Buccal Thin Films as Potent Permeation Enhancers for Cytisine Transbuccal Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CYT-Loaded Buccal Films
2.3. Folding Endurance
2.4. Yield and Uniformity
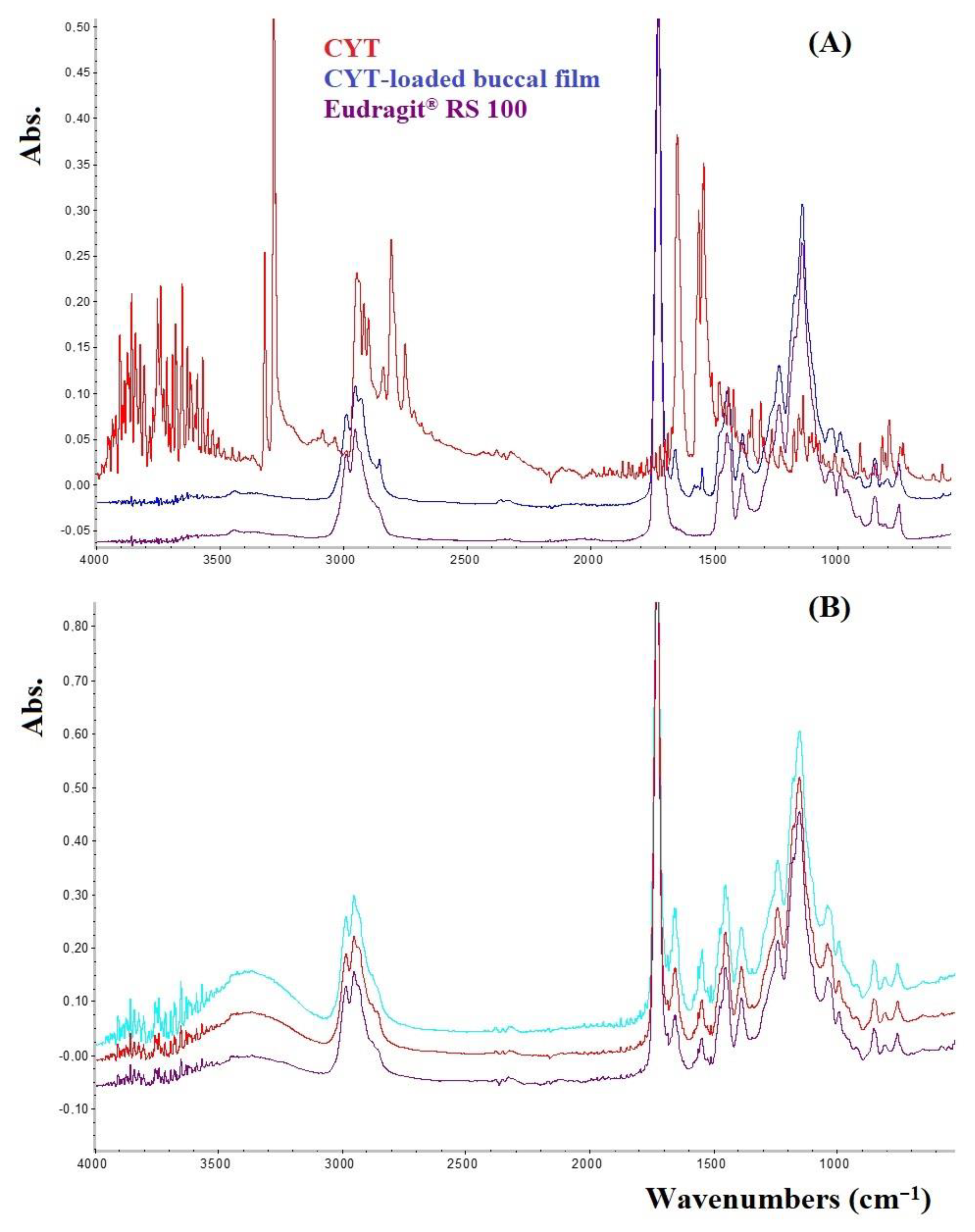
2.5. Fourier Transform Infrared Spectroscopy (FTIR) in Attenuated Total Reflectance (ATR) Mode Analysis
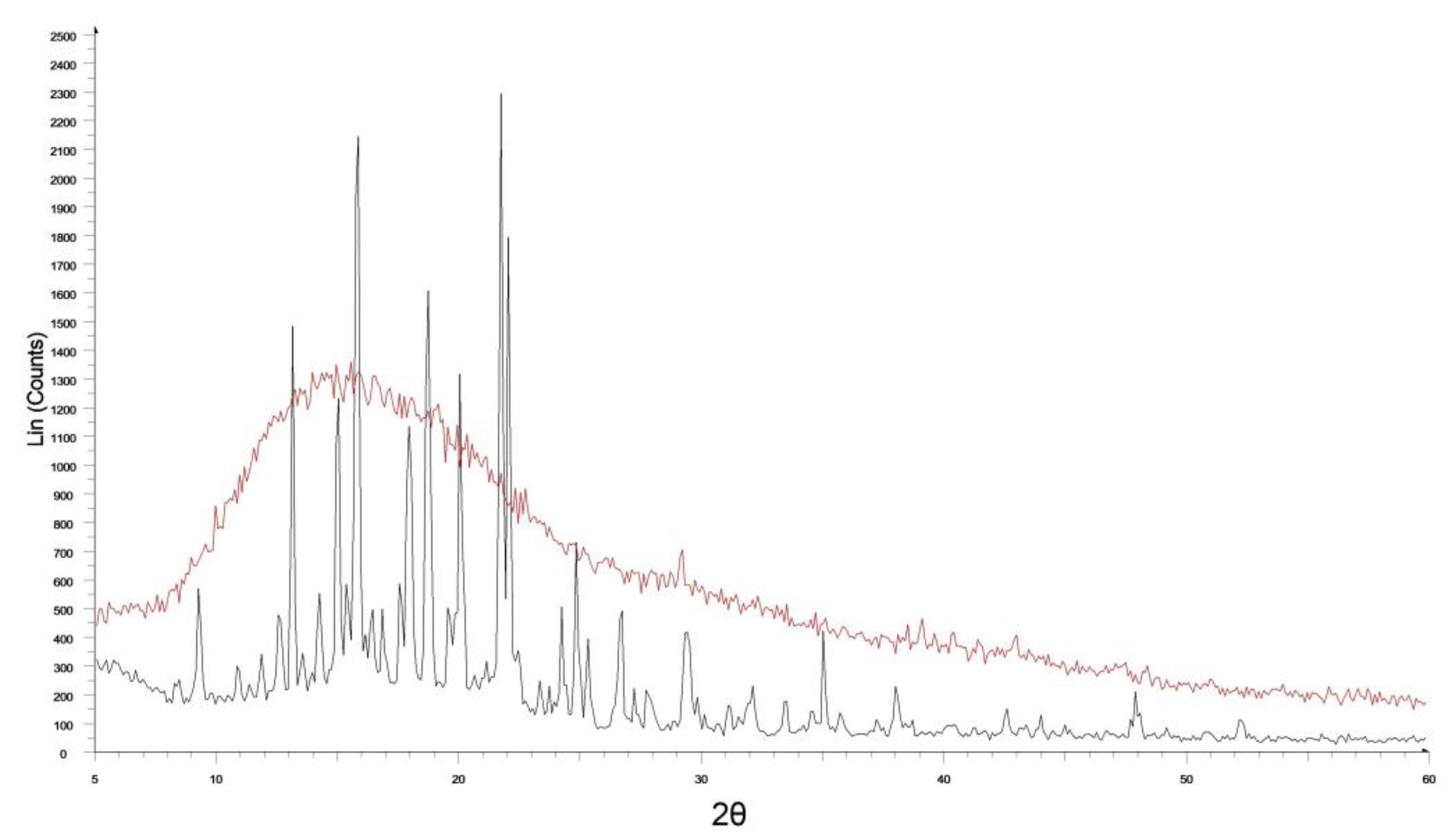
2.6. X-ray Diffraction (XRD) Evaluations
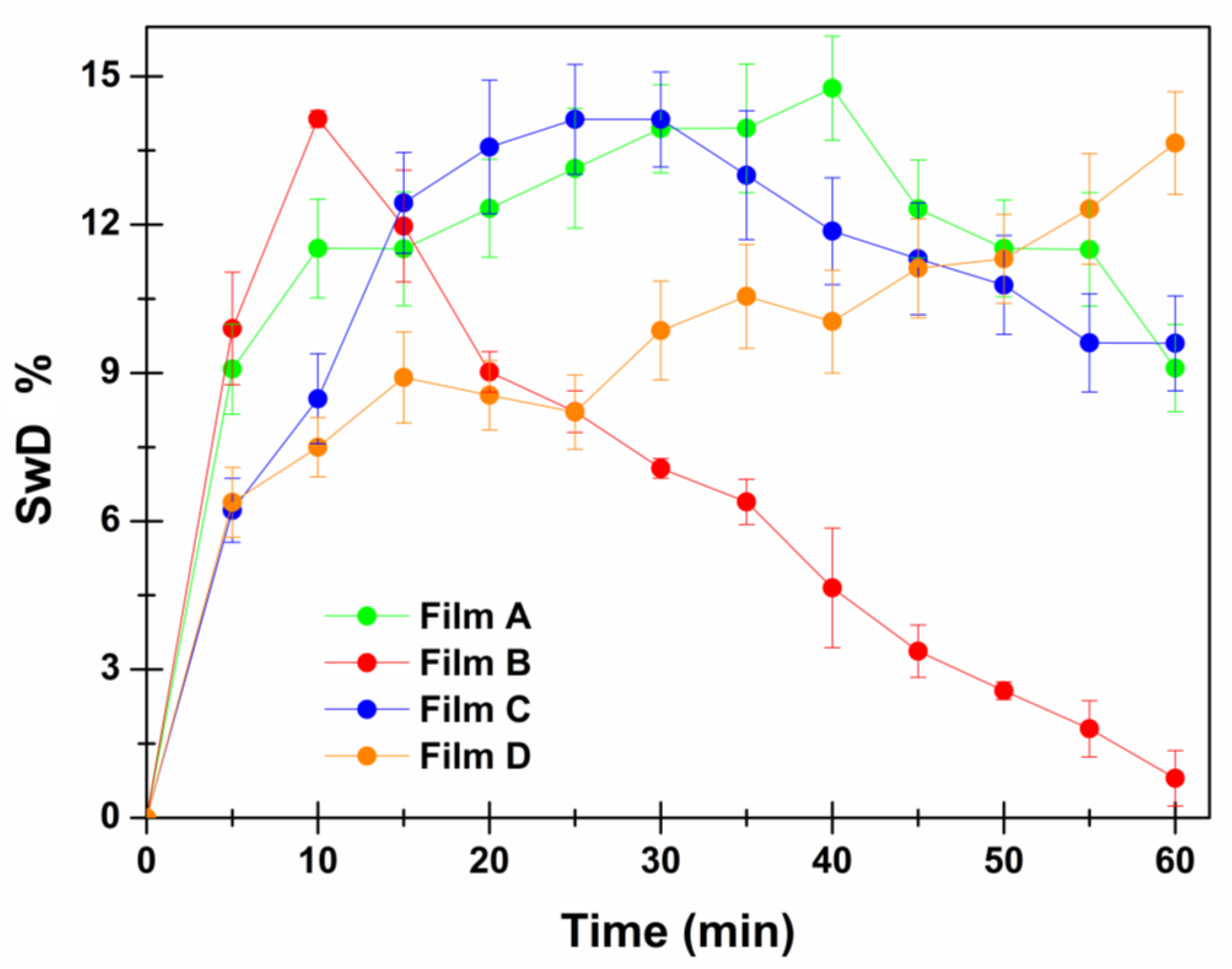
2.7. Swelling Studies
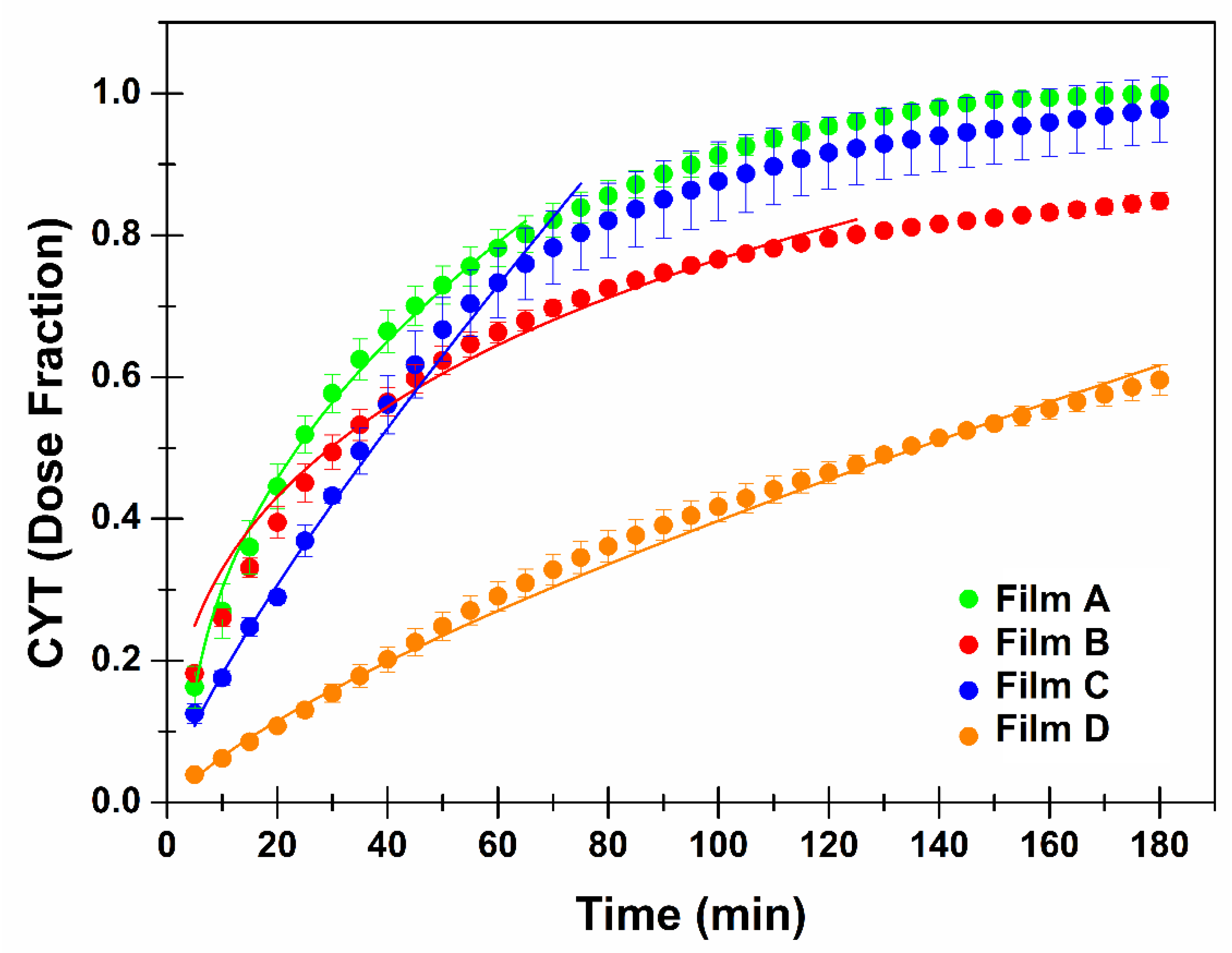
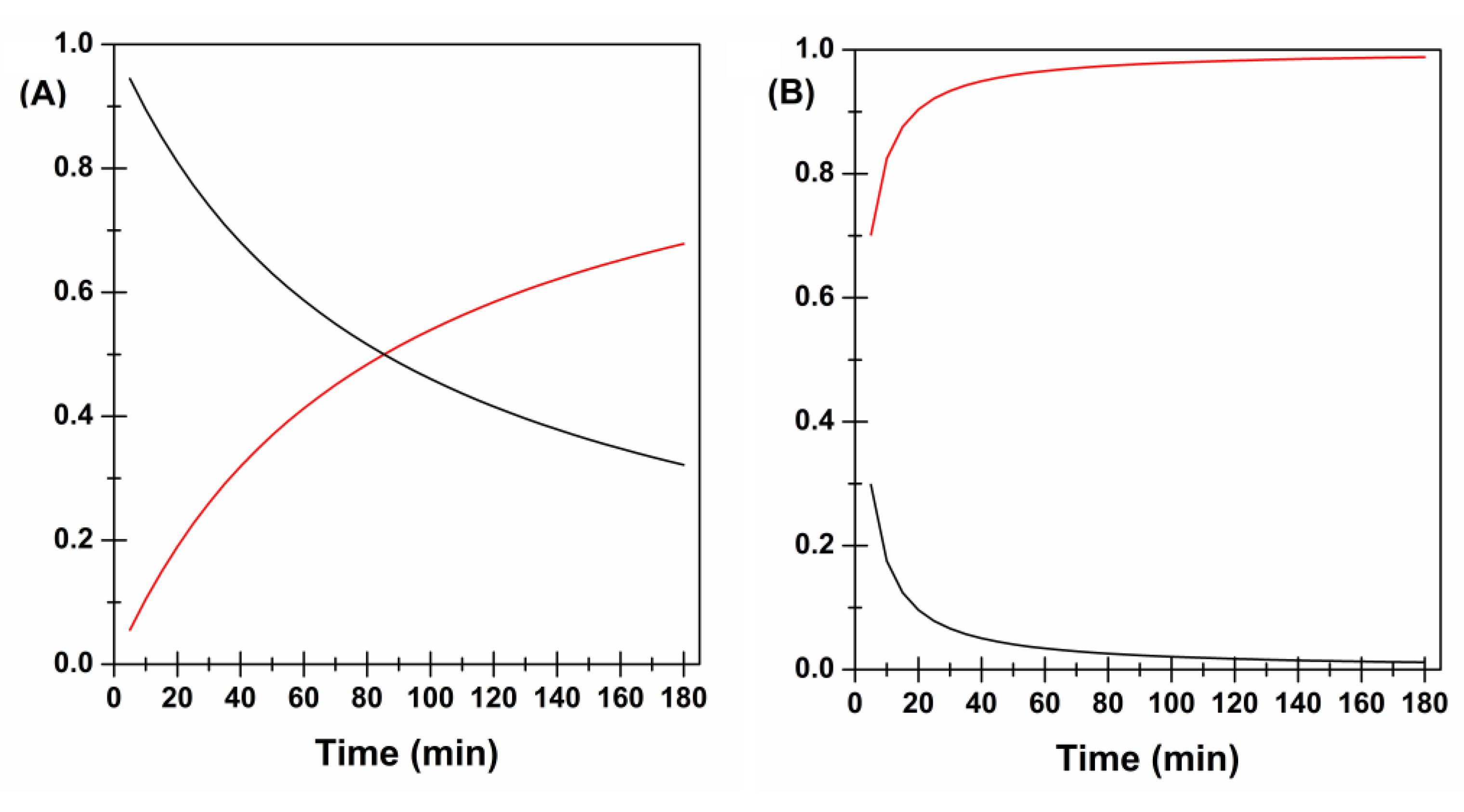
2.8. In Vitro Drug Release Studies and Kinetics Evaluations
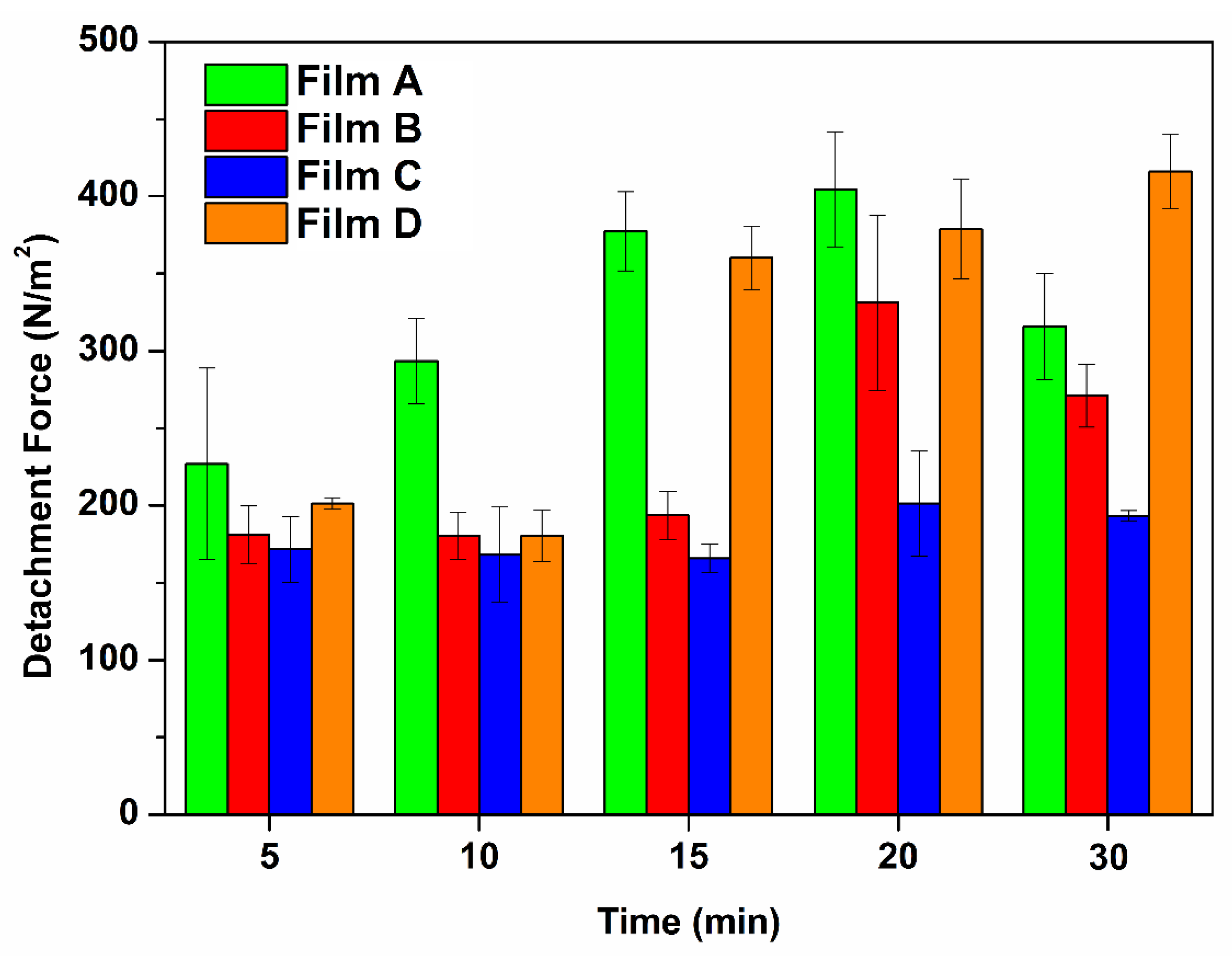
2.9. Ex Vivo Mucoadhesion Tests
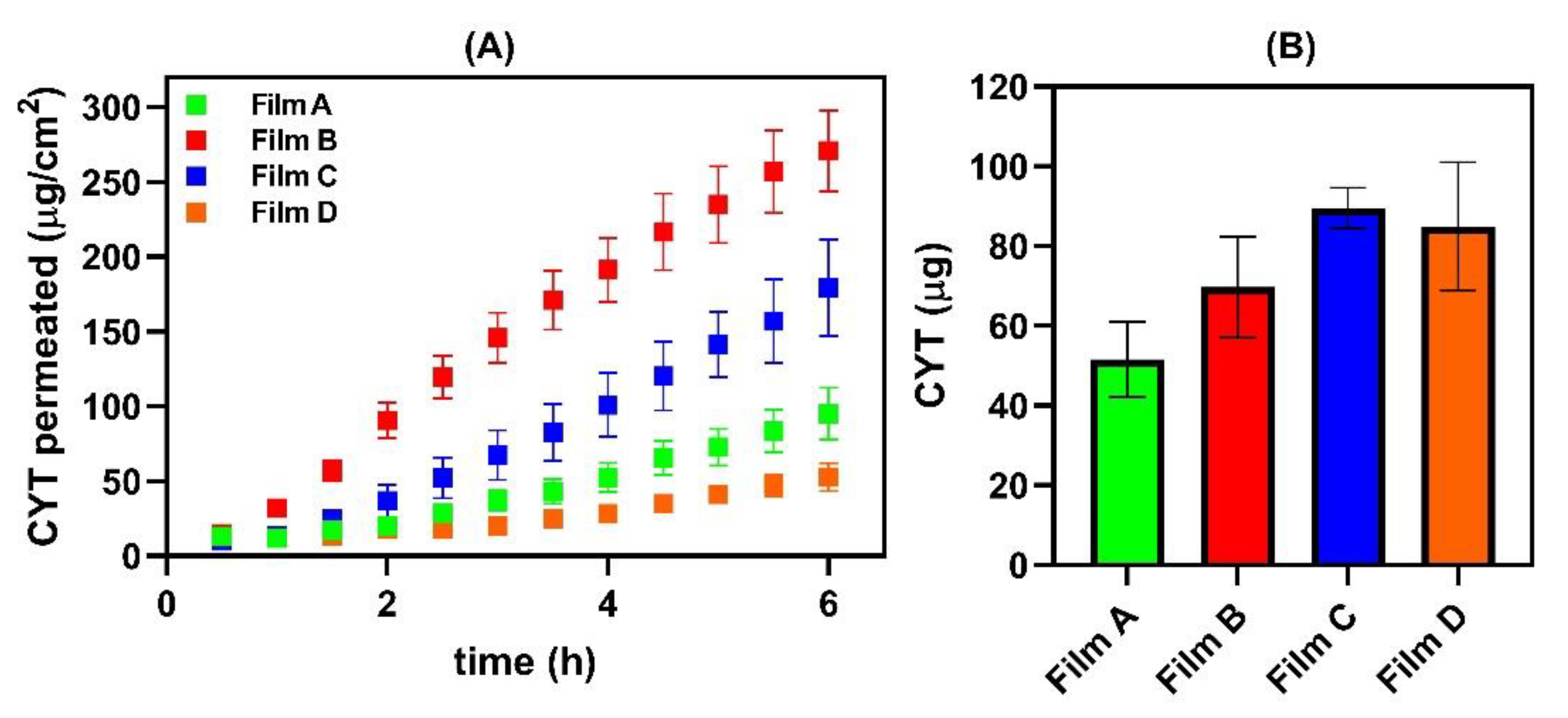
2.10. Ex Vivo Permeation/Penetration Studies through Porcine Buccal Mucosa
2.10.1. Tissue Preparation
2.10.2. Permeation Assay
2.10.3. Entrapment Studies
2.10.4. Biopharmaceutical Parameters
2.11. Data Analysis
3. Results and Discussion
3.1. Design, Preparation and Screening of CYT-Loaded Buccal Films
3.2. Characterization of the Most Promising CYT-Loaded Buccal Films
3.3. Interaction between CYT-Loaded Buccal Films and the Ex Vivo Porcine Mucosal Tissue
- The application of a 2 mg/mL CYT solution into the donor chamber in the same experimental conditions produced a drug flux (Js) of 4.94 µg/cm2∙h−1;
- The maximum CYT flux was obtained by applying a 10 mg/mL CYT solution (46.77 µg/cm2∙h−1), whereas higher concentrations did not produce an increase in flux;
- CYT Kp, when evaluating a 2 mg/mL solution, was underestimated as CYT amount was too low to observe its permeability;
- CYT Kp, when evaluating a 10 mg/mL solution, was truthful and resulted in 0.00468 cm/h.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jacob, S.; Nair, A.B.; Boddu, S.H.S.; Gorain, B.; Sreeharsha, N.; Shah, J. An Updated Overview of the Emerging Role of Patch and Film-Based Buccal Delivery Systems. Pharmaceutics 2021, 13, 1206. [Google Scholar] [CrossRef] [PubMed]
- Wanasathop, A.; Patel, P.B.; Choi, H.A.; Li, S.K. Permeability of Buccal Mucosa. Pharmaceutics 2021, 13, 1814. [Google Scholar] [CrossRef] [PubMed]
