Abstract
There is a global need for optimizing the use of water that has resulted from increased demand due to industrial development, population growth, climate change and the pollution of natural water resources. One of the solutions is to use reclaimed water in industrial applications that do not require water of potable quality, such as cooling water. However, for cooling water, (treated) wastewater’s hardness is too high, apart from having a high load of suspended solids and organic matter. Therefore, a combination of softening with ceramic micro-filtration was proposed for treating wastewater treatment effluent containing fouling agents for potential use in industrial cooling systems. The effectiveness of the softening process on model-treated wastewater with calcium hydroxide in the presence of phosphate and sodium alginate was first evaluated using jar tests. Furthermore, membrane fouling was studied when filtering the softened water. The results showed that the inhibition of calcium carbonate precipitation occurred when inorganic substances, such as phosphate and organic compounds, were present in the water. The fouling of the membranes due to sodium alginate in water was only slightly negatively affected when combined with softening and phosphate. Therefore, this combination of treatments could be potentially helpful for the post-treatment of secondary effluent for cooling systems.
1. Introduction
Water shortages in arid and semi-arid areas are drivers for the use of reclaimed water in industry [1]. Even though the costs of advanced treatment processes for contaminant removal are high, they can be justified by the high value of reclaimed water compared with the use of the scarce natural water sources [2].
Several power plants in the US have already used secondary-treated municipal wastewater treatment plant (WWTP) effluent as make-up water in their recirculating cooling water [3,4]. However, since WWTP effluent typically contains considerable concentrations of hardness, phosphate, ammonia, dissolved solids, and organic matter compared with the concentrations in fresh surface water, for example, extensive treatment is needed [5].
A cooling system relies on water as a heat transfer medium and is the most water-demanding process in industry [6]. Water is utilized to cool pumps and compressors of vacuum systems and steam turbine condensers. Cooling systems can have different configurations but are mainly divided into once-through and recirculating systems [7]. Once-through cooling systems transfer process heat to water to cool the process equipment and then discharge the hot water after a single use. This system requires a large volume of water; typically lake, river-, and seawater are used with little or no treatment [8]. Recirculating cooling systems transfer the heat from warmed water to vapor so that the water can be reused to absorb process heat and recirculated for additional cycles [9,10]. However, as evaporation occurs, the concentration of mineral salts increases, and when the concentration of mineral salts exceeds their solubility, scale formation on heat exchanger surfaces may occur. The level of dissolved solids (mineral salts) is controlled by discharging part of the recirculating water, called blowdown water, from the system and replenishing this volume with fresh make-up water. Previous studies have shown that the major mineral scales formed in recirculating cooling systems using WWTP effluent as make-up water are calcium carbonate and, to a lesser extent, calcium phosphate [5]. To avoid scale formation, hardness in the make-up water should be lower than 1.25 mmol L−1 [11]. Therefore, when using WWTP effluent as cooling water make-up water, additional treatment, such as filtration, chemical precipitation, ion exchange, or reverse osmosis, may be necessary [12].
Ceramic micro-filtration (CMF) is a potential treatment alternative for water reclamation [13]. Ceramic membranes, compared with the most commonly used polymeric membranes, are robust, have high mechanical strength [14], high chemical and thermal resistance [15], high membrane porosity, membrane permeability, and a homogeneous distribution of narrow pores [16]. Ceramic membranes can also be used at a higher flux than polymeric micro-filtration membranes and, therefore, reduce the membrane surface area needed for the same quantity of wastewater [17]. Ceramic membranes are also expected to withstand damage by high pressure, high temperatures or chemicals, enabling processes such as vigorous chemical cleaning of the membrane [18]. Other benefits are the membrane’s long life and the membrane material’s recyclability [14,19]. The disadvantage of ceramic membranes is their relatively high costs compared with polymeric membranes but they could be compensated by the advantages mentioned earlier [20].
MFs are low pressure-driven separation processes that are less energy intensive than traditional treatment methods [19]. They can be used to remove microorganisms and suspended or colloidal particles. However, they do not remove dissolved substances [21]. Nevertheless, previous studies have shown that softening can be promoted by the precipitation of calcium ions (Ca2+) in a membrane system [22,23]. Calcium carbonate particles are formed by dosing a base, and these inorganic particles can be removed from the water by membrane filtration. The findings showed that in a system containing Ca2+ and bicarbonate better softening was achieved in the absence of phosphate (PO43−) due to the inhibitive effect of PO43− on calcium carbonate crystal growth. On the other hand, it is known that calcium promotes the complexation of organic matter, thereby influencing the rate of flux decline in membranes [24].
Therefore, in this work, attention was given to developing a novel application of CMF treatment, focusing on the removal of hardness in model WWTP effluent containing fouling agents, such as Ca2+, Mg2+, HCO3−, and organic matter typically present in WWTP effluent. The effectiveness of calcium hydroxide (Ca(OH)2) as a softening agent was studied during softening with CMF membranes in the presence of PO43−. In addition, the effect of organic compounds on the softening process was analyzed using model water with sodium alginate (SA).
