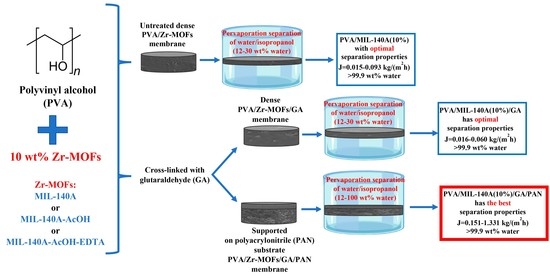
Pervaporation Polyvinyl Alcohol Membranes Modified with Zr-Based Metal Organic Frameworks for Isopropanol Dehydration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Freestanding Membrane Preparation
2.3. Composite Membrane Preparation
2.4. Pervaporation Experiment
2.5. Fourier-Transform Infrared Spectroscopy
2.6. Atomic Force Microscopy
2.7. Scanning Electron Microscopy
2.8. Thermogravimetric Analysis
2.9. Swelling Measurements
2.10. Contact Angle Measurements
2.11. Density Measurements
3. Results and Discussion
3.1. The Development and Investigation of the Freestanding PVA and PVA/Zr-MOFs Membranes
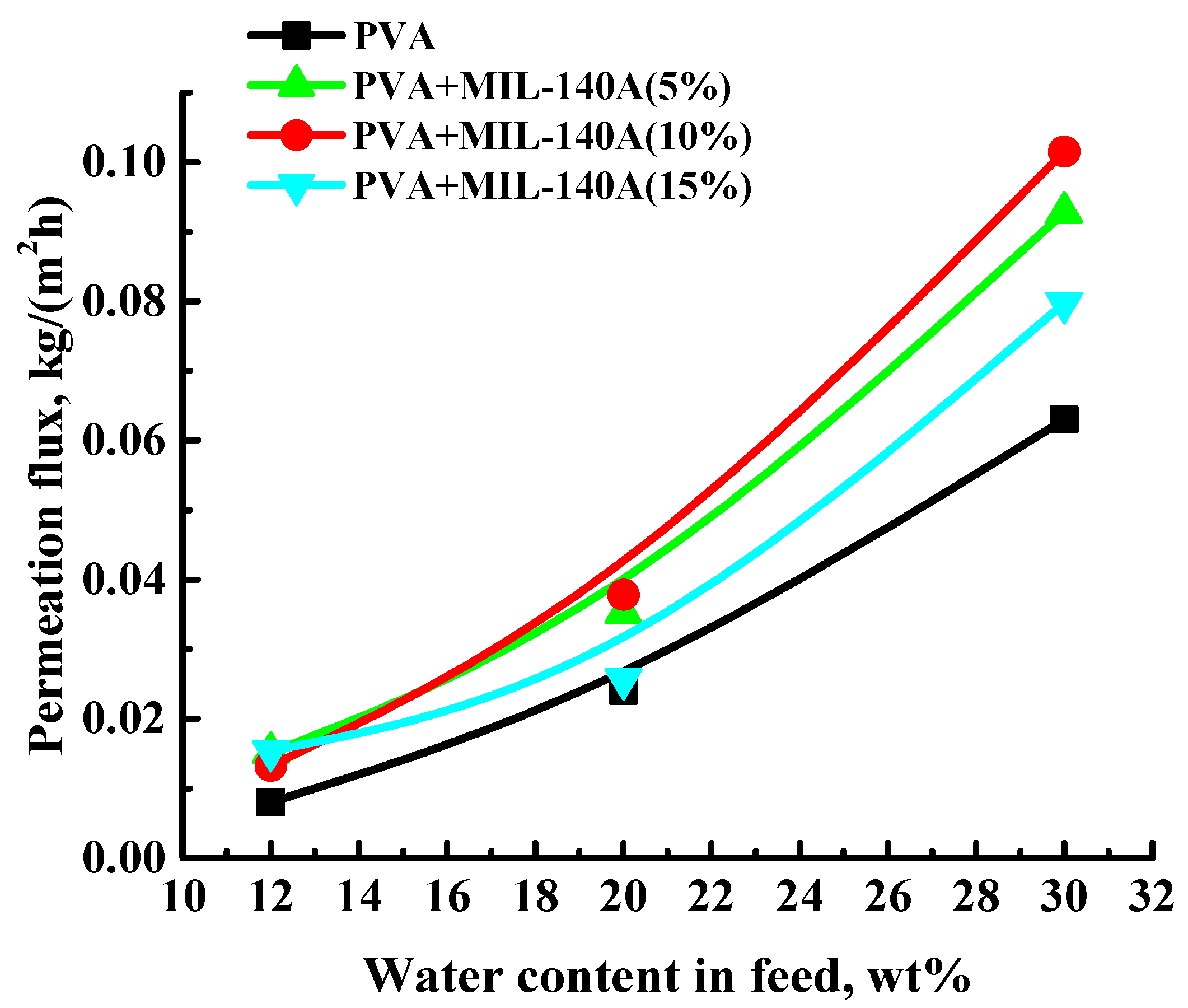
3.1.1. Pervaporation Performance of the Uncross-Linked PVA and PVA/Zr-MOFs Membranes
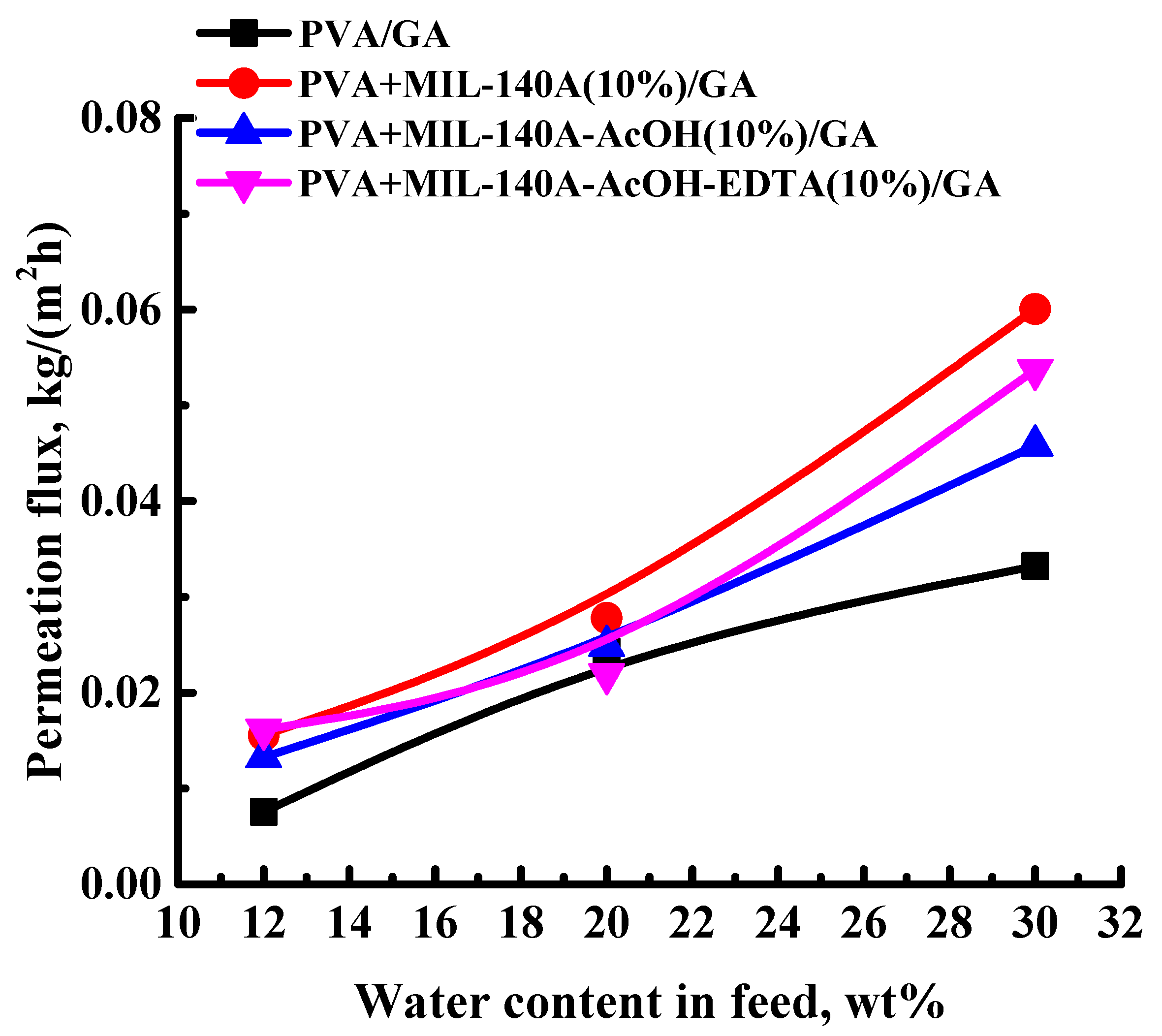
3.1.2. Pervaporation Performance of the Cross-Linked PVA and PVA/Zr-MOFs Membranes
3.1.3. Structure and Physicochemical Properties of the Freestanding PVA and PVA/Zr-MOFs Membranes
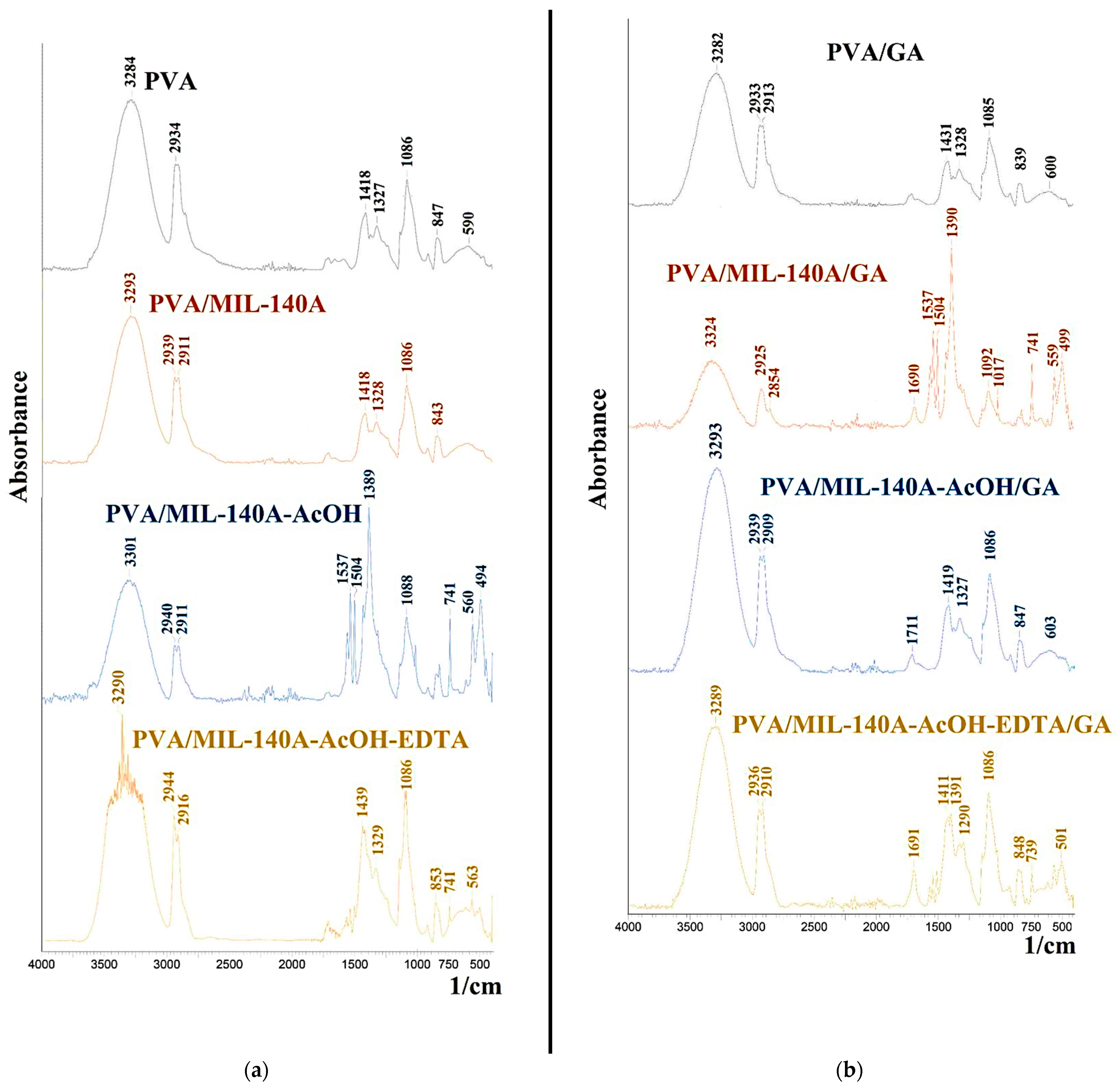
- Fourier-transform infrared spectroscopy
- Scanning electron microscopy
- Atomic force microscopy
- Thermogravimetric analysis
- Swelling degree
- Contact angle measurements
- Density measurements
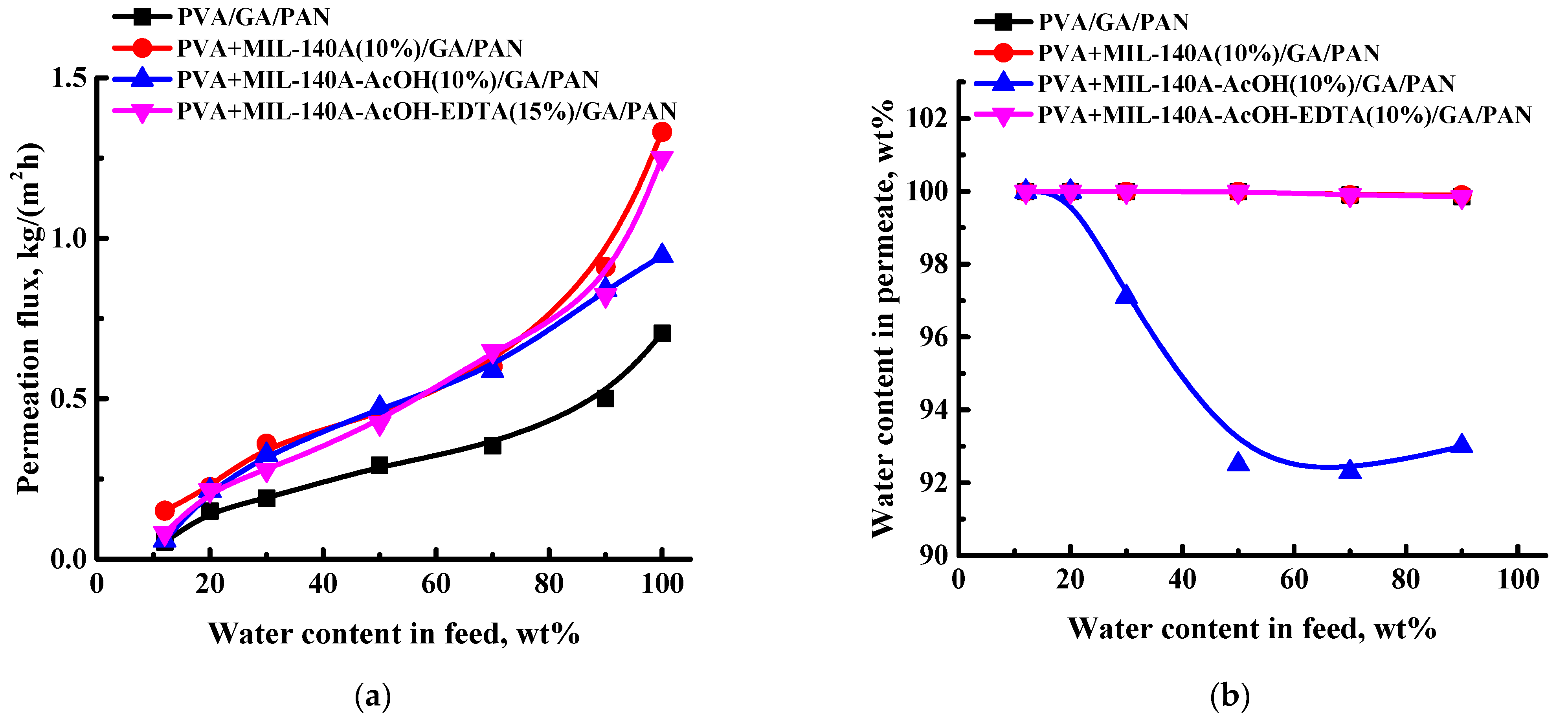
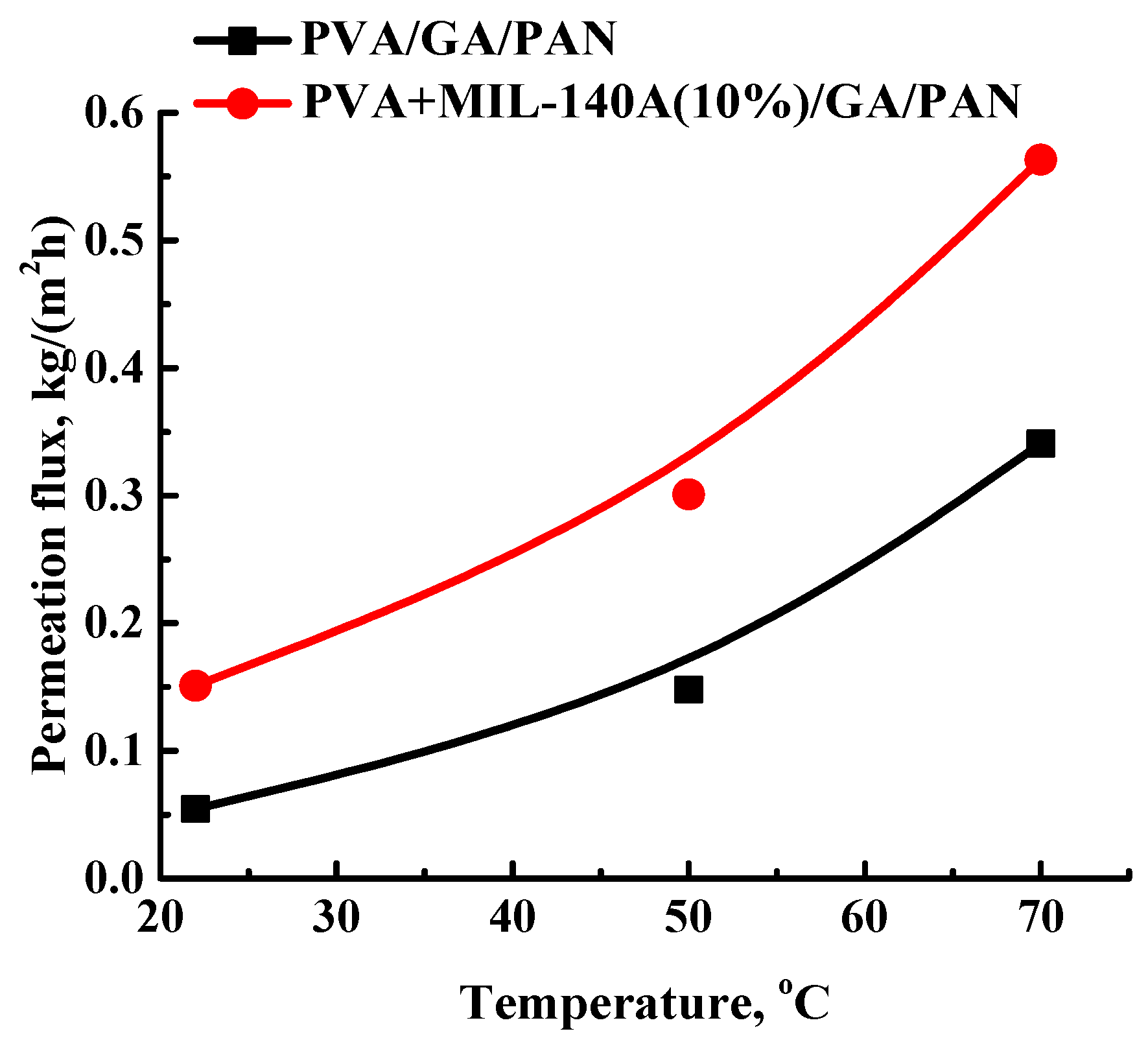
3.2. The Development and Investigation of the Composite PVA and PVA/Zr-MOFs Membranes
3.3. Comparison of the Performance with PVA-Based Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anokhina, T.S.; Pleshivtseva, T.S.; Ignatenko, V.Y.; Antonov, S.V.; Volkov, A.V. Fabrication of composite nanofiltration membranes from cellulose solutions in an [Emim]OAc–DMSO mixture. Pet. Chem. 2017, 57, 477–482. [Google Scholar] [CrossRef]
- Yushkin, A.A.; Efimov, M.N.; Vasil’ev, A.A.; Ivanov, V.I.; Bogdanova, Y.G.; Dolzhikova, V.D.; Karpacheva, G.P.; Bondarenko, G.N.; Volkov, A.V. Effect of IR Radiation on the Properties of Polyacrylonitrile and Membranes on Its Basis. Polym. Sci. Ser. A 2017, 59, 880–890. [Google Scholar] [CrossRef]
- Plisko, T.V.; Bildyukevich, A.V.; Burts, K.S.; Ermakov, S.S.; Penkova, A.V.; Kuzminova, A.I.; Dmitrenko, M.E.; Hliavitskaya, T.A.; Ulbricht, M. One-step preparation of antifouling polysulfone ultrafiltration membranes via modification by a cationic polyelectrolyte based on polyacrylamide. Polymers 2020, 12, 1017. [Google Scholar] [CrossRef] [PubMed]
- Plisko, T.V.; Bildyukevich, A.V.; Volkov, V.V.; Osipov, N.N. Formation of hollow fiber membranes doped with multiwalled carbon nanotube dispersions. Pet. Chem. 2015, 55, 318–332. [Google Scholar] [CrossRef]
- Rostovtseva, V.A.; Pulyalina, A.Y.; Dubovenko, R.R.; Saprykina, N.N.; Vinogradova, L.V.; Polotskaya, G.A. Influence of Ionic Liquid on Transport Properties of Hybrid Membranes in the Lactic Acid Dehydration Process. Membr. Membr. Technol. 2021, 3, 274–281. [Google Scholar] [CrossRef]
- Otvagina, K.; Penkova, A.; Dmitrenko, M.; Kuzminova, A.; Sazanova, T.; Vorotyntsev, A.; Vorotyntsev, I. Novel Composite Membranes Based on Chitosan Copolymers with Polyacrylonitrile and Polystyrene: Physicochemical Properties and Application for Pervaporation Dehydration of Tetrahydrofuran. Membranes 2019, 9, 38. [Google Scholar] [CrossRef]
- Shishov, A.; Penkova, A.; Zabrodin, A.; Nikolaev, K.; Dmitrenko, M.; Ermakov, S.; Bulatov, A. Vapor permeation-stepwise injection simultaneous determination of methanol and ethanol in biodiesel with voltammetric detection. Talanta 2016, 148, 666–672. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Kuzminova, A.; Zolotarev, A.; Ermakov, S.; Roizard, D.; Penkova, A. Enhanced pervaporation properties of PVA-based membranes modified with polyelectrolytes. Application to IPA dehydration. Polymers 2020, 12, 14. [Google Scholar] [CrossRef]
