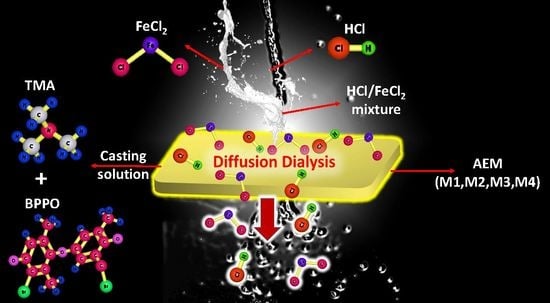
Synthesis of Porous BPPO-Based Anion Exchange Membranes for Acid Recovery via Diffusion Dialysis
Abstract
:1. Introduction
2. Experimental
2.1. Materials
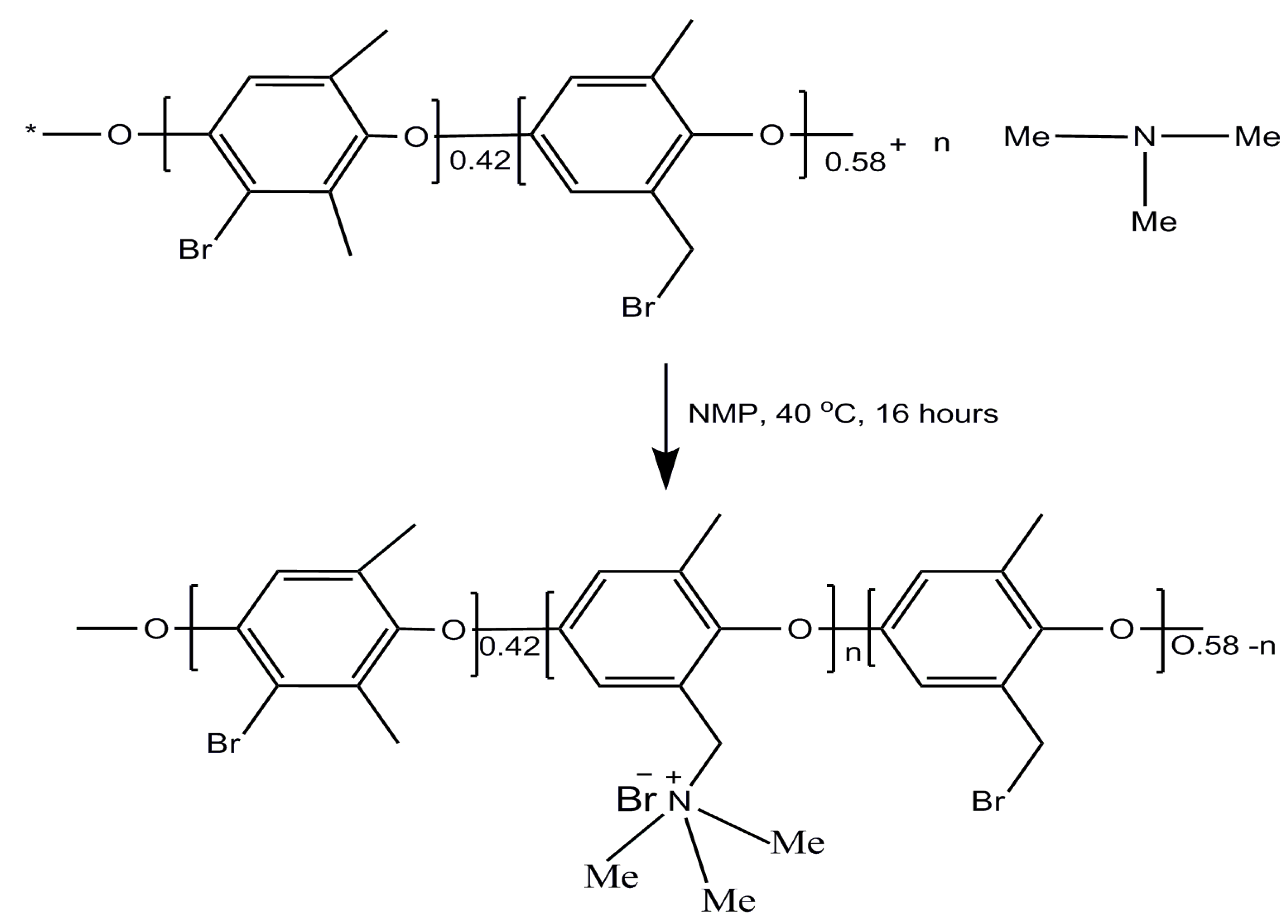
