Design of Laser Photothermal Conversion Membranes Based on Fluorinated Graphene
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
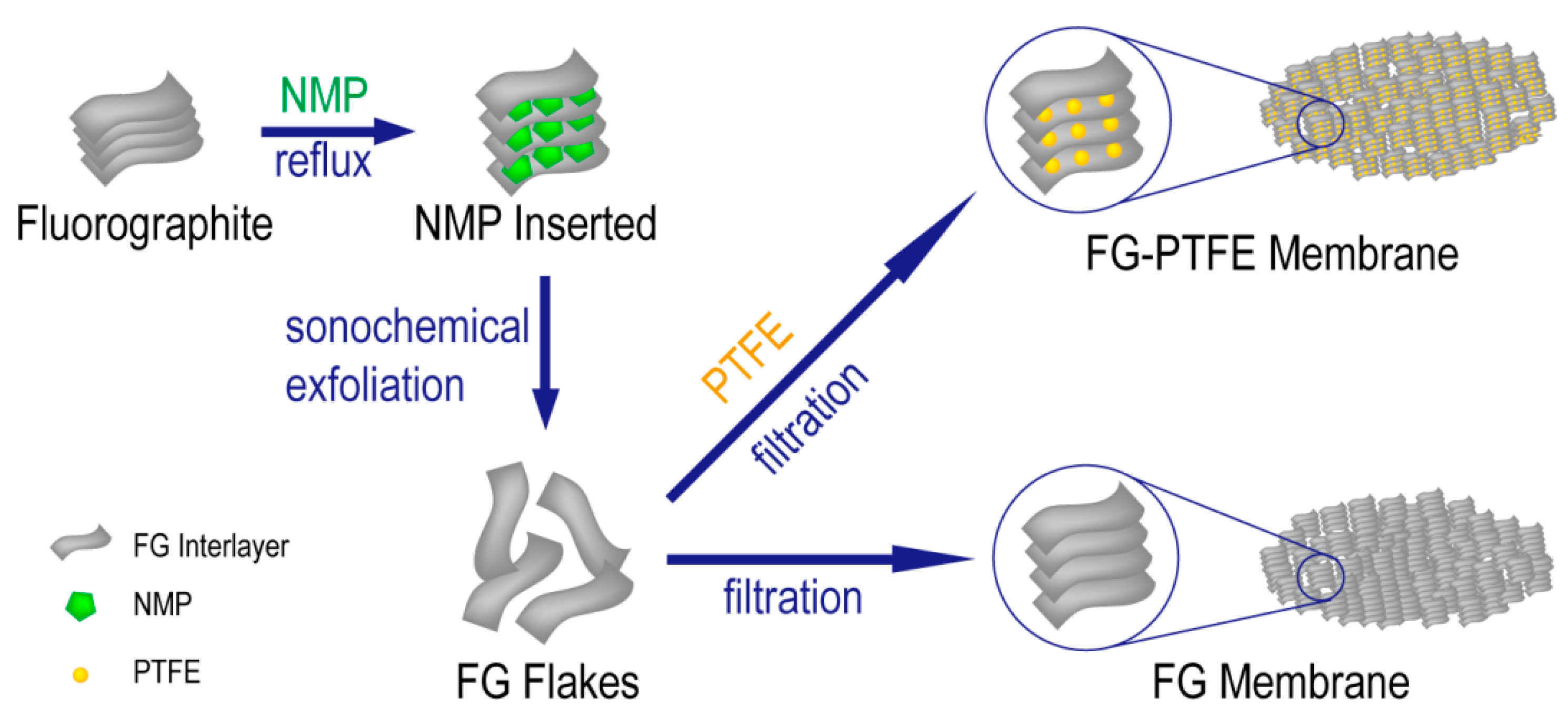
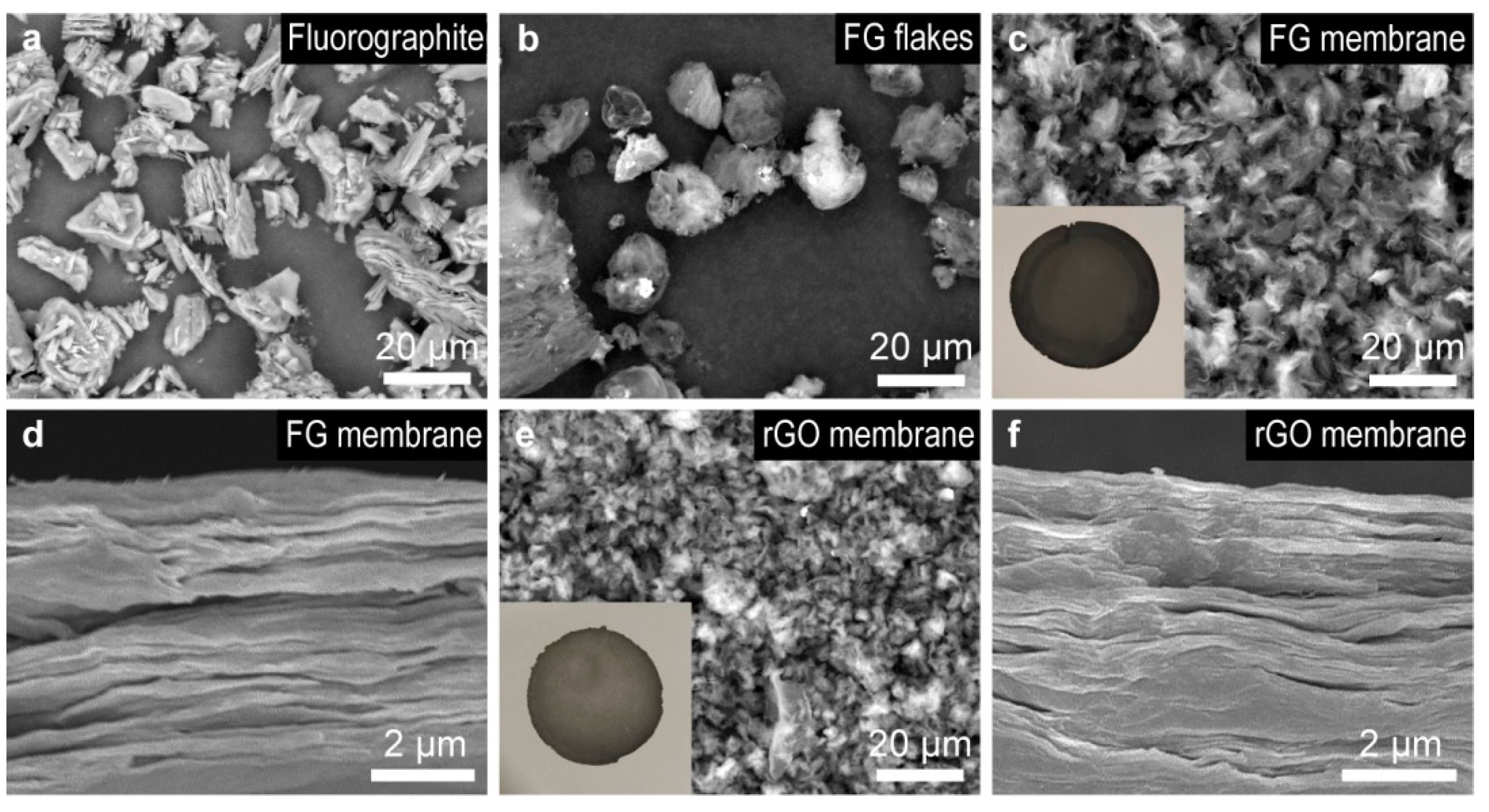
3.1. Morphology and Composition
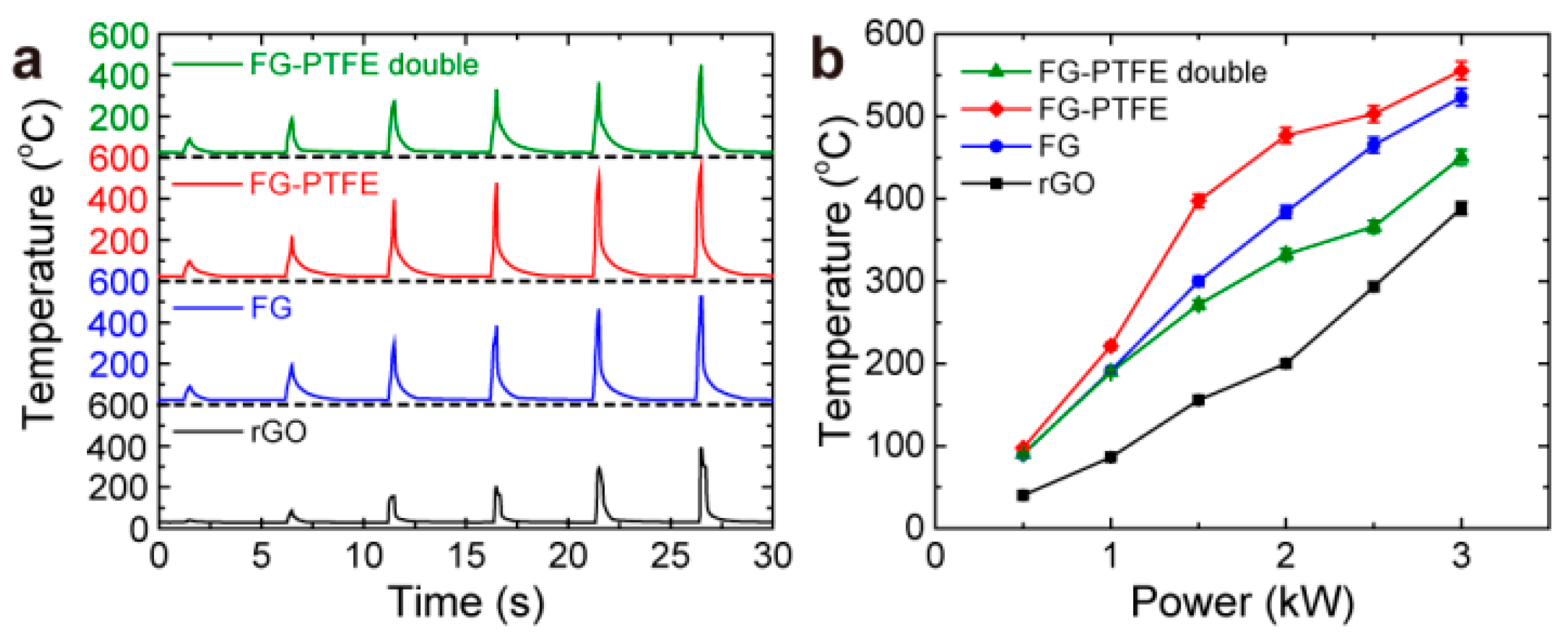
3.2. Photothermal Properties
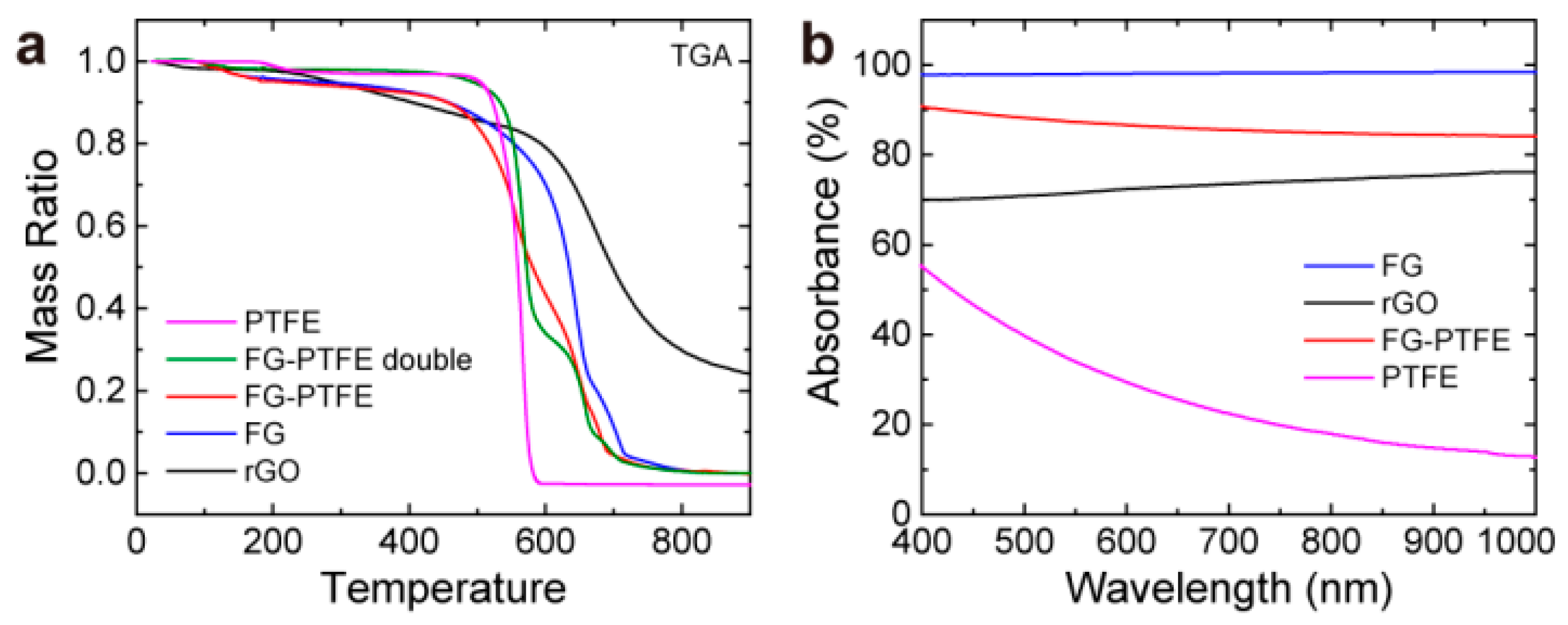
3.3. Mechanism Research
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morsy, M.H. Review and recent developments of laser ignition for internal combustion engines applications. Renew. Sus. Energy Rev. 2012, 16, 4849–4875. [Google Scholar] [CrossRef]
- Ahmad, S.R.; Russell, D.A. Studies into Laser Ignition of Confined Pyrotechnics. Propellants Explos. Pyrotech. 2008, 33, 396–402. [Google Scholar] [CrossRef]
- Gillard, P.; Opdebeck, F. Laser diode ignition of the B/KNO3 pyrotechnic mixture: An experimental study. Combust. Sci. Technol. 2007, 179, 1667–1699. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, G.; Xing, X.; Liu, J.; Cheng, Y.; Ye, T.; Wang, Q.; Xiao, X.; Li, Z.; Deng, H. Near-Infrared Light-Triggered Porous AuPd Alloy Nanoparticles to Produce Mild Localized Heat to Accelerate Bone Regeneration. J. Phys. Chem. Lett. 2019, 10, 4185–4191. [Google Scholar] [CrossRef]
- Ye, T.; Lai, Y.; Wang, Z.; Zhang, X.; Meng, G.; Zhou, L.; Zhang, Y.; Zhou, Z.; Deng, J.; Wang, M.; et al. Precise Modulation of Gold Nanorods for Protecting against Malignant Ventricular Arrhythmias via Near-Infrared Neuromodulation. Adv. Func. Mater. 2019, 29, 1902128. [Google Scholar] [CrossRef]