- Dmitrenko, M.E.; Penkova, A.V.; Missyul, A.B.; Kuzminova, A.I.; Markelov, D.A.; Ermakov, S.S.; Roizard, D. Development and investigation of mixed-matrix PVA-fullerenol membranes for acetic acid dehydration by pervaporation. Sep. Purif. Technol. 2017, 187, 285–293. [Google Scholar] [CrossRef]
- Syrtsova, D.A.; Teplyakov, V.V.; Filistovich, V.A.; Savitskaya, T.A.; Kimlenka, I.M.; Makarevich, S.E.; Grinshpan, D.D. Cellulose-Based Composite Gas Separation Membranes. Membr. Membr. Technol. 2019, 1, 353–360. [Google Scholar] [CrossRef]
- Atlaskin, A.A.; Kryuchkov, S.S.; Yanbikov, N.R.; Smorodin, K.A.; Petukhov, A.N.; Trubyanov, M.M.; Vorotyntsev, V.M.; Vorotyntsev, I.V. Comprehensive experimental study of acid gases removal process by membrane-assisted gas absorption using imidazolium ionic liquids solutions absorbent. Sep. Purif. Technol. 2020, 239, 116578. [Google Scholar] [CrossRef]
- Alentiev, A.Y.; Chirkov, S.V.; Nikiforov, R.Y.; Belov, N.A.; Orlova, A.M.; Kuznetsov, A.A.; Kechekyan, A.S.; Kechekyan, P.A.; Nikolaev, A.Y. Effect of Supercritical CO2 Treatment on Mechanical and Gas Transport Characteristics of Polyimides Based on Diethyl Toluene Diamine Isomers. Membr. Membr. Technol. 2022, 4, 162–169. [Google Scholar] [CrossRef]
- Akhmetshina, A.I.; Petukhov, A.N.; Gumerova, O.R.; Vorotyntsev, A.V.; Nyuchev, A.V.; Vorotyntsev, I.V. Solubility of H2S and CO2 in imidazolium-based ionic liquids with bis(2-ethylhexyl) sulfosuccinate anion. J. Chem. Thermodyn. 2019, 130, 173–182. [Google Scholar] [CrossRef]
- Dmitrenko, M.E.; Penkova, A.V.; Kuzminova, A.I.; Atta, R.R.; Zolotarev, A.A.; Mazur, A.S.; Vezo, O.S.; Lahderanta, E.; Markelov, D.A.; Ermakov, S.S. Development and investigation of novel polyphenylene isophthalamide pervaporation membranes modified with various fullerene derivatives. Sep. Purif. Technol. 2019, 226, 241–251. [Google Scholar] [CrossRef]
- Dmitrenko, M.E.; Penkova, A.V.; Atta, R.R.; Zolotarev, A.A.; Plisko, T.V.; Mazur, A.S.; Solovyev, N.D.; Ermakov, S.S. The development and study of novel membrane materials based on polyphenylene isophthalamide—Pluronic F127 composite. Mater. Des. 2019, 165, 107596. [Google Scholar] [CrossRef]
- Ogorodnikov, S.K.; Lesteva, T.M.; Kogan, V.B. Azeotrope Mixtures; Chemistry: St. Petersburg, Russia, 1971; 848p. [Google Scholar]
- Wozniak, A.I.; Bermesheva, E.V.; Andreyanov, F.A.; Borisov, I.L.; Zarezin, D.P.; Bakhtin, D.S.; Gavrilova, N.N.; Ilyasov, I.R.; Nechaev, M.S.; Asachenko, A.F.; et al. Modifications of addition poly(5-vinyl-2-norbornene) and gas-transport properties of the obtained polymers. React. Funct. Polym. 2020, 149, 104513. [Google Scholar] [CrossRef]
- Polotskaya, G.A.; Penkova, A.V.; Pientka, Z.; Toikka, A.M. Polymer membranes modified by fullerene C60 for pervaporation of organic mixtures. Desalin. Water Treat. 2010, 14, 83–88. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Liamin, V.; Kuzminova, A.; Mazur, A.; Lahderanta, E.; Ermakov, S.; Penkova, A. Novel Mixed Matrix Sodium Alginate–Fullerenol Membranes: Development, Characterization, and Study in Pervaporation Dehydration of Isopropanol. Polymers 2020, 12, 864. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Zolotarev, A.; Liamin, V.; Kuzminova, A.; Mazur, A.; Semenov, K.; Ermakov, S.; Penkova, A. Novel Membranes Based on Hydroxyethyl Cellulose/Sodium Alginate for Pervaporation Dehydration of Isopropanol. Polymers 2021, 13, 674. [Google Scholar] [CrossRef]