2. Materials and Methods
2.1. Experimental Setup
To study the precipitation of calcium carbonate followed by CMF, in the presence of potentially interfering inorganic and organic substances, two configurations were used:
- Jar tests to rapidly evaluate the effectiveness of the softening agent in the presence of PO43− and SA. Solutions containing Ca2+, HCO3−, PO43−, and SA were prepared in demineralized water.
- Membrane tests to study the performance of the membrane when filtering precipitated calcium carbonate in combination with PO43− and SA, all prepared with demineralized water.
Ca(OH)2 in suspension, a common base employed in the softening process [25], was used in this work.
The concentrations of Ca2+ (1.5–3 mmol L−1) and PO43− (0.1 mmol L−1) used during the experiments represent the values found in WWTP effluent [26,27,28]. The concentration of SA that was previously used to simulate the concentration of organic substances in non-treated domestic wastewater is approximately 0.8 g L−1 [27,29,30]. However, to avoid too rapid clogging, only half of the concentration (0.4 g L−1) was used during the experiments.
A chemical cleaning with citric acid (1.5%) and chlorine (0.1%) was performed after the first set of membrane experiments and then after each membrane experiment (according to protocols presented in the sections below), and all experiments were executed in duplicate.
2.2. Jar Tests
Precipitation tests were performed in jars to study the differences between the conditions, with and without PO43− and SA. Table 1 presents the tested conditions. For each condition, samples were collected and filtered over a 0.45 µm pore filter (Whatman, Germany) in order to determine the remaining Ca2+ concentration using ion chromatography (IC, ProfIC 15–AnCat ion chromatograph Metrohm 881 anion). An A Supp 150/4.0 anion column was used with 3.2 mmol L−1 Na2CO3 and 1 mmol L−1 NaHCO3 eluent. To calibrate the IC, 6 standard solutions of Ca2+ (0.0025, 0.025, 0.25, 1.25, 2.5, and 3.75 mmol L−1) were used.

Table 1.
Jar tests softening conditions.
Due to the inhibitive nature of PO43−, its effect was further analyzed considering different dosings of Ca(OH)2 (1.5 and 2.5 mmol L−1).
2.3. Membrane Tests
2.3.1. Effect of Softening Agent on Fouling
The setup depicted in Figure 1 was used for in-line base and in-tank dosings from a neutralization tank to the membrane system.
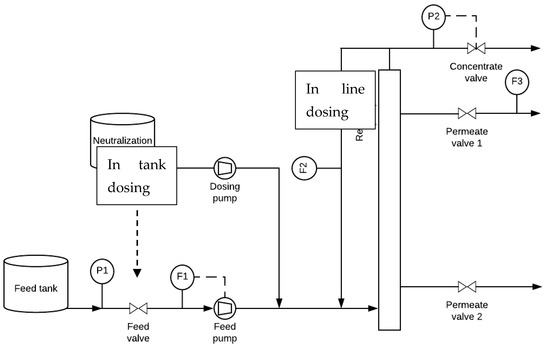
Figure 1.
Schematic representation of the ceramic micro-filtration (CMF) setup. F1—feed flow meter (0–300 L h−1 ± 1 L h−1). F2—recycle flow meter (0–1000 L h−1). F3—permeate flow meter (0–30 L h−1). P1: pressure meter (0–20 ± 0.1 bar). P2—pressure meter (0–20 ± 0.1 bar).
The first two experiments consisted of:
B1 Filtration of the solution containing only a mixture of 3 mmol L−1 of Ca2+ and 6 mmol L−1 of HCO3− to draw the base line and confirm that there is no retention of Ca2+ on the membrane.
B2 Filtration of the solution containing a mixture of 3 mmol L−1 of Ca2+ and 6 mmol L−1 of HCO3− with a constant dosage of Ca(OH)2 (2.5 mmol L−1) to remove the hardness.
The feed flow was 25–30 L h−1 at a constant (transmembrane) pressure of 2 bar and the dosing pump constantly added 3 L h−1 of Ca(OH)2 with a concentration of 22.73 mmol L−1 Ca(OH)2, which resulted in a concentration of 2.5 mmol L−1 Ca(OH)2 in the feed flow. The recovery fluctuated approximately 75–80%. The specification of the used membranes is presented in Table 2.

Table 2.
Ceramic micro-filtration (CMF) membrane specification (Inopor GmbH).
For each experiment, samples of the feed water, permeate, and concentrate flow were taken in duplicate at three points during the tests, after 15 min, 1 h, and at the end of the experiment, respectively. The samples were analyzed for the concentration of Ca2+ using IC.
No flux decline was observed during the two experiments.