- Yang, F.; Tang, D.; Zhang, T.; Qin, W.; Chen, Y.; Wang, L.; Wang, J.; Zhang, H.; Li, Y.; Zhang, L. A free-standing laser energy converter based on energetic graphene oxide for enhanced photothermic ignition. J. Mater. Chem. A 2018, 6, 13761–13768. [Google Scholar] [CrossRef]
- Deng, H.-Y.; Wang, L.; Tang, D.; Zhang, Y.; Zhang, L. Review on the laser-induced performance of photothermal materials for ignition application. Energetic Mater. Front. 2021, 2, 201–217. [Google Scholar] [CrossRef]
- Rodrigo Velez-Cordero, J.; Hernandez-Cordero, J. Heat generation and conduction in PDMS-carbon nanoparticle membranes irradiated with optical fibers. Int. J. Therm. Sci. 2015, 96, 12–22. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, R.; Huang, M.; Lu, W.; Song, S.; Melancon, M.P.; Tian, M.; Liang, D.; Li, C. A Chelator-Free Multifunctional Cu-64 CuS Nanoparticle Platform for Simultaneous Micro-PET/CT Imaging and Photothermal Ablation Therapy. J. Am. Chem. Soc. 2010, 132, 15351–15358. [Google Scholar] [CrossRef] [Green Version]
- Amendola, V.; Pilot, R.; Frasconi, M.; Marago, O.M.; Iati, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef]
- Xin, Q.; Shah, H.; Nawaz, A.; Xie, W.; Akram, M.Z.; Batool, A.; Tian, L.; Jan, S.U.; Boddula, R.; Guo, B.; et al. Antibacterial Carbon-Based Nanomaterials. Adv. Mater. 2019, 31, 1804838. