1. Introduction
Gaseous ethylene is a plant hormone that controls numerous processes in plant growth and development, including seedling growth, leaf and flower senescence, fruit ripening, and plant responses to pathogens [
1,
2,
3]. Many reactions to ethylene are initiated by a group of endoplasmic reticulum (ER)-membrane bound receptors. These ethylene receptors are negative regulators of ethylene responses [
4], which switch off the downstream signal transmission upon ethylene binding, and hence mediate the plant growth and development [
5]. Earlier studies have demonstrated that the ethylene receptors form a complex with constitutive triple response 1 (CTR1) and ethylene insensitive 2 (EIN2) in the response to the plant hormone [
6]. In the absence of ethylene, the ethylene receptor is active, and triggers CTR1 on for inhibiting the plant development; when ethylene binds, the ethylene receptor is inhibited and turns CTR1 off, resulting in the initiation of downstream signaling [
7]. A number of inhibitors, including Ag
+ and some gas molecules, have been reported to block ethylene receptor action to improve the shelf life of fruits, vegetables, and flowers [
8]. The essential roles of ethylene receptors in plant growth and development uncover a great potential of ethylene receptors as a target for practical use in agriculture and horticulture in the future.
Sequence analysis and functional studies of ethylene receptors suggest that ethylene receptors are similar to bacterial two-component histidine kinase receptors [
9]. In
Arabidopsis thaliana, the five ethylene receptors, comprising ETR1, ERS1, ETR2, ERS2, and EIN4, can be classified into two subfamilies, in which ETR1 and ERS1 form the type-I subfamily, and the type-II subfamily contains ETR2, ERS2, and EIN4 [
10]. All members of the receptor family are composed of an N-terminal transmembrane domain (TMD), a middle GAF domain, and a C-terminal catalytic histidine kinase (HK) domain, displaying a similar overall modular structure. ETR1 and ERS1 in subfamily I contain three transmembrane helices, while the subfamily II members have an additional fourth transmembrane helix. The basic functional unit of the ethylene receptors is a disulfide-linked dimer [
11]. The N-terminal TMD is the sensor domain that binds copper ions, and hence binds the ethylene molecules selectively and noncovalently, which is different from the majority of membrane receptors that have a soluble signal-binding domain [
12].
Until today, the ethylene binding of the receptors and the inhibition mechanism in the ethylene signaling network were still obscure. Previously, the structures of the ethylene receptors ERS1 and ETR1 from
Arabidopsis thaliana (At), including GAF, catalytic ATP binding, DHp, and receiver domains, have been obtained by X-ray crystallography and low-resolution, small-angle X-ray scattering (SAXS) [
12]. Together, this has allowed obtaining a model of the entire cytosolic domain. The high-resolution structure of the transmembrane sensor domain (TSD) is yet unknown; a structural model of AtETR1_TSD was recently generated by combining
ab initio protein structure prediction and a coevolutionary relationship [
13]. A dimeric model of AtETR1_TSD with copper(I) was further built based on the experimentally determined copper stoichiometry. These models provided insights into the helix assembly and dimerization for ETR1_TSD [
13]. However, how ethylene receptors are inhibited by sensing ethylene and the conformational change of the dimeric ethylene receptors in response to the inhibitors are still unknown. Therefore, further high-resolution structural studies are needed to understand the mechanistic details of the activation and inhibition of ethylene action.