2.2. Preparation of Porous Membranes Based on BPPO
2.3. Characterizations
2.3.1. Instrumentations
2.3.2. Ion Exchange Capacity
2.3.3. Water Uptake, Linear Expansion Ratio, and Fixed Group Concentration
2.3.4. Diffusion Dialysis for the Mixture of HCl/FeCl2
3. Results and Discussion
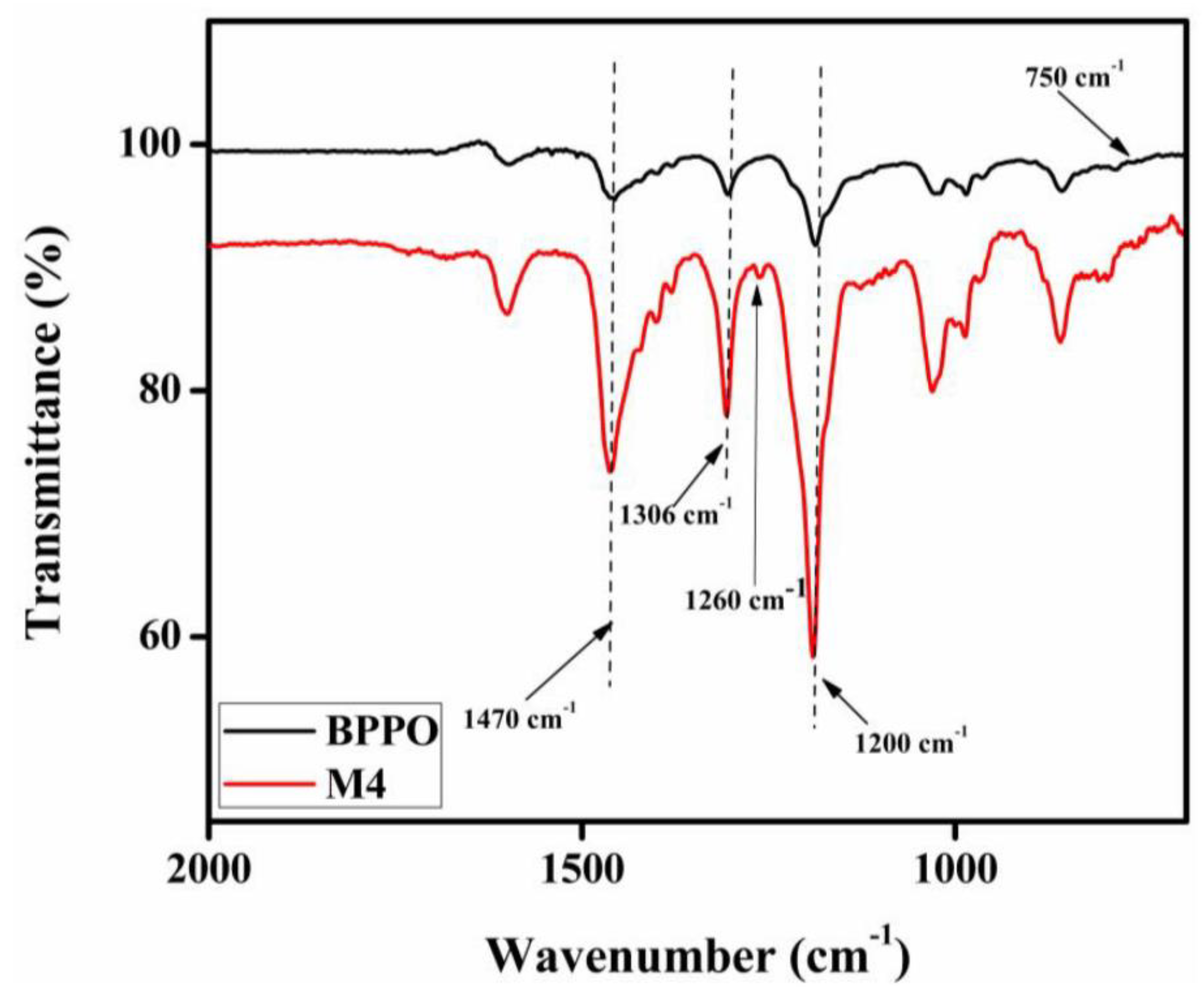
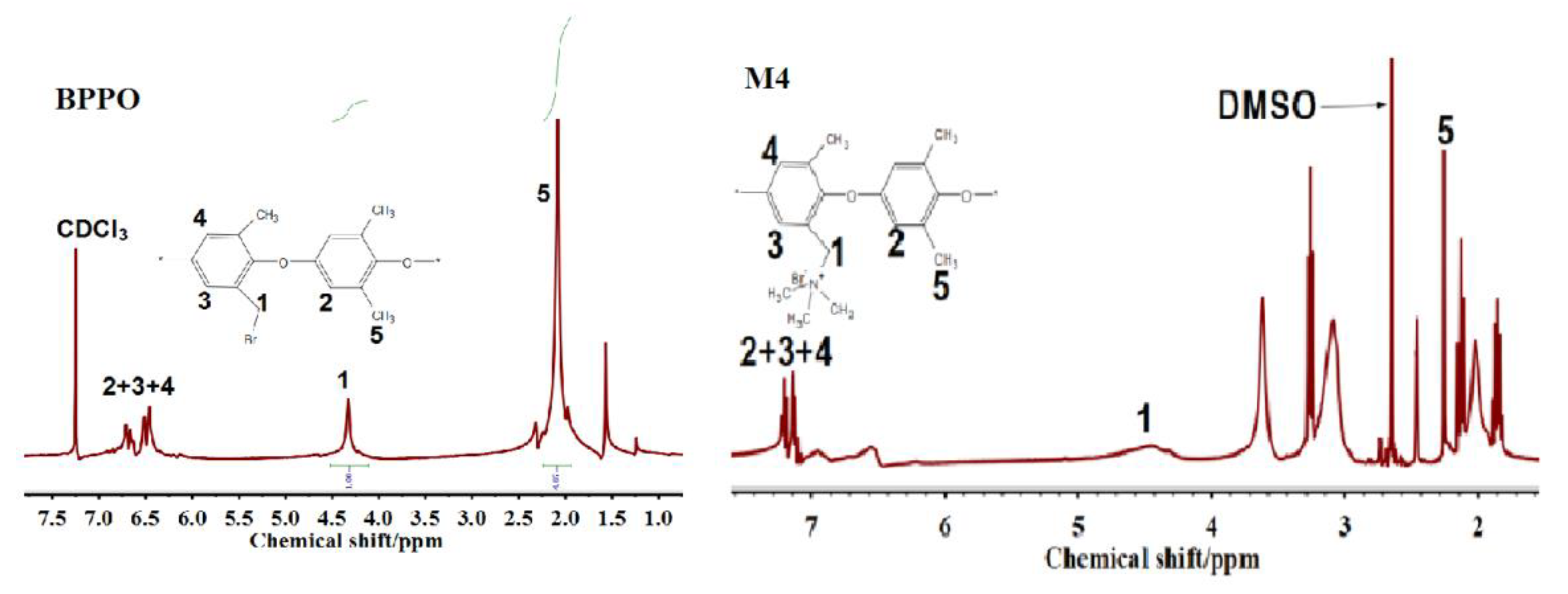
3.1. FTIR and Proton NMR Tests
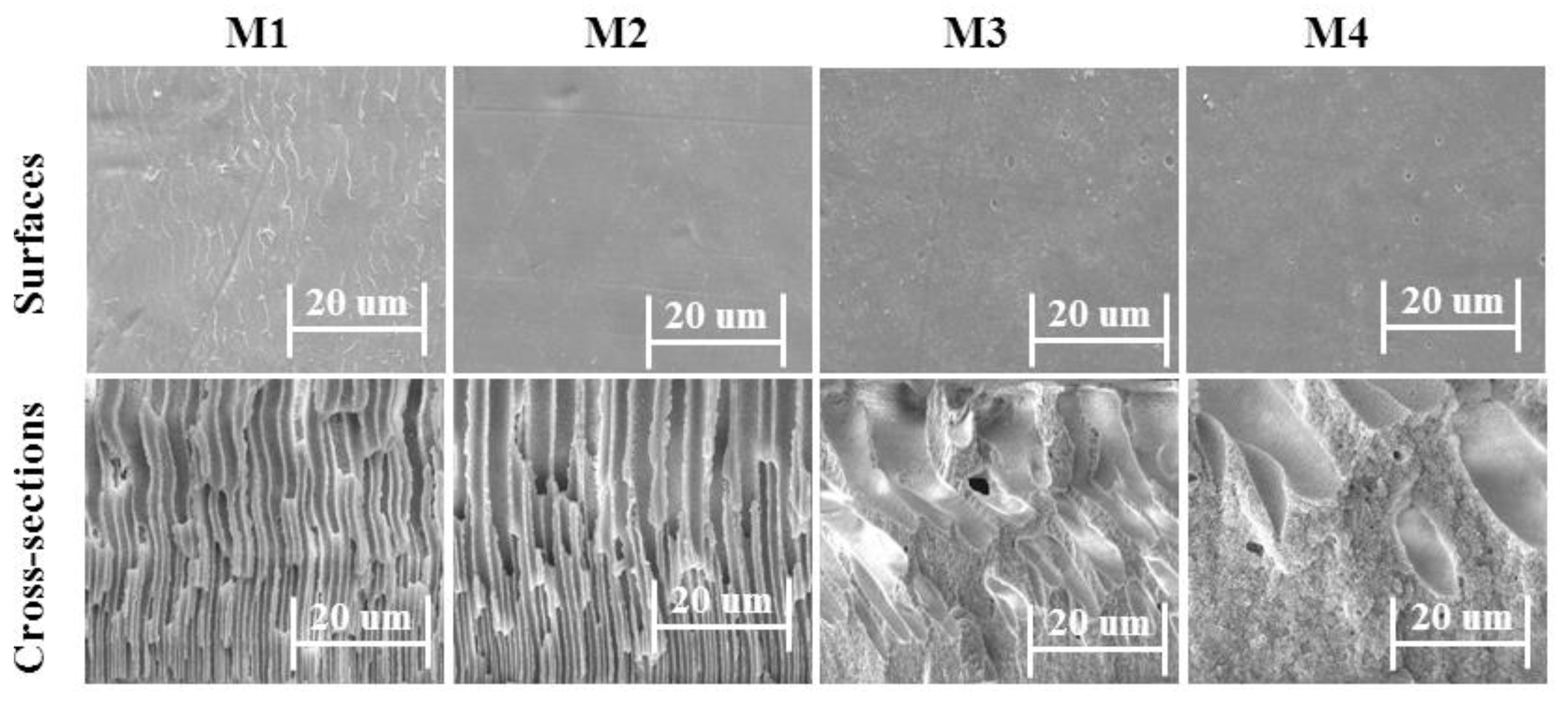
3.2. Morphology
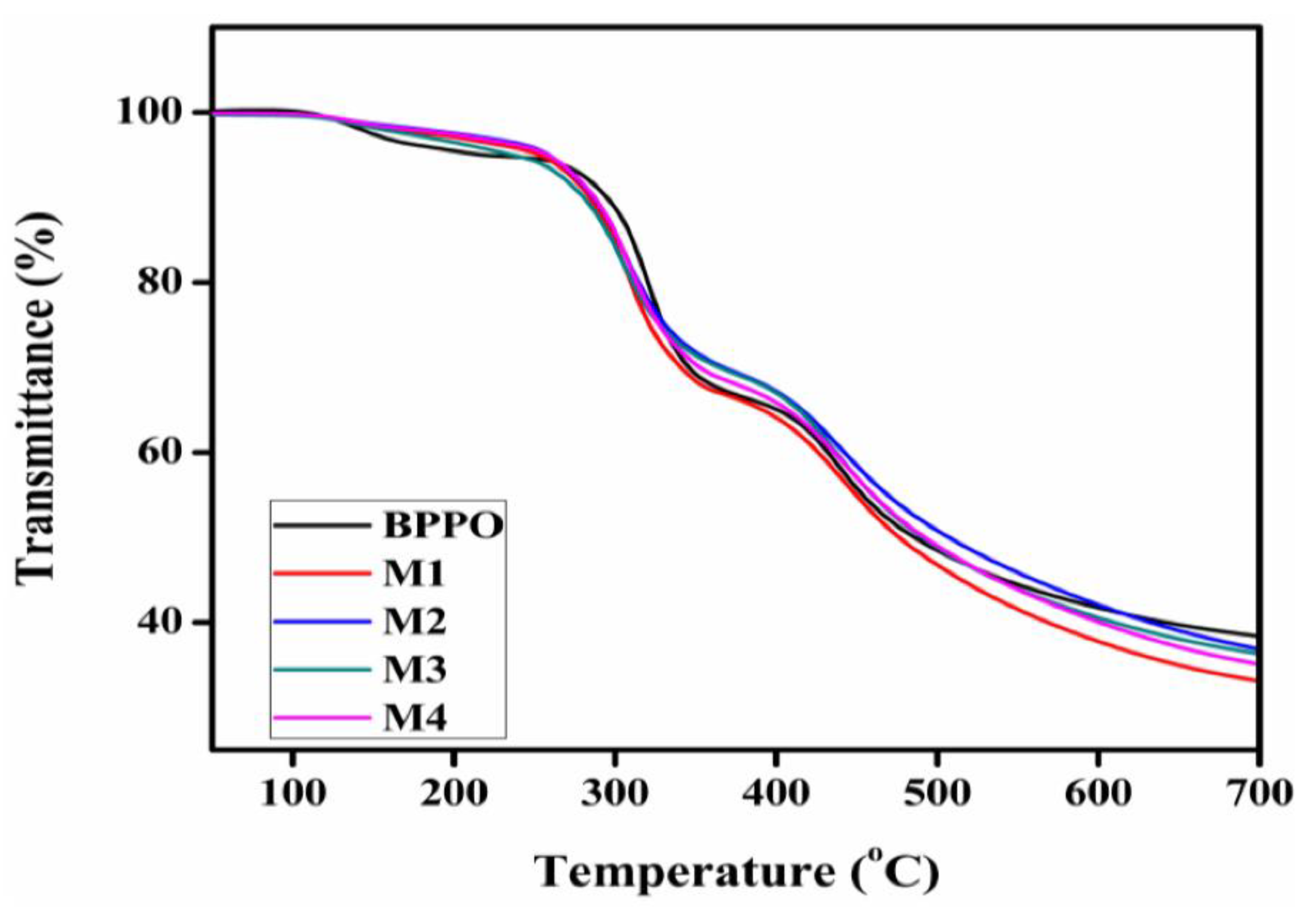
