Interfacial Tailoring of Polyether Sulfone-Modified Silica Mixed Matrix Membranes for CO2 Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Mixed Matrix Membranes (MMMs)
2.3. Characterization Techniques
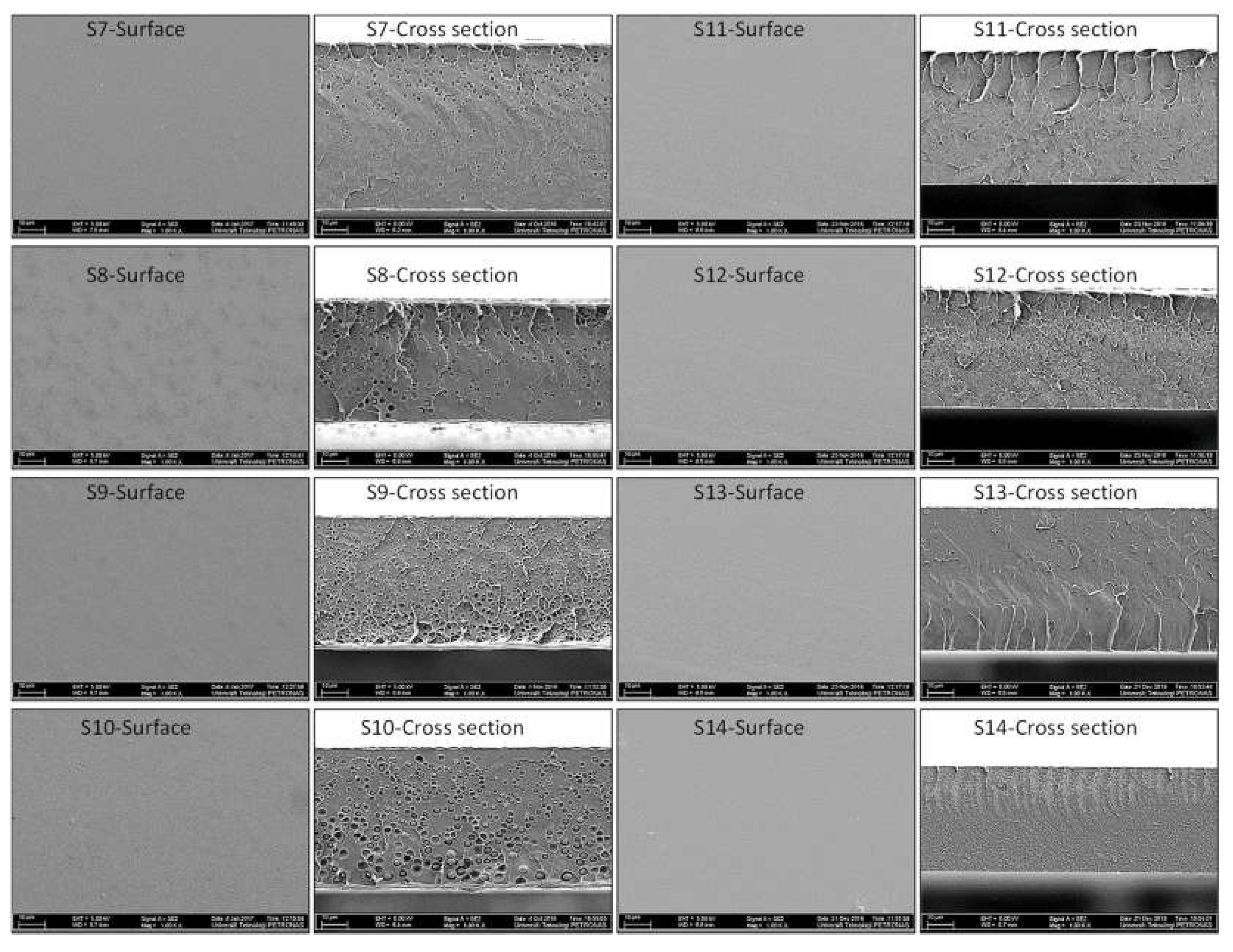
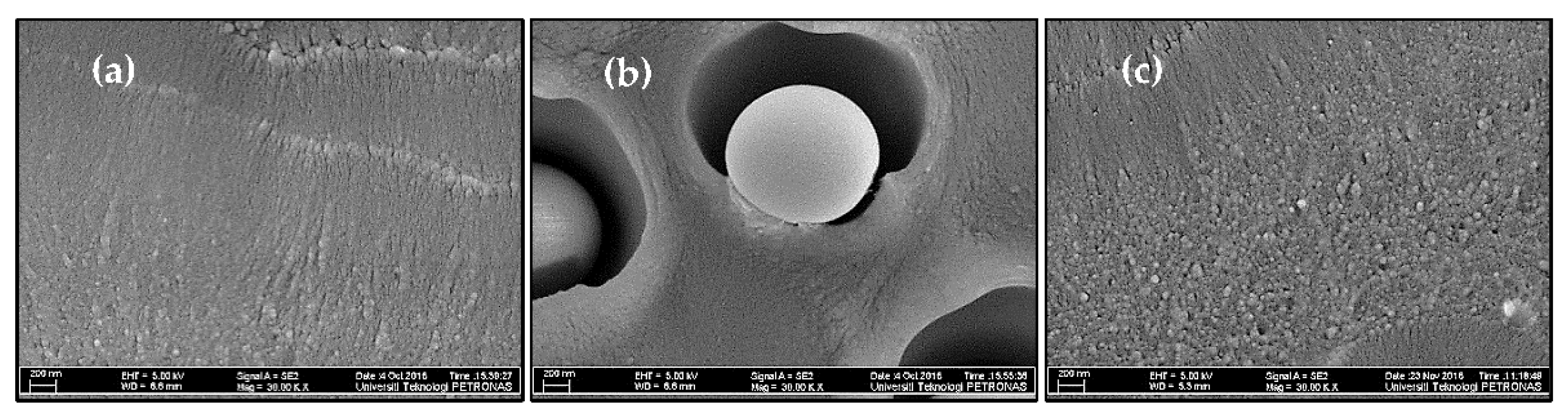
2.3.1. Morphological Analysis
2.3.2. Spectral Analysis
2.3.3. Thermal Analysis
2.3.4. X-ray Diffraction (XRD)
2.4. Performance Analysis
3. Results and Discussion
3.1. Morphological Analysis
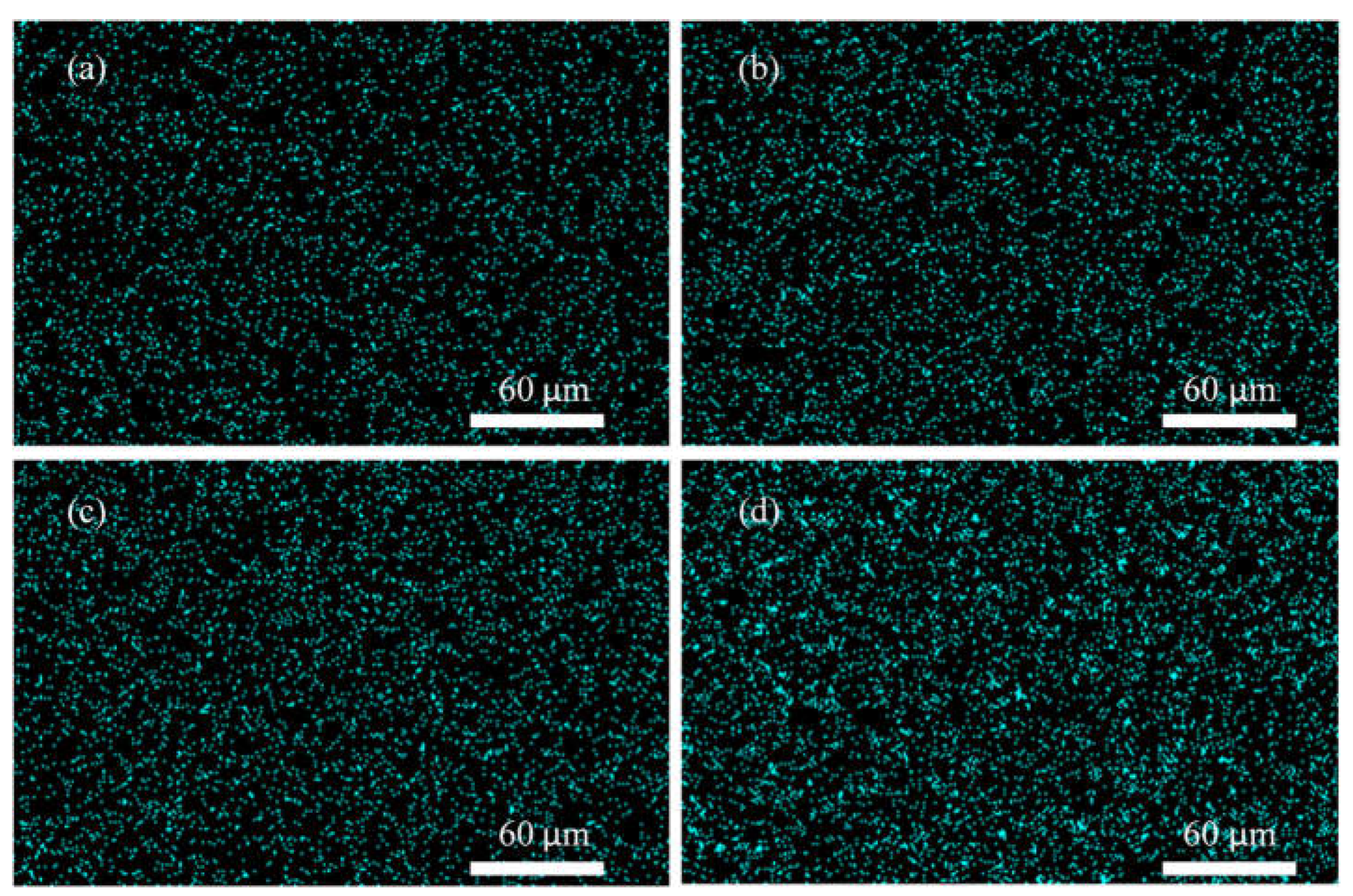
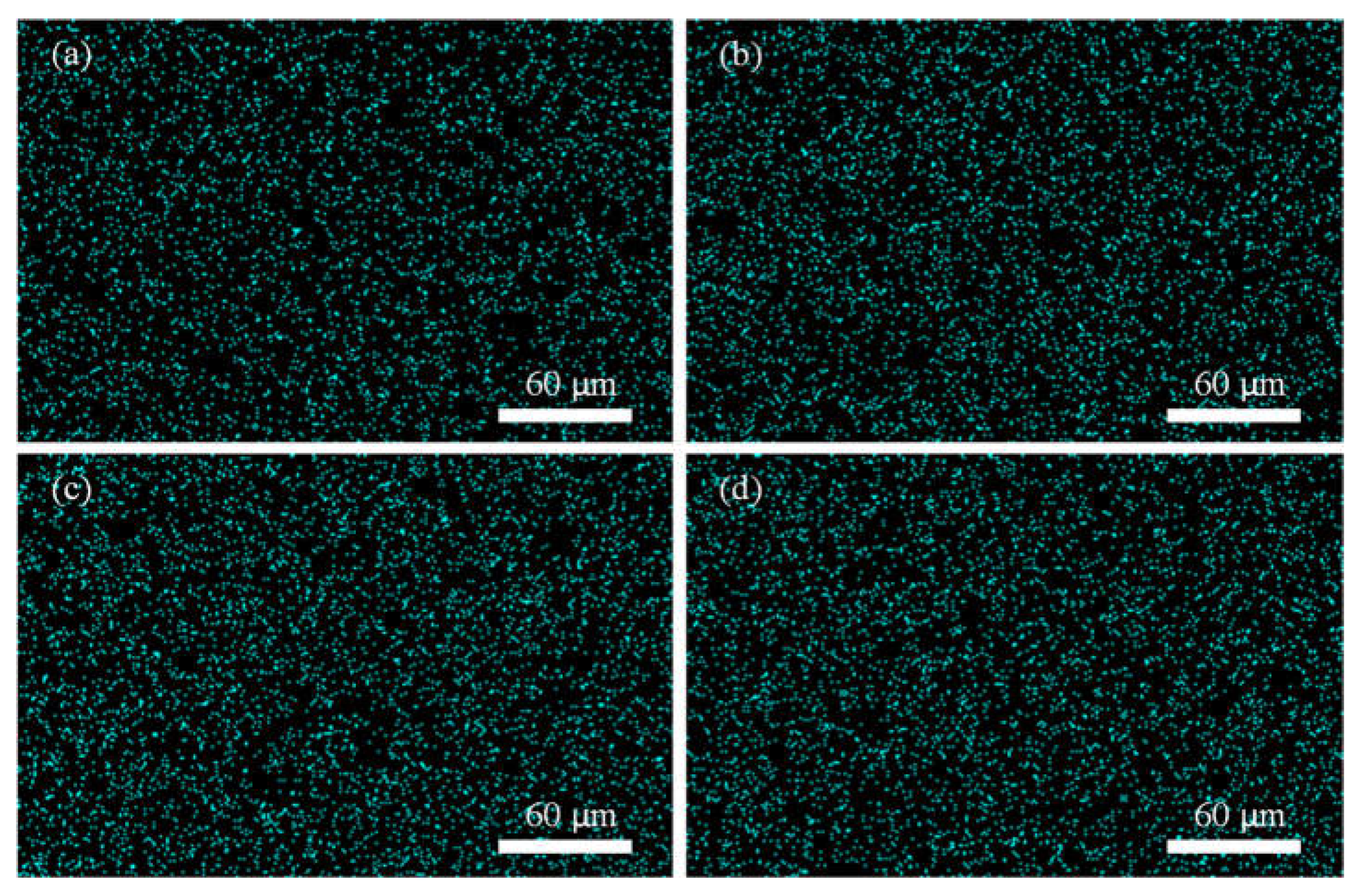
3.2. EDX Mapping Analysis
3.3. Structural Analysis
3.4. Thermal Stability Analysis
3.5. DSC Analysis
3.6. Gas Permeation Performance
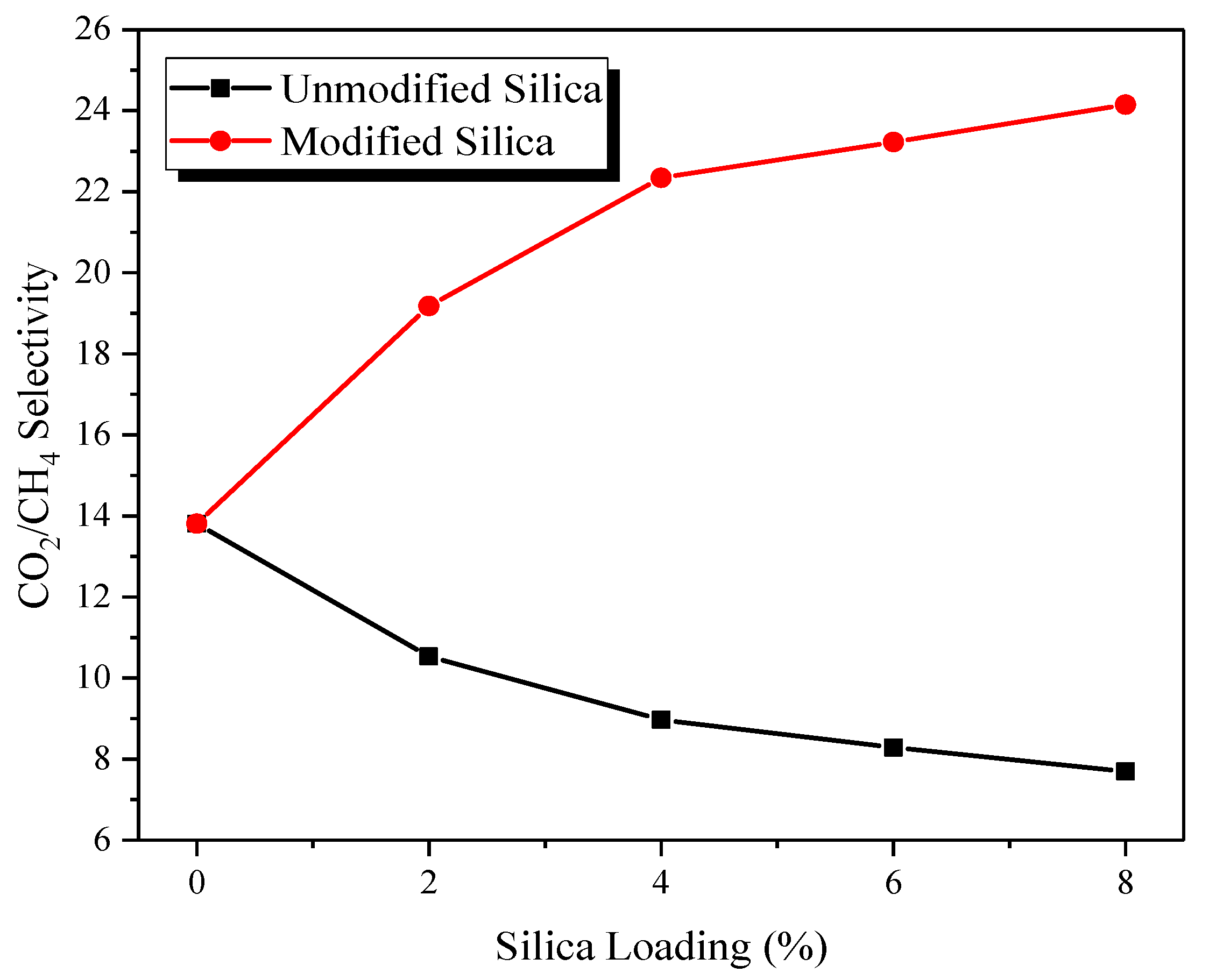
3.6.1. Effect of Modified Silica Loading
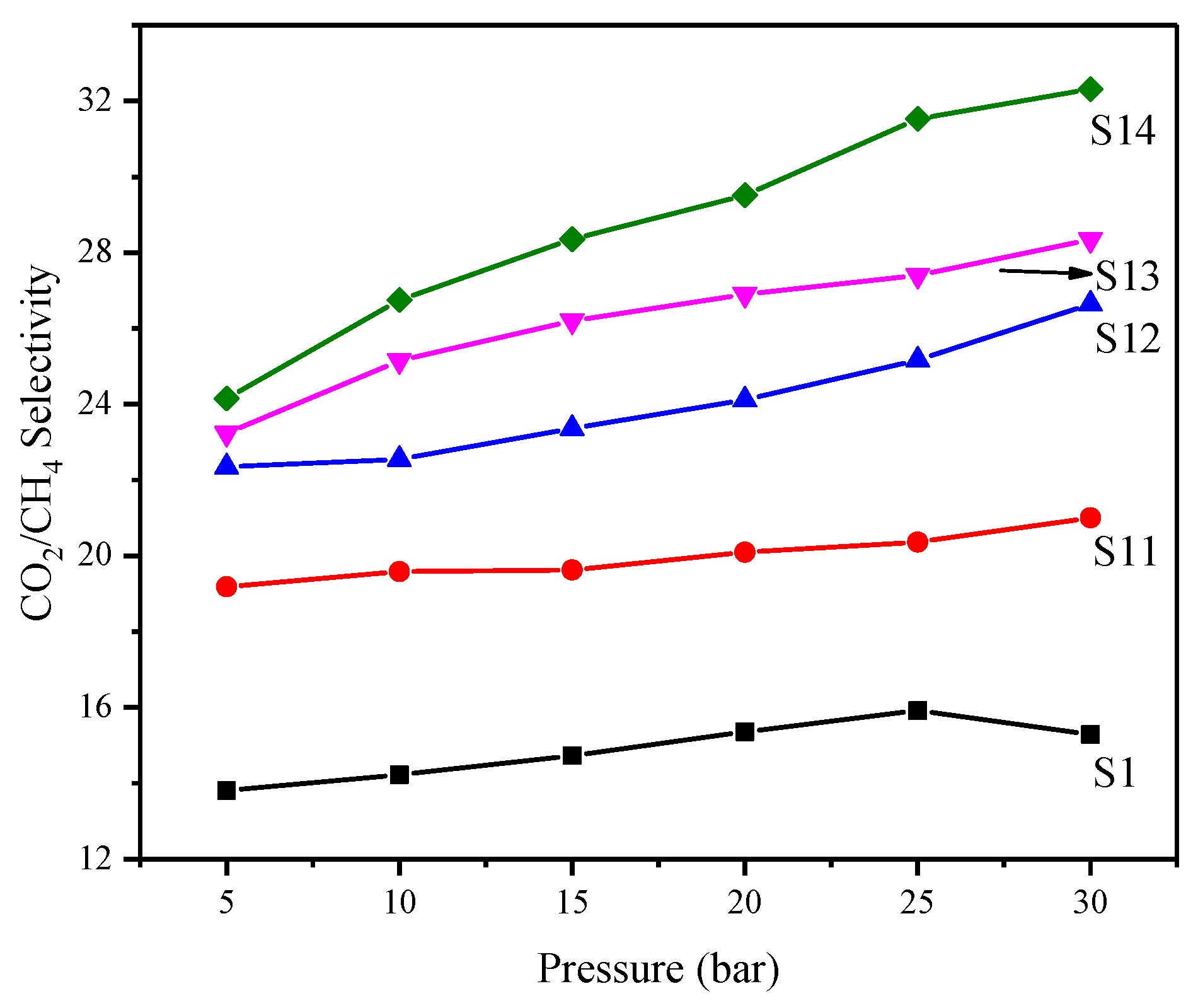
3.6.2. Effect of Feed Pressure
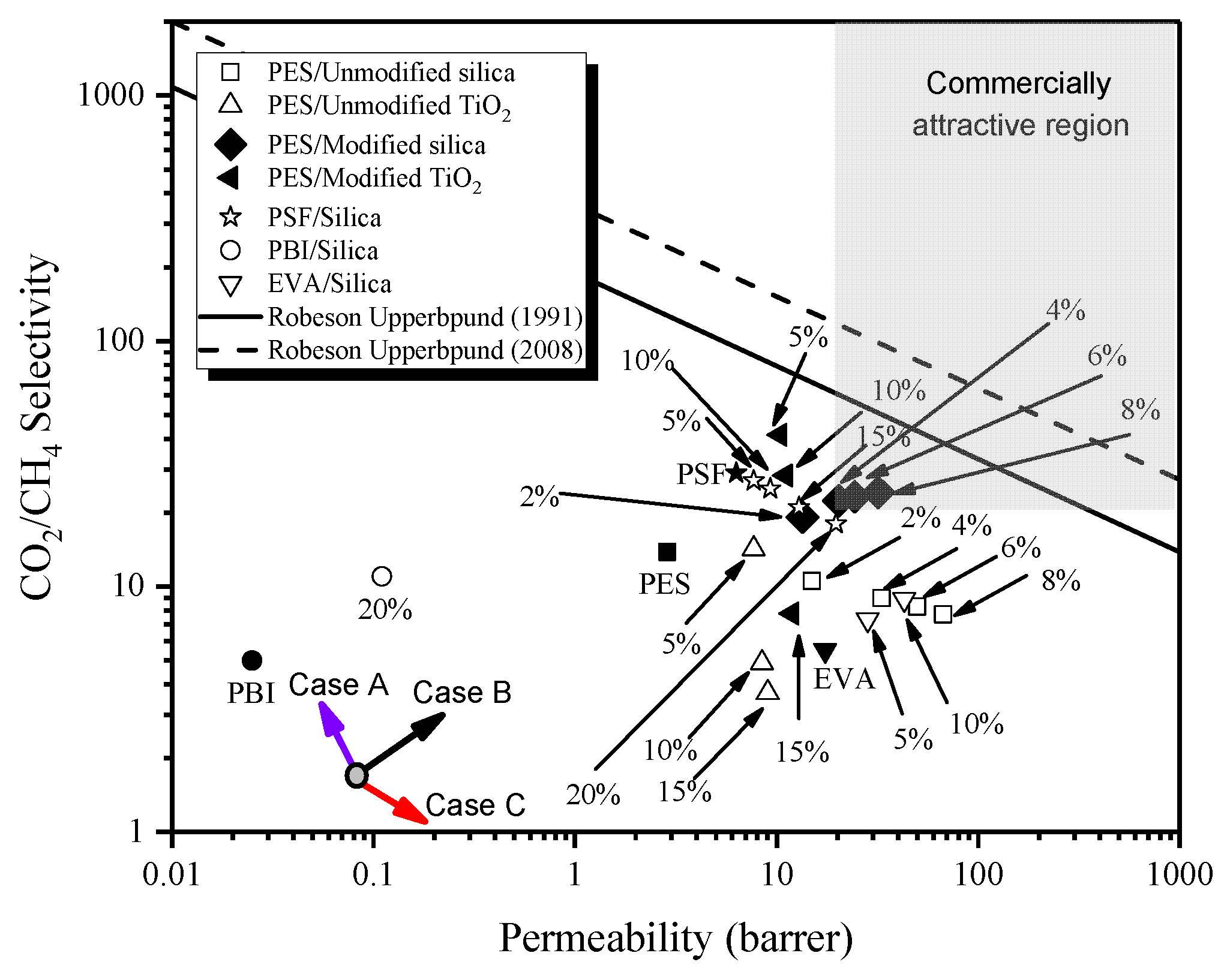
3.6.3. Comparison of MMMs on Robeson Upper Bound Line
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shimekit, B.; Mohd Shariff, A.; Mukhtar, H.; Bustam, M.A.; Elkhalifah, A.E.I.; Ullah, S.; Riaz, N. Interfacial Defects on Mixed Matrix Membranes and Mitigation Techniques for Gas Separation: A Review. Appl. Mech. Mater. 2014, 625, 653–656. [Google Scholar] [CrossRef]
- Idris, A.; Man, Z.; Maulud, A.S.; Uddin, F. Modified Bruggeman models for prediction of CO2 permeance in polycarbonate/silica nanocomposite membranes. Can. J. Chem. Eng. 2017, 95, 2398–2409. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Ebadi Amooghin, A.; Montazer-Rahmati, M.M.; Ismail, A.F.; Matsuura, T. State-of-the-art membrane based CO2 separation using mixed matrix membranes (MMMs): An overview on current status and future directions. Prog. Polym. Sci. 2014, 39, 817–861. [Google Scholar] [CrossRef]