- Courtney, R.J.; McRobbie, H.; Tutka, P.; Weaver, N.A.; Petrie, D.; Mendelsohn, C.P.; Shakeshaft, A.; Talukder, S.; Macdonald, C.; Thomas, D.; et al. Effect of Cytisine vs Varenicline on Smoking Cessation: A Randomized Clinical Trial. JAMA 2021, 326, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Sheridan, J.; Bullen, C.; Newcombe, D.; Walker, N.; Tingle, M. Ascending Single Dose Pharmacokinetics of Cytisine in Healthy Adult Smokers. Xenobiotica 2019, 49, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Walker, N.; Howe, C.; Glover, M.; McRobbie, H.; Barnes, J.; Nosa, V.; Parag, V.; Bassett, B.; Bullen, C. Cytisine versus Nicotine for Smoking Cessation. N. Engl. J. Med. 2014, 371, 2353–2362. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Newcombe, D.; Sheridan, J.; Tingle, M. Pharmacokinetics of Cytisine, an A4β2 Nicotinic Receptor Partial Agonist, in Healthy Smokers Following a Single Dose. Drug Test. Anal. 2015, 7, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Angellotti, G.; Di Prima, G.; Scarpaci, A.G.; D’Agostino, F.; Campisi, G.; De Caro, V. Spray-Dried Cytisine-Loaded Matrices: Development of Transbuccal Sustained-Release Tablets as a Promising Tool in Smoking Cessation Therapy. Pharmaceutics 2022, 14, 1583. [Google Scholar] [CrossRef]
- Hassan, N.; Ahad, A.; Ali, M.; Ali, J. Chemical Permeation Enhancers for Transbuccal Drug Delivery. Expert Opin. Drug Deliv. 2010, 7, 97–112. [Google Scholar] [CrossRef]
- Seydel, J.K.; Coats, E.A.; Cordes, H.P.; Wiese, M. Drug Membrane Interaction and the Importance for Drug Transport, Distribution, Accumulation, Efficacy and Resistance. Arch. Pharm. (Weinheim) 1994, 327, 601–610. [Google Scholar] [CrossRef]
- Tran, P.H.L.; Duan, W.; Tran, T.T.D. Recent Developments of Nanoparticle-Delivered Dosage Forms for Buccal Delivery. Int. J. Pharm. 2019, 571. [Google Scholar] [CrossRef]
- Rossi, S.; Sandri, G.; Caramella, C.M. Buccal Drug Delivery: A Challenge Already Won? Drug Discov. Today. Technol. 2005, 2, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Kraisit, P.; Limmatvapirat, S.; Nunthanid, J.; Sriamornsak, P.; Luangtana-Anan, M. Preparation and Characterization of Hydroxypropyl Methylcellulose/Polycarbophil Mucoadhesive Blend Films Using a Mixture Design Approach. Chem. Pharm. Bull. 2017, 65, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Teaima, M.; Yasser, M.; Elfar, N.; Shoueir, K.; El-Nabarawi, M.; Helal, D. Construction of Sublingual Trilaminated Eszopiclone Fast Dissolving Film for the Treatment of Insomnia: Formulation, Characterization and In Vivo Clinical Comparative Pharmacokinetic Study in Healthy Human Subjects. PLoS ONE 2022, 17. [Google Scholar] [CrossRef] [PubMed]
- De Caro, V.; Scaturro, A.L.; Di Prima, G.; Avellone, G.; Sutera, F.M.; Di Fede, O.; Campisi, G.; Giannola, L.I. Aloin Delivery on Buccal Mucosa: Ex Vivo Studies and Design of a New Locoregional Dosing System. Drug Dev. Ind. Pharm. 2015, 41. [Google Scholar] [CrossRef]