After the first set of experiments, the configuration was changed from in-line dosing to in-tank precipitation, where the base was added in the influent tank for the rest of the experiments.
2.3.2. Individual Effect of Sodium Alginate, Calcium, and Softening on Membrane Fouling
The following set of experiments consisted of evaluating the fouling of CMF in the presence of SA, Ca2+, and HCO3− with and without the addition of Ca(OH)2, using membranes in the same condition leading to a similar initial flux.
The tested conditions are presented in Table 3.

Table 3.
Effect of sodium alginate, calcium, and softening on fouling.
For the experiments, the cleaning followed the same procedure as described in the previous section, except for substituting the citric acid with a solution of sodium hypochlorite 0.1% to remove the organic fouling better [27].
2.3.3. Combined Effect of Sodium Alginate, Phosphate, and Softening on Fouling
The last set of experiments consisted of evaluating the fouling of CMF in the presence of Ca2+, HCO3−, PO43− and SA, with the addition of Ca(OH)2, compared with a solution consisting of SA only, using membranes in the same condition leading to a similar initial flux.
The tested conditions are presented in Table 4.

Table 4.
Effect of organic compounds on softening.
For the experiments, the cleaning followed the same procedure as described in the previous section.
2.3.4. Flux Recovery
During the membrane experiments in the presence of organic fouling (SA), the initial flux varied, indicating that some irreversible fouling occurred during filtration and/or the cleaning protocol did not function efficiently. The membrane flux recovery is the ratio of pure water flux after a filtration and cleaning process, Jpwfn, to the pure water flux of the first filtration, Jpwf1, after either hydraulic or chemical washing.
The flux recovery is calculated using Equation (1) below:
3. Results
3.1. Jar Tests of Softening Process and Influence of PO43− and Organic Compounds
3.1.1. Effect of Ca(OH)2 as Softening Agent
Figure 2 shows the results of the remaining Ca2+ after the precipitation with Ca(OH)2. When the concentration of Ca(OH)2 was low (1.5 mmol L−1), the removal was relatively high as, with the addition of 1.5 mmol L−1, it was expected that the concentration of Ca2+ would be reduced to half (from 3 to 1.5 mmol L−1). The fact that Ca(OH)2 is partially soluble (Kps = 5.5 × 10−6) [31] and was added to the water as a suspension enhanced the nucleation and stimulated the crystallization process. The original solution was most likely already somewhat supersaturated with CaCO3 [32]. When the concentration of Ca(OH)2 was increased to 2.5 mmol L−1, the removal increased, but not linearly. Two explanations can be given: (1) At low supersaturation, the nucleation mechanism is heterogeneous, whereas, at higher supersaturation, homogeneous nucleation prevails [33]. The homogeneous nucleation results in an increasing number of formed nuclei. These nuclei have a relatively lower chance of growing to large crystals compared with the growth of a lower number of formed nuclei during heterogeneous crystallization [34]. Therefore, precipitation is hampered by an excessive increase in Ca(OH)2 concentration. (2) A CaCO3 film is developed on the Ca(OH)2 particles and will eventually be partially or entirely enclosed. This film can inhibit Ca(OH)2 from dissolving any further by a layer of CaCO3 formed on the surface of a particle in water, called the dissolve-precipitate mechanism [35].
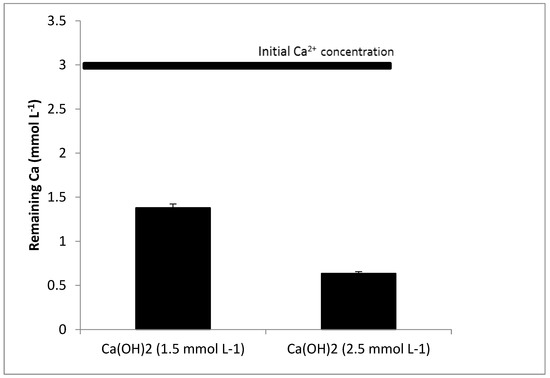
Figure 2.
Calcium removal with calcium hydroxide.
3.1.2. Effect of Phosphate and Sodium Alginate on Softening
Both PO43− and SA inhibited the precipitation of CaCO3 Figure 3. Inhibition by SA was observed only when the concentration of Ca(OH)2 was increased to 2.5 mmol L−1 Figure 3. With a lower dosage of Ca(OH)2 (1.5 mmol L−1) the inhibition was dominated by the presence of PO43− rather than by SA, when considered separately. This was also found earlier, when the growth of CaCO3 took place in the presence of PO43− and interfered with the CaCO3 crystal growth process because of interactions with the lattice ions of CaCO3 at the respective active crystal growth sites [36].