- Mannan, H.A.; Nasir, R.; Mukhtar, H.; Mohshim, D.F.; Shaharun, M.S. 11–Role of ionic liquids in eliminating interfacial defects in mixed matrix membranes. In Interfaces in Particle and Fibre Reinforced Composites; Goh, K.L., Aswathi, M.K., De Silva, R.T., Thomas, S., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 269–309. [Google Scholar] [CrossRef]
- Idris, A.; Man, Z.; Maulud, A.S.; Bustam, M.A.; Mannan, H.A.; Ahmed, I. Investigation on particle properties and extent of functionalization of silica nanoparticles. Appl. Surf. Sci. 2020, 506, 144978. [Google Scholar] [CrossRef]
- Yang, F.; Nelson, G.L. PMMA/silica nanocomposite studies: Synthesis and properties. J. Appl. Polym. Sci. 2004, 91, 3844–3850. [Google Scholar] [CrossRef]
- Nunes, S.P.; Peinemann, K.V.; Ohlrogge, K.; Alpers, A.; Keller, M.; Pires, A.T.N. Membranes of poly(ether imide) and nanodispersed silica. J. Membr. Sci. 1999, 157, 219–226. [Google Scholar] [CrossRef]
- Idris, A.; Man, Z.; Maulud, A.S.; Mannan, H.A.; Shafie, A. Effect of silane coupling agents on properties and performance of polycarbonate/silica MMMs. Polym. Test. 2019, 73, 159–170. [Google Scholar] [CrossRef]
- Sadeghi, F.; Tremblay, A.Y.; Kruczek, B. Synthesis and characterization of emulsion polymerized mixed matrix aluminum silicate/poly(2,6-dimethyl 1,4-phenylene oxide) films. J. Appl. Polym. Sci. 2008, 109, 1454–1460. [Google Scholar] [CrossRef]
- Bissadi, G.; Melo Santos, T.; Kruczek, B. Synthesis and Gas Transport Properties of Poly(2,6-dimethyl-1,4-phenylene oxide)–Silica Nanocomposite Membranes. Membranes 2018, 8, 125. [Google Scholar] [CrossRef]
