Investigation of Phosphatidylserine-Transporting Activity of Human TMEM16C Isoforms
Abstract
1. Introduction
2. Materials and Methods
2.1. Construction and Expression of TMEM16C Isoforms
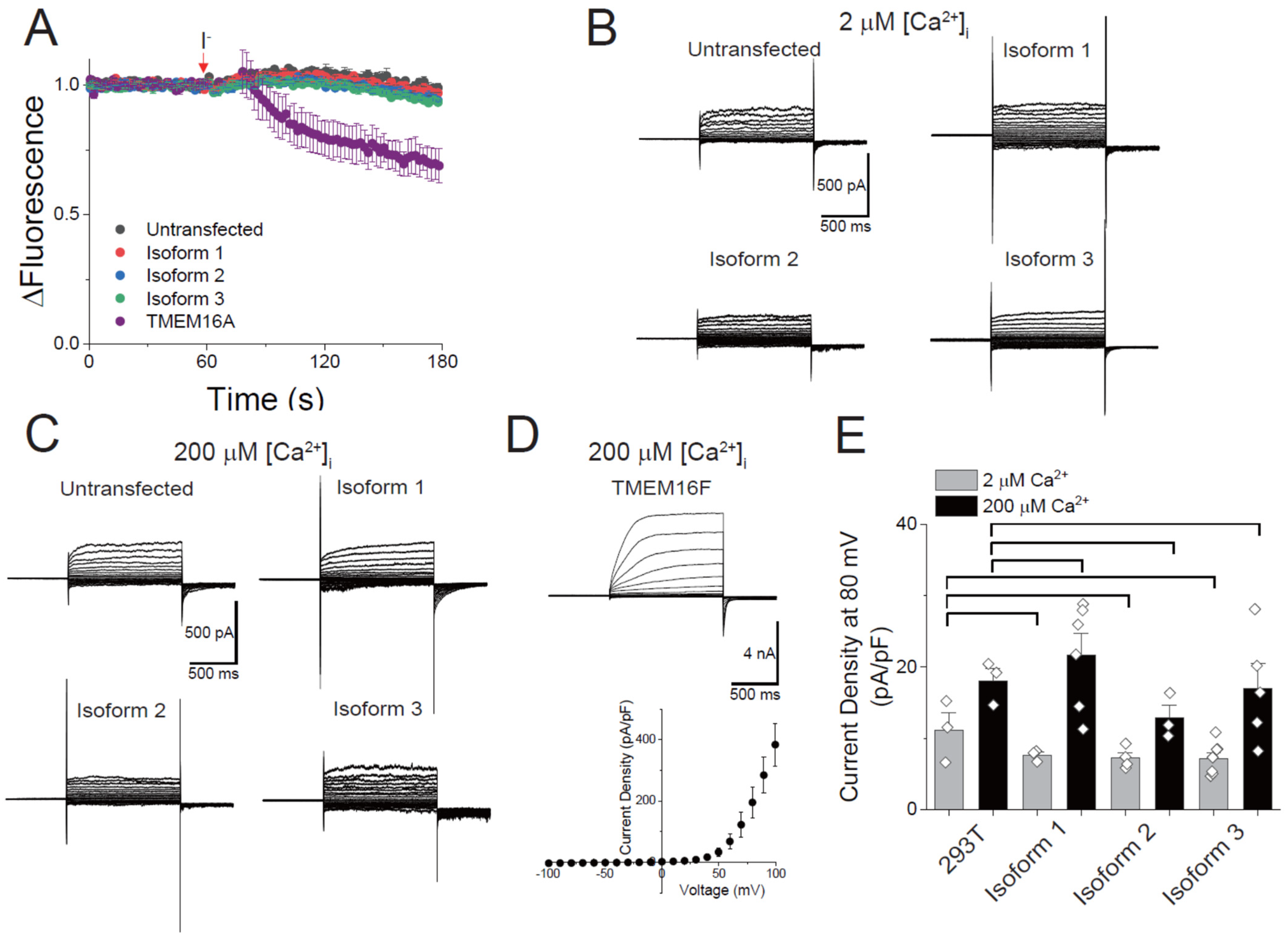
2.2. Scrambling Assay
2.3. Surface Biotinylation and Western Blot
2.4. Halide-Quenching Flux Assay
2.5. Electrophysiology
3. Results
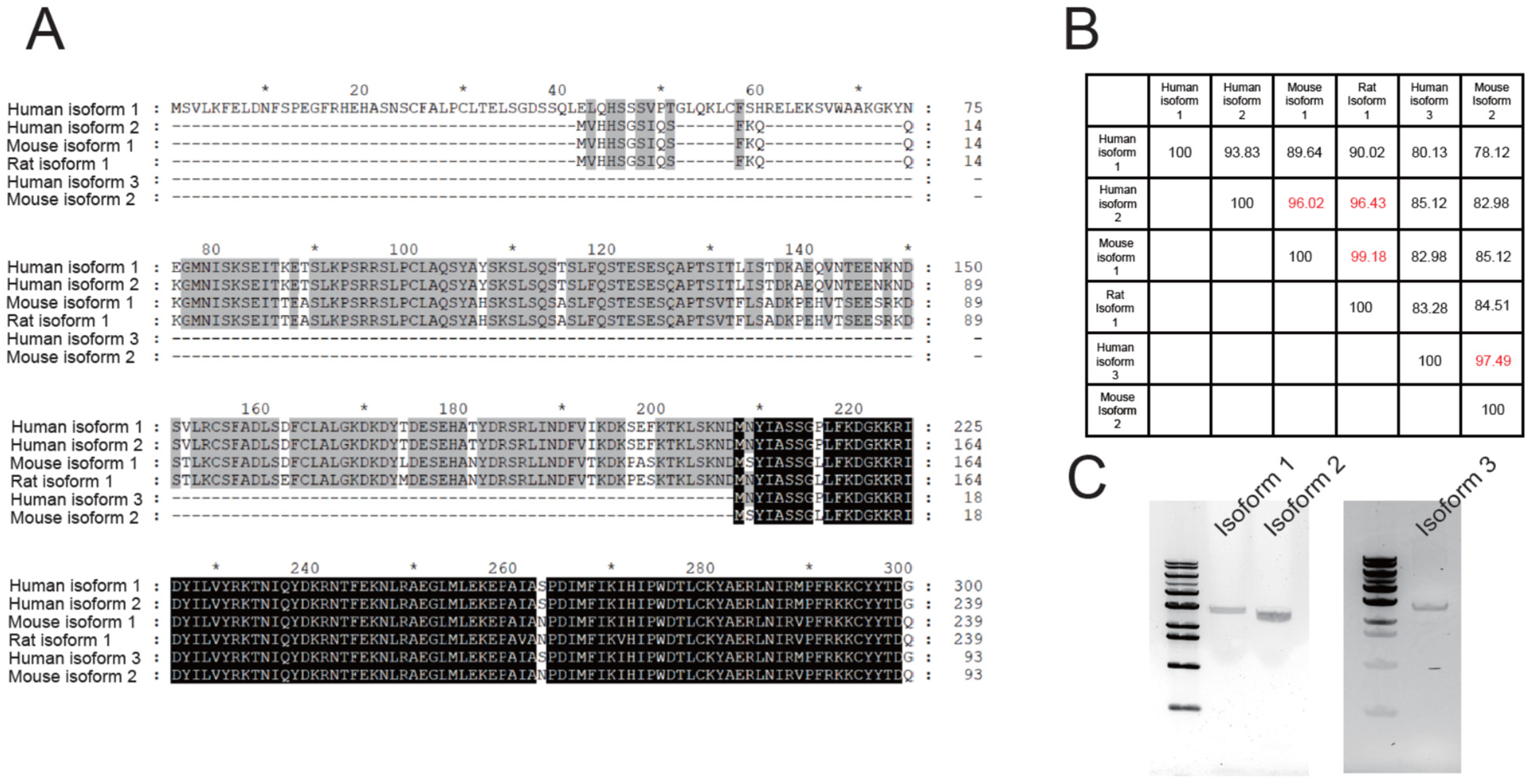
3.1. Human TMEM16C Isoforms Contain Different N-Terminuses
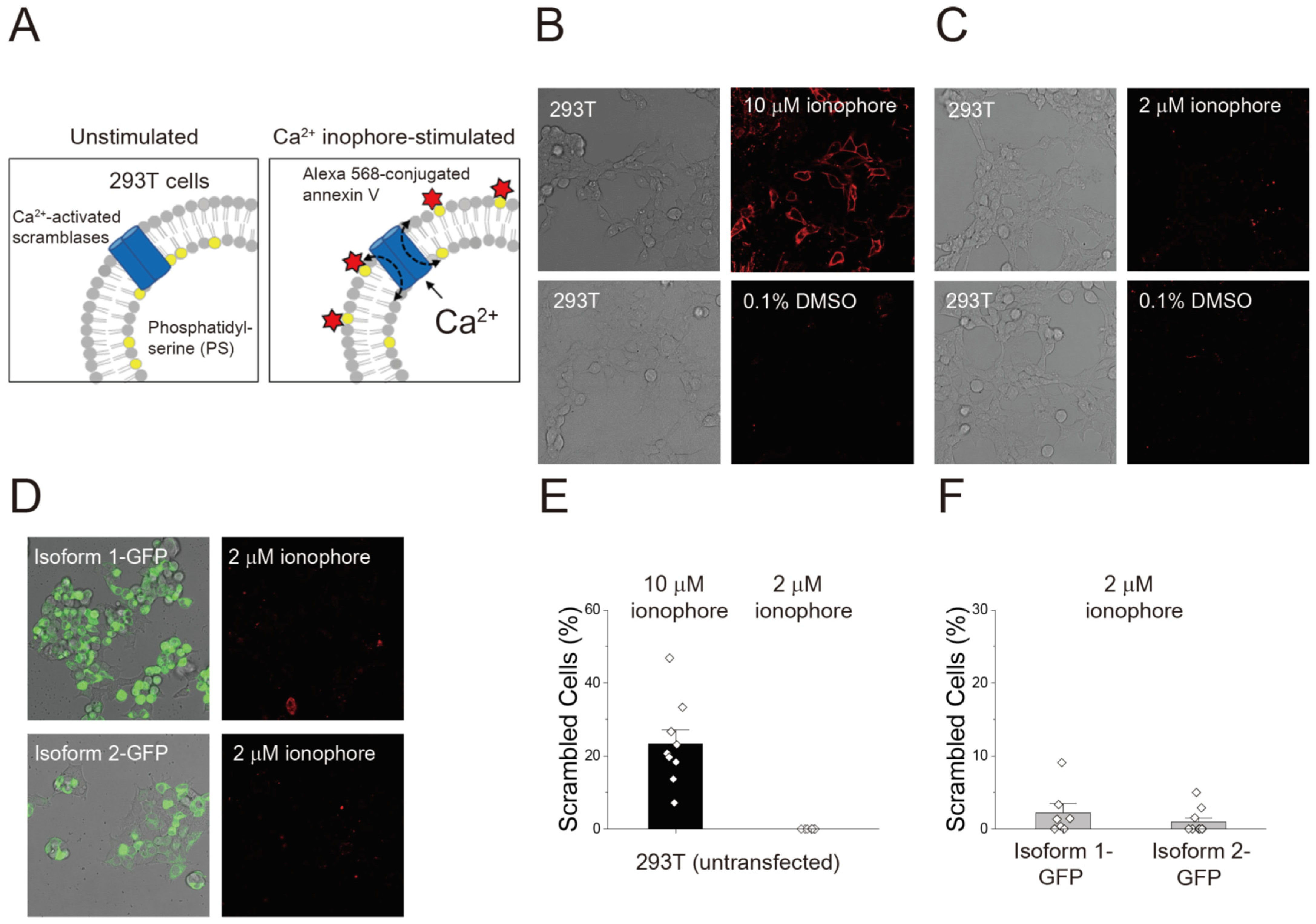
3.2. Endogenous Scrambling Activity of the 293T Cell and the Effects of GFP Tagging on the Scrambling Activity
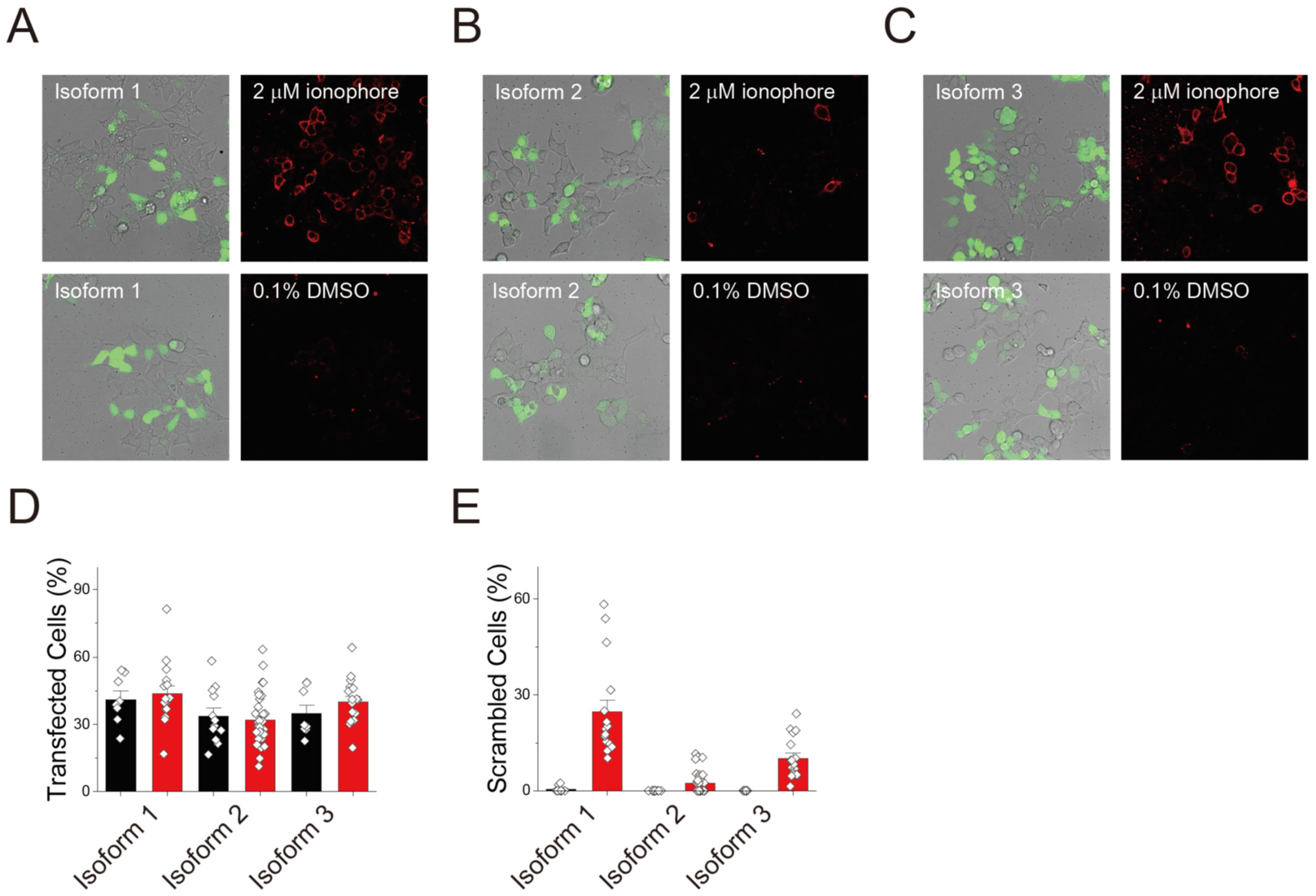
3.3. Scrambling Activity of Three TMEM16C Isoforms
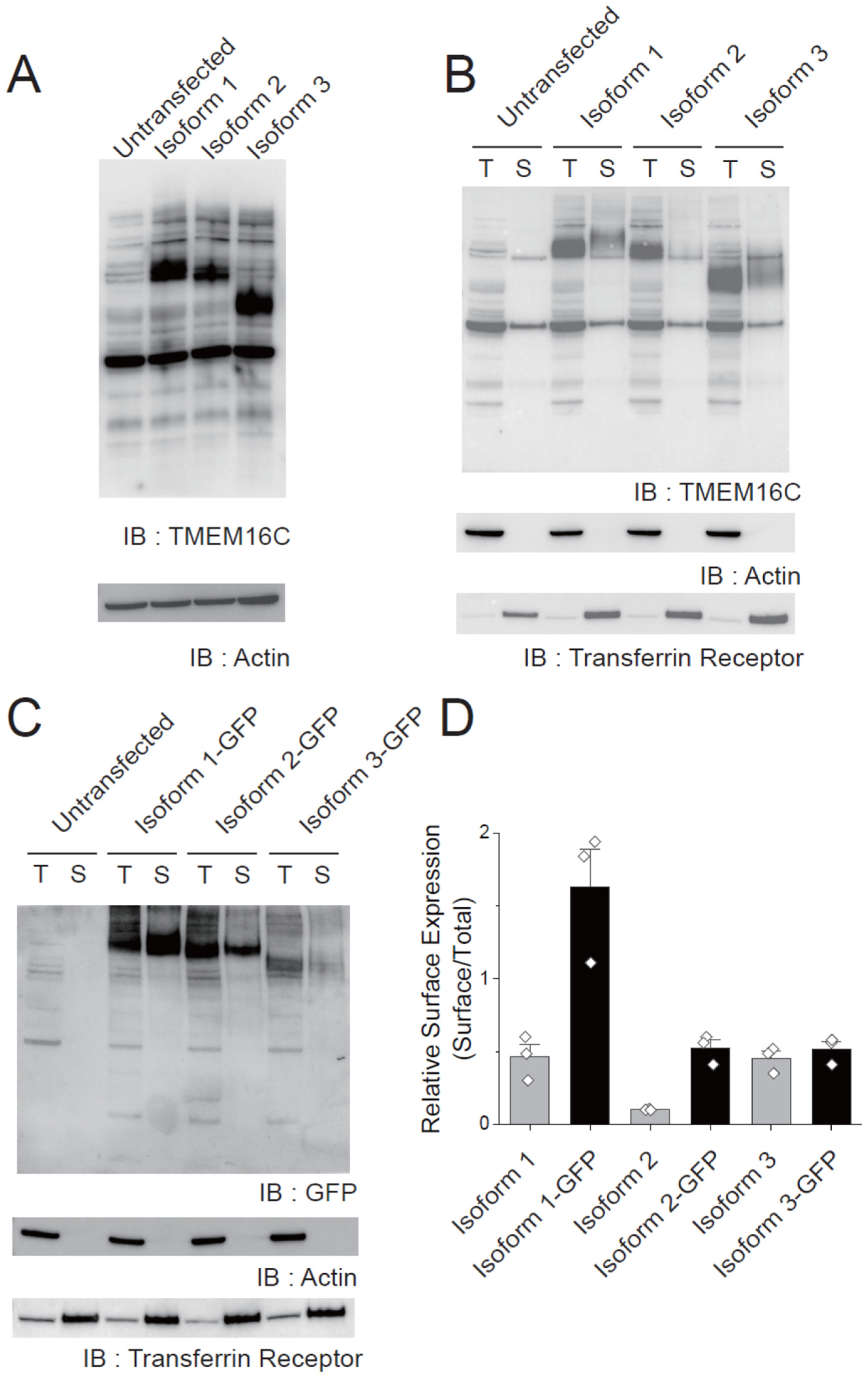
3.4. Surface Expression of Human TMEM16C Isoforms
3.5. Ion Channel Activity of Human TMEM16C Scramblases
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pomorski, T.; Menon, A. Lipid flippases and their biological functions. Experientia 2006, 63, 2908–2921. [Google Scholar] [CrossRef]
- Bevers, E.M.; Williamson, P.L. Phospholipid scramblase: An update. FEBS Lett. 2010, 584, 2724–2730. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, K.; Schroit, A.J. Aminophospholipid Asymmetry: A Matter of Life and Death. Annu. Rev. Physiol. 2003, 65, 701–734. [Google Scholar] [CrossRef] [PubMed]