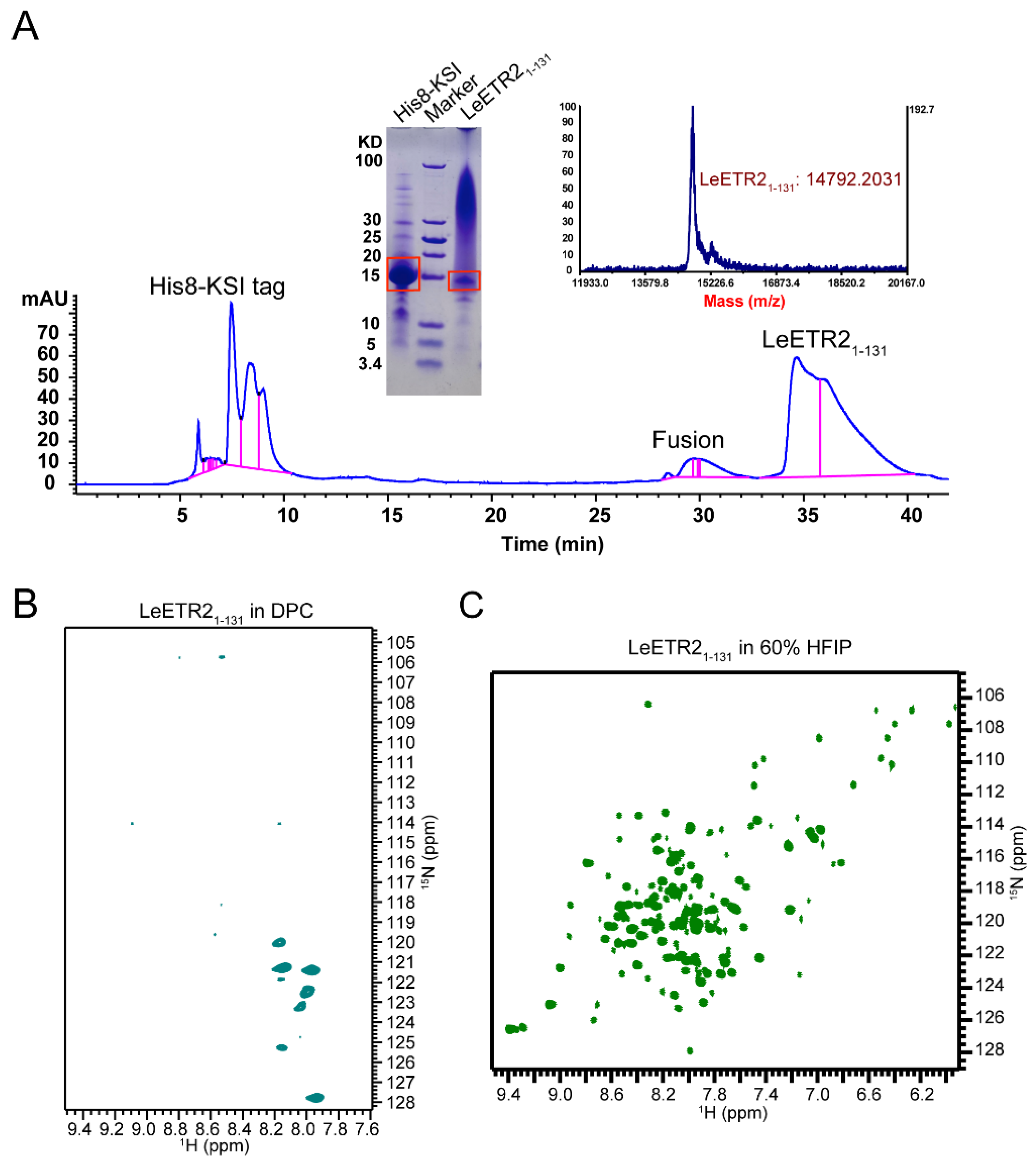
To determine the structure of TSD for ethylene receptors, here we screened the expression and purification of the ethylene receptor’s TSDs in Escherichia coli (E. coli). Different constructs, fusion tags, and detergents were examined, and only the TSD (residues 1–131) from tomato ETR2 (LeETR2) was successfully expressed in the E. coli strain BL21 (DE3) as inclusion bodies, and achieved high yield and purity by Ni-NTA affinity chromatography and high-performance liquid chromatography (HPLC). Unfortunately, only in the organic solvent hexafluoroisopropanol (HFIP) did we obtain a good-quality NMR spectrum for LeETR21–131. Benefiting from the great power of AlphaFold2, we built a dimer model of LeETR21–131, which was highly converged and rigid. Molecular dynamics (MD) simulations of LeETR21–131 with ethylene offered the potential binding sites of ethylene, which shed light on the relationship between the TSD structure and the ethylene binding.
