Unveiling the Impacts of Sodium Hypochlorite on the Characteristics and Fouling Behaviors of Different Commercial Polyvinylidene Fluoride Hollow Fiber Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Membranes and Chemicals
2.2. NaOCl Exposure Experiments
2.3. Membrane Characterization Techniques
2.4. Membrane Separation Performance and Fouling Behavior Evaluation
3. Results and Discussion
3.1. Effects of NaOCl Exposure on Membrane Physicochemical Characteristics
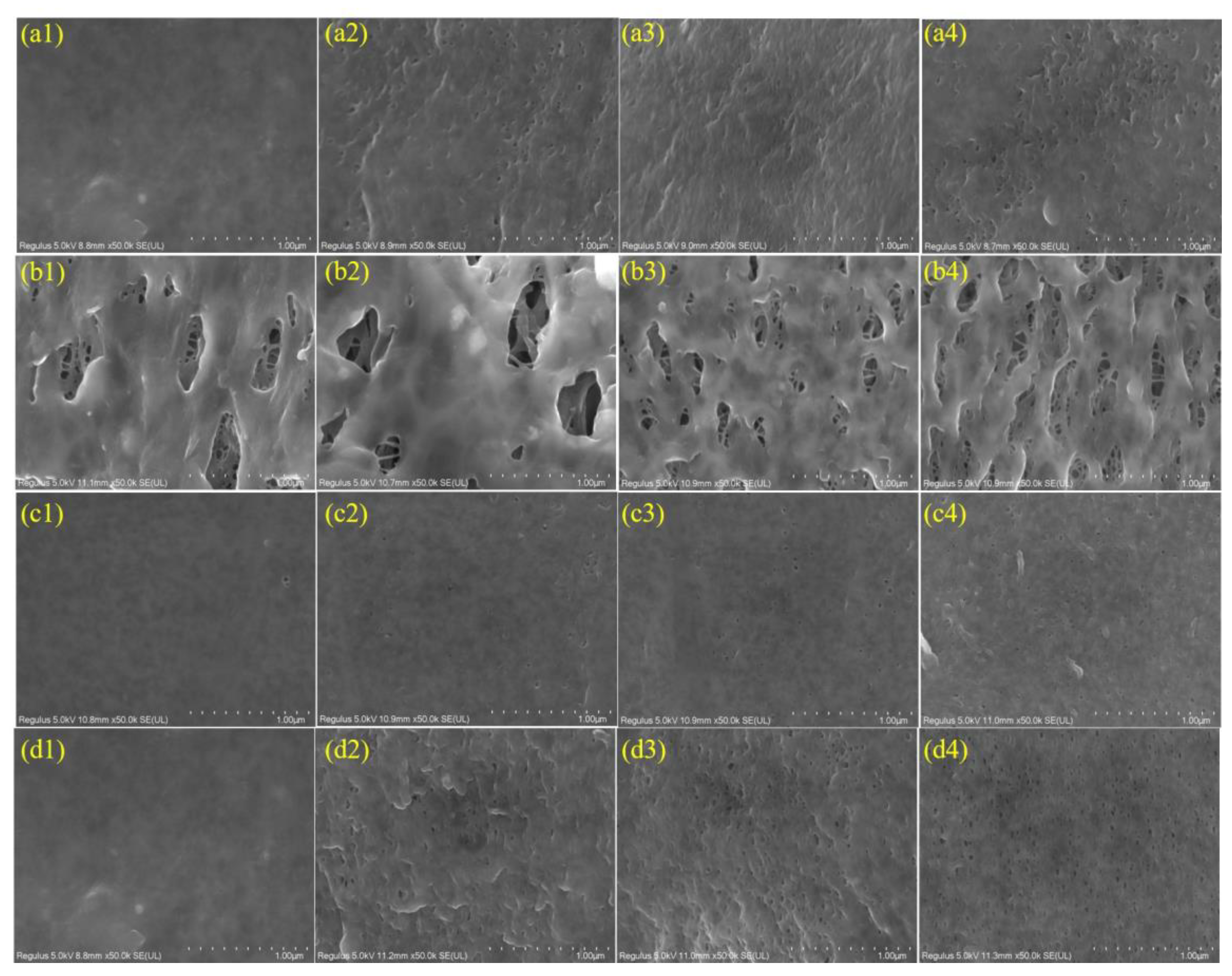
3.1.1. Surface and Cross-Section Morphologies of the NaOCl-Aged Membranes
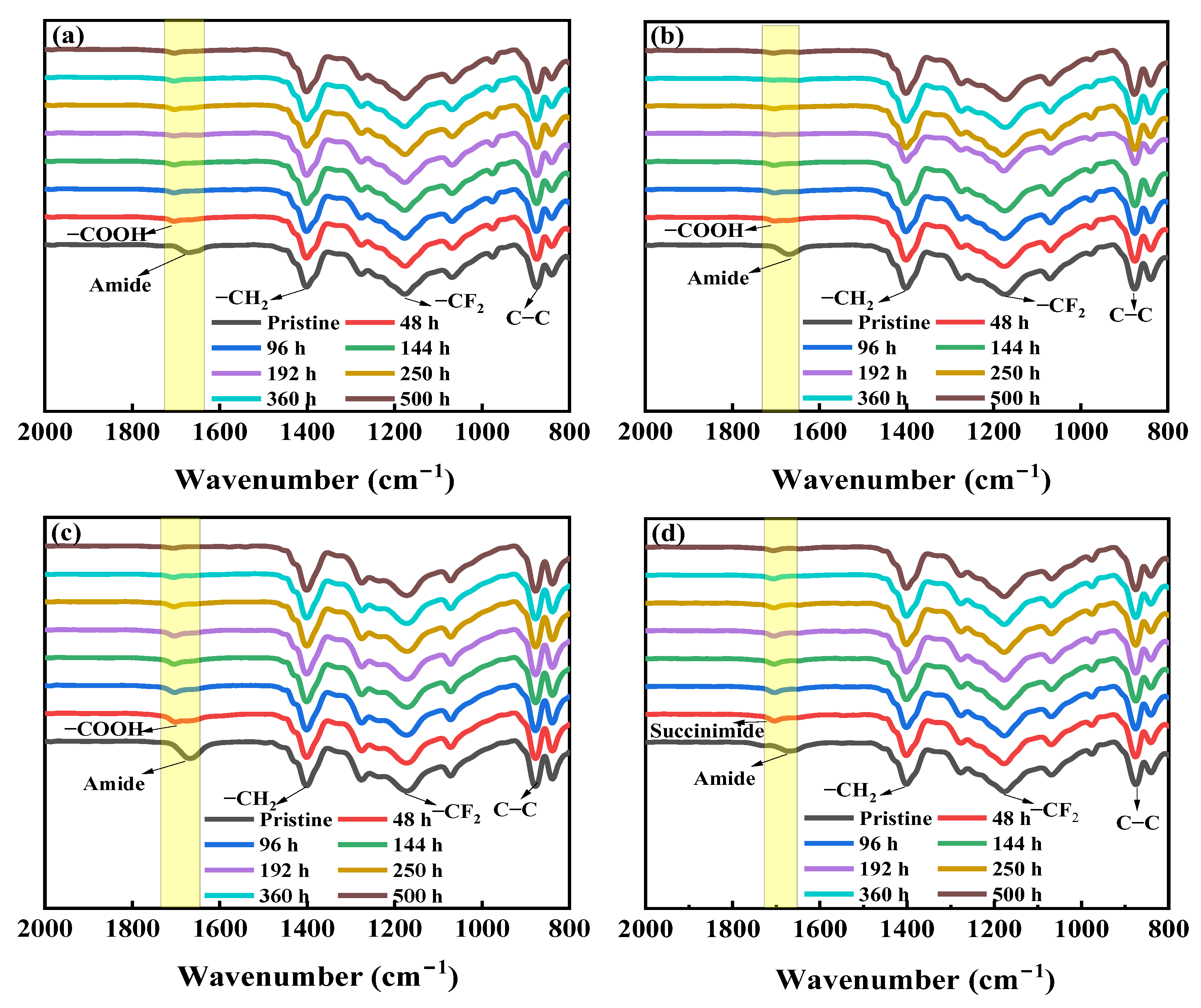
3.1.2. Chemical Compositions of the NaOCl-Aged Membrane Surfaces