- Vilhelmsen, L.B.; Walton, K.S.; Sholl, D.S. Structure and Mobility of Metal Clusters in MOFs: Au, Pd, and AuPd Clusters in MOF-74. J. Am. Chem. Soc. 2012, 134, 12807–12816. [Google Scholar] [CrossRef]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477. [Google Scholar] [CrossRef] [PubMed]
- Paseta, L.; Simón-Gaudó, E.; Gracia-Gorría, F.; Coronas, J. Encapsulation of essential oils in porous silica and MOFs for trichloroisocyanuric acid tablets used for water treatment in swimming pools. Chem. Eng. J. 2016, 292, 28–34. [Google Scholar] [CrossRef]
- Murray, L.J.; Dincă, M.; Long, J.R. Hydrogen storage in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1294. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Nikolaeva, D.; Hartanto, Y.; Luis, P. MOF-based membranes for pervaporation. Sep. Purif. Technol. 2021, 278, 119233. [Google Scholar] [CrossRef]
- De la Iglesia, Ó.; Sorribas, S.; Almendro, E.; Zornoza, B.; Téllez, C.; Coronas, J. Metal-organic framework MIL-101(Cr) based mixed matrix membranes for esterification of ethanol and acetic acid in a membrane reactor. Renew. Energy 2016, 88, 12–19. [Google Scholar] [CrossRef][Green Version]
- Sorribas, S.; Kudasheva, A.; Almendro, E.; Zornoza, B.; de la Iglesia, Ó.; Téllez, C.; Coronas, J. Pervaporation and membrane reactor performance of polyimide based mixed matrix membranes containing MOF HKUST-1. Chem. Eng. Sci. 2015, 124, 37–44. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, Q.; Lv, R.; Soyekwo, F.; Zhu, A.; Liu, Q. Highly efficient polymer–MOF nanocomposite membrane for pervaporation separation of water/methanol/MTBE ternary mixture. Chem. Eng. Res. Des. 2017, 117, 688–697. [Google Scholar] [CrossRef]
- Liu, S.; Liu, G.; Shen, J.; Jin, W. Fabrication of MOFs/PEBA mixed matrix membranes and their application in bio-butanol production. Sep. Purif. Technol. 2014, 133, 40–47. [Google Scholar] [CrossRef]
- Han, G.L.; Zhou, K.; Lai, A.N.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. [Cu2(bdc)2(bpy)]n/SPES-C mixed matrix membranes for separation of methanol/methyl tert-butyl ether mixtures. J. Memb. Sci. 2014, 454, 36–43. [Google Scholar] [CrossRef]
- Vinu, M.; Pal, S.; Chen, J.; Lin, Y.; Lai, Y.; Lee, C.; Lin, C. Microporous 3D aluminum MOF doped into chitosan-based mixed matrix membranes for ethanol/water separation. J. Chin. Chem. Soc. 2019, 66, 1165–1171. [Google Scholar] [CrossRef]
- Vinu, M.; Senthil Raja, D.; Jiang, Y.-C.; Liu, T.-Y.; Xie, Y.-Y.; Lin, Y.-F.; Yang, C.-C.; Lin, C.-H.; Alshehri, S.M.; Ahamad, T.; et al. Effects of structural crystallinity and defects in microporous Al-MOF filled chitosan mixed matrix membranes for pervaporation of water/ethanol mixtures. J. Taiwan Inst. Chem. Eng. 2018, 83, 143–151. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Q.; Zhao, J.; Hua, Y.; Sun, J.; Duan, J.; Jin, W. High efficient water/ethanol separation by a mixed matrix membrane incorporating MOF filler with high water adsorption capacity. J. Memb. Sci. 2017, 544, 68–78. [Google Scholar] [CrossRef]
- Kuzminova, A.; Dmitrenko, M.; Zolotarev, A.; Korniak, A.; Poloneeva, D.; Selyutin, A.; Emeline, A.; Yushkin, A.; Foster, A.; Budd, P.; et al. Novel Mixed Matrix Membranes Based on Polymer of Intrinsic Microporosity PIM-1 Modified with Metal-Organic Frameworks for Removal of Heavy Metal Ions and Food Dyes by Nanofiltration. Membranes 2021, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, J.; Wang, N.; Fan, H.; Zhang, R.; Zhang, G.; Ji, S. Enhanced fl ux of polydimethylsiloxane membrane for ethanol permselective pervaporation via incorporation of MIL-53 particles. J. Memb. Sci. 2015, 492, 322–330. [Google Scholar] [CrossRef]