2. Materials and Methods
2.1. Materials
2.1.1. Reagents
Ammonium chloride (15N, 99% (w/w); Cat. NLM-467), D2O (99.96% (v/v; Cat. DLM-6-PK), and HFIP (99.5%, Cat. H107501) were obtained from Aladdin (Shanghai, China). Other chemicals were sourced from Amresco (Solon, OH, USA), Sigma-Aldrich (St. Louis, MO, USA), and Sangon Biotech (Shanghai, China).
2.1.2. Medium for Cell Culture
Luria–Bertani (LB) (1 L): 10 g tryptone, 5 g yeast extract, 10 g NaCl with 100 μg/mL ampicillin. 15N M9 medium (1 L): 6 g Na2HPO4, 3 g KH2PO4, 1 g NH4Cl (N, 99%, for 15N-labeled samples), 0.5 g NaCl, 4 g glucose, 1 mL 2 M MgSO4, 1 mL 100 mM CaCl2, 4 mL Centrum stock solution (1 tablet of Centrum dissolved in 40 mL ddH2O then filtered).
2.2. Construct Design
To obtain better dissolved target ethylene receptor peptides, we calculated the individual grand average of hydropathicity (GRAVY) (ProtParam tool. Available online:
web.expasy.org/protparam, accessed on 18 December 2021) for every curated peptide sequence from the ethylene receptor family. Four peptide sequences (AtETR1, AtETR2, AtERS2, and LeETR2) were chosen according to their lower GRAVY values, which meant better solubility for the sample preparation. Meanwhile, His8-tag, His9-trpLE, MBP tag, and His8-KSI (ketone steroid isomerase) tag were used for the overexpression, individually.
Synthesized oligonucleotides (Genscript. Available online:
www.genscript.com.cn, accessed on 29 December 2021) corresponding to the membrane-spanning sequence of AtETR1 (
Arabidopsis) (Uniprot ID: P49333), AtETR2 (
Arabidopsis) (Uniprot ID: Q0WPQ2), AtERS2 (
Arabidopsis) (Uniprot ID: P93825), and LeETR2 (
Tomato) (Uniprot ID: O49187) were optimized to
E. coli codons according to the preferred protein translation codon usage in
E. coli.
2.3. Protein Expression
The above constructed plasmids were transformed into BL21 (DE3) cells and plated on LB medium supplemented with appropriate ampicillin resistance, then grown in an incubator at 37 °C overnight.
A single colony of the transformant was inoculated into 5 mL of LB and 100 μg/μL ampicillin. The culture was put in a shaker at 37 °C and shaken at 220 rpm for 8 h. Then, 50 μL of the grown cells were added to 200 mL LB media to continue the growth at 37 °C with shaking at 220 rpm overnight. Then, the cells were spun down and inoculated into 1 L of sterile 15N labelled M9 media in a 3 L baffled flask. The expression of the target proteins was induced by adding 0.2 mM isopropyl-β-D-thiogalactopyranoside (IPTG) at OD600 = 0.7. Then, the cells were grown at 20 °C for 18 h. Finally, the cells were harvested by centrifugation at 4000 rpm for 30 min at room temperature (Avanti J-20XP Centrifuge, Beckman JLA-8.1000 rotor, Beckman Coulter, Brea, CA, USA).
2.4. Protein Purification
The successfully overexpressed LeETR21–131 with a His8-KSI fusion tag was extracted from the inclusion bodies and solubilized in 6 M guanidine HCl, 200 mM NaCl, 1% Triton X-100, and 50 mM Tris-HCl pH 8.0 (Buffer A). The His8-KSI-LeETR21–131 fusion protein was loaded to Ni2+ affinity (NTA) column in Buffer A at room temperature and eluted from the NTA column in the same buffer with 500 mM imidazole. The eluted fusion protein was then cleaved by cyanogen bromide (CNBr) in 80% formic acid (FA) for 1 h to remove the KSI tag. The cleaved mixtures were further loaded onto a Proto-C18 column (Agilent Technology, Santa Clara, CA, USA). A reverse-phase chromatography using the gradient of 55–96% buffer B (100% acetonitrile (ACN) + 0.1% trifluoroacetic acid (TFA)) was performed to obtain highly pure LeETR21–131. NMR samples were prepared by dissolving 1.5 mg of lyophilized peptide in organic solvent (60%HFIP + 30%ddH2O + 10%D2O).