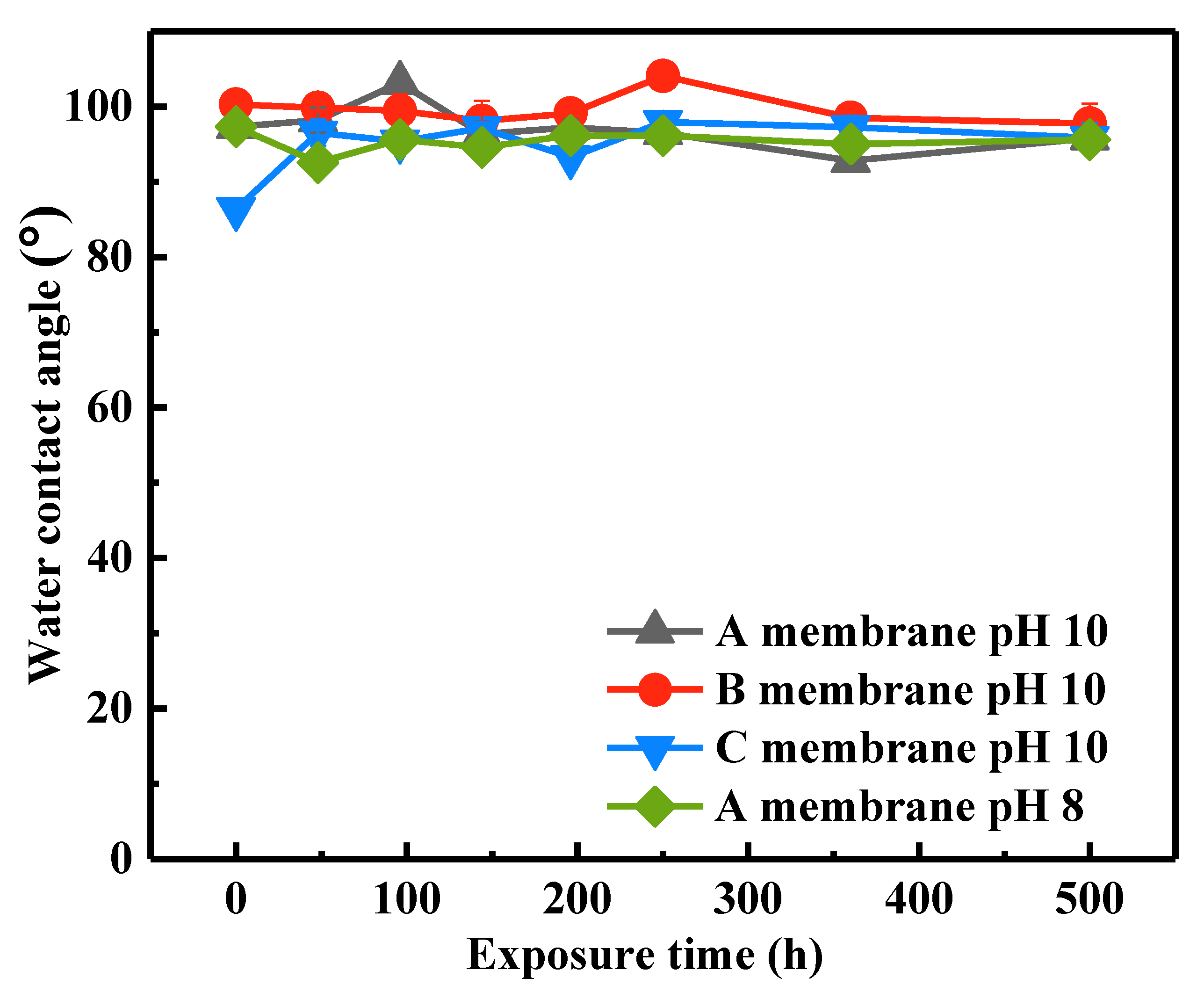
3.1.3. Changes in Surface Hydrophilicity
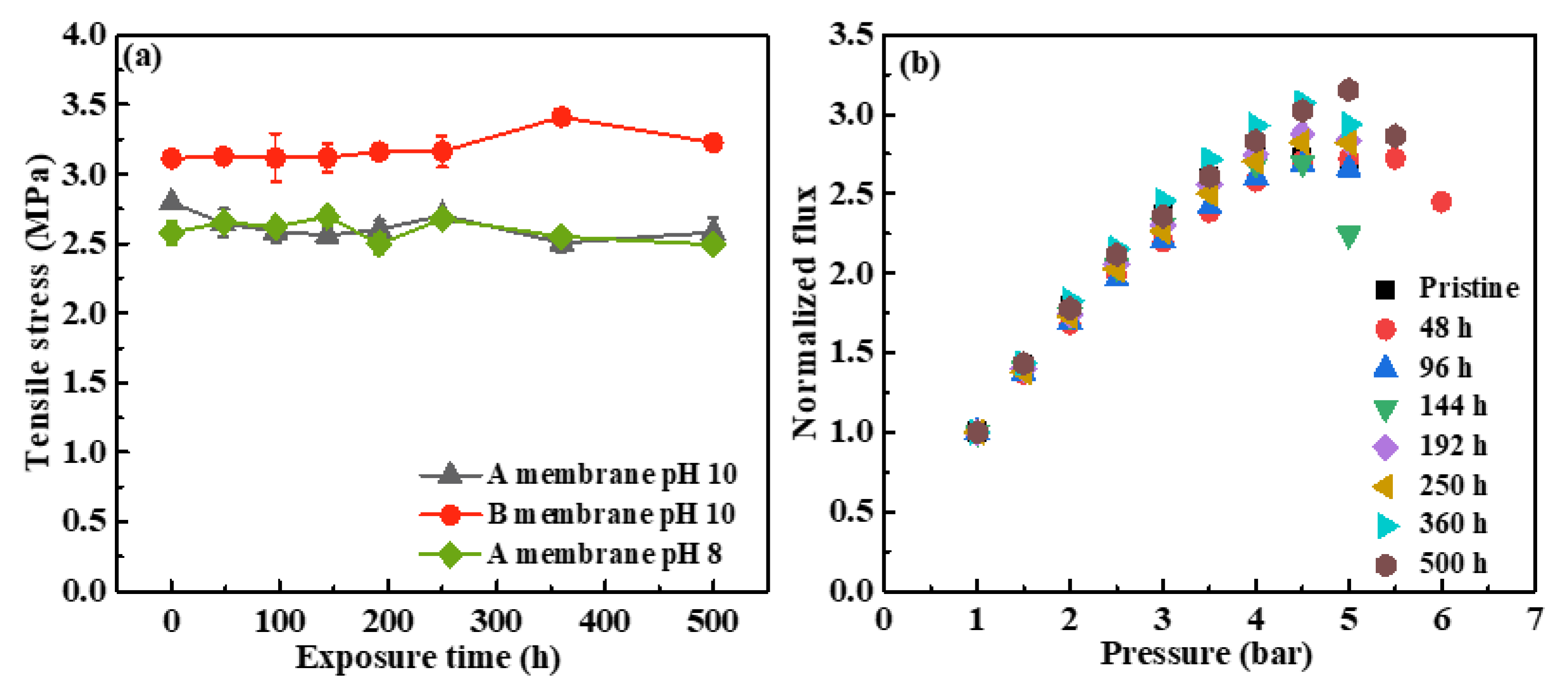
3.1.4. Effects of NaOCl Exposure on Membrane Mechanical Properties
3.2. Separation Performance and Fouling Behavior of NaOCl-Exposed Membranes
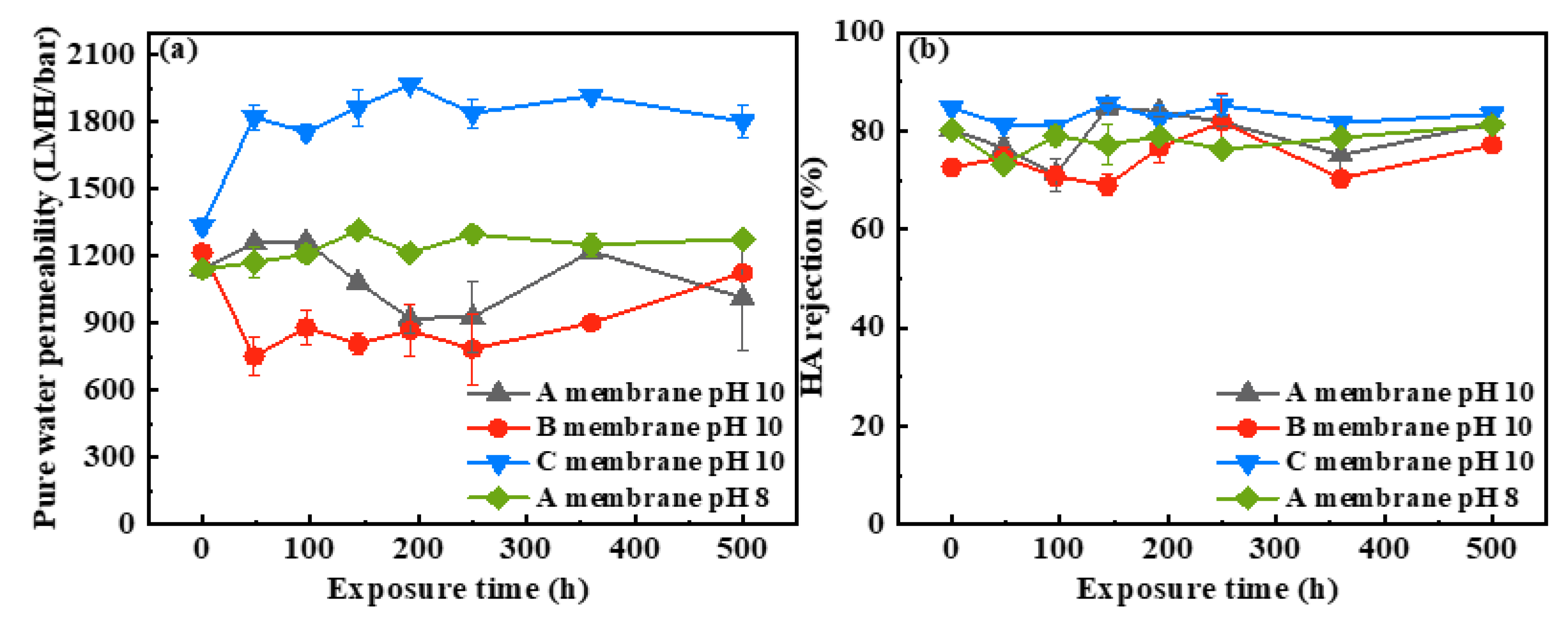
3.2.1. Pure Water Permeability and HA Rejection of the Pristine and NaOCl-Exposed Membranes