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, W.; Ji, W.; Wang, J.; Wang, N.; Wu, W.; Wu, Q.; Hou, X.; Hu, W.; Li, L. Near infrared photothermal conversion materials: Mechanism, preparation, and photothermal cancer therapy applications. J. Mater. Chem. B 2021, 9, 7909–7926. [Google Scholar] [CrossRef] [PubMed]
- Si, S.; Li, W.; Zhao, X.; Han, M.; Yue, Y.; Wu, W.; Guo, S.; Zhang, X.; Dai, Z.; Wang, X.; et al. Significant Radiation Tolerance and Moderate Reduction in Thermal Transport of a Tungsten Nanofilm by Inserting Monolayer Graphene. Adv. Mater. 2017, 29, 1604623. [Google Scholar] [CrossRef] [PubMed]
- Vadhe, P.P.; Pawar, R.B.; Sinha, R.K.; Asthana, S.N.; Rao, A.S. Cast Aluminized Explosives. Combust. Explos. Shock. Waves 2008, 44, 461–477. [Google Scholar] [CrossRef]
- Strankowski, M.; Wlodarczyk, D.; Piszczyk, A.; Strankowska, J. Polyurethane Nanocomposites Containing Reduced Graphene Oxide, FTIR, Raman, and XRD Studies. J. Spectrosc. 2016. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Huang, B.; Li, R.; Zhang, H.-P.; Qin, W.; Qiao, Z.; Liu, Y.; Yang, G. Laser-Ignited Relay-Domino-Like Reactions in Graphene Oxide/CL-20 Films for High-Temperature Pulse Preparation of Bi-Layered Photothermal Membranes. Small 2019, 15, 1900338. [Google Scholar] [CrossRef]
- Nika, D.L.; Balandin, A.A. Phonons and thermal transport in graphene and graphene-based materials. Rep. Prog. Phys. 2017, 80, 036502. [Google Scholar] [CrossRef] [Green Version]