2.5. SDS-PAGE
All the proteins were examined using the Tris–Tricine SDS-PAGE system following a previous protocol [
14]. The samples were mixed with the gel loading buffer and β-mercaptoethanol (β-ME), and stained with Coomassie Blue G250 after the electrophoresis.
2.6. Mass Spectrometry
Approximately 0.2 mg of lyophilized LeETR21–131 was dissolved in 10 μL 50% formic acid (FA), and 1 μL of this protein solution was mixed with 1 μL of matrix solution (10 mg/mL sinnapinic acid (SA), 75% ACN, 25% H2O, 0.1% TFA). The resulting solution was spotted onto a seed layer spot on the MALDI target. The mass spectrum was collected on a 5800 MALDI-TOF/TOF (Applied Biosystems, Waltham, MA, USA).
2.7. NMR Detection for LeETR21–131
Samples were prepared by dissolving 1–2 mg of
15N-labelled protein in 500 μL of NMR buffer (60%HFIP + 30%ddH
2O + 10%D
2O). The solution NMR experiments were performed on an Agilent 700 MHz spectrometer equipped with a triple-resonance 5 mm probe. The two-dimensional (2D)
1H/
15N TROSY-HSQC spectra were obtained at 30 °C. The NMR data were processed using NMRPipe and rendered in NMRDraw on a Dell Precision T7810 Linux workstation [
15].
2.8. AlphaFold2 modeling and relaxation
No structure of LeETR2 was available in the PDB database (PDB. Available online:
https://www.pdbus.org/, accessed on 2 December 2021), so the structural model for LeETR2
1–131 was thus calculated using AlphaFold2 [
16] (AlphaFold2. Available online:
https://github.com/deepmind/alphafold, accessed on 11 October 2021). No homologous templates were used; only the multiple sequence alignment (MSA) was set as true, and the MSA mode was MMseq2. The model procedure was under a homo-dimer construction. The residue confidence score provided by AlphaFold2 (named pIDDT) was above 80% for most residues of LeETR2
1–131. For residues in the alpha-helix regions, pIDDT values were above 90% nearly for all. After analysis, it was shown that the copper binding site containing C64 and H68 of LeETR2
1–131 in the model was well inward-facing inside the channel, and the alpha-helix was well packed. The relaxation of the unrelaxed model of LeETR2
1–131 was conducted using a built-in algorithm for removing the steric clashes of side-chains and incorporating all the hydrogens. Through Ramachandran analysis of these two relaxed models, it was shown that almost all the residue atoms were located in the most favored or allowed regions. Thus, the relaxed model of LeETR2
1–131 was qualified for further docking and MD simulations.
2.9. Molecular Docking Using Autodock 1.2.2
Molecular docking of the ethylene was implemented in the LeETR2
1–131 relaxed model. The two cofactor monovalent copper ions were placed around the copper binding sites, C64 and H68 of LeETR2
1–131. The receptor of LeETR2
1–131 was protonated using the REDUCE algorithm and prepared using the prepare-receptor algorithm, all from the ADFR suite [
17]. The ethylene was protonated and prepared using the mk-prepare-ligand algorithm from the ADFR suite [
17] (ADFR suite. Available online:
https://ccsb.scripps.edu/, accessed on 2 December 2021). The Autodock Vina 1.2.2 software [
18] (Autodock Vina 1.2.2. Available online:
https://github.com/ccsb-scripps/AutoDock-Vina, accessed on 2 December 2021) was utilized for molecular docking, in which vinardo scoring function parameters were selected. The grid dimensions were set to 30 × 30 × 30 Å to allow enough regions to cover the copper binding sites, where the coordinate of the grid center was set to 0 × 0 × 5.844 Å (LeETR2
1–131). In addition, the default values were used for other parameters with the exhaustiveness of 32 for better searching space. The best-fitted poses from docking models were used for further MD simulations.