- Di Prima, G.; Campisi, G.; De Caro, V. Amorphous Ropinirole-Loaded Mucoadhesive Buccal Film: A Potential Patient-Friendly Tool to Improve Drug Pharmacokinetic Profile and Effectiveness. J. Pers. Med. 2020, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- De Caro, V.; Murgia, D.; Seidita, F.; Bologna, E.; Alotta, G.; Zingales, M.; Campisi, G. Enhanced In Situ Availability of Aphanizomenon Flos-Aquae Constituents Entrapped in Buccal Films for the Treatment of Oxidative Stress-Related Oral Diseases: Biomechanical Characterization and In Vitro/Ex Vivo Evaluation. Pharmaceutics 2019, 11, 35. [Google Scholar] [CrossRef]
- De Caro, V.; Giandalia, G.; Siragusa, M.G.; Sutera, F.M.; Giannola, L.I. New Prospective in Treatment of Parkinson’s Disease: Studies on Permeation of Ropinirole through Buccal Mucosa. Int. J. Pharm. 2012, 429, 78–83. [Google Scholar] [CrossRef]
- Del Consuelo, I.D.; Pizzolato, G.P.; Falson, F.; Guy, R.H.; Jacques, Y. Evaluation of Pig Esophageal Mucosa as a Permeability Barrier Model for Buccal Tissue. J. Pharm. Sci. 2005, 94, 2777–2788. [Google Scholar] [CrossRef]
- De Caro, V.; Giannola, L.I.; Di Prima, G. Solid and Semisolid Innovative Formulations Containing Miconazole-Loaded Solid Lipid Microparticles to Promote Drug Entrapment into the Buccal Mucosa. Pharmaceutics 2021, 13, 1361. [Google Scholar] [CrossRef]
- Di Prima, G.; Bongiovì, F.; Palumbo, F.S.; Pitarresi, G.; Licciardi, M.; Giammona, G. Mucoadhesive PEGylated Inulin-Based Self-Assembling Nanoparticles: In Vitro and Ex Vivo Transcorneal Permeation Enhancement of Corticosteroids. J. Drug Deliv. Sci. Technol. 2019, 49, 195–208. [Google Scholar] [CrossRef]
- Di Prima, G.; Licciardi, M.; Bongiovì, F.; Pitarresi, G.; Giammona, G. Inulin-Based Polymeric Micelles Functionalized with Ocular Permeation Enhancers: Improvement of Dexamethasone Permeation/Penetration through Bovine Corneas. Pharmaceutics 2021, 13, 1431. [Google Scholar] [CrossRef] [PubMed]
- Chaves, P.D.S.; Frank, L.A.; Frank, A.G.; Pohlmann, A.R.; Guterres, S.S.; Beck, R.C.R. Mucoadhesive Properties of Eudragit®RS100, Eudragit®S100, and Poly(ε-Caprolactone) Nanocapsules: Influence of the Vehicle and the Mucosal Surface. AAPS Pharm. Sci. Tech. 2018, 19, 1637–1646. [Google Scholar] [CrossRef] [PubMed]
- Patra, C.N.; Priya, R.; Swain, S.; Kumar Jena, G.; Panigrahi, K.C.; Ghose, D. Pharmaceutical Significance of Eudragit: A Review. Futur. J. Pharm. Sci. 2017, 3, 33–45. [Google Scholar] [CrossRef]