- Xu, R.; Wang, B.; Cai, Y. Preparation and structures of PEBA gas separation membrane modified by fumed silica for oil vapor separation. Sci. Rep. 2022, 12, 1025. [Google Scholar] [CrossRef]
- Xin, Q.; Zhang, C.; Zhang, Y.; Liang, Q.; Zhang, L.; Wang, S.; Ye, H.; Ding, X.; Zhang, Y. Constructing superhydrophobic surface of PES/PES-SiO2 mixed matrix membrane contactors for efficient SO2 capture. Sep. Purif. Technol. 2021, 259, 118222. [Google Scholar] [CrossRef]
- Khan, A.A.; Siyal, M.I.; Kim, J.-O. Fluorinated silica–modified anti–oil-fouling omniphobic F–SiO2@PES robust membrane for multiple foulants feed in membrane distillation. Chemosphere 2021, 263, 128140. [Google Scholar] [CrossRef] [PubMed]
- Al-Gamal, A.Q.; Satria, M.; Alghunaimi, F.I.; Aljuryyed, N.W.; Saleh, T.A. Synthesis of thin-film nanocomposite membranes using functionalized silica nanoparticles for water desalination with drastically improved properties. React. Funct. Polym. 2022, 181, 105433. [Google Scholar] [CrossRef]
- Hao, L.; Li, P.; Yang, T.; Chung, T.-S. Room temperature ionic liquid/ZIF-8 mixed-matrix membranes for natural gas sweetening and post-combustion CO2 capture. J. Membr. Sci. 2013, 436, 221–231. [Google Scholar] [CrossRef]
- Chung, T.-S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Ahmad, N.N.R.; Mukhtar, H.; Mohshim, D.F.; Nasir, R.; Man, Z. Effect of different organic amino cations on SAPO-34 for PES/SAPO-34 mixed matrix membranes toward CO2/CH4 separation. J. Appl. Polym. Sci. 2016, 133, 43387. [Google Scholar] [CrossRef]
- Idris, A.; Man, Z.; Maulud, A.S. Polycarbonate/silica nanocomposite membranes: Fabrication, characterization, and performance evaluation. J. Appl. Polym. Sci. 2017, 134, 45310. [Google Scholar] [CrossRef]