- Wee, L.H.; Li, Y.; Zhang, K.; Davit, P.; Bordiga, S.; Jiang, J.; Vankelecom, I.F.J.; Martens, J.A. Submicrometer-Sized ZIF-71 Filled Organophilic Membranes for Improved Bioethanol Recovery: Mechanistic Insights by Monte Carlo Simulation and FTIR Spectroscopy. Adv. Funct. Mater. 2015, 25, 516–525. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, F.-Y.; Zhang, H.-Z.; Xu, Z.-L.; Xue, S.-M.; Ma, X.-H.; Xu, X.-R. In-situ synthetic modified metal-organic framework (MZIF-8) as an interlayer of the composite membranes for ethanol dehydration. J. Memb. Sci. 2020, 601, 117916. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, G.; Wang, L.; Li, J.; An, Q.; Ji, S. Pervaporation dehydration of acetic acid using NH2-UiO-66/PEI mixed matrix membranes. Sep. Purif. Technol. 2017, 186, 20–27. [Google Scholar] [CrossRef]
- Kuzminova, A.I.; Dmitrenko, M.E.; Poloneeva, D.Y.; Selyutin, A.A.; Mazur, A.S.; Emeline, A.V.; Mikhailovskii, V.Y.; Solovyev, N.D.; Ermakov, S.S.; Penkova, A.V. Sustainable composite pervaporation membranes based on sodium alginate modified by metal organic frameworks for dehydration of isopropanol. J. Memb. Sci. 2021, 626, 119194. [Google Scholar] [CrossRef]
- Burts, K.; Plisko, T.; Dmitrenko, M.; Zolotarev, A.; Kuzminova, A.; Bildyukevich, A.; Ermakov, S.; Penkova, A. Novel Thin Film Nanocomposite Membranes Based on Chitosan Succinate Modified with Fe-BTC for Enhanced Pervaporation Dehydration of Isopropanol. Membranes 2022, 12, 653. [Google Scholar] [CrossRef]
- Benzaqui, M.; Semino, R.; Carn, F.; Tavares, S.R.; Menguy, N.; Giménez-Marqués, M.; Bellido, E.; Horcajada, P.; Berthelot, T.; Kuzminova, A.I.; et al. Covalent and Selective Grafting of Polyethylene Glycol Brushes at the Surface of ZIF-8 for the Processing of Membranes for Pervaporation. ACS Sustain. Chem. Eng. 2019, 7, 6629–6639. [Google Scholar] [CrossRef]
- Zhang, W.; Ying, Y.; Ma, J.; Guo, X.; Huang, H.; Liu, D.; Zhong, C. Mixed matrix membranes incorporated with polydopamine-coated metal-organic framework for dehydration of ethylene glycol by pervaporation. J. Memb. Sci. 2017, 527, 8–17. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, N.; Ji, S.; Zhang, R.; Zhao, C.; Li, J.-R. Metal–organic framework/poly(vinyl alcohol) nanohybrid membrane for the pervaporation of toluene/n-heptane mixtures. J. Memb. Sci. 2015, 489, 144–152. [Google Scholar] [CrossRef]
- Wu, G.; Li, Y.; Geng, Y.; Lu, X.; Jia, Z. Adjustable pervaporation performance of Zr-MOF/poly(vinyl alcohol) mixed matrix membranes. J. Chem. Technol. Biotechnol. 2019, 94, 973–981. [Google Scholar] [CrossRef]
- Wu, G.; Jiang, M.; Zhang, T.; Jia, Z. Tunable Pervaporation Performance of Modified MIL-53(Al)-NH2/Poly(vinyl Alcohol) Mixed Matrix Membranes. J. Memb. Sci. 2016, 507, 72–80. [Google Scholar] [CrossRef]
- Burshe, M.C.; Sawant, S.B.; Joshi, J.B.; Pangarkar, V.G. Sorption and permeation of binary water-alcohol systems through PVA membranes crosslinked with multifunctional crosslinking agents. Sep. Purif. Technol. 1997, 12, 145–156. [Google Scholar] [CrossRef]
- Chiang, W.; Lin, Y. Properties of Modified Poly (vinyl alcohol) Membranes Prepared by the Grafting of New Polyelectrolyte Copolymers for Water—Ethanol Mixture Separation. J. Appl. Polym. Sci. 2002, 86, 2854–2859. [Google Scholar] [CrossRef]
- Huang, Z.; Guan, H.; Qiao, X.; Kulprathipanja, S. Pervaporation study of aqueous ethanol solution through zeolite-incorporated multilayer poly (vinyl alcohol) membranes: Effect of zeolites. J. Membr. Sci. 2006, 276, 260–271. [Google Scholar] [CrossRef]
