Growth of the Cycle Life and Rate Capability of LIB Silicon Anodes Based on Macroporous Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Manufacture of Macroporous Si Anodes
2.2. Electrochemical Measurements
2.3. Impedance Spectroscopy Method
3. Results and Discussions
3.1. Electrochemical Characteristics of Anodes
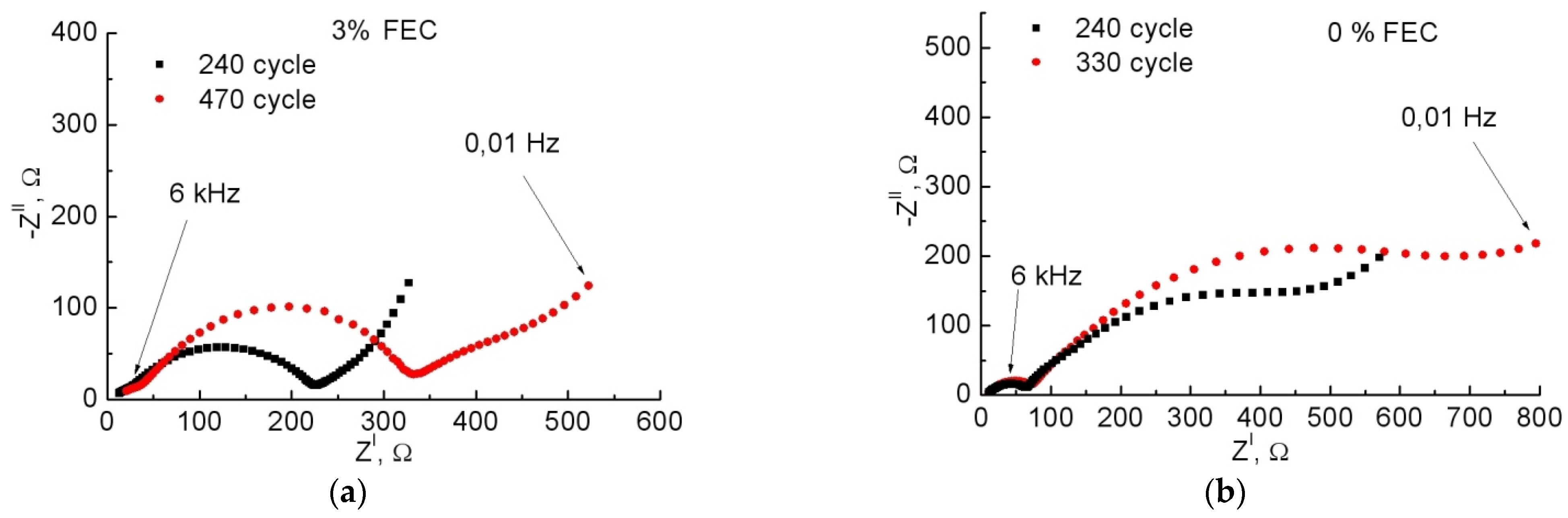
3.2. Study of Anodes by Impedance Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Föll, H.; Hartz, H.; Ossei-Wusu, E.; Carstensen, J.; Riemenschneider, O. Si nanowire arrays as anodes in Li ion batteries. Phys. Status Solidi RRL 2010, 4, 4–6. [Google Scholar] [CrossRef]
- Astrova, E.V.; Fedulova, G.V.; Smirnova, I.A.; Remenyuk, A.D.; Kulova, T.L.; Skundin, A.M. Porous silicon based negative electrodes for lithium ion batteries. Tech. Phys. Lett. 2011, 37, 731–734. [Google Scholar] [CrossRef]
- Thakur, M.; Sinsabaugh, S.L.; Isaacson, M.J.; Wong, M.S.; Biswal, S.L. Inexpensive method for producing macroporous silicon particulates (MPSPs) with pyrolyzed polyacrylonitrile for lithium ion batteries. Sci. Rep. 2012, 2, 795. [Google Scholar] [CrossRef]
- Quiroga-González, E.; Carstensen, J.; Föll, H. Optimal Conditions for Fast Charging and Long Cycling Stability of Silicon Microwire Anodes for Lithium Ion Batteries, and Comparison with the Performance of Other Si Anode Concepts. Energies 2013, 6, 5145–5156. [Google Scholar] [CrossRef]
- Astrova, E.V.; Li, G.V.; Rumyantsev, A.M.; Zhdanov, V.V. Electrochemical characteristics of nanostructured silicon anodes for lithium-ion batteries. Semiconductors 2016, 50, 276–283. [Google Scholar] [CrossRef]
- Luais, E.; Sakai, J.; Desplobain, S.; Gautier, G.; Tran-Van, F.; Ghamouss, F. Thin and flexible silicon anode based on integrated macroporous silicon film onto electrodeposited copper current collector. J. Power Sources 2013, 242, 166–170. [Google Scholar] [CrossRef]