- Bevers, E.M.; Williamson, P.L. Getting to the Outer Leaflet: Physiology of Phosphatidylserine Exposure at the Plasma Membrane. Physiol. Rev. 2016, 96, 605–645. [Google Scholar] [CrossRef] [PubMed]
- Pedemonte, N.; Galietta, L.J. Structure and Function of TMEM16 Proteins (Anoctamins). Physiol. Rev. 2014, 94, 419–459. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Imanishi, E.; Nagata, S. Exposure of Phosphatidylserine by Xk-related Protein Family Members during Apoptosis. J. Biol. Chem. 2014, 289, 30257–30267. [Google Scholar] [CrossRef]
- Goren, M.A.; Morizumi, T.; Menon, I.; Joseph, J.S.; Dittman, J.S.; Cherezov, V.; Stevens, R.C.; Ernst, O.P.; Menon, A.K. Constitutive phospholipid scramblase activity of a G protein-coupled receptor. Nat. Commun. 2014, 5, 5115. [Google Scholar] [CrossRef] [PubMed]
- Menon, I.; Huber, T.; Sanyal, S.; Banerjee, S.; Barré, P.; Canis, S.; Warren, J.D.; Hwa, J.; Sakmar, T.P.; Menon, A.K. Opsin Is a Phospholipid Flippase. Curr. Biol. 2011, 21, 149–153. [Google Scholar] [CrossRef]