- Rao, K.S.V.K.; Subha, M.C.S.; Sairam, M.; Mallikarjuna, N.N.; Aminabhavi, T.M. Blend membranes of chitosan and poly(vinyl alcohol) in pervaporation dehydration of isopropanol and tetrahydrofuran. J. Appl. Polym. Sci. 2007, 103, 1918–1926. [Google Scholar] [CrossRef]
- Lee, K.; Kim, H.; Rhim, J. Pervaporation separation of binary organic–aqueous liquid mixtures using crosslinked PVA membranes. III. Ethanol–water mixtures. Appl. Polym. Sci. 1995, 58, 1707–1712. [Google Scholar] [CrossRef]
- Praptowidodo, V.S. Influence of swelling on water transport through PVA-based membrane. J. Mol. Struct. 2004, 739, 207–212. [Google Scholar] [CrossRef]
- Yamasaki, A.; Iwatsubo, T.; Masuoka, T.; Mizoguchi, K. Pervaporation of ethanol/water through a poly (vinyl alcohol)/cyclodextrin (PVA/CD) membrane. J. Membr. Sci. 1994, 89, 111–117. [Google Scholar] [CrossRef]
- Yeom, C.K.; Lee, S.H.; Lee, J.M. Pervaporative permeations of homologous series of alcohol aqueous mixtures through a hydrophilic membrane. J. Appl. Polym. Sci. 2001, 79, 703–713. [Google Scholar] [CrossRef]
- Xing, Y.; Xue, Y.; Qin, D.; Zhao, P.; Li, P. Microwave-induced ultrafast crosslinking of Poly (vinyl alcohol) blended with nanoparticles as wave absorber for pervaporation desalination. J. Membr. Sci. Lett. 2022, 2, 100021. [Google Scholar] [CrossRef]
- Amirilargani, M.; Sadatnia, B. Poly(vinyl alcohol)/zeolitic imidazolate frameworks (ZIF-8) mixed matrix membranes for pervaporation dehydration of isopropanol. J. Memb. Sci. 2014, 469, 1–10. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Z.; Gao, L.; Gray, S.; Xie, Z. Study of MOF incorporated dual layer membrane with enhanced removal of ammonia and per-/poly-fluoroalkyl substances (PFAS) in landfill leachate treatment. Sci. Total Environ. 2022, 806, 151207. [Google Scholar] [CrossRef]
- Xiong, Y.; Deng, N.; Wu, X.; Zhang, Q.; Liu, S.; Sun, G. De novo synthesis of amino-functionalized ZIF-8 nanoparticles: Enhanced interfacial compatibility and pervaporation performance in mixed matrix membranes applying for ethanol dehydration. Sep. Purif. Technol. 2022, 285, 120321. [Google Scholar] [CrossRef]
- DeCoste, J.B.; Peterson, G.W.; Jasuja, H.; Glover, T.G.; Huang, Y.; Walton, K.S. Stability and degradation mechanisms of metal–organic frameworks containing the Zr6O4(OH)4 secondary building unit. J. Mater. Chem. A 2013, 1, 5642. [Google Scholar] [CrossRef]
- Mondloch, J.E.; Katz, M.J.; Planas, N.; Semrouni, D.; Gagliardi, L.; Hupp, J.T.; Farha, O.K. Are Zr 6-based MOFs water stable? Linker hydrolysis vs. capillary-force-driven channel collapse. Chem. Commun. 2014, 50, 8944. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Feng, D.; Wang, K.; Gu, Z.-Y.; Wei, Z.; Chen, Y.-P.; Zhou, H.-C. An Exceptionally Stable, Porphyrinic Zr Metal–Organic Framework Exhibiting pH-Dependent Fluorescence. J. Am. Chem. Soc. 2013, 135, 13934–13938. [Google Scholar] [CrossRef]
- Guillerm, V.; Ragon, F.; Dan-Hardi, M.; Devic, T.; Vishnuvarthan, M.; Campo, B.; Vimont, A.; Clet, G.; Yang, Q.; Maurin, G.; et al. A Series of Isoreticular, Highly Stable, Porous Zirconium Oxide Based Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2012, 51, 9267–9271. [Google Scholar] [CrossRef]
- Guillerm, V.; Ragon, F.; Dan-Hardi, M.; Devic, T.; Vishnuvarthan, M.; Campo, B.; Vimont, A.; Clet, G.; Yang, Q.; Maurin, G.; et al. CCDC 905026: Experimental Crystal Structure Determination, 2014. Angew. Chem. Int. Ed. 2012, 51, 9267. [Google Scholar] [CrossRef]