- Li, G.V.; Rumyantsev, A.M.; Levitskii, V.S.; Beregulin, E.V.; Zhdanov, V.V.; Terukov, E.I.; Astrova, E.V. Application of Silicon Zig-zag Wall Arrays for Anodes of Li-ion Batteries. Semicond. Sci. Technol. 2016, 31, 014008. [Google Scholar] [CrossRef]
- Astrova, E.V.; Preobrazhenskiy, N.E.; Lihachev, A.I.; Li, G.V. Comparison of n-Si photoanodization in organic and aqueous electrolytes. J. Micromech. Microeng. 2018, 28, 115014. [Google Scholar] [CrossRef]
- Li, G.V.; Astrova, E.V.; Preobrazhenskii, N.E.; Rumyantsev, A.M.; Pavlov, S.I.; Beregulin, E.V. Negative Electrodes for Lithium-Ion Batteries Obtained by Photoanodization of Solar-Grade Silicon. Tech. Phys. 2019, 64, 660–665. [Google Scholar] [CrossRef]
- Astrova, E.V.; Preobrazhenskiy, N.E.; Li, G.V.; Pavlov, S.I. Formation of Macropores in n-Si upon Anodization in an Organic Electrolyte. Fiz. Tekhnika Poluprovodn. 2018, 52, 414–430. (In Russian) [Google Scholar] [CrossRef]
- Yin, Y.X.; Wan, L.J.; Guo, Y.G. Silicon-based nanomaterials for lithium-ion batteries. Chin. Sci. Bull. 2012, 57, 4104–4110. [Google Scholar] [CrossRef]
- Du, F.H.; Wang, K.X.; Chen, J.S. Strategies to succeed in improving the lithium-ion storage properties of silicon nanomaterials. J. Mater. Chem. A 2016, 4, 32–50. [Google Scholar] [CrossRef]
- Manj, R.Z.A.; Zhang, F.; Rehman, W.U.; Luo, W.; Yang, J. Toward understanding the interaction within Silicon-based anodes for stable lithium storage. Chem. Eng. J. 2020, 385, 123821. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, J.; Chen, Y.; Yang, H.; Qin, Y.; Li, F.; Cheng, Q.; Yu, X.; Xu, Z.; Lu, B. Large-scale production of silicon nanoparticles@graphene embedded in nanotubes as ultra-robust battery anodes. J. Mater. Chem. A 2017, 5, 4809–4817. [Google Scholar] [CrossRef]