2.10. Molecular Dynamics Simulation Using Desmond
The MD simulations of LeETR2
1–131 were performed using the Desmond package [
19] (Desmond. Available online:
https://www.deshawresearch.com/, accessed on 2 December 2021) in the presence or absence of ethylene with copper ions. LeETR2
1–131 was placed in a 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) lipid bilayer with an appropriate amount of counterions to balance the net charge of the system solvated in 0.15 M KCl. The membrane of LeETR2
1–131 dimeric complex was defined through helices. The whole system was solvated with the explicit simple point charge (TIP3P) waters under the force field OPLS2005 [
20]. The simulation temperature (300 K) was controlled by Nose–Hoover temperature coupling [
21], and the atmospheric pressure (1 atm) was modulated by the Martina–Tobias–Klein method [
22]. The particle-mesh Ewald (PME) method [
23] was used to calculate electrostatic interactions and van der Waals (VDW) forces. The system was relaxed before the simulation runs using the default setting in Desmond. The initial coordinates of LeETR2
1–131-C
2H
4 for the MD simulation calculations were taken from docking results. The system was subject to 100 ns of a normal pressure and temperature (NPT) production simulation run.
4. Discussion
In the long term, how the plant hormone ethylene is perceived by ethylene receptors and hence regulates the downstream signaling transduction lacks structural visualization. Here, we screened the ETR homologues in the
E. coli expression system for determination of the atomic-level-resolution structure. However, due to the high hydrophobic contents, most of the screened constructs failed to produce a large amount of proteins. The available LeETR2
1–131 proved to be very difficult to reconstitute in detergents for NMR study. Only in organic solvent was an NMR spectrum with good resolution and dispersion available. Therefore, we built a structural model by means of the AlphaFold2 algorithm. Alphafold2 is a recently published powerful computational method to predict protein structures from a protein sequence. To improve the accuracy of protein structure prediction, this method was coupled with existing structure and sequence databases deposited by the experimental community. The model from Alphafold2 displayed that LeETR2
1–131 was an ETR1-like structure with three transmembrane helices, in which H1, H2, and H3 were located in the interface. A previous ETR1-TMD model of
Arabidopsis thaliana (AtETR1-TMD) built by integrating
ab initio Rosetta structure prediction and coevolutionary methods showed that H1 and H2 were the interface helices. The alignment of the LeETR2
1–131 and AtETR1-TMD models showed large differences with RMSD 13.089 Å. The helices in the AtETR1-TMD model were straight and parallel to the center symmetric axis, while the helices in the LeETR2