- Qiao, J.; Fu, J.; Liu, L.; Zhang, J.; Xie, J.; Li, G. Synthesis and properties of chemically cross-linked poly(vinyl alcohol)–poly(acrylamide-co-diallyldimethylammonium chloride) (PVA–PAADDA) for anion-exchange membranes. Solid State Ion. 2012, 214, 6–12. [Google Scholar] [CrossRef]
- Burts, K.S.; Plisko, T.V.; Bildyukevich, A.V.; Li, G.; Kujawa, J.; Kujawski, W. Development of dynamic PVA/PAN membranes for pervaporation: Correlation between kinetics of gel layer formation, preparation conditions, and separation performance. Chem. Eng. Res. Des. 2022, 182, 544–557. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Kuzminova, A.; Zolotarev, A.; Markelov, D.; Komolkin, A.; Loginova, E.; Plisko, T.; Burts, K.; Bildyukevich, A.; Penkova, A. Modification strategies of polyacrylonitrile ultrafiltration membrane using TiO2 for enhanced antifouling performance in water treatment. Sep. Purif. Technol. 2022, 286, 120500. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications; McGraw-Hill: New York, NY, USA, 2000. [Google Scholar]
- Dmitrenko, M.; Kuzminova, A.; Zolotarev, A.; Liamin, V.; Markelov, D.; Semenov, K.; Plisko, T.; Bildyukevich, A.; Penkova, A. Novel pervaporation membranes based on hydroxyethyl cellulose/polyvinyl alcohol modified with fullerene derivatives for enhanced isopropanol dehydration. J. Mater. Res. 2021, 36, 4986–5001. [Google Scholar] [CrossRef]
- Jia, Z.; Wu, G. Microporous and Mesoporous Materials Metal-organic frameworks based mixed matrix membranes for pervaporation. Microporous Mesoporous Mater. 2016, 235, 151–159. [Google Scholar] [CrossRef]
- Penkova, A.V.; Kuzminova, A.I.; Dmitrenko, M.E.; Surkova, V.A.; Liamin, V.P.; Markelov, D.A.; Komolkin, A.V.; Poloneeva, D.Y.; Laptenkova, A.V.; Selyutin, A.A.; et al. Novel pervaporation mixed matrix membranes based on polyphenylene isophtalamide modified by metal–organic framework UiO-66(NH2)-EDTA for highly efficient methanol isolation. Sep. Purif. Technol. 2021, 263, 118370. [Google Scholar] [CrossRef]
- Nordin, N.A.H.; Racha, S.M.; Matsuura, T.; Misdan, N.; Abdullah Sani, N.A.; Ismail, A.F.; Mustafa, A. Facile modification of ZIF-8 mixed matrix membrane for CO2/CH4 separation: Synthesis and preparation. RSC Adv. 2015, 5, 43110–43120. [Google Scholar] [CrossRef]
- Nordin, N.A.H.M.; Ismail, A.F.; Misdan, N.; Nazri, N.A.M. Modified ZIF-8 mixed matrix membrane for CO2/CH4 separation. AIP Conf. Proc. 2017, 1891, 020091. [Google Scholar] [CrossRef]
- Dudek, G.; Krasowska, M.; Turczyn, R.; Strzelewicz, A.; Djurado, D.; Pouget, S. Clustering analysis for pervaporation performance assessment of alginate hybrid membranes in dehydration of ethanol Gabriela Dudek. Chem. Eng. Res. Des. 2019, 144, 483–493. [Google Scholar] [CrossRef]
- Kudasheva, A.; Sorribas, S.; Zornoza, B.; Téllez, C.; Coronas, J. Pervaporation of water/ethanol mixtures through polyimide based mixed matrix membranes containing ZIF-8, ordered mesoporous silica and ZIF-8-silica core-shell spheres. J. Chem. Technol. Biotechnol. 2015, 90, 669–677. [Google Scholar] [CrossRef]
- Dmitrenko, M.E.; Penkova, A.V.; Kuzminova, A.I.; Morshed, M.; Larionov, M.I.; Alem, H.; Zolotarev, A.A.; Ermakov, S.S.; Roizard, D. Investigation of new modification strategies for PVA membranes to improve their dehydration properties by pervaporation. Appl. Surf. Sci. 2018, 450, 527–537. [Google Scholar] [CrossRef]
- Das, P.; Ray, S.K.; Kuila, S.B.; Samanta, H.S.; Singha, N.R. Systematic choice of crosslinker and filler for pervaporation membrane: A case study with dehydration of isopropyl alcohol–water mixtures by polyvinyl alcohol membranes. Sep. Purif. Technol. 2011, 81, 159–173. [Google Scholar] [CrossRef]