- Ma, F.; Zheng, H.B.; Sun, Y.J.; Yang, D.; Xu, K.W.; Chu, P.K. Strain effect on lattice vibration, heat capacity, and thermal conductivity of graphene. Appl. Phys. Lett. 2012, 101. [Google Scholar] [CrossRef] [Green Version]
- Raj, A.M.E.; Victoria, S.G.; Jothy, V.B.; Ravidhas, C.; Wollschlaeger, J.; Suendorf, M.; Neumann, M.; Jayachandran, M.; Sanjeeviraja, C. XRD and XPS characterization of mixed valence Mn3O4 hausmannite thin films prepared by chemical spray pyrolysis technique. Appl. Sur. Sci. 2010, 256, 2920–2926. [Google Scholar] [CrossRef]
- Apte, S.K.; Naik, S.D.; Sonawane, R.S.; Kale, B.B.; Pavaskar, N.; Mandale, A.B.; Das, B.K. Nanosize Mn3O4 (Hausmannite) by microwave irradiation method. Mater. Res. Bull. 2006, 41, 647–654. [Google Scholar] [CrossRef]
- Ng, L.Y.; Chua, H.S.; Ng, C.Y. Incorporation of graphene oxide-based nanocomposite in the polymeric membrane for water and wastewater treatment: A review on recent development. J Environ. Chem. Eng. 2021, 9. [Google Scholar] [CrossRef]
- Ye, Y.; Shu, L.; Shen, R. Effect of Phenolic Resin on Laser Ablation of B/KNO3. Chin. J. Energetic Mater. 2007, 15, 33–35. [Google Scholar] [CrossRef]
- Wang, J.; Qiao, Z.; Yang, Y.; Shen, J.; Long, Z.; Li, Z.; Cui, X.; Yang, G. Core-Shell Al-Polytetrafluoroethylene (PTFE) Configurations to Enhance Reaction Kinetics and Energy Performance for Nanoenergetic Materials. Chem. Eur. J. 2016, 22, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, T.; Ding, W.; Yao, M.; Song, J. Study on Disposal Ability of Al/MnOx Flame Jet Thermite for Simulated Metal Shell of Ammunition. Initiat. Pyrotech. 2018, 14–18. [Google Scholar] [CrossRef]
