A High Performance Polyacrylonitrile Composite Separator with Cellulose Acetate and Nano-Hydroxyapatite for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Pure PAN Separator
2.3. Preparation of PAN/CA/HAP Composite Separator
2.4. Characterization
3. Results and Discussion
3.1. FT-IR Analysis
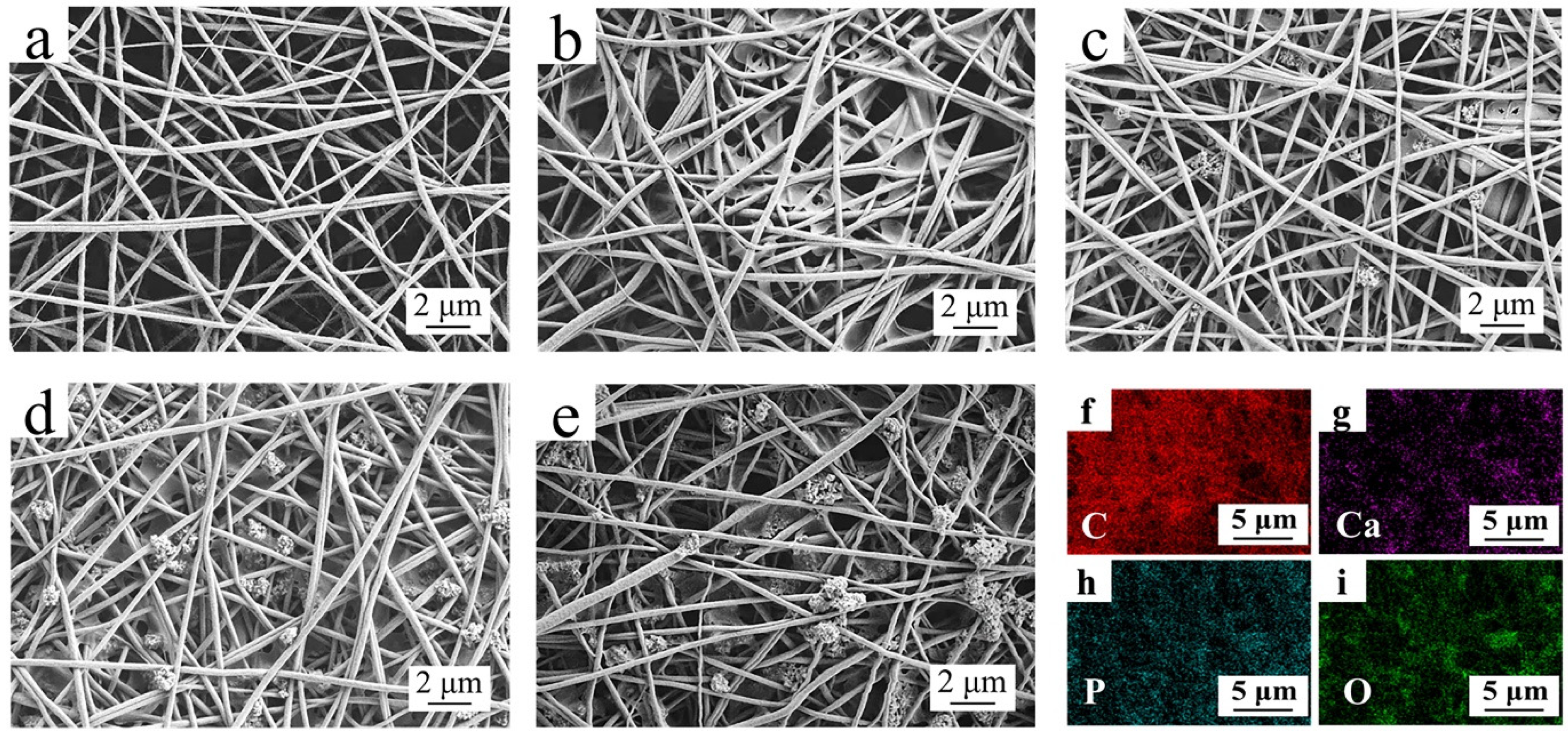
3.2. Micromorphological Analysis
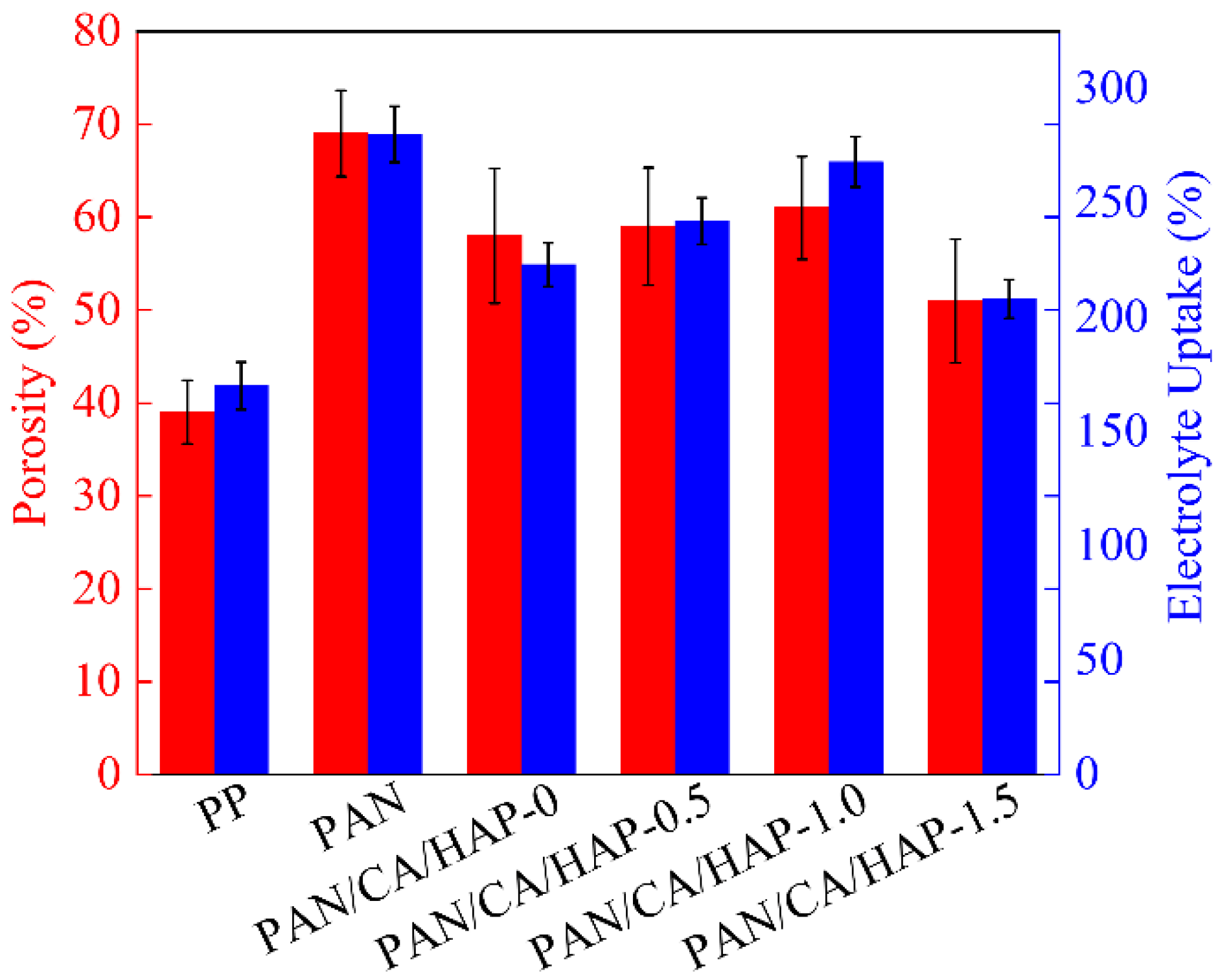
3.3. Porosity and Electrolyte Uptake Analysis
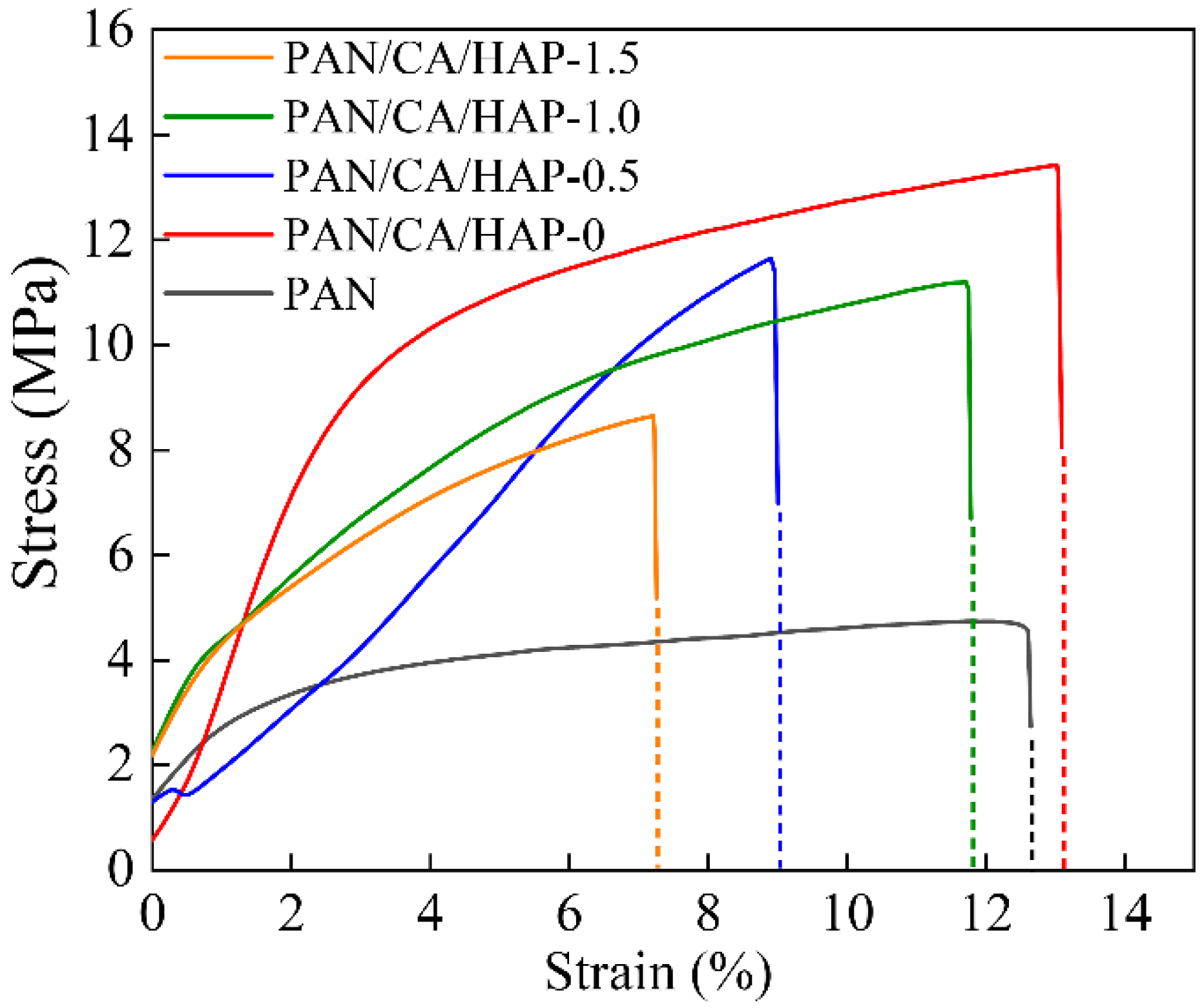
3.4. Mechanical Performance Analysis
3.5. Ionic Conductivity Analysis
3.6. Thermal Performance Analysis
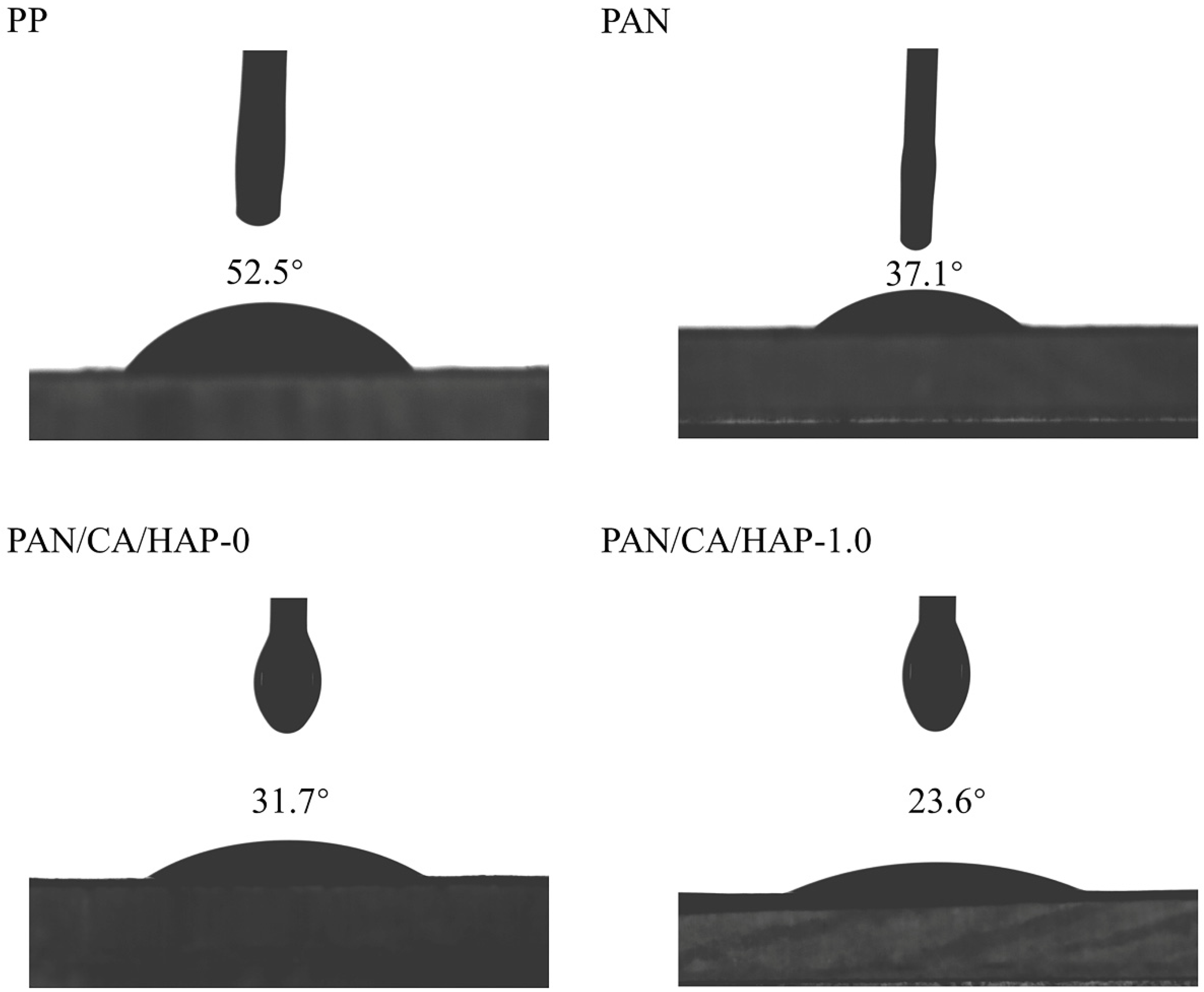
3.7. Contact Angle Analysis
3.8. Electrochemical Performance Analysis
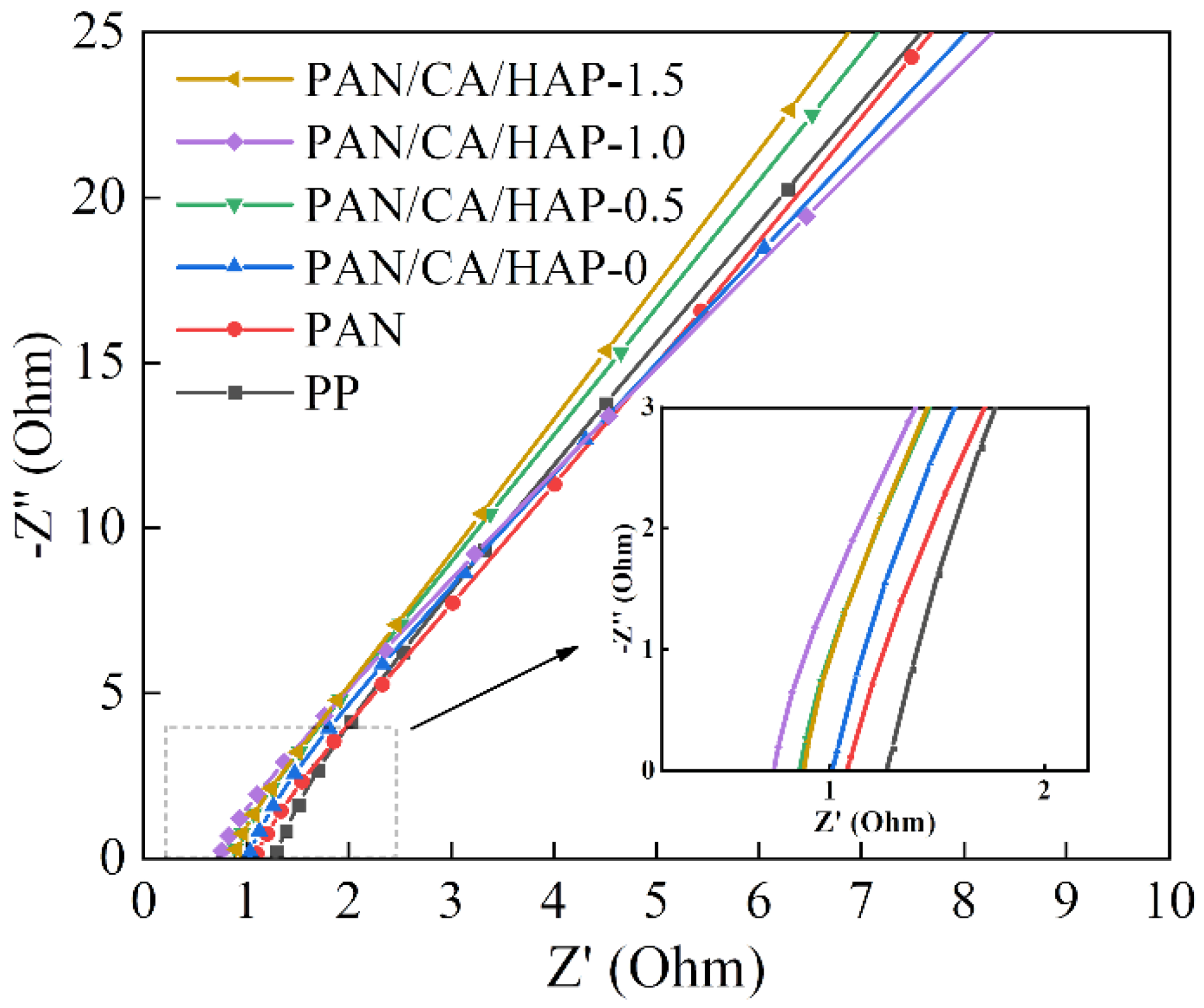
3.8.1. Interfacial AC Impedance and LSV
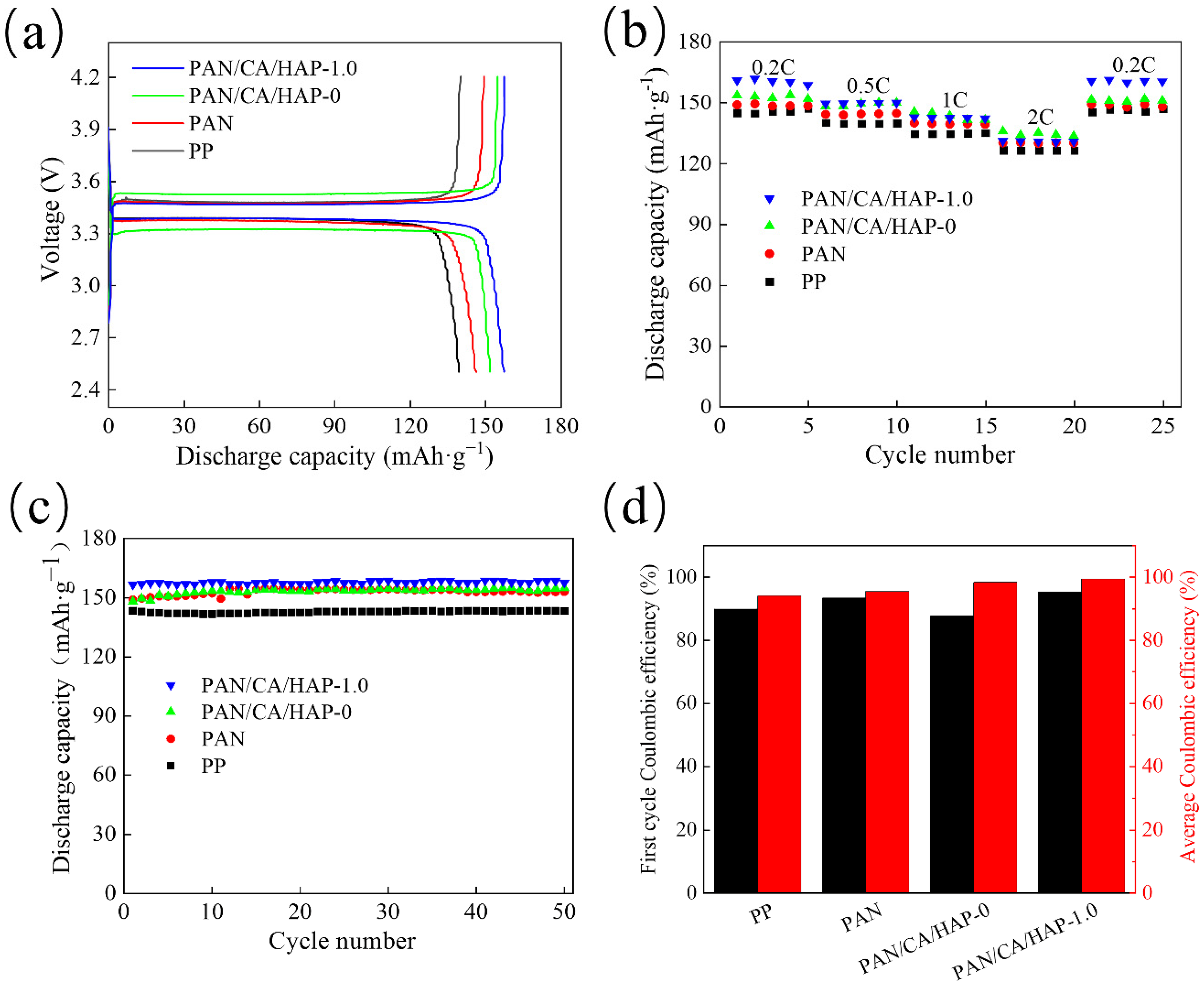
3.8.2. Charge and Discharge Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhandari, B.; Poudel, S.R.; Lee, K.-T.; Ahn, S.-H. Mathematical modeling of hybrid renewable energy system: A review on small hydro-solar-wind power generation. Int. J. Precis. Eng. Manuf.-Green Technol. 2014, 1, 157–173. [Google Scholar] [CrossRef]
- Lu, J.; Wang, W.; Zhang, Y.; Ye, S. Techno-economic feasibility of PV-wind-diesel-battery hybrid energy system in a remote island in the South China sea. Model. Meas. Control A 2017, 90, 162–182. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, W.; Niu, S. Emission modeling for new-energy buses in real-world driving with a deep learning-based approach. Atmos. Pollut. Res. 2021, 12, 101195. [Google Scholar] [CrossRef]