1–131 model were tilted with a 25° angle to the axis (
Figure S5). These differences could have been caused by the sequence or homologue differences, or the prediction algorithm. Further experimental investigations are required to clarify these differences.
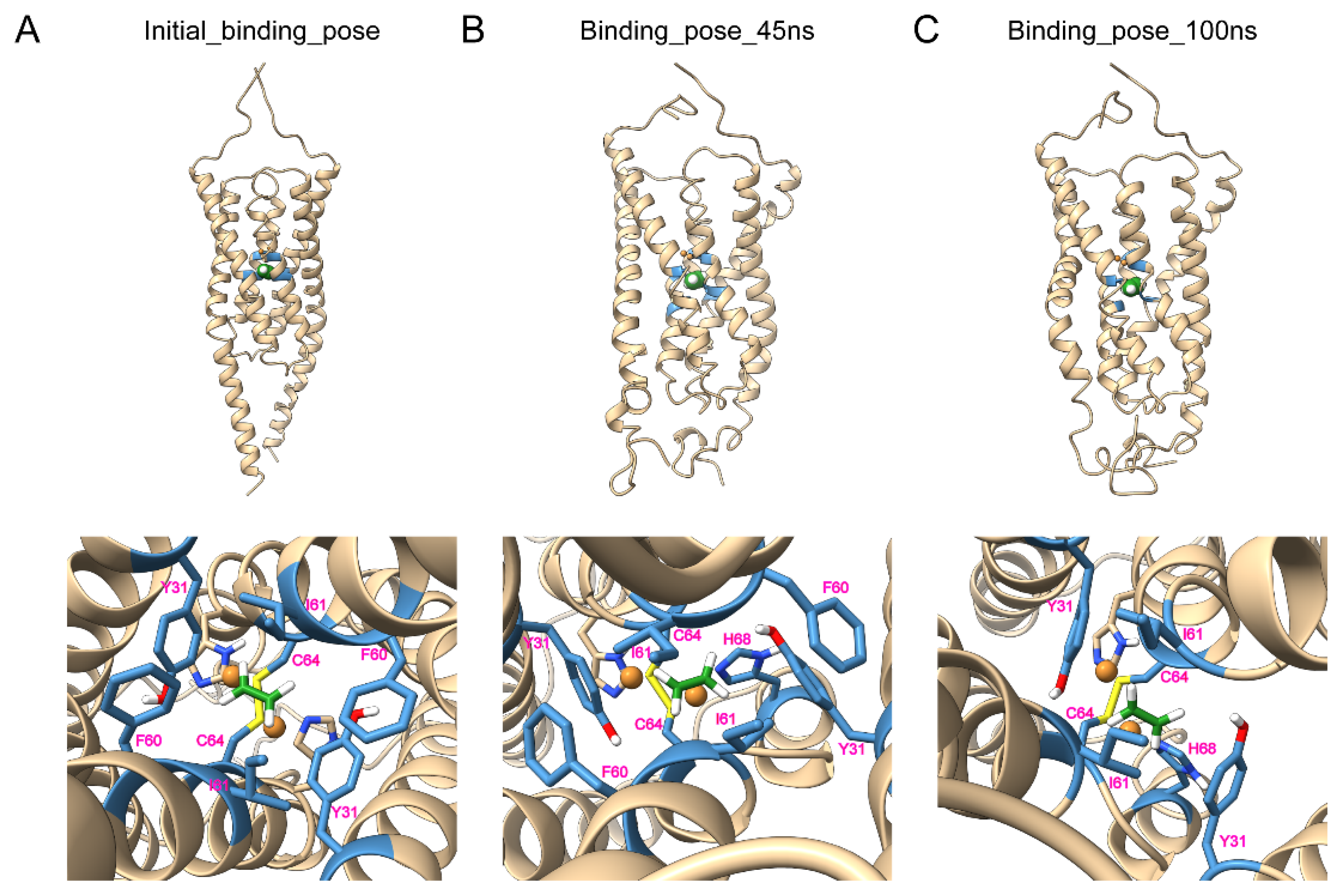
The ethylene binding model showed small differences between the initial model and after the MD simulation with the RMSD 3.106 Å (
Figure 4). The largest changes came from the membrane-proximal region at the C-terminus, which lost its helical structure and became a flexible loop; as a result, it provided the flexibility of the membrane-proximal region to interact with the membranes. The top of the potential ethylene binding site in the model contained C64 and H68 to bind with copper ions, and the bottom of the binding site consisted of large hydrophobic residues Y31, F60, and I61; thus together, they could catch and lock the small gas molecule ethylene in the binding pocket tightly, which was consistent with previous results showing that AtETR1 had a very low
Kd at 2.4 nM [
24] or a dissociation constant of the response (
Kr) of 0.1 μL/L [
25] to ethylene. In accordance, an earlier study mutated the corresponding binding residues of AtETR1, including Y32A, F61A, I62A, C65Y, and H69A, and led to the diminishment of ethylene binding activity [
26]. The ethylene binding residues (Y31, I61, C64, and H68) of LeETR2
1–131 maintained their binding to ethylene through the whole MD simulation, while F60 did not appear to interact with ethylene at the last snapshot, possibly due to the dynamics. The ethylene binding model of LeETR2
1–131 after the MD simulation showed slightly larger differences from the apo model after the MD simulation with the RMSD 3.954 Å (
Figure S6), which also displayed obvious changes at the C-terminal parts, including part of the transmembrane helices, indicating the C-terminal part was more dynamic during the signaling transduction.
Collectively, our exploration of the expression and purification of the ethylene receptors provided preliminary information for the structural studies of ethylene receptors. The dimeric model of LeETR2
1–131 built by the AlphaFold2 algorithm [
16] provided a good template for further molecular docking and MD simulations to decipher the potential binding sites of ethylene. The identified key binding residues of ethylene in LeETR2
1–131 agreed well with previous findings in the ethylene receptor homologues. Our data also provided a comprehensive insight into subtle conformational changes of LeETR2
1–131 in the presence of ethylene, which provided clues to understand the activity of ethylene receptors and enhance the activator/inhibitor development of ethylene receptors in the future, which is particularly important from both ecological and agricultural points of view.