- Di Prima, G.; Conigliaro, A.; De Caro, V. Mucoadhesive Polymeric Films to Enhance Barbaloin Penetration Into Buccal Mucosa: A Novel Approach to Chemoprevention. AAPS Pharm. Sci. Tech. 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Innocenzi, P.; Malfatti, L.; Costacurta, S.; Kidchob, T.; Piccinini, M.; Marcelli, A. Evaporation of Ethanol and Ethanol-Water Mixtures Studied by Time-Resolved Infrared Spectroscopy. J. Phys. Chem. A 2008, 112, 6512–6516. [Google Scholar] [CrossRef] [PubMed]
- Semalty, M.; Semalty, A.; Kumar, G. Formulation and Characterization of Mucoadhesive Buccal Films of Glipizide. Indian J. Pharm. Sci. 2008, 70, 43–48. [Google Scholar] [CrossRef]
- Kurakula, M.; Rao, G.S.N.K. Pharmaceutical Assessment of Polyvinylpyrrolidone (PVP): As Excipient from Conventional to Controlled Delivery Systems with a Spotlight on COVID-19 Inhibition. J. Drug Deliv. Sci. Technol. 2020, 60. [Google Scholar] [CrossRef] [PubMed]
- Di Prima, G.; Angellotti, G.; Scarpaci, A.G.; Murgia, D.; D’agostino, F.; Campisi, G.; De Caro, V. Improvement of Resveratrol Permeation through Sublingual Mucosa: Chemical Permeation Enhancers versus Spray Drying Technique to Obtain Fast-Disintegrating Sublingual Mini-Tablets. Pharmaceutics 2021, 13, 1370. [Google Scholar] [CrossRef]
- Wu, X.; Desai, K.G.H.; Mallery, S.R.; Holpuch, A.S.; Phelps, M.P.; Schwendeman, S.P. Mucoadhesive Fenretinide Patches for Site-Specific Chemoprevention of Oral Cancer: Enhancement of Oral Mucosal Permeation of Fenretinide by Coincorporation of Propylene Glycol and Menthol. Mol. Pharm. 2012, 9, 937–945. [Google Scholar] [CrossRef]
- Serra, L.; Doménech, J.; Peppas, N.A. Design of Poly(Ethylene Glycol)-Tethered Copolymers as Novel Mucoadhesive Drug Delivery Systems. Eur. J. Pharm. Biopharm. 2006, 63, 11–18. [Google Scholar] [CrossRef]
- Safdari, F.; Carreau, P.J.; Heuzey, M.C.; Kamal, M.R. Effects of Poly(Ethylene Glycol) on the Morphology and Properties of Biocomposites Based on Polylactide and Cellulose Nanofibers. Cellulose 2017, 24, 2877–2893. [Google Scholar] [CrossRef]
- Salli, K.; Lehtinen, M.J.; Tiihonen, K.; Ouwehand, A.C. Xylitol’s Health Benefits beyond Dental Health: A Comprehensive Review. Nutrients 2019, 11, 1813. [Google Scholar] [CrossRef] [PubMed]
- Przybył, A.K.; Grzeskiewicz, A.M.; Kubicki, M. Weak Interactions in the Structures of Newly Synthesized (–)-Cytisine Amino Acid Derivatives. Crystals 2021, 11, 146. [Google Scholar] [CrossRef]