- Ehlen, H.W.; Chinenkova, M.; Moser, M.; Munter, H.-M.; Krause, Y.; Gross, S.; Brachvogel, B.; Wuelling, M.; Kornak, U.; Vortkamp, A. Inactivation of anoctamin-6/Tmem16f, a regulator of phosphatidylserine scrambling in osteoblasts, leads to decreased mineral deposition in skeletal tissues. J. Bone Miner. Res. 2012, 28, 246–259. [Google Scholar] [CrossRef]
- Caputo, A.; Caci, E.; Ferrera, L.; Pedemonte, N.; Barsanti, C.; Sondo, E.; Pfeffer, U.; Ravazzolo, R.; Zegarra-Moran, O.; Galietta, L.J.V. TMEM16A, A Membrane Protein Associated with Calcium-Dependent Chloride Channel Activity. Science 2008, 322, 590–594. [Google Scholar] [CrossRef]
- Schroeder, B.C.; Cheng, T.; Jan, Y.N.; Jan, L.Y. Expression Cloning of TMEM16A as a Calcium-Activated Chloride Channel Subunit. Cell 2008, 134, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.D.; Cho, H.; Koo, J.Y.; Tak, M.H.; Cho, Y.; Shim, W.-S.; Park, S.P.; Lee, J.; Lee, B.; Kim, B.-M.; et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 2008, 455, 1210–1215. [Google Scholar] [CrossRef]
- Suzuki, J.; Fujii, T.; Imao, T.; Ishihara, K.; Kuba, H.; Nagata, S. Calcium-dependent Phospholipid Scramblase Activity of TMEM16 Protein Family Members. J. Biol. Chem. 2013, 288, 13305–13316. [Google Scholar] [CrossRef]
