Evaluation of Gas-to-Liquid Transfer with Ceramic Membrane Sparger for H2 and CO2 Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pilot Plant Description
2.2. Experimental Setup
2.2.1. Preliminary Tests with Water
2.2.2. Gas-to-Liquid Mass Transfer Coefficient Estimation with Microorganisms
2.3. Analytical Procedure
2.4. Theoretical Calculations
3. Results and Discussion
3.1. Preliminary Tests
3.2. Impact of Liquid Pressure
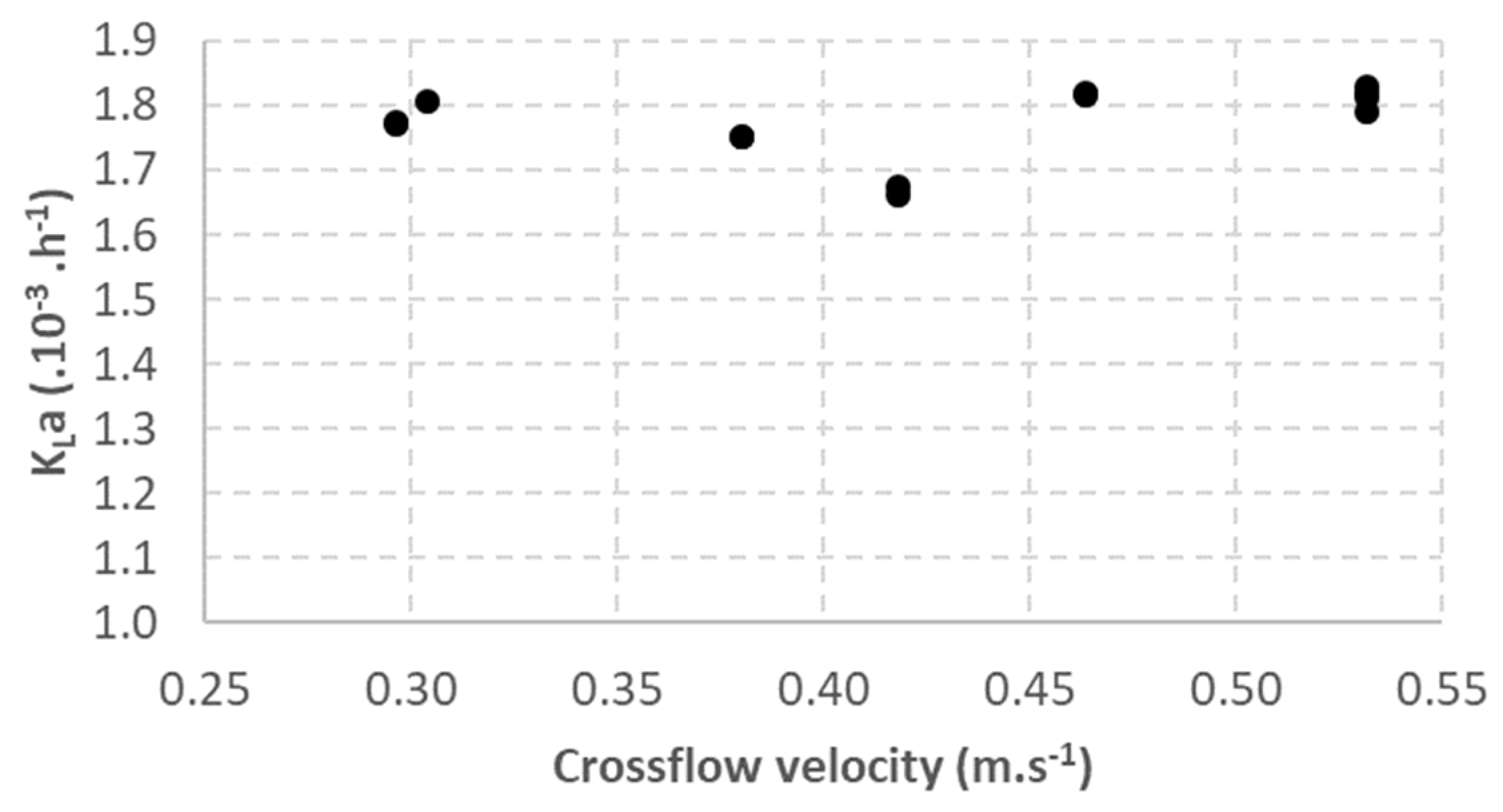
3.3. Impact of Liquid Flow Velocity
3.4. Impact of Gas Flow Rate
3.5. Impact of Broth Characteristics
3.6. Impact of Hydrogen Partial Pressure
3.7. Impact of Membrane Hydrophobicity
3.8. Impact of Membrane Surface
3.9. Impact of Membrane Pore Size
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Acronyms
References
- Ghaib, K.; Ben-Fares, F.-Z. Power-to-Methane: A state-of-the-art review. Renew. Sustain. Energy Rev. 2018, 81, 433–446. [Google Scholar] [CrossRef]
- Zabranska, J.; Pokorna, D. Bioconversion of carbon dioxide to methane using hydrogen and hydrogenotrophic methanogens. Biotechnol. Adv. 2018, 36, 707–720. [Google Scholar] [CrossRef]
- Sander, R. Compilation of Henry's law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef] [Green Version]
- Jensen, M.; Ottosen, L.; Kofoed, M. H2 gas-liquid mass transfer: A key element in biological Power-to-Gas methanation. Renew. Sustain. Energy Rev. 2021, 147, 111209. [Google Scholar] [CrossRef]
- Díaz, I.; Pérez, C.; Alfaro, N.; Fdz-Polanco, F. A feasibility study on the bioconversion of CO2 and H2 to biomethane by gas sparging through polymeric membranes. Bioresour. Technol. 2015, 185, 246–253. [Google Scholar] [CrossRef]
- Alfaro, N.; Fdz-Polanco, M.; Fdz-Polanco, F.; Díaz, I. Evaluation of process performance, energy consumption and microbiota characterization in a ceramic membrane bioreactor for ex-situ biomethanation of H2 and CO2. Bioresour. Technol. 2018, 258, 142–150. [Google Scholar] [CrossRef]