- De Caro, V.; Ajovalasit, A.; Sutera, F.; Murgia, D.; Sabatino, M.; Dispenza, C. Development and Characterization of an Amorphous Solid Dispersion of Furosemide in the Form of a Sublingual Bioadhesive Film to Enhance Bioavailability. Pharmaceutics 2017, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A Simple Equation for the Description of Solute Release. III. Coupling of Diffusion and Relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of Solute Release from Porous Hydrophilic Polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Alaei, S.; Omidian, H. Mucoadhesion and Mechanical Assessment of Oral Films. Eur. J. Pharm. Sci. 2021, 159, 105727. [Google Scholar] [CrossRef]
- Itin, C.; Domb, A.J.; Hoffman, A. On the Suitability of Porcine Labial Mucosa as a Model for Buccal Mucosal Drug Delivery Research. J. Pharm. Sci. 2021, 110, 1863–1864. [Google Scholar] [CrossRef]
- Meng-Lund, E.; Marxen, E.; Pedersen, A.M.L.; Müllertz, A.; Hyrup, B.; Holm, R.; Jacobsen, J. Ex Vivo Correlation of the Permeability of Metoprolol Across Human and Porcine Buccal Mucosa. J. Pharm. Sci. 2014, 103, 2053–2061. [Google Scholar] [CrossRef]

| Mucoadhesion Agents | Plasticizers | Others | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Formula Code | Eudragit® RS100 | PVP K90 | PVP K30 | PVP CLM | PVA | GL | TRC | PG | PEG200 | PEG1000 | SOR | XYL | CYT | EtOH |
| FE-1 | 790 | 60 | - | - | - | 100 | - | - | - | - | - | - | 50 | 15 |
| FE-2 | 790 | 60 | - | - | - | 50 | 50 | - | - | - | - | - | 50 | 12.5 |
| FE-3 | 740 | - | - | - | 60 | 100 | 50 | - | - | - | - | - | 50 | 20 |
| FE-4 | 540 | - | 260 | - | - | - | 50 | 100 | - | - | - | - | 50 | 12 |
| FE-5 | 500 | - | 260 | - | - | - | 50 | 100 | - | - | 40 | - | 50 | 12 |
| FE-6 | 550 | - | 260 | - | - | - | - | 100 | - | - | 40 | - | 50 | 12 |
| FE-7 | 400 | - | 410 | - | - | - | - | 100 | - | - | 40 | - | 50 | 10 |
| FE-8 | 550 | - | - | 260 | - | - | - | 100 | - | - | 40 | - | 50 | 10 |
| FE-9 | 638 | - | - | - | 212 | - | - | 100 | - | - | - | - | 50 | 10 |
| FE-10 | 750 | 60 | - | - | - | - | - | 100 | - | - | 40 | - | 50 | 15 |
| FE-11 | 750 | 40 | - | - | - | - | - | 100 | 60 | - | - | - | 50 | 15 |
| FE-12 | 750 | - | 40 | - | - | - | - | 100 | 60 | - | - | - | 50 | 16 |
| FE-13 | 750 | - | - | - | - | - | - | 100 | 100 | - | - | - | 50 | 10 |
| FE-14 | 425 | - | - | - | - | - | - | 100 | - | 425 | - | - | 50 | 10 |
| FE-15 | 567 | - | - | - | - | - | - | 100 | - | 283 | - | - | 50 | 10 |
| FE-16 | 638 | - | - | - | - | - | - | 100 | - | 212 | - | - | 50 | 10 |
