | [68] |
| B | 300 | 350 | [69] |
| C | 250 | 300 | [70] |
| D | 270 (at pH = 6.0) | [71] | |
| 200 (at pH = 8.0) |
| Disinfectant | Redox Potential (V) | Feed | Permeate | Concentrate | Discharge from CIP Sanitization |
|---|---|---|---|---|---|
| Chloramine | 0.75 | ✓ | — | ✓ | — |
| Chlorine dioxide | 0.95 | ✓ | In case | ✓ | — |
| DBNPA | ─ | — | NA | ✓ | ✓ |
| Scenario | Related Key Reactions | Formed Chloramines Bromamines |
|---|---|---|
| Prechloirnated SW + NH4 salts injection | BrȒ + HOCl → Cl− + HOBr NH3 + HOBr → NH2Br + H2O NH2Br + HOBr → NHBr2 + H2O | Monobromamine Dibromamine |
| NH4 salts first or NH4 salts and NaOCl injection to SW together | NH3 + HOCl → NH2Cl + H2O | Monochloramine |
| NH2Cl + HOCl → NHCl2 + H2O | Dichloramine | |
| NH3 + HOBr → NH2Br + H2O | Bromamine | |
| NH2Br + HOBr → NHBr2 + H2O | Dibromamine | |
| Preformed chloramine injection to SW | NH2Cl (stable for an hour in SW) | Monochloramine |
| NH2Cl + Br− + H+ → NHBrCl + NH4 + Cl− | Bromochloramine |
| Sulfites | Molecular Weight | Theoretical Dosage to Remove 1 mg ClO2 (mg) |
|---|---|---|
| Sodium sulfite | 126.1 | 4.67 |
| SBS | 104.1 | 3.85 |
| SMBS | 190.2 | 3.52 |
| Sodium thiosulfate | 158.11 | 1.46 |
| Chemical Species | Standard Oxidation Potential (V) | Relative Strength |
|---|---|---|
| Hydroxy radical (∙OH) | 2.8 | 2.0 |
| Sulfate radical (∙SO4−) | 2.6 | 1.8 |
| Ozone (O3) | 2.1 | 1.5 |
| Persulfate Anion (S2O82−) | 2.0 | 1.4 |
| Peroxymonosulfate (HSO5−) [307] | 1.8 | 1.3 |
| Chlorine (Cl2) | 1.4 | 1.0 |
| Peroxymonosulfate radical (∙SO5−) at pH 7.0 [308] | 1.1 | 0.8 |
| Sulfite radical (∙SO3−) at pH > 7.0 [308] | 0.63 | 0.5 |
| Factors Affecting Membrane Degradation by SBS | References |
|---|---|
| Heavy metals | [47,248,252,309] |
| SBS concentration | [248,309] |
| Dissolved oxygen concentration | [248] |
| Feed pH | [47,197,309] |
| Bicarbonate ion concentration | [47,197,309] |
| Chloride ion concentration | [248] |
| Salinity or feed TDS | [47,197] |
| Temperature | [47,197] |
| Chelating Agent | Initial | 100 h | 1000 h | |||
|---|---|---|---|---|---|---|
| SP (%) | Flux (m/d) | SP (%) | Flux (m/d) | SP (%) | Flux (m/d) | |
| None | 0.52 | 0.68 | 0.78 | 0.69 | 1.60 | 0.81 |
| SHMP 10 ppm | 0.50 | 0.67 | 0.49 | 0.66 | 0.53 | 0.65 |
| EDTA 5 ppm | 0.49 | 0.69 | 0.51 | 0.68 | 0.54 | 0.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeda, Y. Roles of Sulfites in Reverse Osmosis (RO) Plants and Adverse Effects in RO Operation. Membranes 2022, 12, 170. https://doi.org/10.3390/membranes12020170
Maeda Y. Roles of Sulfites in Reverse Osmosis (RO) Plants and Adverse Effects in RO Operation. Membranes. 2022; 12(2):170. https://doi.org/10.3390/membranes12020170
Chicago/Turabian StyleMaeda, Yasushi. 2022. "Roles of Sulfites in Reverse Osmosis (RO) Plants and Adverse Effects in RO Operation" Membranes 12, no. 2: 170. https://doi.org/10.3390/membranes12020170
APA StyleMaeda, Y. (2022). Roles of Sulfites in Reverse Osmosis (RO) Plants and Adverse Effects in RO Operation. Membranes, 12(2), 170. https://doi.org/10.3390/membranes12020170