- Gyobu, S.; Miyata, H.; Ikawa, M.; Yamazaki, D.; Takeshima, H.; Suzuki, J.; Nagata, S. A Role of TMEM16E Carrying a Scrambling Domain in Sperm Motility. Mol. Cell. Biol. 2016, 36, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Bushell, S.R.; Pike, A.C.W.; Falzone, M.E.; Rorsman, N.J.G.; Ta, C.M.; Corey, R.A.; Newport, T.D.; Christianson, J.C.; Scofano, L.F.; Shintre, C.A.; et al. The structural basis of lipid scrambling and inactivation in the endoplasmic reticulum scramblase TMEM16K. Nat. Commun. 2019, 10, 3956. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Whitlock, J.M.; Lee, K.; Ortlund, E.A.; Cui, Y.Y.; Hartzell, H.C. Identification of a lipid scrambling domain in ANO6/TMEM16F. eLife 2015, 4, e06901. [Google Scholar] [CrossRef]
- Brunner, J.D.; Lim, N.K.; Schenck, S.; Duerst, A.; Dutzler, R. X-ray structure of a calcium-activated TMEM16 lipid scramblase. Nature 2014, 516, 207–212. [Google Scholar] [CrossRef]
- Dang, S.; Feng, S.; Tien, J.; Peters, C.; Bulkley, D.; Lolicato, M.; Zhao, J.; Zuberbühler, K.; Ye, W.; Qi, L.; et al. Cryo-EM structures of the TMEM16A calcium-activated chloride channel. Nature 2017, 552, 426–429. [Google Scholar] [CrossRef]
- Alvadia, C.; Lim, N.K.; Mosina, V.C.; Oostergetel, G.T.; Dutzler, R.; Paulino, C. Cryo-EM structures and functional characterization of the murine lipid scramblase TMEM16F. eLife 2019, 8, e44365. [Google Scholar] [CrossRef]