- Moore, T.T.; Koros, W.J. Non-ideal effects in organic–inorganic materials for gas separation membranes. J. Mol. Struct. 2005, 739, 87–98. [Google Scholar] [CrossRef]
- Shen, Y.; Lua, A.C. Structural and transport properties of BTDA-TDI/MDI co-polyimide (P84)–silica nanocomposite membranes for gas separation. Chem. Eng. J. 2012, 188, 199–209. [Google Scholar] [CrossRef]
- Sadeghi, M.; Khanbabaei, G.; Dehaghani, A.H.S.; Sadeghi, M.; Aravand, M.A.; Akbarzade, M.; Khatti, S. Gas permeation properties of ethylene vinyl acetate–silica nanocomposite membranes. J. Membr. Sci. 2008, 322, 423–428. [Google Scholar] [CrossRef]
- Gopiraman, M.; Fujimori, K.; Zeeshan, K.; Kim, B.S.; Kim, I.S. Structural and mechanical properties of cellulose acetate/graphene hybrid nanofibers: Spectroscopic investigations. Express Polym. Lett. 2013, 7, 554–563. [Google Scholar] [CrossRef]
- Sabir, A.; Shafiq, M.; Islam, A.; Sarwar, A.; Dilshad, M.R.; Shafeeq, A.; Zahid Butt, M.T.; Jamil, T. Fabrication of tethered carbon nanotubes in cellulose acetate/polyethylene glycol-400 composite membranes for reverse osmosis. Carbohydr. Polym. 2015, 132, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.; Sabir, A.; Islam, A.; Khan, S.M.; Gull, N.; Hussain, S.N.; Butt, M.T.Z. Cellulaose acetate based thin film nanocomposite reverse osmosis membrane incorporated with TiO2 nanoparticles for improved performance. Carbohydr. Polym. 2018, 186, 367–376. [Google Scholar] [CrossRef]
- Khdary, N.H.; Abdelsalam, M.E. Polymer-silica nanocomposite membranes for CO2 capturing. Arab. J. Chem. 2020, 13, 557–567. [Google Scholar] [CrossRef]
- Sadeghi, M.; Semsarzadeh, M.A.; Moadel, H. Enhancement of the gas separation properties of polybenzimidazole (PBI) membrane by incorporation of silica nano particles. J. Membr. Sci. 2009, 331, 21–30. [Google Scholar] [CrossRef]