- Luo, G.; Angelidaki, I. Hollow fiber membrane based H2 diffusion for efficient in situ biogas upgrading in an anaerobic reactor. Appl. Microbiol. Biotechnol. 2013, 97, 3739–3744. [Google Scholar] [CrossRef]
- Wang, W.; Xie, L.; Luo, G.; Zhou, Q.; Angelidaki, I. Performance and microbial community analysis of the anaerobic reactor with coke oven gas biomethanation and in situ biogas upgrading. Bioresour. Technol. 2013, 146, 234–239. [Google Scholar] [CrossRef] [Green Version]
- Alfaro, N.; Fdz-Polanco, M.; Fdz-Polanco, F.; Díaz, I. H2 addition through a submerged membrane for in-situ biogas upgrading in the anaerobic digestion of sewage sludge. Bioresour. Technol. 2019, 280, 1–8. [Google Scholar] [CrossRef]
- Deschamps, L.; Imatoukene, N.; Lemaire, J.; Mounkaila, M.; Filali, R.; Lopez, M.; Theoleyre, M.-A. In-situ biogas upgrading by bio-methanation with an innovative membrane bioreactor combining sludge filtration and H2 injection. Bioresour. Technol. 2021, 337, 125444. [Google Scholar] [CrossRef]
- Khirani, S.; Kunwapanitchakul, P.; Augier, F.; Guigui, C.; Guiraud, P.; Hébrard, G. Microbubble Generation through Porous Membrane under Aqueous or Organic Liquid Shear Flow. Ind. Eng. Chem. Res. 2011, 51, 1997–2009. [Google Scholar] [CrossRef]
- Kukizaki, M.; Goto, M. Size control of nanobubbles generated from Shirasu-porous-glass (SPG) membranes. J. Membr. Sci. 2006, 281, 386–396. [Google Scholar] [CrossRef]
- Kukizaki, M.; Baba, Y. Effect of surfactant type on microbubble formation behavior using Shirasu porous glass (SPG) membranes. Colloids Surf. A Physicochem. Eng. Asp. 2008, 326, 129–137. [Google Scholar] [CrossRef]
- Kukizaki, M. Microbubble formation using asymmetric Shirasu porous glass (SPG) membranes and porous ceramic membranes—A comparative study. Colloids Surf. A Physicochem. Eng. Asp. 2009, 340, 20–32. [Google Scholar] [CrossRef]
- Moletta, R. La Méthanisation, 3rd ed.; Lavoisier Tec & Doc: Paris, France, 2015; Available online: https://books.google.fr/books?id=T44_rgEACAAJ (accessed on 10 January 2022).