- Falzone, E.M.; Rheinberger, J.; Lee, B.-C.; Peyear, T.; Sasset, L.; Raczkowski, A.M.; Eng, E.T.; Di Lorenzo, A.; Andersen, O.S.; Nimigean, C.M.; et al. Structural basis of Ca2+-dependent activation and lipid transport by a TMEM16 scramblase. eLife 2019, 8, e43229. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, G.; Plagnol, V.; Holmström, K.M.; Bras, J.; Sheerin, U.-M.; Preza, E.; Rubio-Agusti, I.; Ryten, M.; Schneider, S.A.; Stamelou, M.; et al. Mutations in ANO3 Cause Dominant Craniocervical Dystonia: Ion Channel Implicated in Pathogenesis. Am. J. Hum. Genet. 2012, 91, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Zhang, C.; Méar, L.; Zhong, W.; Digre, A.; Katona, B.; Sjöstedt, E.; Butler, L.; Odeberg, J.; Dusart, P.; et al. A single–cell type transcriptomics map of human tissues. Sci. Adv. 2021, 7, eabh2169. [Google Scholar] [CrossRef]
- Huang, F.; Wang, X.; Ostertag, E.M.; Nuwal, T.; Huang, B.; Jan, Y.-N.; Basbaum, A.I.; Jan, L.Y. TMEM16C facilitates Na+-activated K+ currents in rat sensory neurons and regulates pain processing. Nat. Neurosci. 2013, 16, 1284–1290. [Google Scholar] [CrossRef]
- Gadotti, V.M.; Zamponi, G.W. TMEM16C cuts pain no SLACK. Nat. Neurosci. 2013, 16, 1165–1166. [Google Scholar] [CrossRef]