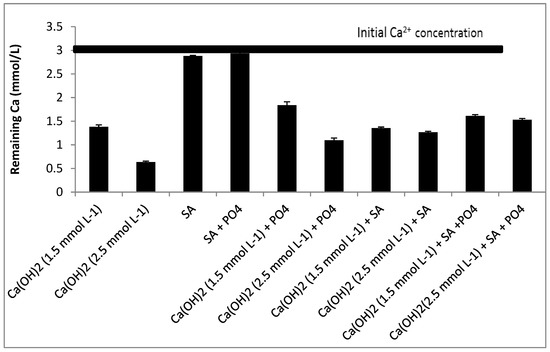
Figure 3.
Effect of phosphate and sodium alginate in softening with calcium hydroxide.
Two processes have been accepted to explain the mechanisms of potential inhibition by organic molecules. First is the formation of chelate complexes of dissolved Ca2+ ions with organic molecules, which reduces the effective supersaturation of CaCO3, thereby decreasing the rate of nucleation and crystal growth, depending on the saturation state [37]. Second is the adsorption of organic compounds on the specific surface of the CaCO3 crystals. The active growth sites on the CaCO3 surface may be blocked by the adsorption reactions, thus preventing the further growth of the crystals of CaCO3 [38,39]. The negative charge of SA, due to deprotonated carboxylic functional groups, may then induce repulsive inter- and intramolecular electrostatic forces, decreasing the chances of CaCO3 precipitation [24].
When phosphate and SA were considered simultaneously, the precipitation of Ca2+ was less influenced compared with the condition where PO43− was considered separately, especially when the concentration of Ca(OH)2 was 1.5 mmol L−1. The chelate complexes formed after adding SA may thus have masked Ca2+ and inactivated the interaction between Ca2+ and PO43− [37].
3.1.3. Extent of Inhibition Effect of Phosphate
From Figure 4, it can be observed that an increment in the concentration of PO43− from 0.05 to 0.15 mmol L−1 led to a small increase in the inhibition of CaCO3 precipitation, but it was slightly reduced when 0.2 mmol L−1 of PO43− was added. Further, when the concentration of Ca(OH)2 was increased from 1.5 to 2.5 mmol L−1, the effect of PO43− was similar. Gebauer et al. [40] studied the role of additives in CaCO3 precipitation. They also found that the addition of 10 mg/L sodium triphosphate (approximately 0.1 mmol L−1) solution performed only slightly weaker than the addition of 100 mg/L sodium triphosphate (1 mmol L−1). Therefore, this suggests that the mechanism of colloidal stability of the intermediate cluster structures is the most relevant in suppressing nucleation in this system and not the stoichiometric binding events (as expected by an ion-binding-like mechanism).
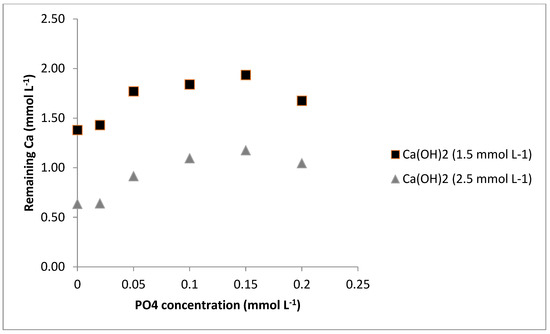
Figure 4.
Inhibition effect of PO43− on precipitation of CaCO3.
3.2. Ceramic Membrane Tests Results
3.2.1. Effect of Softening on Fouling
During the CMF tests without adding a base, the IC results (Table 5) showed a constant Ca2+ feed concentration of 1.6 mmol L−1. The first two samples of the retentate were not taken because too little was produced. The retentate showed values of approximately 0.1 mmol L−1 lower than the feed. Since the base was not added in this experiment, no difference in concentration was expected from the collected samples, as the pores of the CMF are too large to retain dissolved salts (i.e., Ca(HO3)2) without previous precipitation [41], which was confirmed by the obtained results.

Table 5.
Calcium concentrations in the feed, permeate, and concentrate samples.
In the experiment with the addition of Ca(OH)2, the Ca2+ concentration in the feed flow was approximately 1.9 mmol L−1 (Table 5). The chemical dosing in this experiment was 2.5 mmol L−1 of Ca(OH)2, directly dosed into the feed flow. The Ca2+ concentration in the retentate decreased to a value of 0.5 mmol L−1 and, near the end of the experiment, even reached a concentration of less than 0.1 mmol L−1, having precipitated 1.4 to 1.8 mmol L−1. Since the base was stoichiometrically overdosed, it was expected that almost all Ca2+ would be removed by precipitation. These results were in accordance with the results of the jar tests, where we found that when increasing the concentration of Ca(OH)2 to 2.5 mmol L−1, substantial removal of Ca2+ was observed. The results were also in accordance with the results of Zeppenfeld [42] and Heinsbroek [22], who found that by increasing the carbonate concentration, promoted by the addition of the base, thereby increasing supersaturation, the rate constant increased linearly and consequently increased the Ca2+ removal.