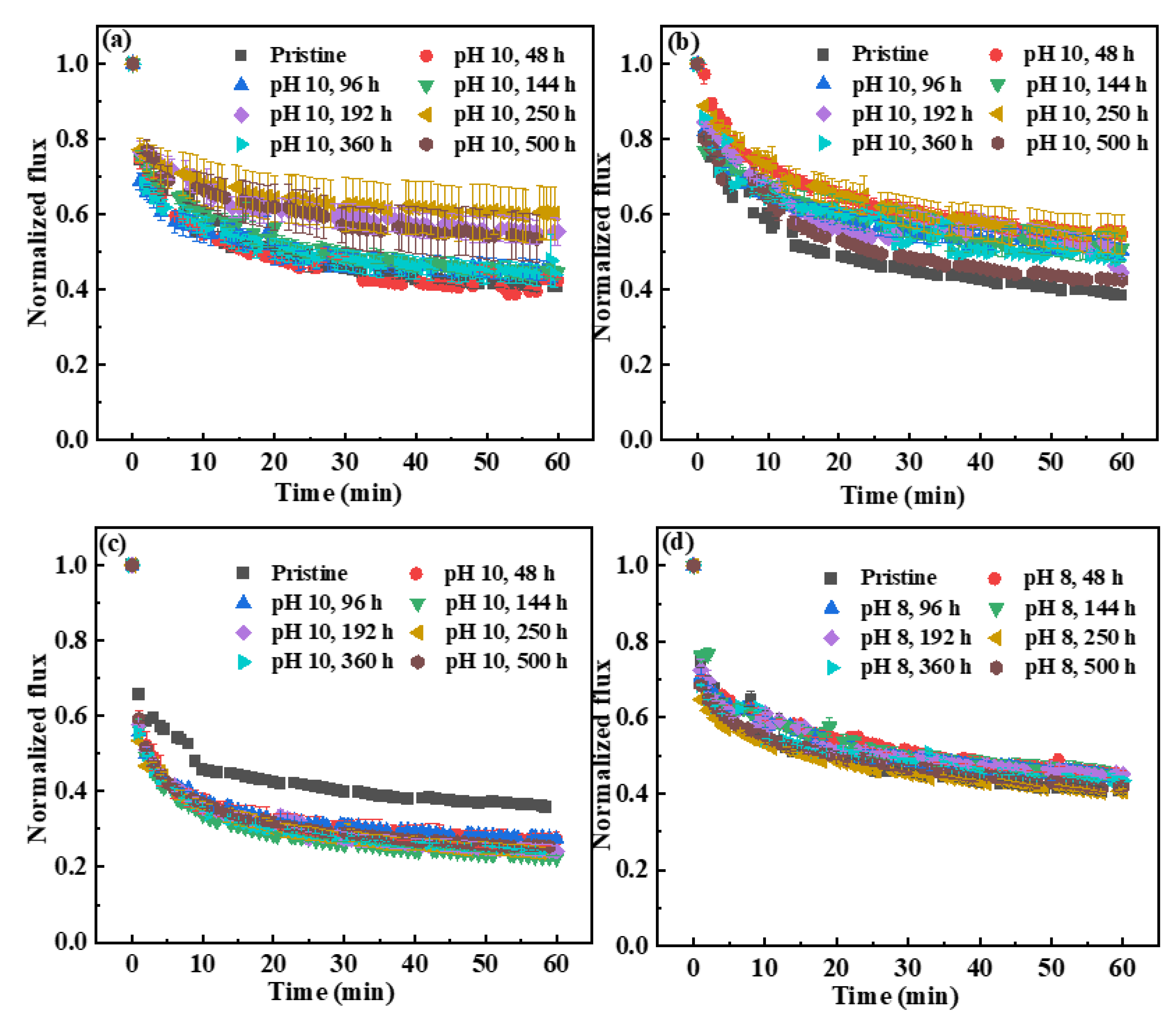
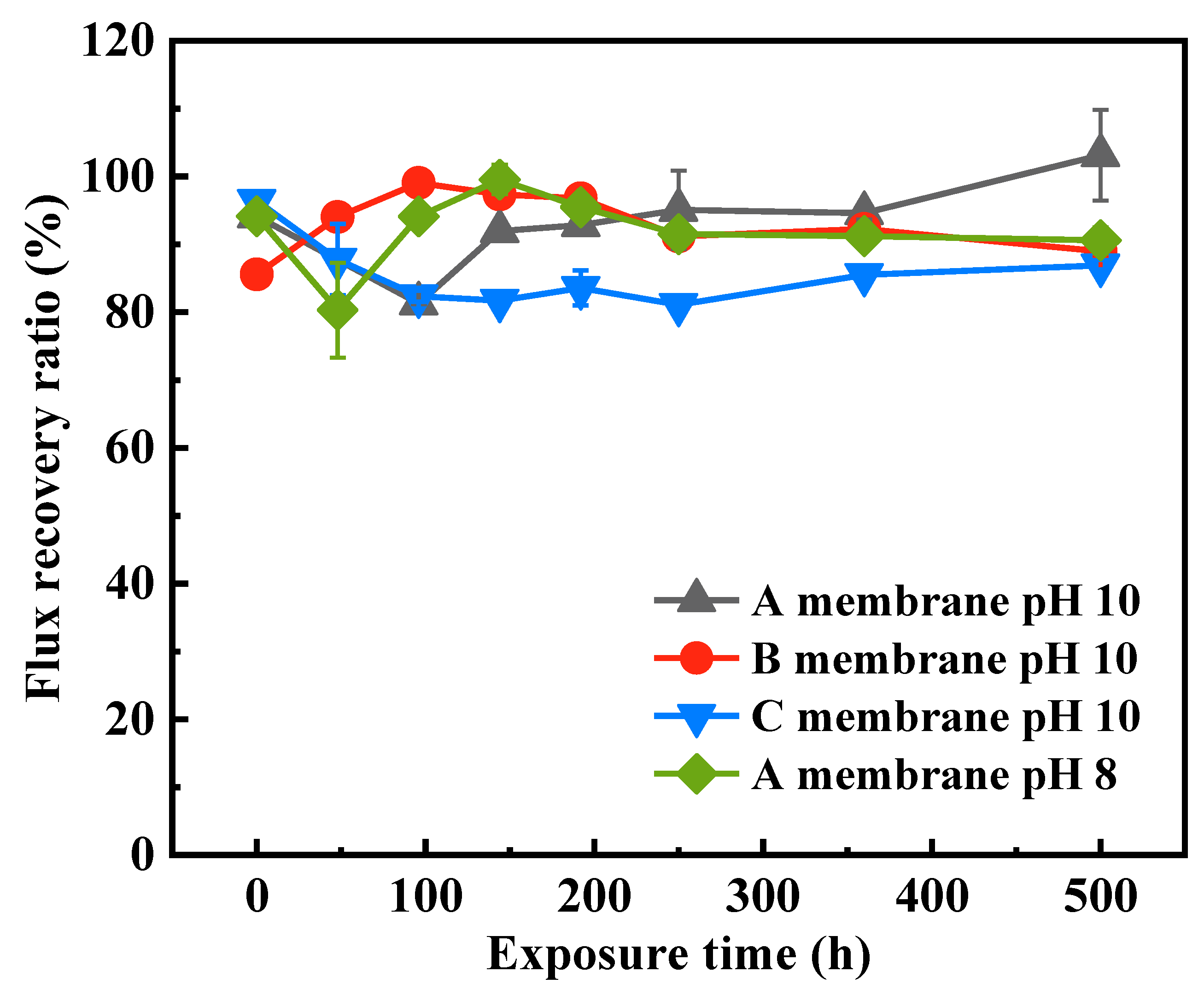
3.2.2. Fouling Behaviors of the Pristine and NaOCl-Exposed Membranes
4. Conclusions
- (1)
- The FESEM and ATR-FTIR results demonstrated the degradation and leaching out of hydrophilic additive PVP from the membranes after exposure to NaOCl. The number of pores on the membrane surfaces increased with a prolonged exposure time. The ATR-FTIR results indicated that the degradation and dissolution of PVP by NaOCl in alkaline condition led to the formation of carboxylic acid group through ring opening of pyrrolidone, while a succinimide group was formed at pH 8 condition.
- (2)
- The WCAs of the SMM-1010 and MEMCOR® CS II membranes showed insignificant change with the various exposure time, whereas the WCA of the ZeeWeed 500 membrane increased after exposure to NaOCl. These three membranes presented robust mechanical properties during NaOCl aging process, which is independent of exposure dose and solution pH, ascribing to the stable C-F bonds.