- Elia, G.; Bernhard, R.; Hassoun, J. A lithium-ion oxygen battery using a polyethylene glyme electrolyte mixed with an ionic liquid. RSC Adv. 2015, 5, 21360–21365. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, Z.; Sun, X.G.; Hu, Y.S.; Xing, H.; Dai, S. Ionic liquids and derived materials for lithium and sodium batteries. Chem. Soc. Rev. 2018, 47, 2020–2064. [Google Scholar] [CrossRef]
- Tarascon, J.M. Key challenges in future Li-battery research. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 3227–3241. [Google Scholar] [CrossRef] [Green Version]
- Ji, L.; Lin, Z.; Alcoutlabi, M.; Zhang, X. Recent developments in nanostructured anode materials for rechargeable lithium-ion batteries. Energy Environ. Sci. 2011, 4, 2680–2682. [Google Scholar] [CrossRef]
- Pathak, A.D.; Samanta, K.; Sahu, K.K.; Pati, S. Mechanistic insight into the performance enhancement of Si anode of a lithium-ion battery with a fluoroethylene carbonate electrolyte additive. J. Appl. Electrochem. 2021, 51, 143–154. [Google Scholar] [CrossRef]
- Zuo, X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y.J. Silicon based lithium-ion battery anodes: A chronicle perspective review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Hui, W.; Zhuo, D.; Kong, D.; Yi, C. Improving battery safety by early detection of internal shorting with a bifunctional separator. Nat. Commun. 