During the experiment, the flux built up rapidly in the first few minutes of the test. Still, it stayed near a constant value (of 190 L m−2 h−1) for the rest of the experiment, which is represented by the example in Figure 5, indicating that scaling of the membrane did not occur during the experiment [22].
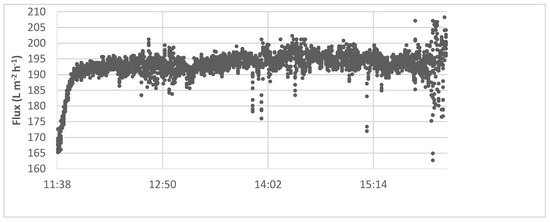
Figure 5.
Membrane performance of the softening with calcium hydroxide.
3.2.2. Individual Effect of Sodium Alginate, Calcium, and Softening Agent on Fouling
When filtering a solution with Ca2+, bicarbonate, and 0.4 g L−1 of SA, the initial retentate flux was 270 L m−2 h−1, pronouncedly decreased, and reached a flux of approximately 50 L m−2 h−1 in more than 30 min (experiment C1) (Figure 6). The accentuated decrease in the retentate flux can be explained by the complexation of Ca2+ with alginate, forming a compacted gel layer on the membrane surface. Compression of the electric double layers of the alginate on the membrane results in a lower electrostatic repulsion and a denser fouling layer with a higher absolute resistance [43,44]. Not much difference (the flux also reached 50 L m−2 h−1 in more than 30 min) was observed when 1.5 mmol L−1 Ca(OH)2 was added to the previous mixture (experiment C2). From the jar tests, it was observed that, approximately 50% of Ca2+ was removed in the presence of SA, and apparently, this removal did not influence the performance of the membrane, e.g., through scaling.
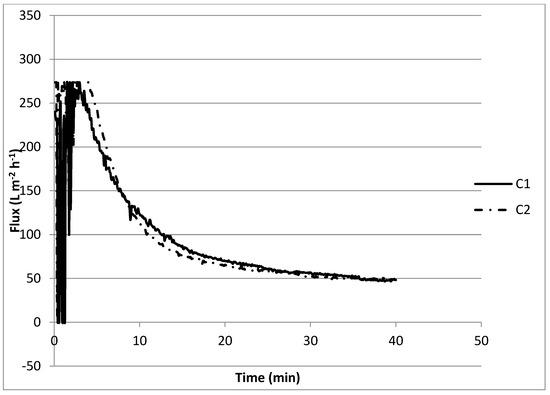
Figure 6.
Membrane performance for the effect of sodium alginate, calcium, and softening agent on fouling.
3.2.3. Combined Effect of Sodium Alginate, Calcium, Phosphate, and Softening Agent on Fouling
During experiment D1, where a solution containing only 0.4 g L−1 SA was filtered, it took approximately 10 min to lower the retentate flux from 150 L m−2 h−1 to approximately 80 L m−2 h−1, 2/3 (Figure 7) and then stayed more or less constant. In all experiments, a steep decline in permeability at the beginning of filtration was observed and was most likely caused by the loading effect [45] being a direct interaction between the membrane surface and the alginate molecules that could then create a firm fouling layer. This interaction is mostly dominated by the adsorption of foulant to the membrane surface, which leads to pore constriction [24].
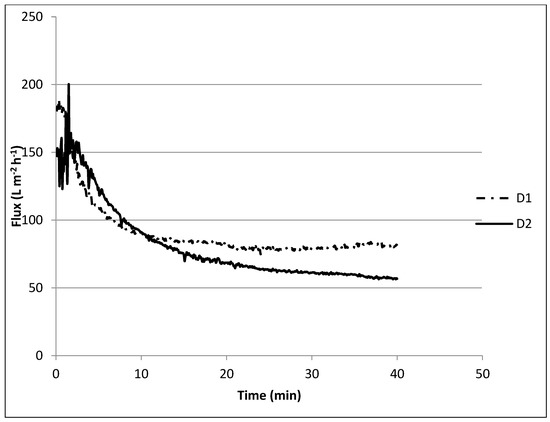
Figure 7.
Membrane performance for the combined effect of sodium alginate, calcium, phosphate, and softening agent on fouling.
Similar behavior was observed when dosing Ca(OH)2 in the presence of SA, Ca2+, HCO3−, and PO43− (D2), although the final flux further decreased to approximately 60 L m−2 h−1. This could be explained by the complexation of Ca2+ with alginate forming a compacted gel layer on the membrane surface and some scaling of CaCO3 on the surface or in the pores [46]. Although flux decline was low compared with the first experiment, it is likely that the Ca2+ removal was negatively affected, as was observed during the jar tests.