- Jiang, M.; Mu, P.; Zhang, H.; Dong, T.; Tang, B.; Qiu, H.; Chen, Z.; Cui, G. An Endotenon Sheath-Inspired Double-Network Binder Enables Superior Cycling Performance of Silicon Electrodes. Nano-Micro Lett. 2022, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Haregewoin, A.M.; Wotango, A.S.; Hwang, B.J. Electrolyte additives for lithium ion battery electrodes: Progress and perspectives. Energy Environ. Sci. 2016, 9, 1955–1988. [Google Scholar] [CrossRef]
- Markevich, E.; Salitra, G.; Aurbach, D. Fluoroethylene carbonate as an important component for the formation of an effective solid electrolyte interphase on anodes and cathodes for advanced Li-ion batteries. ACS Energy Lett. 2017, 2, 1337–1345. [Google Scholar] [CrossRef]
- Suo, L.; Xue, W.; Gobet, M.; Greenbaum, S.G.; Wang, C.; Chen, Y.; Yang, W.; Li, Y.; Li, J. Fluorine-donating electrolytes enable highly reversible 5-V-class Li metal batteries. Proc. Natl. Acad. Sci. USA 2018, 115, 1156–1161. [Google Scholar] [CrossRef]
- Bordes, A.; Eom, K.; Fuller, T.F. The effect of fluoroethylene carbonate additive content on the formation of the solid–electrolyte interphase and capacity fade of Li-ion full-cell employing nano Si–graphene composite anodes. J. Power Sources 2014, 257, 163–169. [Google Scholar] [CrossRef]
- Domi, Y.; Usui, H.; Shimizu, M.; Morishita, S.; Yodoya, S.; Sakata, T.; Sakaguchi, H. Effect of film-forming additive on electrochemical performance of silicon negative-electrode in lithium-ion batteries. Int. J. Electrochem. Sci. 2015, 10, 9678–9686. [Google Scholar]