2014, 5, 5193. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Yuan, W.; Li, L. Enhanced wettability and thermal stability of nano-SiO2/poly(vinyl alcohol)-coated polypropylene composite separators for lithium-ion batteries. Particuology 2018, 37, 91–98. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z.J. Battery Separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef]
- Gao, K.; Hu, X.; Dai, C.; Yi, T. Crystal structures of electrospun PVDF membranesand its separator application for rechargeable lithium metal cells. Mater. Sci. Eng. B 2006, 131, 100–105. [Google Scholar] [CrossRef]
- Huang, X.; Bahroloomi, D.; Xiao, X. A multilayer composite separator consisting of non-woven mats and ceramic particles for use in lithium ion batteries. J. Solid State Electrochem. 2014, 18, 133–139. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, G.; Zong, W.; Ouyang, Y.; Liu, T. Porous polymer composite separators with three-dimensional ion-selective nanochannels for high-performance Li–S batteries. Compos. Commun. 2021, 25, 100679. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Low, T.R. An inorganic composite membrane as the separator of Li-ion batteries. J. Power Sources 2005, 140, 361–364. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Liang, J.; Wu, K.; Wang, J. Design of a high performance zeolite/Polyimide composite separator for Lithium-Ion batteries. Polymers 2020, 12, 764. [Google Scholar] [CrossRef] [Green Version]