- (3)
- The PWP of the SMM-1010 and MEMCOR® CS II membranes slightly declined after exposure to NaOCl pH 10 for 500 h, while that of the NaOCl aged-ZeeWeed 500 membrane increased by ~1.4-fold. The HA rejections of these membranes had insignificant changes during NaOCl aging process. The antifouling performances of SMM-1010 and MEMCOR® CS II membranes were slightly improved after exposure to NaOCl in the alkaline condition, whereas NaOCl aging aggravated ZeeWeed 500 membrane fouling.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meng, F.; Chae, S.-R.; Drews, A.; Kraume, M.; Shin, H.-S.; Yang, F. Recent advances in membrane bioreactors (MBRs): Membrane fouling and membrane material. Water Res. 2009, 43, 1489–1512. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zhang, S.; Oh, Y.; Zhou, Z.; Shin, H.-S.; Chae, S.-R. Fouling in membrane bioreactors: An updated review. Water Res. 2017, 114, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Liang, S.; Wang, X.; Chen, C.; Huang, X. Current state and challenges of full-scale membrane bioreactor applications: A critical review. Bioresour. Technol. 2019, 271, 473–481. [Google Scholar] [CrossRef]
- She, Q.; Wang, R.; Fane, A.G.; Tang, C.Y. Membrane fouling in osmotically driven membrane processes: A review. J. Membr. Sci. 2016, 499, 201–233. [Google Scholar] [CrossRef]
- Li, D.; Yang, X.; Zhou, Z.; Jiang, B.; Tawfik, A.; Zhao, S.; Meng, F. Molecular traits of phenolic moieties in dissolved organic matter: Linkages with membrane fouling development. Environ. Int. 2019, 133, 105202. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, J.; Wang, Z.; Chen, H.; Liu, M.; Wu, Z. Reinvestigation of membrane cleaning mechanisms using NaOCl: Role of reagent diffusion. J. Membr. Sci. 2018, 550, 278–285. [Google Scholar] [CrossRef]
- Lin, J.C.-T.; Lee, D.-J.; Huang, C. Membrane fouling mitigation: Membrane cleaning. Sep. Sci. Technol. 2010, 45, 858–872. [Google Scholar] [CrossRef]
- Gul, A.; Hruza, J.; Yalcinkaya, F. Fouling and chemical cleaning of microfiltration membranes: A mini-review. Polymers 2021, 13, 846. [Google Scholar] [CrossRef]
- Shi, W.; Gao, F.; Li, X.; Wang, Z. High zeolite loading mixed matrix membrane for effective removal of ammonia from surface water. Water Res. 2022, 221, 118849. [Google Scholar] [CrossRef]
- Arkhangelsky, E.; Kuzmenko, D.; Gitis, V. Impact of chemical cleaning on properties and functioning of polyethersulfone membranes. J. Membr. Sci. 2007, 305, 176–184. [Google Scholar] [CrossRef]
- Abdullah, S.Z.; Bérubé, P.R. Assessing the effects of sodium hypochlorite exposure on the characteristics of PVDF based membranes. Water Res. 2013, 47, 5392–5399. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Wang, J.; Zhang, H.; Zhang, Y.; Hang, M.A. Effects of sodium hypochlorite on structural/surface characteristics, filtration performance and fouling behaviors of PVDF membranes. J. Membr. Sci. 2016, 519, 22–31. [Google Scholar] [CrossRef]
- Hanafi, Y.; Szymczyk, A.; Rabiller-Baudry, M.; Baddari, K. Degradation of poly (ether sulfone)/polyvinylpyrrolidone membranes by sodium hypochlorite: Insight from advanced electrokinetic characterizations. Environ. Sci. Technol. 2014, 48, 13419–13426. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Su, Q.; Li, S.; Wen, G.; Huang, T. Aging of PVDF and PES ultrafiltration membranes by sodium hypochlorite: Effect of solution pH. J. Environ. Manage. 2021, 104, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Puspitasari, V.; Granville, A.; Le-Clech, P.; Chen, V. Cleaning and ageing effect of sodium hypochlorite on polyvinylidene fluoride (PVDF) membrane. Sep. Purif. Technol. 2010, 72, 301–308. [Google Scholar] [CrossRef]
- Ravereau, J.; Fabre, A.; Brehant, A.; Bonnard, R.; Sollogoub, C.; Verdu, J. Ageing of polyvinylidene fluoride hollow fiber membranes in sodium hypochlorite solutions. J. Membr. Sci. 2016, 505, 174–184. [Google Scholar] [CrossRef]
- Ren, L.; Yu, S.; Yang, H.; Li, L.; Cai, L.; Xia, Q.; Shi, Z.; Liu, G. Chemical cleaning reagent of sodium hypochlorite eroding polyvinylidene fluoride ultrafiltration membranes: Aging pathway, performance decay and molecular mechanism. J. Membr. Sci. 2021, 625, 119141. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Gao, F.; Chen, Y.; Zhang, H. A comparison study: The different impacts of sodium hypochlorite on PVDF and PSF ultrafiltration (UF) membranes. Water Res. 2017, 109, 227–236. [Google Scholar] [CrossRef]