- Xia, L.L.; Li, C.L.; Wang, Y. In-situ crosslinked PVA/organosilica hybrid membranes for pervaporation separations. J. Memb. Sci. 2016, 498, 263–275. [Google Scholar] [CrossRef]
- Sun, D.; Yang, P.; Sun, H.-L.; Li, B.-B. Preparation and characterization of cross-linked poly (vinyl alcohol)/hyperbranched polyester membrane for the pervaporation dehydration of ethylene glycol solution. Eur. Polym. J. 2015, 62, 155–166. [Google Scholar] [CrossRef]
- Zhang, Q.G.; Liu, Q.L.; Shi, F.F.; Xiong, Y. Structure and permeation of organic—inorganic hybrid membranes composed of poly (vinyl alcohol) and polysilisesquioxane. J. Mater. Chem. 2008, 18, 4646–4653. [Google Scholar] [CrossRef]
- Shahid, S.; Nijmeijer, K. Performance and plasticization behavior of polymer—MOF membranes for gas separation at elevated pressures. J. Memb. Sci. 2014, 470, 166–177. [Google Scholar] [CrossRef]
- Penkova, A.V.; Dmitrenko, M.E.; Savon, N.A.; Missyul, A.B.; Mazur, A.S.; Kuzminova, A.I.; Zolotarev, A.A.; Mikhailovskii, V.; Lahderanta, E.; Markelov, D.A.; et al. Novel mixed-matrix membranes based on polyvinyl alcohol modified by carboxyfullerene for pervaporation dehydration. Sep. Purif. Technol. 2018, 204, 1–12. [Google Scholar] [CrossRef]
- Yeo, S.J.; Chaudhari, S.; Kim, U.; Shin, H.; Cho, K.Y.; Kwon, H.T.; Shon, M.; Nam, S.; Park, Y. Robust and water-selective natural-cellulose-nanofiber-reinforced polyvinyl alcohol composite membranes for pervaporation of isopropanol/water mixtures. Chem. Eng. Process.—Process Intensif. 2022, 179, 109046. [Google Scholar] [CrossRef]
- Choi, S.Y.; Chaudhari, S.; Shin, H.T.; Cho, K.Y.; Lee, D.U.; Shon, M.Y.; Nam, S.E.; Park, Y.I. Polydopamine-modified halloysite nanotube-incorporated polyvinyl alcohol membrane for pervaporation of water-isopropanol mixture. J. Ind. Eng. Chem. 2022, 105, 158–170. [Google Scholar] [CrossRef]
- Chaudhari, S.; Baek, M.; Kwon, Y.; Shon, M.; Nam, S.; Park, Y. Surface-modified halloysite nanotube-embedded polyvinyl alcohol/polyvinyl amine blended membranes for pervaporation dehydration of water/isopropanol mixtures. Appl. Surf. Sci. 2019, 493, 193–201. [Google Scholar] [CrossRef]
- Kwon, Y.; Chaudhari, S.; Kim, C.; Son, D.; Park, J. Ag-exchanged NaY zeolite introduced polyvinyl alcohol/polyacrylic acid mixed matrix membrane for pervaporation separation of water/isopropanol mixture. RSC Adv. 2018, 8, 20669–20678. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Atta, R.; Zolotarev, A.; Kuzminova, A.; Ermakov, S.; Penkova, A. Development of Novel Membranes Based on Polyvinyl Alcohol Modified by Pluronic F127 for Pervaporation Dehydration of Isopropanol. Sustainability 2022, 14, 3561. [Google Scholar] [CrossRef]
- Lecaros, R.L.G.; Deseo, K.M.; Hung, W.-S.; Tayo, L.L.; Hu, C.-C.; An, Q.-F.; Tsai, H.-A.; Lee, K.-R.; Lai, J.-Y. Influence of integrating graphene oxide quantum dots on the fine structure characterization and alcohol dehydration performance of pervaporation composite membrane. J. Memb. Sci. 2019, 576, 36–47. [Google Scholar] [CrossRef]


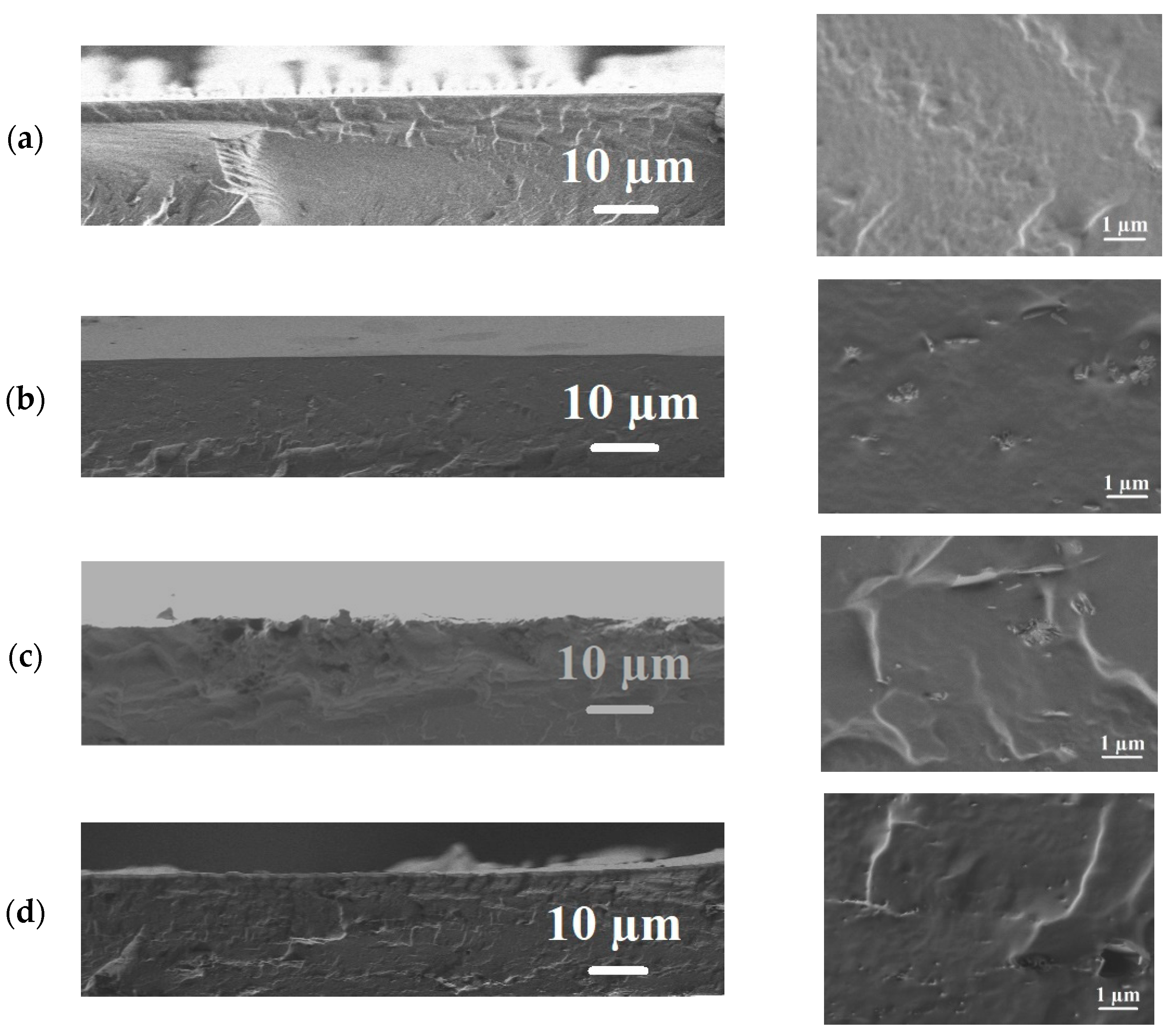
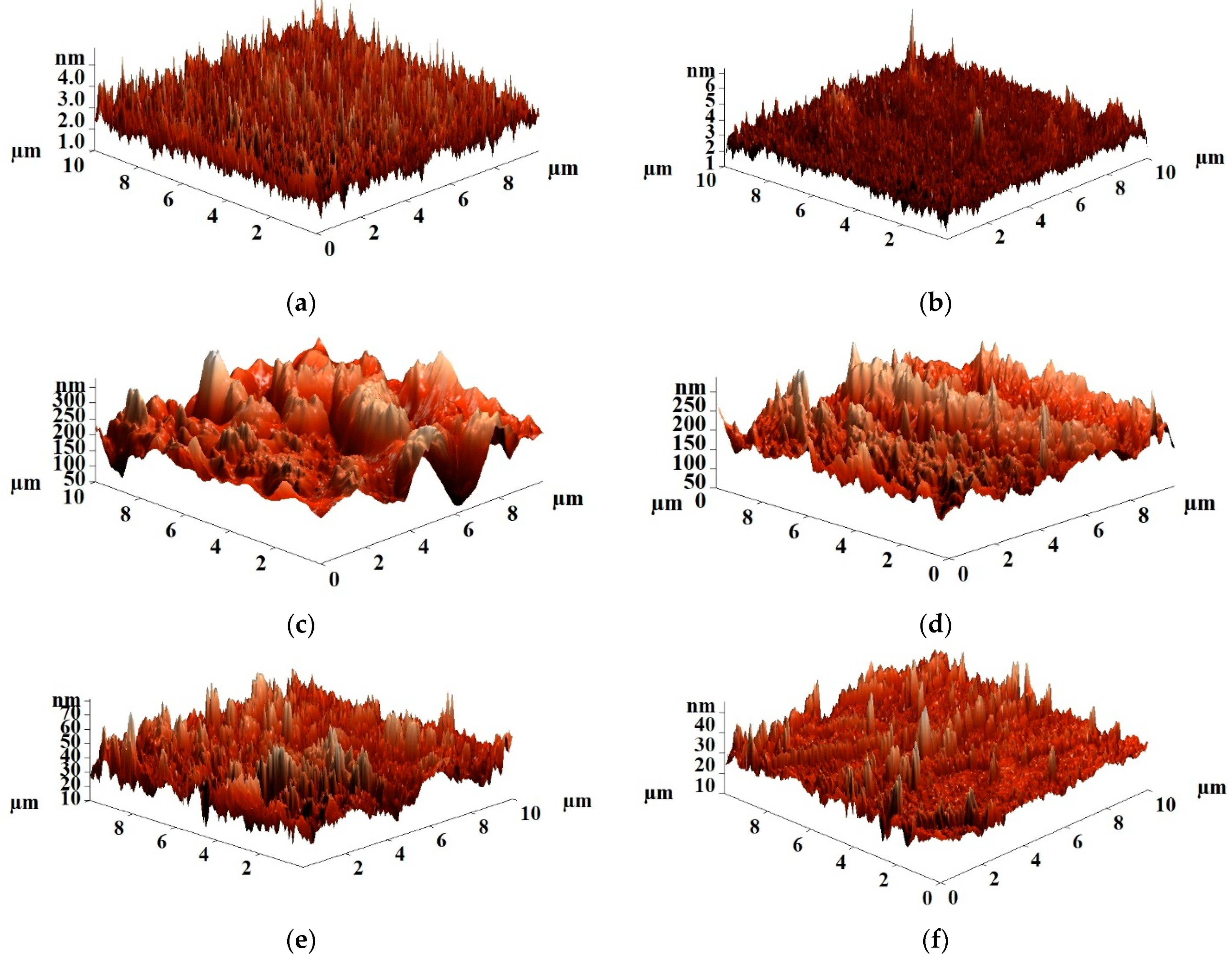
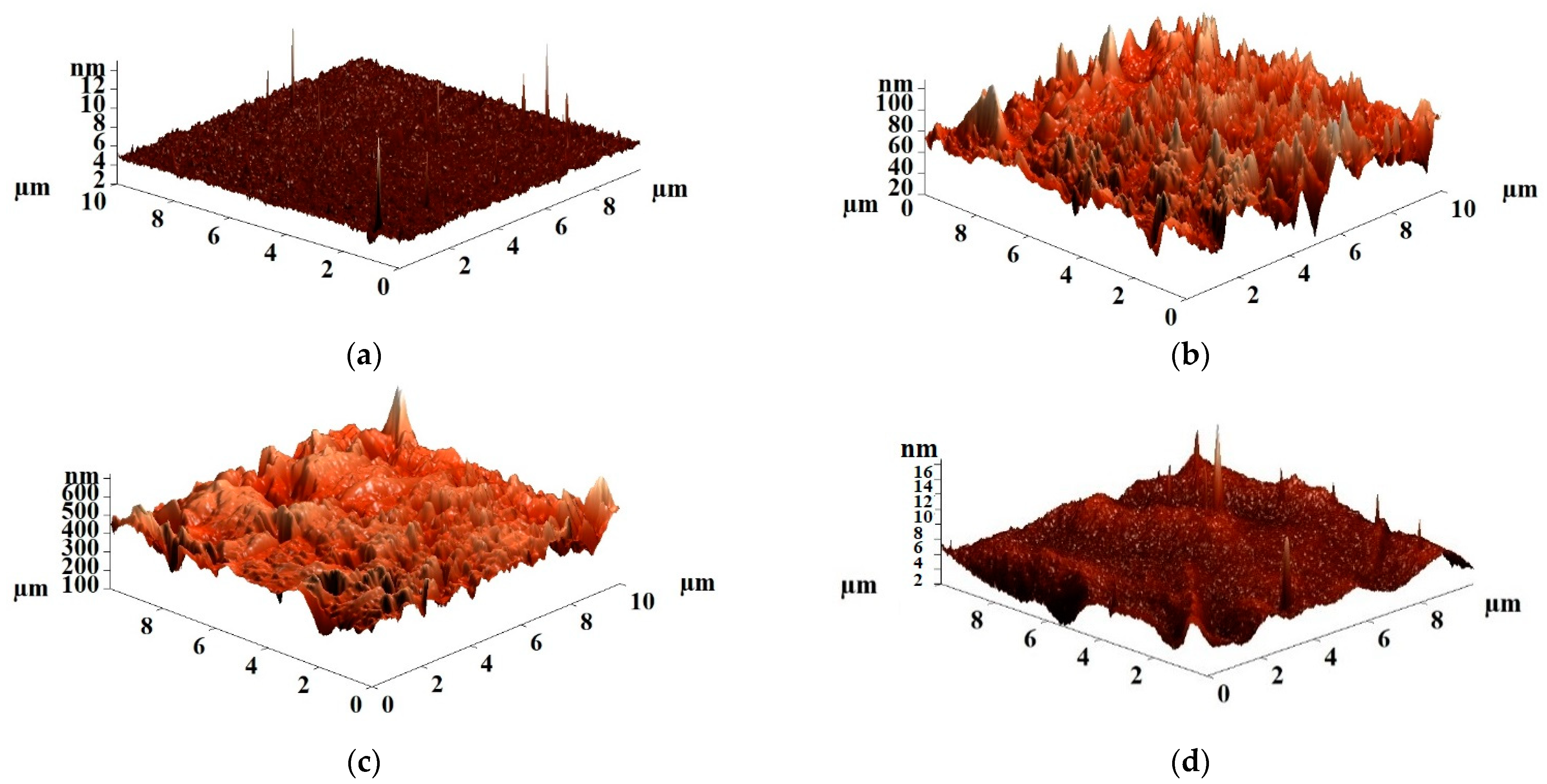



| Membranes | Ra, nm | Rq, nm |
|---|---|---|
| PVA | 0.38 | 0.68 |
| PVA+MIL-140A(5%) | 1.79 | 2.52 |
| PVA+MIL-140A(10%) | 33.76 | 36.61 |
| PVA+MIL-140A(15%) | 41.89 | 52.89 |
| PVA+MIL-140A-AcOH(10%) | 13.54 | 17.71 |
| PVA+MIL-140A-AcOH-EDTA(10%) | 14.62 | 21.01 |
| Membranes | Ra, nm | Rq, nm |
|---|---|---|
| PVA/GA | 0.35 | 1.31 |
| PVA+MIL-140A(10%)/GA | 19.24 | 24.72 |
| PVA+MIL-140A-AcOH(10%)/GA | 10.54 | 15.21 |