- Feenstra, B.; Pasternak, B.; Geller, F.; Carstensen, L.; Wang, T.; Huang, F.; Eitson, J.L.; Hollegaard, M.V.; Svanström, H.; Vestergaard, M.; et al. Common variants associated with general and MMR vaccine–related febrile seizures. Nat. Genet. 2014, 46, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Briones, N.; Dinu, V. Data mining of high density genomic variant data for prediction of Alzheimer’s disease risk. BMC Med. Genet. 2012, 13, 7. [Google Scholar] [CrossRef]
- Hargis, E.K.; Blalock, E.M. Transcriptional signatures of brain aging and Alzheimer’s disease: What are our rodent models telling us? Behav. Brain Res. 2016, 322, 311–328. [Google Scholar] [CrossRef]
- Jiang, T.; Yu, K.; Hartzell, H.C.; Tajkhorshid, E. Lipids and ions traverse the membrane by the same physical pathway in the nhTMEM16 scramblase. eLife 2017, 6, e28671. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, L.; Li, J.; Wang, C.; Chen, Y.; Yuan, Y.; Xie, K.; Wang, G.; Yu, Y. A Role for Transmembrane Protein 16C/Slack Impairment in Excitatory Nociceptive Synaptic Plasticity in the Pathogenesis of Remifentanil-induced Hyperalgesia in Rats. Neurosci. Bull. 2021, 37, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Le, S.C.; Yang, H. Drosophila Subdued is a moonlighting transmembrane protein 16 (TMEM16) that transports ions and phospholipids. J. Biol. Chem. 2019, 294, 4529–4537. [Google Scholar] [CrossRef]