- Boributh, S.; Assabumrungrat, S.; Laosiripojana, N.; Jiraratananon, R. A modeling study on the effects of membrane characteristics and operating parameters on physical absorption of CO2 by hollow fiber membrane contactor. J. Membr. Sci. 2011, 380, 21–33. [Google Scholar] [CrossRef]
- Bakeri, G.; Matsuura, T.; Ismail, A.; Rana, D. A novel surface modified polyetherimide hollow fiber membrane for gas–liquid contacting processes. Sep. Purif. Technol. 2012, 89, 160–170. [Google Scholar] [CrossRef]
- Capodici, M.; Corsino, S.F.; Di Trapani, D.; Torregrossa, M.; Viviani, G. Effect of biomass features on oxygen transfer in conventional activated sludge and membrane bioreactor systems. J. Clean. Prod. 2019, 240, 118071. [Google Scholar] [CrossRef]
- Tanaka, S.; Kastens, S.; Fujioka, S.; Schlüter, M.; Terasaka, K. Mass transfer from freely rising microbubbles in aqueous solutions of surfactant or salt. Chem. Eng. J. 2020, 387, 121246. [Google Scholar] [CrossRef]
- Schröder, V.; Schubert, H. Production of emulsions using microporous, ceramic membranes. Colloids Surf. A Physicochem. Eng. Asp. 1999, 152, 103–109. [Google Scholar] [CrossRef]
- Joscelyne, S.M.; Trägårdh, G. Membrane emulsification—A literature review. J. Membr. Sci. 2000, 169, 107–117. [Google Scholar] [CrossRef]
- Kunacheva, C.; Le, C.; Soh, Y.N.A.; Stuckey, D.C. Chemical Characterization of Low Molecular Weight Soluble Microbial Products in an Anaerobic Membrane Bioreactor. Environ. Sci. Technol. 2017, 51, 2254–2261. [Google Scholar] [CrossRef]
- Sardeing, R.; Painmanakul, P.; Hébrard, G. Effect of surfactants on liquid-side mass transfer coefficients in gas–liquid systems: A first step to modeling. Chem. Eng. Sci. 2006, 61, 6249–6260. [Google Scholar] [CrossRef]