- Sivan, J.; Haas, Y. Spectroscopic Characterization of B/KNO3 Diode-Laser Induced Combustion. J. Phys. Chem. A 2013, 117, 11808–11814. [Google Scholar] [CrossRef]
- Du, J.; Liu, J.; Gong, P.; Tian, M.; Sun, L.; Ji, S.; Zhang, L.; Liu, Z. Construction of a novel fluorinated graphene-based magnetic nanocomposite and its application in cancer photo-chemotherapy. Mater. Lett. 2017, 196, 165–167. [Google Scholar] [CrossRef]
- Gong, P.; Du, J.; Wang, D.; Cao, B.; Tian, M.; Wang, Y.; Sun, L.; Ji, S.; Liu, Z. Fluorinated graphene as an anticancer nanocarrier: An experimental and DFT study. J. Mater. Chem. B 2018, 6, 2769–2777. [Google Scholar] [CrossRef]
- Gong, P.; Zhao, Q.; Dai, D.; Zhang, S.; Tian, Z.; Sun, L.; Ren, J.; Liu, Z. Functionalized Ultrasmall Fluorinated Graphene with High NIR Absorbance for Controlled Delivery of Mixed Anticancer Drugs. Chem. Eur. J. 2017, 23, 17531–17541. [Google Scholar] [CrossRef]
- Gong, P.; Zhang, L.; Yuan, X.-A.; Liu, X.; Diao, X.; Zhao, Q.; Tian, Z.; Sun, J.; Liu, Z.; You, J. Multifunctional fluorescent PEGylated fluorinated graphene for targeted drug delivery: An experiment and DFT study. Dye. Pigment. 2019, 162, 573–582. [Google Scholar] [CrossRef]
- Tang, P.; Zhu, W.; Qiao, Z.; Yang, G.; Li, X.; Tang, Y.; Zhang, H. Laser-induced energetic material ignition with various fluorinated graphenes: Theoretical and experimental studies. Appl. Sur. Sci. 2021, 570, 151187. [Google Scholar] [CrossRef]