- Hajji, P.; David, L.; Gerard, J.F.; Pascault, J.P.; Vigier, G. Synthesis, structure, and morphology of polymer–silica hybrid nanocomposites based on hydroxyethyl methacrylate. J. Polym. Sci. Part B: Polym. Phys. 1999, 37, 3172–3187. [Google Scholar] [CrossRef]
- Rafiq, S.; Man, Z.; Maulud, A.; Muhammad, N.; Maitra, S. Separation of CO2 from CH4 using polysulfone/polyimide silica nanocomposite membranes. Sep. Purif. Technol. 2012, 90, 162–172. [Google Scholar] [CrossRef]
- Naghsh, M.; Sadeghi, M.; Moheb, A.; Chenar, M.P.; Mohagheghian, M. Separation of ethylene/ethane and propylene/propane by cellulose acetate–silica nanocomposite membranes. J. Membr. Sci. 2012, 423, 97–106. [Google Scholar] [CrossRef]
- Şen, D.; Kalıpçılar, H.; Yilmaz, L. Development of polycarbonate based zeolite 4A filled mixed matrix gas separation membranes. J. Membr. Sci. 2007, 303, 194–203. [Google Scholar] [CrossRef]
- Ahmad, J.; Hägg, M.-B. Preparation and characterization of polyvinyl acetate/zeolite 4A mixed matrix membrane for gas separation. J. Membr. Sci. 2013, 427, 73–84. [Google Scholar] [CrossRef]
- Zou, H.; Wu, S.; Shen, J. Polymer/Silica Nanocomposites: Preparation, Characterization, Properties, and Applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef] [PubMed]
- Hassanajili, S.; Masoudi, E.; Karimi, G.; Khademi, M. Mixed matrix membranes based on polyetherurethane and polyesterurethane containing silica nanoparticles for separation of CO2/CH4 gases. Sep. Purif. Technol. 2013, 116, 1–12. [Google Scholar] [CrossRef]
- Takahashi, S.; Paul, D.R. Gas permeation in poly(ether imide) nanocomposite membranes based on surface-treated silica. Part 1: Without chemical coupling to matrix. Polymer 2006, 47, 7519–7534. [Google Scholar] [CrossRef]