- Le, T.; Jia, Z.; Le, S.C.; Zhang, Y.; Chen, J.; Yang, H. An inner activation gate controls TMEM16F phospholipid scrambling. Nat. Commun. 2019, 10, 1846. [Google Scholar] [CrossRef]
- Lee, B.-C.; Menon, A.K.; Accardi, A. The nhTMEM16 Scramblase Is Also a Nonselective Ion Channel. Biophys. J. 2016, 111, 1919–1924. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Kim, E.; Lee, B.-C. Investigation of Phosphatidylserine-Transporting Activity of Human TMEM16C Isoforms. Membranes 2022, 12, 1005. https://doi.org/10.3390/membranes12101005
Kim H, Kim E, Lee B-C. Investigation of Phosphatidylserine-Transporting Activity of Human TMEM16C Isoforms. Membranes. 2022; 12(10):1005. https://doi.org/10.3390/membranes12101005
Chicago/Turabian StyleKim, Hanggu, Eunyoung Kim, and Byoung-Cheol Lee. 2022. "Investigation of Phosphatidylserine-Transporting Activity of Human TMEM16C Isoforms" Membranes 12, no. 10: 1005. https://doi.org/10.3390/membranes12101005
APA StyleKim, H., Kim, E., & Lee, B.-C. (2022). Investigation of Phosphatidylserine-Transporting Activity of Human TMEM16C Isoforms. Membranes, 12(10), 1005. https://doi.org/10.3390/membranes12101005