- Rana, M.; Li, M.; He, Q.; Luo, B.; Wang, L.; Gentle, I.; Knibbe, R. Separator coatings as efficient physical and chemical hosts of polysulfides for high-sulfur-loaded rechargeable lithium–sulfur batteries. J. Energy Chem. 2020, 44, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Wang, X.; Liang, J.; Zhang, Z.; Chen, W. A novel electrospinning Polyacrylonitrile separator with dip-coating of zeolite and Phenoxy Resin for Li-ion batteries. Membranes 2021, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gao, K. The study on methyl methacrylate graft-copolymerized composite separator prepared by pre-irradiation method for Li-ion batteries. Surf. Coat. Technol. 2010, 204, 2822–2828. [Google Scholar] [CrossRef]
- Iwata, T. Characterization of Ca2+ Doped Lanthanum chromate film prepared by plasma-spray process for solid oxide fuel cell separator. Nippon Kagaku Kaishi 2001, 2001, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Anilkumar, K.M.; Jinisha, B.; Manoj, M.; Jayalekshmi, S. Poly(ethylene oxide) (PEO)—Poly(vinyl pyrrolidone) (PVP) blend polymer based solid electrolyte membranes for developing solid state magnesium ion cells. Eur. Polym. J. 2017, 89, 249–262. [Google Scholar] [CrossRef]