- Laghaei, M.; Sadeghi, M.; Ghalei, B.; Shahrooz, M. The role of compatibility between polymeric matrix and silane coupling agents on the performance of mixed matrix membranes: Polyethersulfone/MCM-41. J. Membr. Sci. 2016, 513, 20–32. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, Y.M. Gas permeation properties of poly(amide-6-b-ethylene oxide)–silica hybrid membranes. J. Membr. Sci. 2001, 193, 209–225. [Google Scholar] [CrossRef]
- Zornoza, B.; Téllez, C.; Coronas, J. Mixed matrix membranes comprising glassy polymers and dispersed mesoporous silica spheres for gas separation. J. Membr. Sci. 2011, 368, 100–109. [Google Scholar] [CrossRef]
- El-Dakkony, S.R.; Mubarak, M.F.; Ali, H.R.; Gaffer, A.; Moustafa, Y.M.; Abdel-Rahman, A.H. Composite thin-film membrane of an assembled activated carbon thin film with autoself-healing and high-efficiency water desalination. Environ. Dev. Sustain. 2022, 24, 2514–2541. [Google Scholar] [CrossRef]
- Mubashir, M.; Yeong, Y.F.; Lau, K.K.; Chew, T.L.; Norwahyu, J. Efficient CO2/N2 and CO2/CH4 separation using NH2-MIL-53(Al)/cellulose acetate (CA) mixed matrix membranes. Sep. Purif. Technol. 2018, 199, 140–151. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, H.; Zhang, X.-F.; Jia, M.; Yao, J. Polyethylenimine grafted ZIF-8@cellulose acetate membrane for enhanced gas separation. J. Membr. Sci. 2022, 662, 120996. [Google Scholar] [CrossRef]
- Mannan, H.A. Development of Ionic Liquid Nanocomposite Membranes (ILNCMs) For CO2/CH4 Separation. Ph.D. Thesis, Universiti Teknologi PETRONAS, Seri Iskandar, Perak, Malaysia, 2018. [Google Scholar]