- Gong, P.; Wang, Z.; Wang, J.; Wang, H.; Li, Z.; Fan, Z.; Xu, Y.; Han, X.; Yang, S. One-pot sonochemical preparation of fluorographene and selective tuning of its fluorine coverage. J. Mater. Chem. 2012, 22, 16950–16956. [Google Scholar] [CrossRef]
- Ng, L.Y.; Mohammad, A.W.; Rohani, R.; Hairom, N.H.H. Development of a nanofiltration membrane for humic acid removal through the formation of polyelectrolyte multilayers that contain nanoparticles. Desalin. Water Treat. 2016, 57, 7627–7636. [Google Scholar] [CrossRef]
- Piao, J.; Duan, S.; Zhang, Y.; Wang, N.; Zhou, Q.; He, S.; Liu, J.; Liao, J.; Zhang, L. Design of a graphene-based core–shell structure for the improvement of photothermic performance. J. Phys. D Appl. Phys. 2020, 53, 025303. [Google Scholar] [CrossRef]
- Wang, N.; Liao, J.; Liu, J.; Zhang, Y.; Piao, J.; Zhang, L. Freestanding graphene oxide-polytetrafluoroethylene membranes with excellent photothermic performance for laser ignition. Mater. Lett. 2020, 270, 127691. [Google Scholar] [CrossRef]
- Xu, W.; Hang, S.; Li, Y.; Han, Z.; Wang, B. Effects of PTFE Content and Sintering Temperature on the Morphology and Combustion Performances of Al/PTFE Composites. Chin. J. Energetic Mater. 2020, 28, 1061–1067. [Google Scholar] [CrossRef]
- Golden, J.H. The degradation of polytetrafluoroethylene by ionizing radiation. J. Polym, Sci. 1960, 45, 534–536. [Google Scholar] [CrossRef]