- Sohn, J.Y.; Im, J.S.; Gwon, S.J.; Choi, J.H.; Shin, J.; Nho, Y.C. Preparation and characterization of a PVDF-HFP/PEGDMA-coated PE separator for lithium-ion polymer battery by electron beam irradiation—ScienceDirect. Radiat. Phys. Chem. 2009, 78, 505–508. [Google Scholar] [CrossRef]
- He, H.; Wang, X.; Liu, W. Effects of PEGDMA on a PET non-woven fabric embedded PAN lithium-ion power battery separator. Solid State Ion. 2016, 294, 31–36. [Google Scholar] [CrossRef]
- Shi, J.L.; Fang, L.F.; Li, H.; Zhang, H.; Zhu, B.K.; Zhu, L.P. Improved thermal and electrochemical performances of PMMA modified PE separator skeleton prepared via dopamine-initiated ATRP for lithium ion batteries. J. Membr. Sci. 2013, 437, 160–168. [Google Scholar] [CrossRef]
- Min, H.S.; Ko, J.M.; Kim, D.W. Preparation and characterization of porous polyacrylonitrile membranes for lithium-ion polymer batteries. J. Power Sources 2003, 119, 469–472. [Google Scholar] [CrossRef]
- Lee, J.; Lee, C.L.; Park, K.; Kim, I.D. Synthesis of an Al2O3-coated polyimide nanofiber mat and its electrochemical characteristics as a separator for lithium ion batteries. J. Power Sources 2014, 248, 1211–1217. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, X.; Shang, Y.; Xu, P.; Liu, C. Highly thermally conductive polyvinyl alcohol/boron nitride nanocomposites with interconnection oriented boron nitride nanoplatelets. Compos. Sci. Technol. 2021, 201, 108521. [Google Scholar] [CrossRef]