3.2.4. Flux Recovery Results
From Figure 6 and Figure 7 it can be observed that the initial flux declined from 270 to 200 L m−2 h−1, a flux recovery of 74%. These results are in accordance with the first set of experiments (Figure 5) with recoveries between 75 and 80%. Katsoufidou et al. [47] also analyzed a fouling of membranes with SA and found that the fouling was due to two mechanisms; (1) a rapid irreversible fouling due to internal pore constriction, followed by (2) cake development on the membrane surface, which becomes the dominant fouling mechanism when calcium concentration increases. This may explain why we observed a low flux recovery in the last experiments.
4. Conclusions
A novel method of treatment of the model WWTP effluent with ceramic micro-filtration (CMF) for use in cooling systems was developed with a focus on scale formation by removing hardness in the presence of other fouling agents, such as organic matter. The effectiveness of Ca(OH)2 as a softening agent was studied using jar tests, and the effect of organic compounds on the softening process was analyzed using model water with SA. During the CMF tests, the effect on fouling (through flux decline) was studied.
It was found that PO43− inhibited the precipitation of CaCO3 when using Ca(OH)2 as a softening agent. In conditions where PO43− was present, the concentration of Ca(OH)2 needed to be increased to compensate for the precipitation of PO43− and the inhibition of the calcite crystallization process. Organic compounds also affected the removal of Ca2+ on the softening process, although in the presence of PO43−, the alginate inhibition of the crystallization process was reduced.
Finally, it was concluded that the fouling of the membranes due to sodium alginate in water was only slightly negatively affected by PO43− and when combined with softening.
Author Contributions
Conceptualization, N.J.G., L.C.R., A.M.A. and S.G.J.H.; investigation, N.J.G. and D.D.; writing—original draft preparation, N.J.G. and L.C.R.; writing—review and editing, A.M.A., S.G.J.H. and L.C.R.; funding acquisition, L.C.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NWO-WOTRO, grant number W 07.69.109.
Acknowledgments
The research presented in this manuscript was supported by an NWO-WOTRO grant (Project number W 07.69.109) as part of the Urbanizing Deltas of the World program. The authors acknowledge the PhD scholarship awarded to Noor Jehan Gulamussen by Nuffic. Furthermore, the authors would like to thank Ivan Machava, Sarah Almahfoodh, and the staff of the water laboratory for their contribution to this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gulamussen, N.J.; Arsénio, A.M.; Matsinhe, N.P.; Rietveld, L.C. Water reclamation for industrial use in sub-Saharan Africa-A critical review. Drink. Water Eng. Sci. 2019, 12, 45–58. [Google Scholar] [CrossRef]
- UNEP. Economic Valuation of Wastewater: The Cost of Action and the Cost of No Action; United Nations: New York, NY, USA, 2015; ISBN 9789280734744. [Google Scholar]
- Ehrhardt, R.F.; Tucker, R.C.; Fabian, T.R. Analysis of Alternative Sources of Cooling Water; Final Report; No. EPRI-EA-4732; Dames and Moore: Bethesda, MD, USA, 1986. [Google Scholar]
- Difilippo, M.N. Use of Alternate Water Sources for Power Plant Cooling; Palo Alto: Santa Clara, CA, USA, 2008; Volume 1014935. [Google Scholar]
- Li, H.; Hsieh, M.K.; Chien, S.H.; Monnell, J.D.; Dzombak, D.A.; Vidic, R.D. Control of mineral scale deposition in cooling systems using secondary-treated municipal wastewater. Water Res. 2011, 45, 748–760. [Google Scholar] [CrossRef]
- EUROSTAT: Water Use in Industry. 2014. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Archive:Water_use_in_industry (accessed on 3 June 2016).
- Veil, J.A. Use of Reclaimed Water for Power Plant Cooling; No. ANL/EVS/R-07/3; Argonne National Lab. (ANL): Argonne, IL, USA, 2007. [Google Scholar]
- Lens, P.; Pol, L.H.; Wilderer, P.; Asano, T. Water Recycling and Resource Recovery in Industry: Analysis, Technologies and Implementation; IWA Publishing: London, UK, 2002; ISBN 1843390051. [Google Scholar]
- San Jose Environmental Service Department. Guidelines for Managing Water in Cooling Systems for Owners, Operators, and Environmental Managers; San Jose Environmental Service Department: San Jose, CA, USA, 2002. Available online: https://www.sanjoseca.gov/home/showdocument?id=37061 (accessed on 3 June 2016).
- Joint Research Council. Integrated Pollution Prevention and Control (IPPC) Reference Document on the application of Best Available Techniques to Industrial Cooling Systems; Joint Research Council: Brussels, Belgium, 2001. [Google Scholar]
- Mech Co., Ltd. Water Quality Standard for Cooling Water, Cold Water, Hot Water, Makeup Water (JRA GL02-1994). 1994. Available online: http://www.mech.co.jp/web-en/01/02/img/kijun02.pdf (accessed on 19 June 2019).