- Etacheri, V.; Haik, O.; Goffer, Y.; Roberts, G.A.; Stefan, I.C.; Fasching, R.; Aurbach, D. Effect of Fluoroethylene Carbonate (FEC) on the Performance and Surface Chemistry of Si-Nanowire Li-Ion Battery Anodes. ACS Langmuir 2012, 28, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.M.; Klavetter, K.C.; Abel, P.R.; Davy, N.C.; Snider, J.L.; Heller, A.; Mullins, C.B. High performance silicon nanoparticle anode in fluoroethylene carbonate-based electrolyte for Li-ion batteries. Chem. Commun. 2012, 48, 7268–7270. [Google Scholar] [CrossRef] [PubMed]
- Eom, K.S.; Joshi, T.; Bordes, A.; Do, I.; Fuller, T.F. The design of a Li-ion full cell battery using a nano silicon and nano multi-layer graphene composite anode. J. Power Sources 2014, 249, 118–124. [Google Scholar] [CrossRef]
- Nguyen, C.C.; Lucht, B.L. Comparative Study of Fluoroethylene Carbonate and Vinylene Carbonate for Silicon Anodes in Lithium Ion Batteries. J. Electrochem. Soc. 2014, 161, A1933–A1938. [Google Scholar] [CrossRef]
- Jin, Y.; Kneusels, N.-J.H.; Marbella, L.E.; Castillo-Martínez, E.; Magusin, P.C.M.M.; Weatherup, R.S.; Jónsson, E.; Liu, T.; Paul, S.; Grey, C.P. Understanding Fluoroethylene Carbonate and Vinylene Carbonate Based Electrolytes for Si Anodes in Lithium Ion Batteries with NMR Spectroscopy. J. Am. Chem. Soc. 2018, 140, 9854–9867. [Google Scholar] [CrossRef]
- Li, Q.; Liu, X.; Han, X.; Xiang, Y.; Zhong, G.; Wang, J.; Zheng, B.; Zhou, J.; Yang, Y. Identification of the Solid Electrolyte Interface on the Si/C Composite Anode with FEC as the Additive. ACS Appl. Mater. Interfaces 2019, 11, 14066–14075. [Google Scholar] [CrossRef]
- Pathak, A.D.; Samanta, K.; Sahu, K.K.; Pati, S. Mechanistic insight into the performance enhancement of Si anode of a lithium-ion battery with a fuoroethylene carbonate electrolyte additive. J. Appl. Electrochem. 2021, 51, 143–154. [Google Scholar] [CrossRef]