- Li, L.; Liu, P.; Fu, Q.S.; Gong, Y.; Zhang, S.R.; He, H.J.; Chen, J. Study on preparation of polyacrylonitrile/polyimide composite lithium-ion battery separator by electrospinning. J. Mater. Res. 2019, 34, 1–10. [Google Scholar] [CrossRef]
- Gopalan, A.I.; Santhosh, P.; Manesh, K.M.; Jin, H.N.; Lee, K.P. Development of electrospun PVDF–PAN membrane-based polymer electrolytes for lithium batteries. J. Membr. Sci. 2008, 325, 683–690. [Google Scholar] [CrossRef]
- Jiang, F.; Yu, N.; Lei, Y.; Yuan, F.; Zhong, C. Core-shell-structured nanofibrous membrane as advanced separator for lithium-ion batteries. J. Membr. Sci. 2016, 510, 1–9. [Google Scholar] [CrossRef]
- Xiao, K.; Zhai, Y.; Yu, J.; Ding, B. Nanonet-structured poly(m-phenylene isophthalamide)-polyurethane membranes with enhanced thermostability and wettability for high power lithium ion batteries. RSC Adv. 2015, 5, 55478–55485. [Google Scholar] [CrossRef]
- Lee, J.H.; Manuel, J.; Choi, H.; Park, W.H.; Ahn, J.H. Partially oxidized polyacrylonitrile nanofibrous membrane as a thermally stable separator for lithium ion batteries. Polymer 2015, 68, 335–343. [Google Scholar] [CrossRef]
- Shin, W.K.; Ji, H.Y.; Choi, W.; Chung, K.Y.; Jang, S.S.; Kim, D.W. Cycling performance of lithium-ion polymer cells assembled with a cross-linked composite polymer electrolyte using a fibrous polyacrylonitrile membrane and vinyl-functionalized SiO2 nanoparticles. J. Mater. Chem. A 2015, 3, 12163–12170. [Google Scholar] [CrossRef]