- Scott, K. Membrane materials, preparation and characterization. In Handbook of Industrial Membranes; Elsevier: Amsterdam, The Netherlands, 1995; pp. 187–269. [Google Scholar] [CrossRef]
- Xie, B.; Zhou, C.; Sang, L.; Ma, X.; Zhang, J. Preparation and characterization of microbubbles with a porous ceramic membrane. Chem. Eng. Process. Process Intensif. 2021, 159, 108213. [Google Scholar] [CrossRef]
- Zare, S.; Kargari, A. 4–Membrane properties in membrane distillation. In Emerging Technologies for Sustainable Desalination Handbook; Elsevier: Amsterdam, The Netherlands, 2018; pp. 107–156. [Google Scholar] [CrossRef]
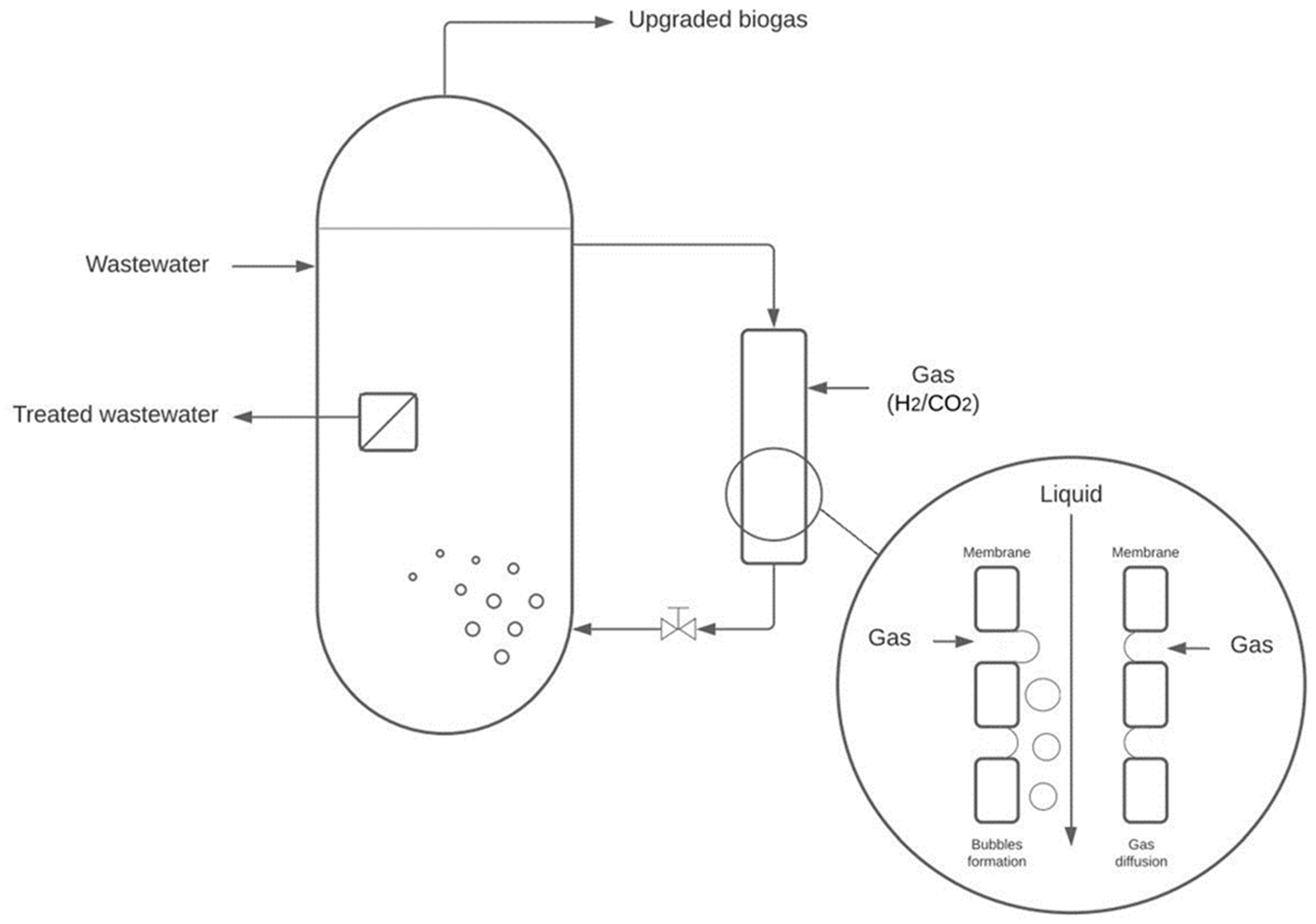



| Parameter Studied | Liquid Pressure (barg) | Crossflow Velocity (m·s−1) | H2 Flow Rate (NL·h−1) | CO2 Injection | Membrane Hydrophobicity | Membrane Surface (m²) | Membrane Pore Size (µm) | Results Part |
|---|---|---|---|---|---|---|---|---|
| Preliminary tests | Atmospheric pressure | 1.5 | Measured for different gas pressures | None a | Hydrophobic | 0.25 | 0.1 | 3.1 |
| Liquid pressure | From 0.5 to 1.5 | 0.30 | 15 | Separated a | Hydrophobic | 0.25 | 0.1 | 3.2 |
| Crossflow velocity | 1.5 | From 0.30 to 0.53 | 15 | Separated a | Hydrophobic | 0.25 | 0.1 | 3.3 |
| Gas flow rate | 1.5 | 0.30–0.53 | From 11 to 15 | Separated a | Hydrophobic | 0.25 | 0.1 | 3.4 |
| Broth characteristics | 1.5 | 0.3 | 15 | Separated a | Hydrophobic | 0.25 | 0.1 | 3.5 |
| Hydrogen partial pressure | 1.5 | 0.3 | 15 | Together with H2 b | Hydrophobic | 0.25 | 0.1 | 3.6 |
| Membrane hydrophobicity | 1.5 | 0.3 | 15 | Together with H2 b | Hydrophobic and hydrophilic | 0.25 | 0.1 | 3.7 |
| Membrane surface | 1.5 | 0.3 | 15 | Together with H2 b | Hydrophilic | 0.5 | 0.1 | 3.8 |