- Asano, T. Wastewater Reclamation and Reuse; CRC Press: Boca Raton, FL, USA, 1998; ISBN 1566766206. [Google Scholar]
- Kramer, F.C.; Shang, R.; Heijman, S.G.J.; Scherrenberg, S.M.; Van Lier, J.B.; Rietveld, L.C. Direct water reclamation from sewage using ceramic tight ultra- and nanofiltration. Sep. Purif. Technol. 2015, 147, 329–336. [Google Scholar] [CrossRef]
- Manohar. Development & Characterization of Ceramic Membranes. Int. J. Mod. Eng. Res. 2012, 2, 1492–1506. [Google Scholar]
- Hu, Z.; Yang, Y.; Chang, Q.; Liu, F.; Wang, Y.; Rao, J. Preparation of a high-performance porous ceramic membrane by a two-step coating method and one-step sintering. Appl. Sci. 2018, 9, 52. [Google Scholar] [CrossRef]
- Ahmad, R.; Kim, J.K.; Kim, J.H. Nanostructured Ceramic Photocatalytic Membrane Modified with a Polymer Template for Textile Wastewater Treatment. Appl. Sci. 2017, 7, 12. [Google Scholar] [CrossRef]
- Mouratib, R.; Achiou, B.; El Krati, M.; Younssi, S.A.; Tahiri, S. Low-cost ceramic membrane made from alumina- and silica-rich water treatment sludge and its application to wastewater fi ltration. J. Eur. Ceram. Soc. 2020, 40, 5942–5950. [Google Scholar] [CrossRef]
- Perez-Galvez, R.; Guadix, E.M.; Berge, J.; Guadix, A. Archimer. J. Membr. Sci. 2011, 384, 142–148. [Google Scholar] [CrossRef]
- Lee, M.; Wu, Z.; Li, K. Advances in Ceramic Membranes for Water Treatment; Woodhead Publishing: Sawston, UK, 2015; ISBN 9781782421269. [Google Scholar]
- Aryanti, P.T.P.; Subroto, E.; Mangindaan, D.; Widiasa, I.N.; Wenten, I.G. Semi-industrial high-temperature ceramic membrane clarification during starch hydrolysis. J. Food Eng. 2020, 274, 109844. [Google Scholar] [CrossRef]
- Ha, C. Ozonation and/or Coagulation-Ceramic Membrane Hybrid for Filtration of Impaired-Quality Source Waters. Ph.D. Thesis, King Abdullah University of Science and Technology, Thuwal, Saudi Arabia, 2013. [Google Scholar]
- Heinsbroek, A.R. Calcium Hydroxide as Precipitative Antiscalant for Nanofiltration. Master’s Thesis, Delft University of Tecnology, Delft, The Netherlands, 2016. [Google Scholar]
- Pokrovsky, O.S. Precipitation of calcium and magnesium carbonates from homogeneous supersaturated solutions. J. Cryst. Growth 1998, 186, 233–239. [Google Scholar] [CrossRef]
- Katsoufidou, K.; Yiantsios, S.G.; Karabelas, A.J. Experimental study of ultrafiltration membrane fouling by sodium alginate and flux recovery by backwashing. J. Memb. Sci. 2007, 300, 137–146. [Google Scholar] [CrossRef]
- Ratnayaka, D.D.; Brandt, M.J.; Johnson, M. (Eds.) Specialised and advanced water treatment processes. In Water Supply; Elsevier Ltd.: Amsterdam, The Netherlands, 2009; pp. 370–428. ISBN 9780080940847. [Google Scholar]
- Dudziak, M.; Kudlek, E. Removal of hardness in wastewater effluent using membrane filtration. Archit. Civ. Eng. Environ. 2019, 2, 141–147. [Google Scholar] [CrossRef]
- Kramer, F. Ceramic Nanofiltration for Direct Filtration of Municipal Sewage. Ph.D. Thesis, Delft University of Tecnology, Delft, The Netherlands, 2019. [Google Scholar]
- Lei, Y.; Hidayat, I.; Saakes, M.; van der Weijden, R.; Buisman, C.J. Fate of calcium, magnesium and inorganic carbon in electrochemical phosphorus recovery from domestic wastewater. Chem. Eng. J. 2019, 362, 453–459. [Google Scholar] [CrossRef]
- Charfi, A.; Tibi, F.; Kim, J.; Hur, J.; Cho, J. Organic Fouling Impact in a Direct Contact Membrane Distillation System Treating Wastewater: Experimental Observations and Modeling Approach. Membranes 2021, 11, 493. [Google Scholar] [CrossRef] [PubMed]
- Arndt, F.; Roth, U.; Nirschl, H.; Schutz, S.; Guthausen, G. New Insights into Sodium Alginate Fouling of Ceramic Hollow Fiber Membranes by NMR Imaging. AIChE J. 2016, 62, 2459–2467. [Google Scholar] [CrossRef]