- Wang, W.; Yang, S. Enhanced overall electrochemical performance of silicon/carbon anode for lithium-ion batteries using fluoroethylene carbonate as an electrolyte additive. J. Alloys Compd. 2017, 695, 3249–3255. [Google Scholar] [CrossRef]
- Kennedy, T.; Brandon, M.; Laffir, F.; Ryan, K.M. Understanding the influence of electrolyte additives on the electrochemical performance and morphology evolution of silicon nanowire based lithium-ion battery anodes. J. Power Sources 2017, 359, 601–610. [Google Scholar] [CrossRef]
- Kulova, T.L.; Emets, V.V.; Skundin, A.M. The dynamic character of processes taking place at aging of electrodes based on silicon composites. Elektrokhimicheskaya Enegetika 2016, 16, 3–9. (In Russian) [Google Scholar] [CrossRef]
- Kamenskii, M.A.; Eliseeva, S.N.; Kondratiev, V.V. Effect of long-term cycling on impedance spectra of LiMn2O4-electrodes. ECS Trans. 2019, 95, 121–127. [Google Scholar] [CrossRef]
- Li, J.; Dahn, J.R. An In Situ X-Ray Diffraction Study of the Reaction of Li with Crystalline Si. J. Electrochem. Soc. 2007, 154, A156–A161. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Krause, L.J. Reversible. Cycling of Crystalline Silicon Powder. J. Electrochem. Soc. 2007, 154, A103–A108. [Google Scholar] [CrossRef]
- Key, B.; Morcrette, M.; Tarascon, J.-M.; Grey, C.P. Pair Distribution Function Analysis and Solid State NMR Studies of Silicon Electrodes for Lithium Ion Batteries: Understanding the (De)lithiation Mechanisms. J. Am. Chem. Soc. 2011, 133, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Li, G.V.; Astrova, E.V.; Rumyantsev, A.M. Electrochemical Amorphization As a Method to Increase the Rate Capability of Crystalline Silicon Anodes for Lithium-Ion Batteries. Tech. Phys. Lett. 2019, 45, 1131–1135. [Google Scholar] [CrossRef]