- Zhou, Z.; Huang, G.; Xiong, Y.; Zhou, M.; Zhang, S.; Tang, C.Y.; Meng, F. Unveiling the susceptibility of functional groups of poly (ether sulfone)/polyvinylpyrrolidone membranes to NaOCl: A two-dimensional correlation spectroscopic study. Environ. Sci. Technol. 2017, 51, 14342–14351. [Google Scholar] [CrossRef]
- Kang, G.-D.; Cao, Y.-M. Application and modification of poly (vinylidene fluoride) (PVDF) membranes—A review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Yong, M.; Zhang, Y.; Sun, S.; Liu, W. Properties of polyvinyl chloride (PVC) ultrafiltration membrane improved by lignin: Hydrophilicity and antifouling. J. Membr. Sci. 2019, 575, 50–59. [Google Scholar] [CrossRef]
- Li, J.-H.; Shao, X.-S.; Zhou, Q.; Li, M.-Z.; Zhang, Q.-Q. The double effects of silver nanoparticles on the PVDF membrane: Surface hydrophilicity and antifouling performance. Appl. Surf. Sci. 2013, 265, 663–670. [Google Scholar] [CrossRef]
- Choudhury, M.R.; Anwar, N.; Jassby, D.; Rahaman, M.S. Fouling and wetting in the membrane distillation driven wastewater reclamation process–A review. Adv. Colloid Interface Sci. 2019, 269, 370–399. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tian, J.; Luo, C.; Chen, C.; Liu, J.; Ma, Z.; Lu, X. Research on in situ continuous off-line chemical cleaning in full-scale membrane bioreactors. Bioresour. Technol. Rep. 2018, 4, 186–192. [Google Scholar] [CrossRef]
- Sharma, V.K. Oxidative transformations of environmental pharmaceuticals by Cl2, ClO2, O3, and Fe (VI): Kinetics assessment. Chemosphere 2008, 73, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Emerson, D.W. Microdetermination of bromine, chlorine, and chlorine dioxide in water in any combination. Microchem. J. 1994, 50, 116–124. [Google Scholar] [CrossRef]
- Qiu, S.; Fuentes, C.A.; Zhang, D.; Van Vuure, A.W.; Seveno, D. Wettability of a single carbon fiber. Langmuir 2016, 32, 9697–9705. [Google Scholar] [CrossRef]
- Zhao, S.; Tao, Z.; Han, M.; Huang, Y.-X.; Zhao, B.; Wang, L.; Tian, X.; Meng, F. Hierarchical Janus membrane with superior fouling and wetting resistance for efficient water recovery from challenging wastewater via membrane distillation. J. Membr. Sci. 2021, 618, 118676. [Google Scholar] [CrossRef]
- Askari, M.; Liang, C.Z.; Choong, L.T.S.; Chung, T.-S. Optimization of TFC-PES hollow fiber membranes for reverse osmosis (RO) and osmotically assisted reverse osmosis (OARO) applications. J. Membr. Sci. 2021, 625, 119156. [Google Scholar] [CrossRef]
- Sun, H.; Rhee, K.B.; Kitano, T.; Mah, S.I. HDPE hollow-fiber membrane via thermally induced phase separation. II. Factors affecting the water permeability of the membrane. J. Appl. Polym. Sci. 2000, 75, 1235–1242. [Google Scholar] [CrossRef]
| No | Manufacturer | Model number | Structure | Outer Diameter (mm) a | Inner Diameter (mm) a | Mean Pore Size (μm) b |
|---|---|---|---|---|---|---|
| A | Memstar, Singapore | SMM-1010 | Self-supported membrane | 1.22 ± 0.02 | 0.63 ± 0.03 | <0.1 |
| B | DuPont, Wilmington, USA | MEMCOR® CS II (S10N) | Self-supported membrane | 1.08 ± 0.02 | 0.50 ± 0.02 | 0.04 |
| C | Suez, Paris, France | ZeeWeed 500 | Reinforced membrane | 1.98 ± 0.01 | 0.95 ± 0.02 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, M.; Han, Q.; Wu, S.; Xiao, H.; Zhang, L.; Lin, Y.; Meng, F.; Zhao, S. Unveiling the Impacts of Sodium Hypochlorite on the Characteristics and Fouling Behaviors of Different Commercial Polyvinylidene Fluoride Hollow Fiber Membranes. Membranes 2022, 12, 965. https://doi.org/10.3390/membranes12100965
Han M, Han Q, Wu S, Xiao H, Zhang L, Lin Y, Meng F, Zhao S. Unveiling the Impacts of Sodium Hypochlorite on the Characteristics and Fouling Behaviors of Different Commercial Polyvinylidene Fluoride Hollow Fiber Membranes. Membranes. 2022; 12(10):965. https://doi.org/10.3390/membranes12100965
Chicago/Turabian StyleHan, Muqiao, Qi Han, Shanwei Wu, Hu Xiao, Lei Zhang, Yibo Lin, Fangang Meng, and Shanshan Zhao. 2022. "Unveiling the Impacts of Sodium Hypochlorite on the Characteristics and Fouling Behaviors of Different Commercial Polyvinylidene Fluoride Hollow Fiber Membranes" Membranes 12, no. 10: 965. https://doi.org/10.3390/membranes12100965
APA StyleHan, M., Han, Q., Wu, S., Xiao, H., Zhang, L., Lin, Y., Meng, F., & Zhao, S. (2022). Unveiling the Impacts of Sodium Hypochlorite on the Characteristics and Fouling Behaviors of Different Commercial Polyvinylidene Fluoride Hollow Fiber Membranes. Membranes, 12(10), 965. https://doi.org/10.3390/membranes12100965