- Kellner, R.A.; Mermet, J.-M.; Otto, M.; Varcarcel, M.; Widmer, H.M. (Eds.) Analytical Chemistry: A Modern Approach to Analytical Science, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2004; ISBN 3-527-30590-4. [Google Scholar]
- Spanos, N.; Koutsoukos, P.G. Kinetics of Precipitation of Calcium Carbonate in Alkaline pH at Constant Supersaturation. Spontaneous and Seeded Growth. J. Phys. Chem. B 1998, 102, 6679–6684. [Google Scholar] [CrossRef]
- Van Driessche, A.E.S.; Kellermeier, M.; Benning, L.G.; Gebauer, D. (Eds.) New Perspectives on Mineral Nucleation and Growth; Springer International Publishing: Cham, Switzerland, 2017; ISBN 9783319456676. [Google Scholar]
- El-Shall, H.; Rashad, M.M.; Abel-Aal, E.A. Effect of phosphonate additive on crystallization of gypsum in phosphoric and sulfuric acid medium. Cryst. Res. Technol. 2002, 37, 1264–1273. [Google Scholar] [CrossRef]
- Van Eekeren, M.W.M.; Van Paassen, J.A.M.; Merks, C.W.A.M. Improved milk-of-lime for softening of drinking water-the answer to the carry-over problem. Aqua 1994, 43, 1–10. [Google Scholar]
- Tadier, S.; Rokidi, S.; Rey, C.; Combes, C.; Koutsoukos, P.G. Crystal growth of aragonite in the presence of phosphate. J. Cryst. Growth 2017, 458, 44–52. [Google Scholar] [CrossRef]
- Sundarrajan, P.; Eswaran, P.; Marimuthu, A.; Subhadra, L.B.; Kannaiyan, P. One pot synthesis and characterization of alginate stabilized semiconductor nanoparticles. Bull. Korean Chem. Soc. 2012, 33, 3218–3224. [Google Scholar] [CrossRef]
- Kawano, M.; Tokonami, M. Effect of organic acids on the precipitation rate and polymorphism of calcium carbonate minerals in solutions with Mg2+ ions. Clay Sci. 2014, 18, 1–10. [Google Scholar] [CrossRef]
- Haidari, A.H.; Gonzales-Olmos, R.; Heijman, S.G.J. Scaling after remineralisation of reverse osmosis permeate. Desalination 2019, 467, 57–63. [Google Scholar] [CrossRef]
- Gebauer, D.; Colfen, H.; Verch, A.; Antonietti, M. The Multiple Roles of Additives in CaCO3 Crystallization: A Quantitative Case Study. Adv. Mater. 2009, 27, 435–439. [Google Scholar] [CrossRef]
- Serhiienko, A.O.; Dontsova, T.A.; Yanushevska, O.I.; Nahirniak, S.V.; Ahmad, H. Ceramic Membranes: New Trends and Prospects (Short Review). Water Water Purif. Technol. Tech. News 2020, 27, 4–31. [Google Scholar] [CrossRef]
- Zeppenfeld, K. Calcite precipitation from aqueous solutions with different calcium and hydrogen carbonate concentrations. J. Water Supply Res. Technol. 2014, 59, 482–491. [Google Scholar] [CrossRef][Green Version]
- van de Ven, W.J.C.; van Sant, K.; Pünt, I.G.M.; Zwijnenburg, A.; Kemperman, A.J.B.; van der Meer, W.G.J.; Wessling, M. Hollow fiber dead-end ultrafiltration: Influence of ionic environment on filtration of alginates. J. Memb. Sci. 2008, 308, 218–229. [Google Scholar] [CrossRef]
- Van Den Brink, P.; Zwijnenburg, A.; Smith, G.; Temmink, H.; Loosdrecht, M. Van Effect of free calcium concentration and ionic strength on alginate fouling in cross-flow membrane filtration. J. Membr. Sci. 2009, 345, 207–216. [Google Scholar] [CrossRef]
- Abdelrasoul, A.; Doan, H.; Lohi, A. Fouling in Membrane Filtration and Remediation Methods. In Mass Transfer-Advances in Suitable Energy and Environment Oriented Numerical Modeling, 1st ed.; Nakajima, H., Ed.; InTech: Rikeja, Croatia, 2013; pp. 195–218. [Google Scholar]
- Hashino, M.; Katagiri, T.; Kubota, N.; Ohmukai, Y.; Maruyama, T.; Matsuyama, H. Effect of membrane surface morphology on membrane fouling with sodium alginate. J. Memb. Sci. 2011, 366, 258–265. [Google Scholar] [CrossRef]
- Katsoufidou, K.; Yiantsios, S.G.; Karabelas, A.J. An experimental study of UF membrane fouling by humic acid and sodium alginate solutions: The effect of backwashing on flux recovery. Desalination 2008, 220, 214–227. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).