| FE-17 | 750 | - | - | - | - | - | - | 100 | - | 100 | - | - | 50 | 12 |
| FE-18 | 700 | - | - | - | - | - | - | 100 | - | 100 | - | 50 | 50 | 12 |
| Mucoadhesive Agent | Plasticizers | Others | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Formula Code | Eudragit® RS100 | PVP K90 | GL | TRC | TC | PG | PEG200 | PEG1000 | SOR | XYL | CYT | MeOH |
| FM-1 | 790 | 60 | 100 | - | - | - | - | - | - | - | 50 | 9 |
| FM-2 | 740 | 60 | 150 | - | - | - | - | - | - | - | 50 | 8 |
| FM-3 | 740 | 60 | 100 | - | 50 | - | - | - | - | - | 50 | 17.5 |
| FM-4 | 740 | 60 | 100 | 50 | - | - | - | - | - | - | 50 | 17.5 + 0.2 mL acetic acid |
| FM-5 | 740 | 60 | 50 | 100 | - | - | - | - | - | - | 50 | 15 |
| FM-6 | 740 | 60 | 100 | 50 | - | - | - | - | - | - | 50 | 12 |
| FM-7 | 740 | 20 | 120 | 70 | - | - | - | - | - | - | 50 | 15 |
| FM-8 | 740 | 60 | 100 | 50 | - | - | - | - | - | - | 50 | 20 |
| FM-9 | 790 | 60 | 50 | 50 | - | - | - | - | - | - | 50 | 7.5 |
| FM-10 | 790 | 60 | - | 100 | - | - | - | - | - | - | 50 | 9 |
| FM-11 | 590 | 60 | - | 100 | - | - | 200 | - | - | - | 50 | 9 |
| FM-12 | 590 | 60 | - | 100 | - | 200 | - | - | - | - | 50 | 6 |
| FM-13 | 690 | 60 | - | 100 | - | 100 | - | - | - | - | 50 | 9 |
| FM-14 | 790 | 60 | - | 50 | - | 50 | - | - | - | - | 50 | 5 |
| FM-15 | 740 | 60 | - | - | - | 150 | - | - | - | - | 50 | 7 |
| FM-16 | 570 | 100 | - | - | - | 200 | - | - | 80 | - | 50 | 7 |
| FM-17 | 650 | 50 | - | - | - | 100 | - | 100 | - | 50 | 50 | 23 + 2 mL H2O |
| FM-18 | 700 | 50 | - | - | - | 100 | - | 100 | - | 50 | 23 + 2 mL H2O | |
| Formula Code | Denomination | Eudragit® RS100 | PVP K90 | Propylene Glycol | PEG1000 | Xylitol | Cytisine | Solvent(s) |
|---|---|---|---|---|---|---|---|---|
| FE-17 | Film A | 750 | - | 100 | 100 | - | 50 | 12 mL EtOH |
| FE-18 | Film B | 700 | - | 100 | 100 | 50 | 50 | 12 mL EtOH |
| FM-17 | Film C | 650 | 50 | 100 | 100 | 50 | 50 | 23 mL MeOH + 2 mL H2O |
| FM-18 | Film D | 700 | 50 | 100 | 100 | - | 50 | 23 mL MeOH + 2 mL H2O |
| Formulation | Yield (%) | Weight (mg) | Thickness (mm) | CYT Content (mg) | DL% * | LE % |
|---|---|---|---|---|---|---|
| Film A | 93.9 ± 2.1 | 15.56 ± 1.09 | 0.59 ± 0.04 | 0.75 ± 0.05 | 4.82 ± 0.14 | 96.48 ± 2.83 |
| Film B | 95.8 ± 1.8 | 17.87 ± 0.42 | 0.48 ± 0.04 | 0.84 ± 0.02 | 4.71 ± 0.13 | 94.27 ± 2.57 |
| Film C | 95.8 ± 2.2 | 17.35 ± 1.17 | 0.48 ± 0.04 | 0.96 ± 0.06 | 5.53 ± 0.15 | 110.60 ± 3.00 |
| Film D | 94.9 ± 1.6 | 18.12 ± 0.33 | 0.44 ± 0.08 | 0.83 ± 0.02 | 4.60 ± 0.10 | 92.00 ± 2.00 |