| Membrane pore size | 1.5 | 0.3 | 15 | Together with H2 b | Hydrophilic | 0.5 | 50 kDa, 300 kDa and 0.1 µm | 3.9 |
| Membrane Used | Gas Injected | KLa (×10−3·h−1) | TMP (bar) | H2 Consumption Yield (%) |
|---|---|---|---|---|
| Hydrophobic-0.25 m²-0.1 µm | H2 * | 1.45 ± 0.01 | <0.1 | 76 ± 1 |
| Hydrophobic-0.25 m²-0.1 µm | H2 + CO2 | 1.44 ± 0.09 | <0.1 | 63 ± 4 |
| Hydrophilic-0.25 m²-0.1 µm | H2 + CO2 | 1.69 ± 0.01 | 0.5 | 74 ± 1 |
| Hydrophilic-0.5 m²-0.1 µm | H2 + CO2 | 1.89 ± 0.10 | <0.1 | 83 ± 4 |
| Hydrophilic-0.5 m²-300 kDa | H2 + CO2 | 1.61 ± 0.05 | <0.1 | 70 ± 2 |
| Hydrophilic-0.5 m²-50 kDa | H2 + CO2 | 1.60 ± 0.03 | <0.1 | 70 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deschamps, L.; Lemaire, J.; Imatoukene, N.; Lopez, M.; Theoleyre, M.-A. Evaluation of Gas-to-Liquid Transfer with Ceramic Membrane Sparger for H2 and CO2 Fermentation. Membranes 2022, 12, 1220. https://doi.org/10.3390/membranes12121220
Deschamps L, Lemaire J, Imatoukene N, Lopez M, Theoleyre M-A. Evaluation of Gas-to-Liquid Transfer with Ceramic Membrane Sparger for H2 and CO2 Fermentation. Membranes. 2022; 12(12):1220. https://doi.org/10.3390/membranes12121220
Chicago/Turabian StyleDeschamps, Laure, Julien Lemaire, Nabila Imatoukene, Michel Lopez, and Marc-André Theoleyre. 2022. "Evaluation of Gas-to-Liquid Transfer with Ceramic Membrane Sparger for H2 and CO2 Fermentation" Membranes 12, no. 12: 1220. https://doi.org/10.3390/membranes12121220
APA StyleDeschamps, L., Lemaire, J., Imatoukene, N., Lopez, M., & Theoleyre, M.-A. (2022). Evaluation of Gas-to-Liquid Transfer with Ceramic Membrane Sparger for H2 and CO2 Fermentation. Membranes, 12(12), 1220. https://doi.org/10.3390/membranes12121220