- Lau, K.K.S.; Caulfield, J.A.; Gleason, K.K. Structure and Morphology of Fluorocarbon Films Grown by Hot Filament Chemical Vapor Deposition. Chem. Mater. 2000, 12, 3032–3037. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Li, Z.; Del Cul, G.D.; Yan, W.; Liang, C.; Dai, S. Fluorinated Carbon with Ordered Mesoporous Structure. J. Am. Chem. Soc. 2004, 126, 12782–12783. [Google Scholar] [CrossRef]

| Sample | rGO | FG | FG-PTFE | FG-PTFE Double | PTFE |
|---|---|---|---|---|---|
| 5% weight loss temperature (°C) | 288.7 | 268.7 | 213.8 | 393.0 | 503.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piao, J.; Li, K.; Zhang, Y.; Zhang, L. Design of Laser Photothermal Conversion Membranes Based on Fluorinated Graphene. Membranes 2022, 12, 135. https://doi.org/10.3390/membranes12020135
Piao J, Li K, Zhang Y, Zhang L. Design of Laser Photothermal Conversion Membranes Based on Fluorinated Graphene. Membranes. 2022; 12(2):135. https://doi.org/10.3390/membranes12020135
Chicago/Turabian StylePiao, Junyu, Keding Li, Yong Zhang, and Long Zhang. 2022. "Design of Laser Photothermal Conversion Membranes Based on Fluorinated Graphene" Membranes 12, no. 2: 135. https://doi.org/10.3390/membranes12020135
APA StylePiao, J., Li, K., Zhang, Y., & Zhang, L. (2022). Design of Laser Photothermal Conversion Membranes Based on Fluorinated Graphene. Membranes, 12(2), 135. https://doi.org/10.3390/membranes12020135