- Xu, C.; Lindgren, F.; Philippe, B.; Gorgoi, M.; Björefors, F.; Edström, K.; Gustafsson, T. Improved Performance of the Silicon Anode for Li-Ion Batteries: Understanding the Surface Modification Mechanism of Fluoroethylene Carbonate as an Effective Electrolyte Additive. Chem. Mater. 2015, 27, 2591–2603. [Google Scholar] [CrossRef]
- Bintang, H.M.; Lee, S.; Shin, S.; Kim, B.G.; Jung, H.G.; Whang, D.; Lim, H.D. Stabilization effect of solid-electrolyte interphase by electrolyte engineering for advanced Li-ion batteries. Chem. Eng. J. 2021, 424, 130524. [Google Scholar] [CrossRef]
- Churikov, A.V.; Pridatko, K.I.; Ivanishchev, A.V.; Ivanishcheva, I.A.; Gamayunova, I.M.; Zapsis, K.V.; Sycheva, V.O. Impedance spectroscopy of lithium-tin film electrodes. Rus. J. Electrochem. 2008, 44, 550–557. [Google Scholar] [CrossRef]
- Ivanishchev, A.V.; Churikov, A.V.; Ivanishcheva, I.A.; Zapsis, K.V.; Gamayunova, I.M. Impedance spectroscopy of lithium-carbon electrodes. Rus. J. Electrochem. 2008, 44, 510–524. [Google Scholar] [CrossRef]
- Kulova, T.L.; Pleskov, Y.V.; Skundin, A.M.; Terukov, E.I.; Kon’kov, O.I. Lithium intercalation into amorphous-silicon thin films: An electrochemical-impedance study. Rus. J. Electrochem. 2006, 42, 708–714. [Google Scholar] [CrossRef]
- Umeda, M.; Dokko, K.; Fujita, Y.; Mohamedi, M.; Uchida, I.; Selman, J. Electrochemical impedance study of Li-ion insertion into mesocarbon microbead single particle electrode: Part I. Graphitized carbon. Electrochim. Acta 2001, 47, 885–890. [Google Scholar] [CrossRef]