- Wang, Q.; Song, W.L.; Fan, L.Z.; Song, Y. Facile fabrication of polyacrylonitrile/alumina composite membranes based on triethylene glycol diacetate-2-propenoic acid butyl ester gel polymer electrolytes for high-voltage lithium-ion batteries. J. Membr. Sci. 2015, 486, 21–28. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Nagai, K.; Nakagawa, T.; Mau, W.H. Effect of polyethyleneglycol (PEG) on gas permeabilities and permselectivities in its cellulose acetate (CA) blend membranes. J. Membr. Sci. 1998, 138, 143–152. [Google Scholar] [CrossRef]
- Lee, H.J.; Kang, S.W. Cellulose acetate containing CaO coated on polypropylene for enhanced thermal stability of separator. Chem. Commun. 2021, 57, 4388–4391. [Google Scholar] [CrossRef]
- Dong, Y.; Lou, Y.; Han, Y.; Wickramaratne, M.N.; Dai, H.; Wang, X. Controllable synthesis of poly(acrylic acid)-stabilized nano-hydroxyapatite suspension by an ultrasound-assisted precipitation method. Mater. Lett. 2018, 227, 9–12. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Liu, Y.L. Crosslinked electrospun poly(vinylidene difluoride) fiber mat as a matrix of gel polymer electrolyte for fast-charging lithium-ion battery. Electrochim. Acta 2017, 258, 1329–1335. [Google Scholar] [CrossRef]
- Kapoor, S.; Batra, U.; Kohli, S.; Tripathi, S.K.; Dharamvir, K.; Kumar, R.; Saini, G. Sintering effects on morphology, thermal stability and surface area of sol-gel derived nano-hydroxyapatite powder. Am. Inst. Phys. 2011, 1393, 375–376. [Google Scholar] [CrossRef]