- Nasir, R.; Mukhtar, H.; Man, Z.; Dutta, B.K.; Shaharun, M.S.; Abu Bakar, M.Z. Mixed matrix membrane performance enhancement using alkanolamine solution. J. Membr. Sci. 2015, 483, 84–93. [Google Scholar] [CrossRef]
- Shahid, S.; Nijmeijer, K. High pressure gas separation performance of mixed-matrix polymer membranes containing mesoporous Fe(BTC). J. Membr. Sci. 2014, 459, 33–44. [Google Scholar] [CrossRef]
- Saedi, S.; Madaeni, S.S.; Shamsabadi, A.A. Fabrication of asymmetric polyethersulfone membranes for separation of carbon dioxide from methane using polyetherimide as polymeric additive. Chem. Eng. Res. Des. 2014, 92, 2431–2438. [Google Scholar] [CrossRef]
- Khan, A.L.; Sree, S.P.; Martens, J.A.; Raza, M.T.; Vankelecom, I.F.J. Mixed matrix membranes comprising of matrimid and mesoporous COK-12: Preparation and gas separation properties. J. Membr. Sci. 2015, 495, 471–478. [Google Scholar] [CrossRef]
- Eltahir Mustafa, S.G.E.; Mannan, H.A.; Nasir, R.; Mohshim, D.F.; Mukhtar, H. Synthesis, characterization, and performance evaluation of PES/EDA-functionalized TiO2 mixed matrix membranes for CO2/CH4 separation. J. Appl. Polym. Sci. 2017, 134, 45346. [Google Scholar] [CrossRef]
- Ahn, J.; Chung, W.-J.; Pinnau, I.; Guiver, M.D. Polysulfone/silica nanoparticle mixed-matrix membranes for gas separation. J. Membr. Sci. 2008, 314, 123–133. [Google Scholar] [CrossRef]
- Sanaeepur, H.; Amooghin, A.E.; Moghadassi, A.; Kargari, A. Preparation and characterization of acrylonitrile–butadiene–styrene/poly(vinyl acetate) membrane for CO2 removal. Sep. Purif. Technol. 2011, 80, 499–508. [Google Scholar] [CrossRef]




| Description | Membrane ID | Silica Composition (wt.%) |
|---|---|---|
| Pure PES membrane | S1 | 0 |
| PES/unmodified silica MMMs | S7 | 2 |
| S8 | 4 | |
| S9 | 6 | |
| S10 | 8 | |