3.3. Thermal Decomposition and Chemical Stability of the Prepared Membranes
3.4. Ion Exchange Capacity (IEC) and Fixed Group Concentration (CR)
3.5. Water Uptake and Linear Expansion Ratio
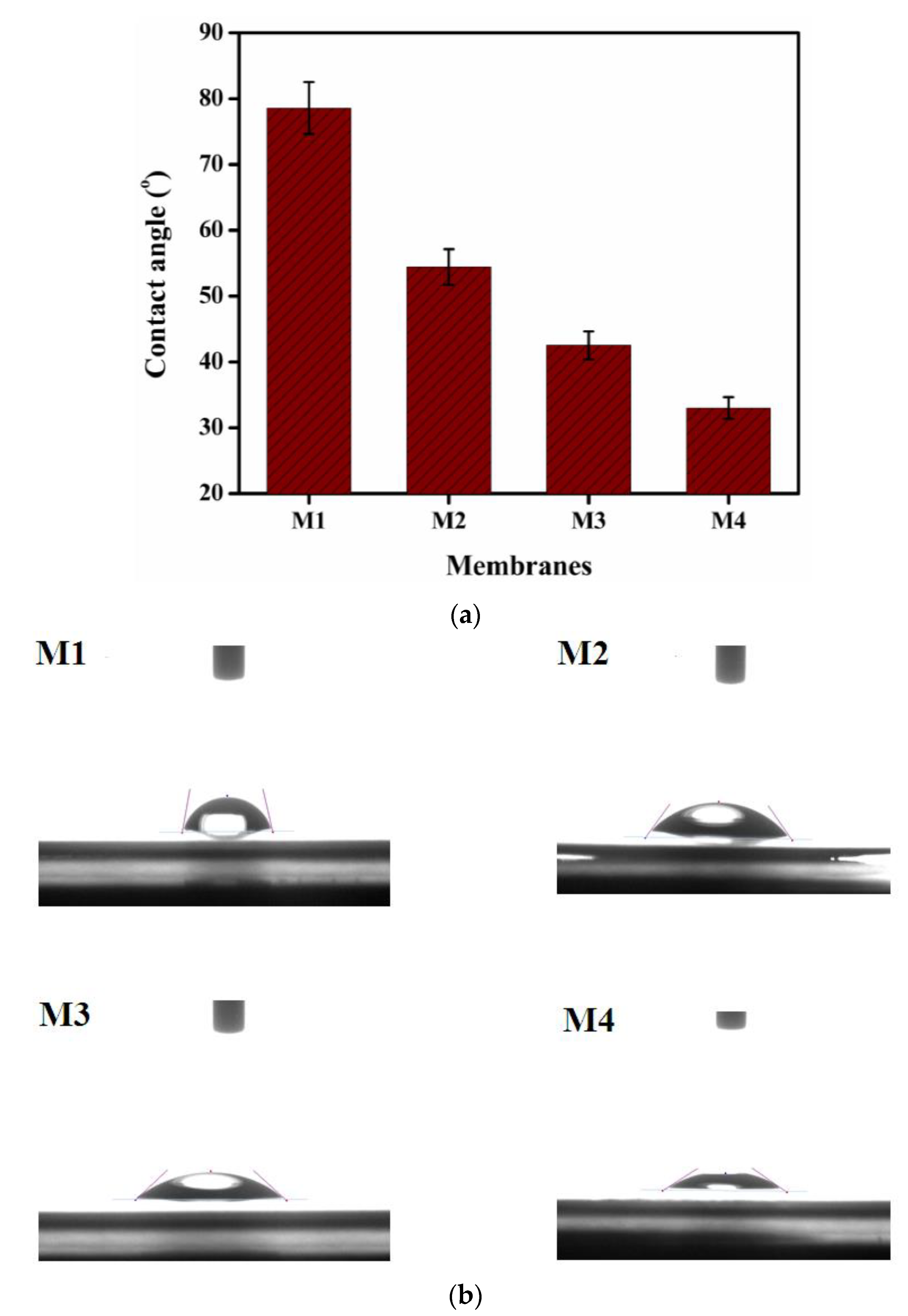
3.6. Water Contact Angle
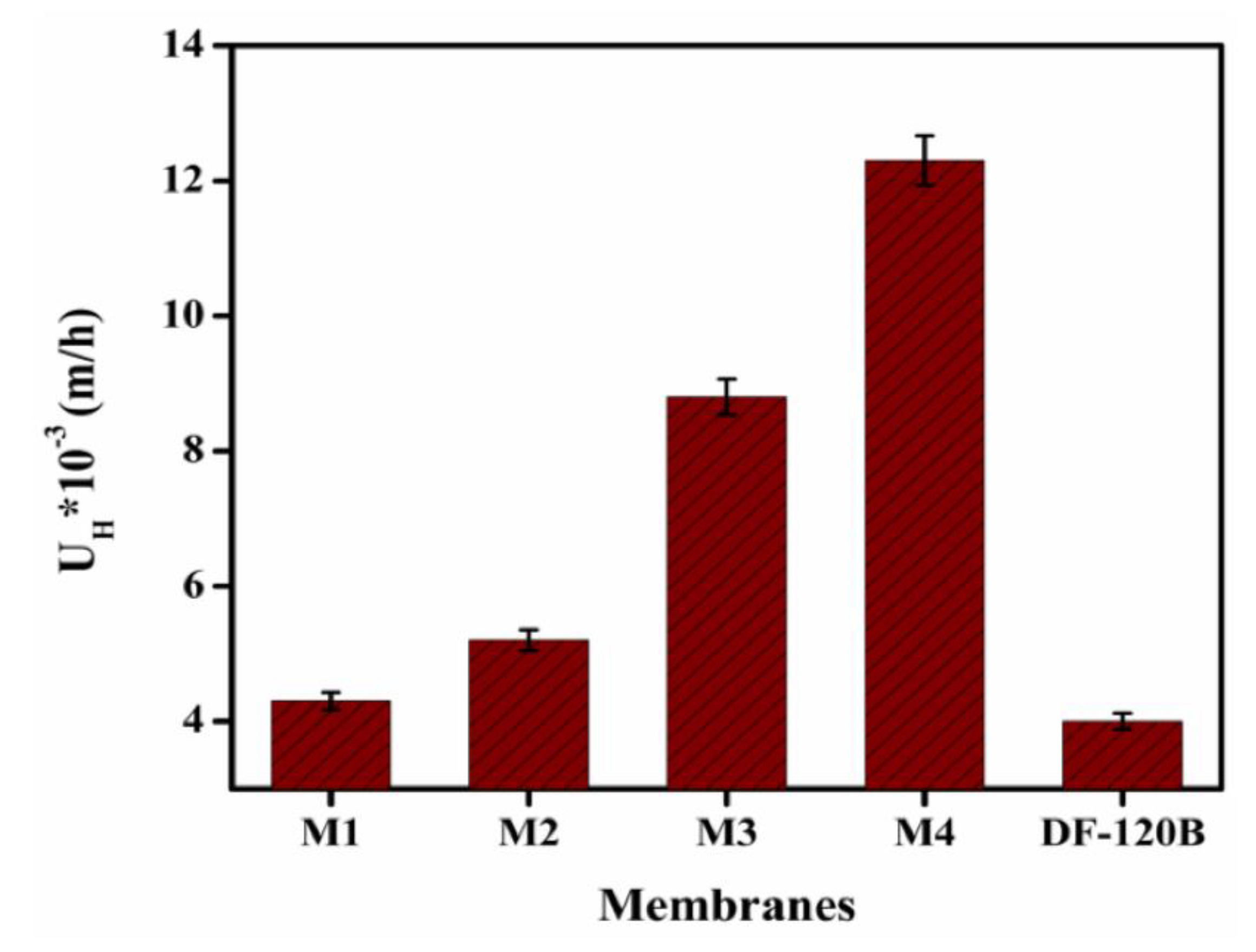