| Sample | Thickness, μm | Ionic Conductivity, mS·cm−1 |
|---|---|---|
| PP | 20 | 0.86 |
| PAN | 39 | 1.76 |
| PAN/CA/HAP-0 | 46 | 2.21 |
| PAN/CA/HAP-0.5 | 47 | 2.81 |
| PAN/CA/HAP-1.0 | 46 | 3.02 |
| PAN/CA/HAP-1.5 | 48 | 2.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Wang, X.; Liang, J.; Chen, Y.; Ma, W.; Zhang, S. A High Performance Polyacrylonitrile Composite Separator with Cellulose Acetate and Nano-Hydroxyapatite for Lithium-Ion Batteries. Membranes 2022, 12, 124. https://doi.org/10.3390/membranes12020124
Chen W, Wang X, Liang J, Chen Y, Ma W, Zhang S. A High Performance Polyacrylonitrile Composite Separator with Cellulose Acetate and Nano-Hydroxyapatite for Lithium-Ion Batteries. Membranes. 2022; 12(2):124. https://doi.org/10.3390/membranes12020124
Chicago/Turabian StyleChen, Weiping, Xiang Wang, Jianyu Liang, Yao Chen, Wei Ma, and Siyuan Zhang. 2022. "A High Performance Polyacrylonitrile Composite Separator with Cellulose Acetate and Nano-Hydroxyapatite for Lithium-Ion Batteries" Membranes 12, no. 2: 124. https://doi.org/10.3390/membranes12020124
APA StyleChen, W., Wang, X., Liang, J., Chen, Y., Ma, W., & Zhang, S. (2022). A High Performance Polyacrylonitrile Composite Separator with Cellulose Acetate and Nano-Hydroxyapatite for Lithium-Ion Batteries. Membranes, 12(2), 124. https://doi.org/10.3390/membranes12020124