| Swelling Degree % | ||||
|---|---|---|---|---|
| Time (min) | Film A | Film B | Film C | Film D |
| 5 | 9.08 ± 0.91 | 9.90 ± 1.14 | 6.22 ± 0.65 | 6.38 ± 0.71 |
| 10 | 11.52 ± 1.00 | 14.14 ± 0.17 | 8.48 ± 0.91 | 5.50 ± 0.60 |
| 15 | 11.51 ± 1.15 | 11.97 ± 1.13 | 12.44 ± 1.02 | 8.91 ±0.92 |
| 20 | 12.33 ± 0.99 | 9.02 ± 0.41 | 13.57 ± 1.35 | 8.55 ± 0.70 |
| 25 | 13.14 ± 1.21 | 8.22 ± 0.42 | 14.13 ± 1.11 | 8.21 ± 0.75 |
| 30 | 13.94 ± 0.89 | 7.07 ± 0.20 | 14.13 ± 0.96 | 9.86 ± 1.00 |
| 35 | 13.95 ± 1.30 | 6.39 ± 0.46 | 13.00 ± 1.30 | 10.55 ± 1.05 |
| 40 | 14.76 ± 1.05 | 4.65 ± 1.21 | 11.87 ± 1.08 | 10.04 ± 1.04 |
| 45 | 12.32 ± 0.99 | 3.37 ± 0.59 | 11.31 ± 1.13 | 11.12 ± 1.00 |
| 50 | 11.52 ± 0.98 | 0.57 ± 0.18 | 10.78 ± 1.00 | 11.31 ± 0.90 |
| 55 | 11.50 ± 1.15 | 1.80 ± 0.57 | 9.61 ± 0.99 | 12.32 ± 1.12 |
| 60 | 9.10 ± 0.88 | 0.80 ± 0.56 | 9.60 ± 0.96 | 13.65 ± 1.04 |
| Applied Mathematical Model | Film A | Film B | Film C | Film D |
|---|---|---|---|---|
| Zero Order Mt/M∞ = k × t | k = 0.0147 ± 0.0008 R2 = 0.655 | k = 0.0077 ± 0.0001 R2 = 0.472 | k = 0.0138 ± 0.0005 R2 = 0.917 | k = 0.0036 ± 0.0001 R2 = 0.9942 |
| First Order Mt/M∞ = FMAX × (1 − e −k × t) | k = 0.0272 ± 0.0005 R2 = 0.986 | k = 0.0232 ± 0.0002 R2 = 0.975 | k = 0.0190 ± 0.0004 R2 = 0.979 | k = 0.0052 ± 0.0001 R2 = 0.9990 |
| Higuchi Mt/M∞ = k × t0.5 | k = 0.1013 ± 0.0011 R2 = 0.981 | k = 0.0766 ± 0.0002 R2 = 0.942 | k = 0.0742 ± 0.0033 R2 = 0.861 | k = 0.0435 ± 0.0002 R2 = 0.9720 |
| Korsmeyer-Peppas (Power Law) Mt/M∞ = k × tn | k = 0.0951 ± 0.0097 n = 0.516 ± 0.026 R2 = 0.980 | k = 0.1295 ±0.0138 n = 0.386 ± 0.023 R2 = 0.969 | k = 0.0296 ± 0.0024 n = 0.781 ± 0.023 R2 = 0.988 | k = 0.0109 ± 0.0004 n = 0.778 ± 0.008 R2 = 0.9996 |
| Korsmeyer-Peppas (Power Law) considering Tlag Mt/M∞ = k × (t − Tlag)n | k = 0.1324 ± 0.0112 Tlag = 3.58 ± 0.48 n = 0.443 ± 0.021 R2 = 0.993 | k = 0.3745 ± 0.1705 Tlag = 25.87 ± 20.42 n = 0.166 ± 0.097 R2 = 0.848 | k = 0.0249 ± 0.0065 Tlag = -1.24 ± 1.75 n = 0.821 ± 0.061 R2 = 0.988 | k = 0.0134 ± 0.0012 Tlag = 1.59 ± 0.59 n = 0.739 ± 0.017 R2 = 0.9997 |
| Hixson-Crowell Mt/M∞ = 1 × [1 − (1 − k × t)3] | k = 0.0074 ± 0.0003 R2 = 0.935 | k = 0.0038 ± 0.0001 R2 = 0.853 | k = 0.0058 ± 0.0001 R2 = 0.985 | k = 0.0015 ± 0.0001 R2 = 0.9983 |
| Peppas-Sahlin Mt/M∞ = k1 × tm + k2 × t2m (m = 0.43) * | k1 = 0.0984 ± 0.0104 k2 = 0.0067 ± 0.0020 R2 = 0.977 | k1 = 0.1321 ± 0.0067 k2 =-0.0036 ± 0.0009 R2 = 0.979 | k1 = 0.0170 ± 0.0050 k2 = 0.0186 ± 0.0012 R2 = 0.989 | k1 = 0.0070 ± 0.0009 k2 = 0.0065 ± 0.0001 R2 = 0.9995 |