| PES/modified silica MMMs | S11 | 2 |
| S12 | 4 | |
| S13 | 6 | |
| S14 | 8 |
| Membrane | Description | Tg (°C) |
|---|---|---|
| S1 | Pure PES membrane | 204.6 |
| S11 | Silica = 2 wt.% | 205.3 |
| S12 | Silica = 4 wt.% | 205.9 |
| S13 | Silica = 6 wt.% | 206.3 |
| S14 | Silica = 8 wt.% | 206.6 |
| Silica Loading (%) | FFV | Percentage Increase in FFV (%) | CO2 Permeability (Barrer) | CH4 Permeability (Barrer) |
|---|---|---|---|---|
| 0 | 0.141 | 0 | 2.86 | 0.20 |
| 2 | 0.163 | 15.60 | 13.42 | 0.70 |
| 4 | 0.170 | 20.57 | 20.33 | 0.91 |
| 6 | 0.173 | 22.70 | 24.39 | 1.05 |
| 8 | 0.179 | 26.95 | 31.88 | 1.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mannan, H.A.; Idris, A.; Nasir, R.; Mukhtar, H.; Qadir, D.; Suleman, H.; Basit, A. Interfacial Tailoring of Polyether Sulfone-Modified Silica Mixed Matrix Membranes for CO2 Separation. Membranes 2022, 12, 1129. https://doi.org/10.3390/membranes12111129
Mannan HA, Idris A, Nasir R, Mukhtar H, Qadir D, Suleman H, Basit A. Interfacial Tailoring of Polyether Sulfone-Modified Silica Mixed Matrix Membranes for CO2 Separation. Membranes. 2022; 12(11):1129. https://doi.org/10.3390/membranes12111129
Chicago/Turabian StyleMannan, Hafiz Abdul, Alamin Idris, Rizwan Nasir, Hilmi Mukhtar, Danial Qadir, Humbul Suleman, and Abdul Basit. 2022. "Interfacial Tailoring of Polyether Sulfone-Modified Silica Mixed Matrix Membranes for CO2 Separation" Membranes 12, no. 11: 1129. https://doi.org/10.3390/membranes12111129
APA StyleMannan, H. A., Idris, A., Nasir, R., Mukhtar, H., Qadir, D., Suleman, H., & Basit, A. (2022). Interfacial Tailoring of Polyether Sulfone-Modified Silica Mixed Matrix Membranes for CO2 Separation. Membranes, 12(11), 1129. https://doi.org/10.3390/membranes12111129