- Dokko, K.; Fujita, Y.; Mohamedi, M.; Umeda, M.; Uchida, I.; Selman, J.R. Electrochemical impedance study of Li-ion insertion into mesocarbon microbead single particle electrode Part II. Disordered carbon. Electrochim. Acta 2001, 47, 933–938. [Google Scholar] [CrossRef]
- Pan, K.; Zou, F.; Canova, M.; Zhu, Y.; Kim, J.H. Comprehensive electrochemical impedance spectroscopy study of Si-Based anodes using distribution of relaxation times analysis. J. Power Sources 2020, 479, 229083. [Google Scholar] [CrossRef]
- Kang, Y.M.; Go, J.Y.; Lee, S.M.; Choi, W.U. Impedance study on the correlation between phase transition and electrochemical degradation of Si-based materials. Electrochem. Commun. 2007, 9, 1276–1281. [Google Scholar] [CrossRef]
- Aurbach, D.; Markovsky, B.; Weissman, I.; Levi, E.; Ein-Eli, Y. On the correlation between surface chemistry and performance of graphite negative electrodes for Li ion batteries. Electrochim. Acta 1999, 45, 67–86. [Google Scholar] [CrossRef]
- Dimov, N.; Fukuda, K.; Umeno, T.; Kugino, S.; Yoshio, M. Characterization of carbon-coated silicon structural evolution and possible limitations. J. Power Source 2003, 114, 88–95. [Google Scholar] [CrossRef]
- Stoynov, Z.B.; Grafov, B.M.; Savova-Stoynov, B.S.; Elkin, V.V. Electrochemical Impedance; Nauka Publishing: Moscow, Russia, 1991; pp. 21–51. (In Russian) [Google Scholar]
- Yoshimura, K.; Suzuki, J.; Sekine, K.; Takamura, T. Evaluation of the Li insertion/extraction reaction rate at a vacuum-deposited silicon film anode. J. Power Sources 2005, 146, 445–447. [Google Scholar] [CrossRef]



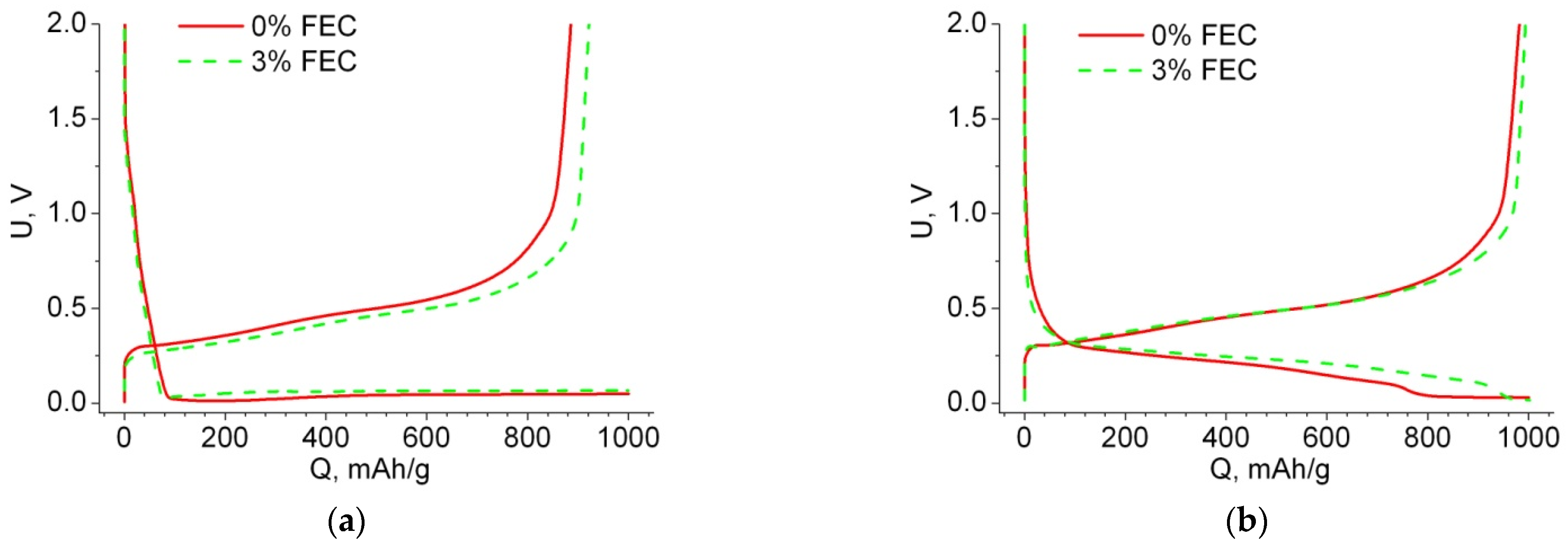
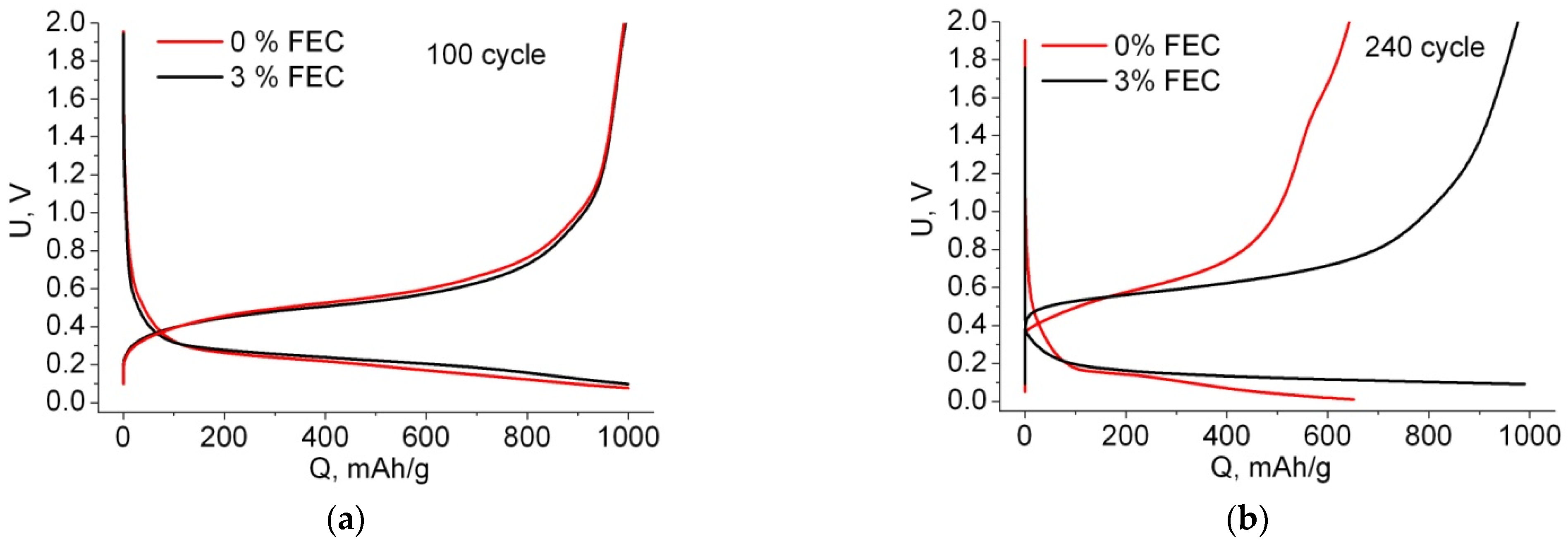
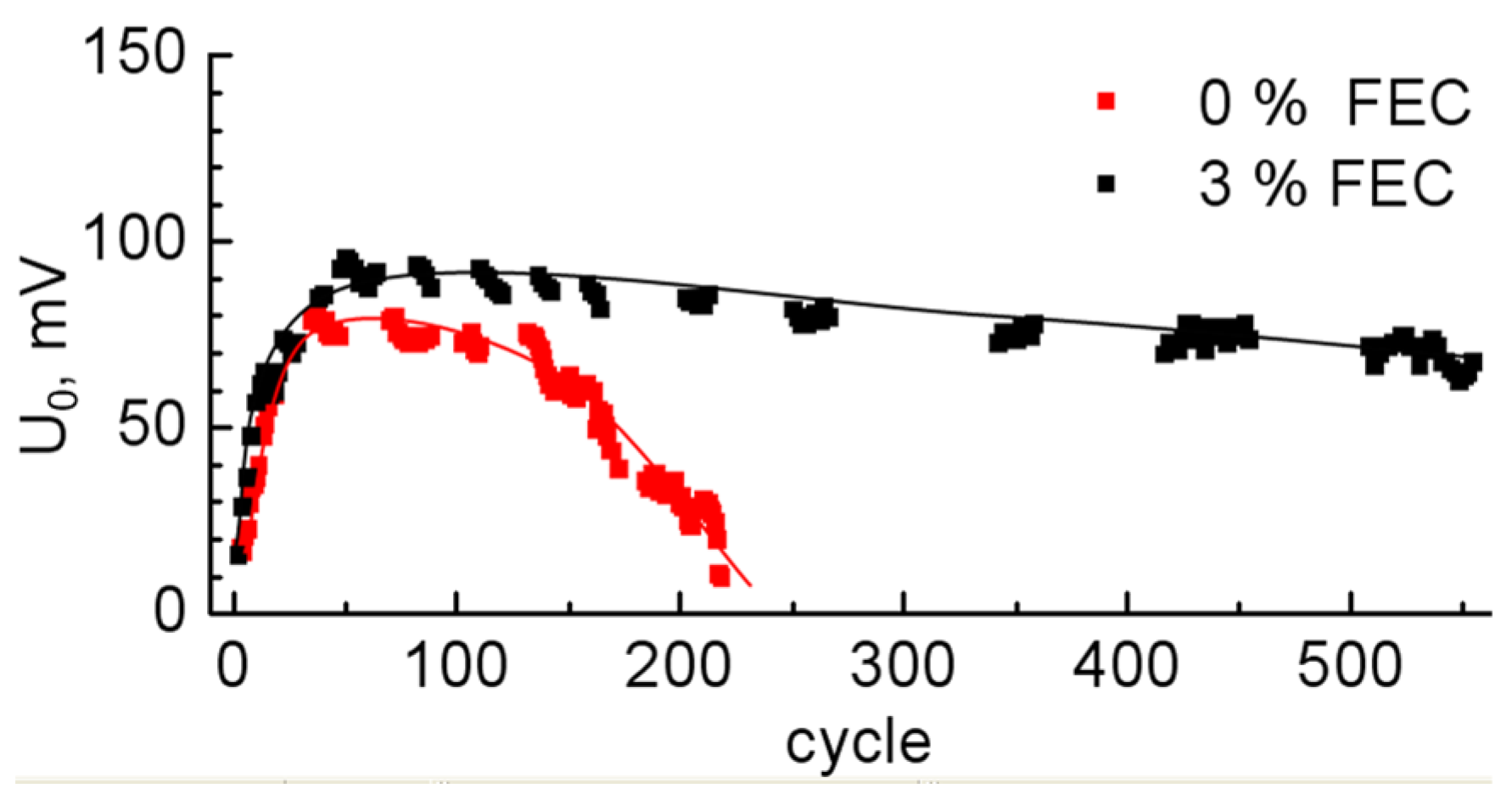


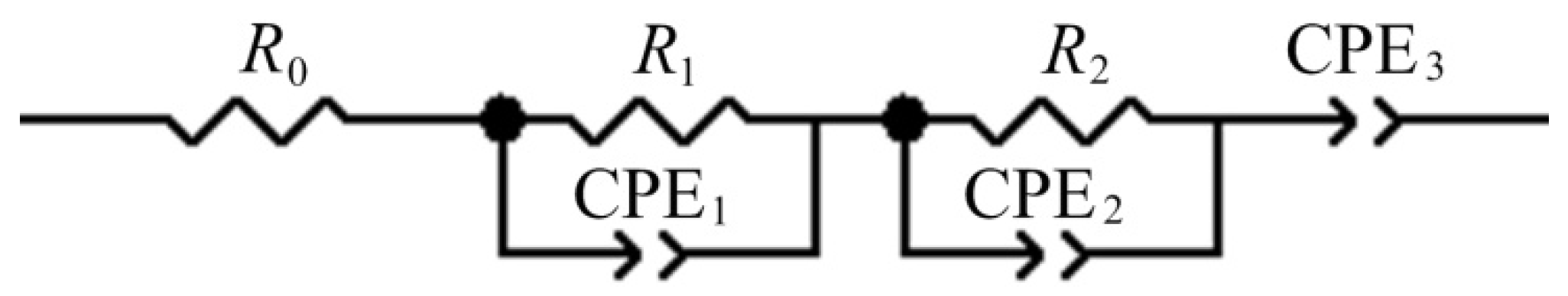
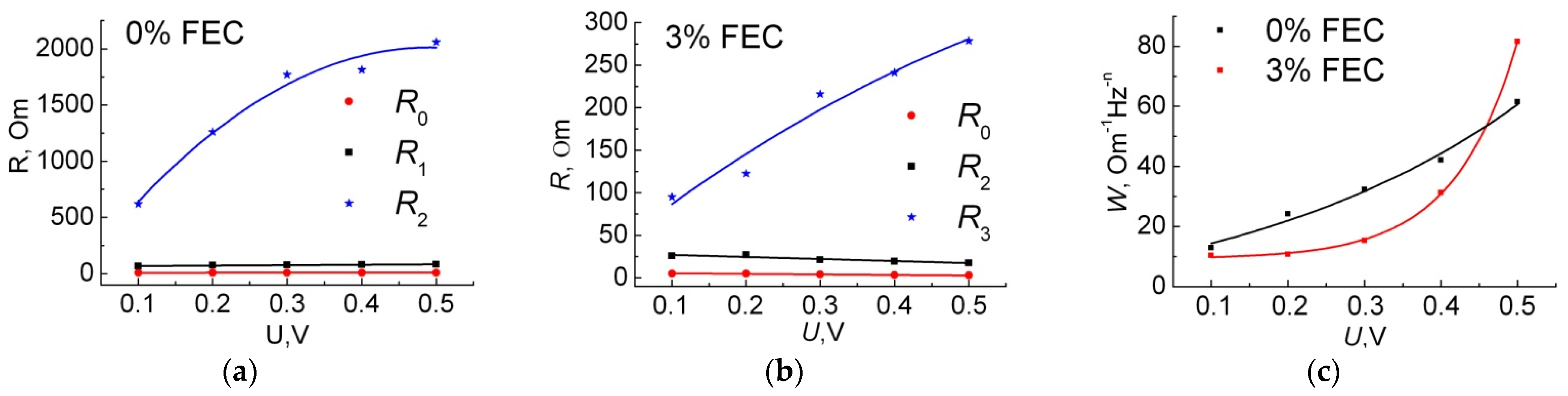
| 0% FEC | 3% FEC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| U, V | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 |