| Contact Preload Time (min) | Formulation | ||||
|---|---|---|---|---|---|
| Film A | Film B | Film C | Film D | ||
| 5 | N | 0.0302 ± 0.0082 | 0.0241 ± 0.0025 | 0.0228 ± 0.0028 | 0.0268 ± 0.0005 |
| N/m2 | 226.85 ± 61.92 | 181.01 ± 18.82 | 171.64 ± 21.07 | 201.36 ± 3.69 | |
| 10 | N | 0.0390 ± 0.0037 | 0.0240 ± 0.0020 | 0.0224 ± 0.0041 | 0.0240 ± 0.0022 |
| N/m2 | 293.44 ± 27.77 | 180.47 ± 15.05 | 168.12 ± 30.61 | 180.38 ± 16.63 | |
| 15 | N | 0.0502 ± 0.0034 | 0.0258 ± 0.0021 | 0.0221 ± 0.0013 | 0.0479 ± 0.0027 |
| N/m2 | 377.43 ± 25.53 | 193.59 ± 15.57 | 165.89 ± 9.44 | 360.28 ± 20.54 | |
| 20 | N | 0.0538 ± 0.0050 | 0.0441 ± 0.0076 | 0.0268 ± 0.0045 | 0.0504 ± 0.0043 |
| N/m2 | 404.50 ± 37.32 | 331.25 ± 56.89 | 201.27 ± 33.93 | 378.76 ± 32.45 | |
| 30 | N | 0.0420 ± 0.0046 | 0.0361 ± 0.0027 | 0.0257 ± 0.0004 | 0.0553 ± 0.0032 |
| N/m2 | 315.73 ± 34.19 | 271.10 ± 20.54 | 193.36 ± 3.28 | 416.10 ± 24.04 | |
| Sample | Js (µg/cm2∙h−1) | Kp (cm/h) | De (µg/cm2) | [CYT]DONOR (mg/mL) | Lag Time (min) |
|---|---|---|---|---|---|
| Film A | 18.61 ± 3.23 | 0.01918 ± 0.00465 | 81.03 ± 70.80 | 0.97 ± 0.12 | 59 |
| Film B | 52.33 ± 6.45 | 0.04961 ± 0.00611 | 109.59 ± 19.89 | 1.06 ± 0.15 | 17 |
| Film C | 35.70 ± 5.97 | 0.03143 ± 0.06780 | 140.78 ± 8.03 | 1.14 ± 0.20 | 64 |
| Film D | 10.40 ± 2.60 | 0.01040 ± 0.00230 | 133.65 ± 40.97 | 0.81 ± 0.11 | NO |
| CYT 2 mg/mL solution * | 4.94 ± 0.16 | 0.00247 ± 0.00001 | 164.72 ± 13.12 | 2.00 | NO |
| CYT 10 mg/mL solution * | 46.77 ± 6.18 | 0.00468 ± 0.00062 | 559.30 ± 93.66 | 10.00 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Caro, V.; Angellotti, G.; D’Agostino, F.; Di Prima, G. Buccal Thin Films as Potent Permeation Enhancers for Cytisine Transbuccal Delivery. Membranes 2022, 12, 1169. https://doi.org/10.3390/membranes12111169
De Caro V, Angellotti G, D’Agostino F, Di Prima G. Buccal Thin Films as Potent Permeation Enhancers for Cytisine Transbuccal Delivery. Membranes. 2022; 12(11):1169. https://doi.org/10.3390/membranes12111169
Chicago/Turabian StyleDe Caro, Viviana, Giuseppe Angellotti, Fabio D’Agostino, and Giulia Di Prima. 2022. "Buccal Thin Films as Potent Permeation Enhancers for Cytisine Transbuccal Delivery" Membranes 12, no. 11: 1169. https://doi.org/10.3390/membranes12111169
APA StyleDe Caro, V., Angellotti, G., D’Agostino, F., & Di Prima, G. (2022). Buccal Thin Films as Potent Permeation Enhancers for Cytisine Transbuccal Delivery. Membranes, 12(11), 1169. https://doi.org/10.3390/membranes12111169