| PVA+MIL-140A-AcOH-EDTA(10%)/GA | 14.64 | 21.84 |
| Membrane | Swelling Degree, % | ||
|---|---|---|---|
| Water/Isopropanol Mixture | Water | ||
| 12/88 wt% | 30/70 wt% | ||
| PVA | 15 | 53 | - |
| PVA+MIL-140A(5%) | 18 | 58 | - |
| PVA+MIL-140A(10%) | 20 | 61 | - |
| PVA+MIL-140A(15%) | 19 | 58 | - |
| PVA+MIL-140A-AcOH(10%) | 16 | 52 | - |
| PVA+MIL-140A-AcOH-EDTA(10%) | 17 | 53 | - |
| PVA/GA | 14 | 50 | 236 |
| PVA+MIL-140A(10%)/GA | 19 | 56 | 293 |
| PVA+MIL-140A-AcOH(10%)/GA | 15 | 51 | 248 |
| PVA+MIL-140A-AcOH-EDTA(10%)/GA | 16 | 52 | 250 |
| Membranes | Contact Angle of Water, ° |
|---|---|
| PVA/GA | 67 |
| PVA+MIL-140A(10%)/GA | 64 |
| PVA+MIL-140A-AcOH(10%)/GA | 66 |
| PVA+MIL-140A-AcOH-EDTA(10%)/GA | 65 |
| Membrane | Density, g/cm3 |
|---|---|
| PVA | 1.26 |
| PVA+MIL-140A(10%) | 1.31 |
| PVA+MIL-140A-AcOH(10%) | 1.29 |
| PVA+MIL-140A-AcOH-EDTA(10%) | 1.30 |
| PVA/GA | 1.27 |
| PVA+MIL-140A(10%)/GA | 1.32 |
| PVA+MIL-140A-AcOH(10%)/GA | 1.31 |
| PVA+MIL-140A-AcOH-EDTA(10%)/GA | 1.30 |
| Membranes | Ra, nm | Rq, nm |
|---|---|---|
| PVA/GA/PAN | 18.07 | 22.77 |
| PVA+MIL-140A(10%)/GA/PAN | 45.01 | 78.88 |
| PVA+MIL-140A-AcOH(10%)/GA/PAN | 37.51 | 51.35 |
| PVA+MIL-140A-AcOH-EDTA(10%)/GA/PAN | 26.04 | 34.55 |
| Membranes | Thickness, μm | Water Content in the Feed, wt% | Temperature, °C | Permeation Flux, g/(m2h) | Water Content in Permeate, wt% | Reference |
|---|---|---|---|---|---|---|
| PVA+MIL-140A(10%)/GA/PAN | 0.9 | 20 | 22 | 225 | 99.9 | This study |
| PERVAPTM 1201 | - | 20 | 22 | 34 | 99.9 | [75] |
| PVA+cellulose nanofiber (6 wt%) | 53-54 | 20 | 40 | ~65 | ~99.9 | [82] |
| PVA+Polydopamine coated halloysite nanotube (5 wt%) | ~70 | 20 | 40 | 190 | ~99.2 | [83] |
| PVA+PVAm (polyvinyl amine)+Surface-modified halloysite nanotube (5 wt%) | 75 | 20 | 40 | 130 | ~99.1 | [84] |
| PVA+poly(acrylic acid)+Ag-modified zeolite incorporation (12.5 g) | 50 | 20 | 40 | 84 | ~99.9 | [85] |
| PVA+MIL-140A(10%)/GA/PAN | 0.9 | 30 | 22 | 360 | 99.9 | This study |
| PERVAPTM 1201 | - | 30 | 22 | 28 | ~99.9 | [40] |
| PVA+ Pluronic F127 (3 wt%) cross-linked with maleic acid deposited on polyamide (17 wt%) support | 1.5 | 30 | 22 | 620 | 97.7 | [86] |
| PVA/hydroxyethyl cellulose (70/30 wt%)+ carboxyfullerene (5 wt%) | 30 | 30 | 22 | 193 | ~98.7 | [68] |
| PVA+Graphene oxide quantum dots (GOQDs) (300 ppm) | 3 | 30 | 25 | 463.5 | ~99.5 | [87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuzminova, A.; Dmitrenko, M.; Zolotarev, A.; Myznikov, D.; Selyutin, A.; Su, R.; Penkova, A. Pervaporation Polyvinyl Alcohol Membranes Modified with Zr-Based Metal Organic Frameworks for Isopropanol Dehydration. Membranes 2022, 12, 908. https://doi.org/10.3390/membranes12100908
Kuzminova A, Dmitrenko M, Zolotarev A, Myznikov D, Selyutin A, Su R, Penkova A. Pervaporation Polyvinyl Alcohol Membranes Modified with Zr-Based Metal Organic Frameworks for Isopropanol Dehydration. Membranes. 2022; 12(10):908. https://doi.org/10.3390/membranes12100908
Chicago/Turabian StyleKuzminova, Anna, Mariia Dmitrenko, Andrey Zolotarev, Danila Myznikov, Artem Selyutin, Rongxin Su, and Anastasia Penkova. 2022. "Pervaporation Polyvinyl Alcohol Membranes Modified with Zr-Based Metal Organic Frameworks for Isopropanol Dehydration" Membranes 12, no. 10: 908. https://doi.org/10.3390/membranes12100908
APA StyleKuzminova, A., Dmitrenko, M., Zolotarev, A., Myznikov, D., Selyutin, A., Su, R., & Penkova, A. (2022). Pervaporation Polyvinyl Alcohol Membranes Modified with Zr-Based Metal Organic Frameworks for Isopropanol Dehydration. Membranes, 12(10), 908. https://doi.org/10.3390/membranes12100908