| R0, Ohm | 7.5 | 7.7 | 7.9 | 8.0 | 8.1 | 5.0 | 5.0 | 3.8 | 3.3 | 2.8 |
| R1, Ohm | 66 | 73 | 76 | 78 | 82 | 26 | 27 | 21 | 19 | 18 |
| A1, μF | 45 | 38 | 32 | 29 | 27 | 15 | 15 | 13 | 13 | 12 |
| n1 | 0.62 | 0.64 | 0.65 | 0.66 | 0.67 | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 |
| R2, Ohm | 620 | 1300 | 1800 | 1800 | 2100 | 95 | 120 | 220 | 240 | 280 |
| A2, μF | 1400 | 1200 | 1100 | 1100 | 1100 | 49 | 43 | 39 | 45 | 56 |
| n2 | 0.65 | 0.68 | 0.70 | 0.72 | 0.71 | 0.69 | 0.70 | 0.66 | 0.64 | 0.62 |
| A3, Ohm−1·Hz−n3 | 0.019 | 0.012 | 0.007 | 0.006 | 0.005 | 0.043 | 0.028 | 0.031 | 0.026 | 0.008 |
| n3 | 0.50 | 0.50 | 0.50 | 0.50 | 0.55 | 0.46 | 0.50 | 0.55 | 0.56 | 0.40 |
| W, Ohm/√s | 10 | 11 | 15 | 31 | 82 | 13 | 24 | 32 | 42 | 62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Rumyantsev, A.; Astrova, E.; Maximov, M. Growth of the Cycle Life and Rate Capability of LIB Silicon Anodes Based on Macroporous Membranes. Membranes 2022, 12, 1037. https://doi.org/10.3390/membranes12111037
Li G, Rumyantsev A, Astrova E, Maximov M. Growth of the Cycle Life and Rate Capability of LIB Silicon Anodes Based on Macroporous Membranes. Membranes. 2022; 12(11):1037. https://doi.org/10.3390/membranes12111037
Chicago/Turabian StyleLi, Galina, Aleksander Rumyantsev, Ekaterina Astrova, and Maxim Maximov. 2022. "Growth of the Cycle Life and Rate Capability of LIB Silicon Anodes Based on Macroporous Membranes" Membranes 12, no. 11: 1037. https://doi.org/10.3390/membranes12111037
APA StyleLi, G., Rumyantsev, A., Astrova, E., & Maximov, M. (2022). Growth of the Cycle Life and Rate Capability of LIB Silicon Anodes Based on Macroporous Membranes. Membranes, 12(11), 1037. https://doi.org/10.3390/membranes12111037