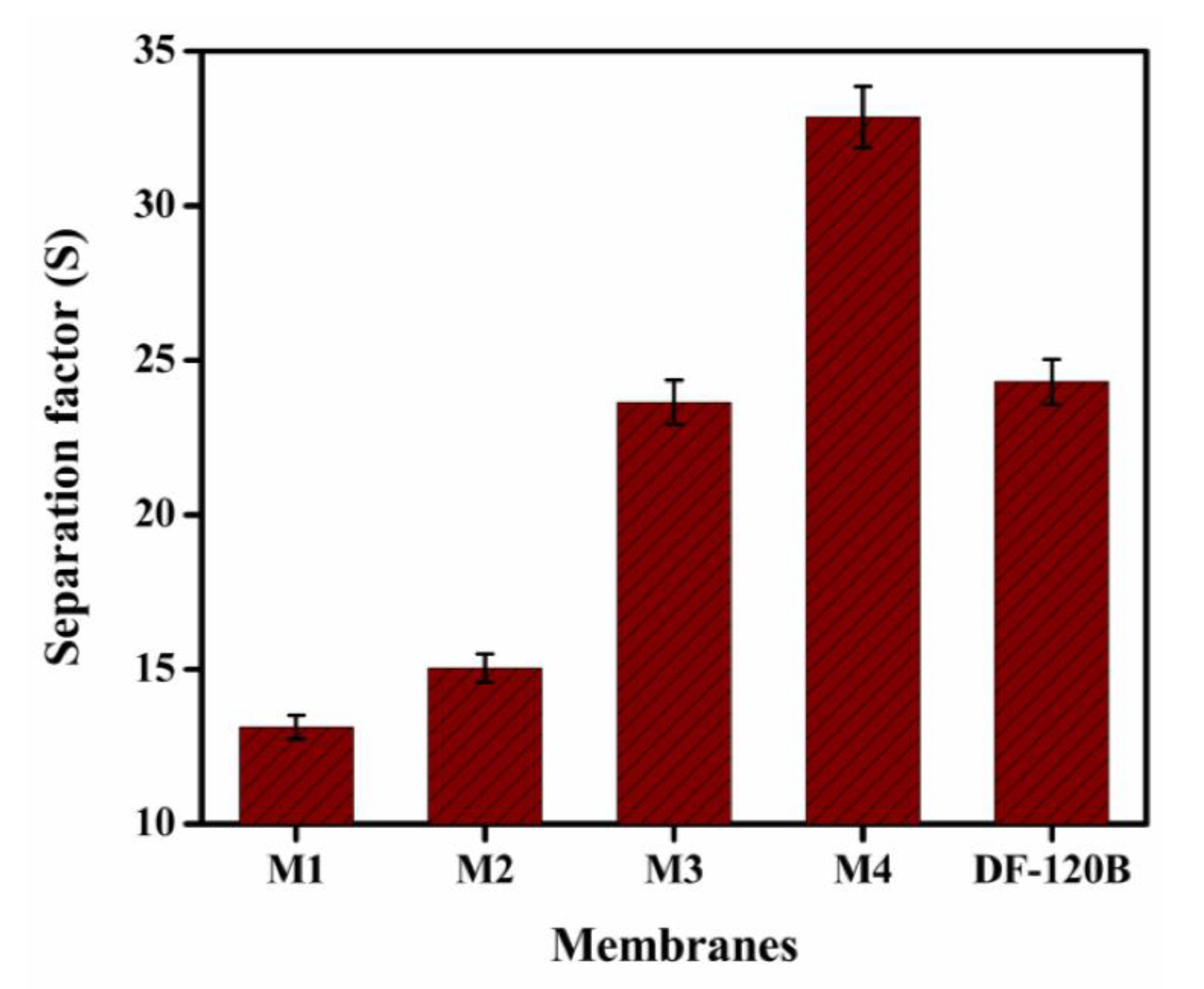
3.7. DD for HCl/FeCl2 Solution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AEM | Anion exchange membrane |
| BPPO | Brominated poly(2,6-dimethyl-1,4-phenylene oxide) |
| CR | Fixed group concentration |
| DD | Diffusion dialysis |
| IEC | Ion exchange capacity |
| IEM | Ion exchange membrane |
| NMP | N-Methyl-2-pyrrolidone |
| SEM | Scanning electron microscopy |
| S | Separation factor |
| TGA | Thermogravimetric analysis |
| TMA | Trimethylamine |
| UH | Diffusion dialysis coefficient of HCl |
| UFe | Diffusion dialysis coefficient of FeCl2 |
| WR | Water uptake |
References
- Xu, T.; Yang, W. Industrial recovery of mixed acid (HF+HNO3) from the titanium spent leaching solutions by diffusion dialysis with a new series of anion exchange membranes. J. Membr. Sci. 2003, 220, 89–95. [Google Scholar] [CrossRef]
- Agrawal, A.; Sahu, K.K. An overview of the recovery of acid from spent acidic solutions from steel and electroplating industries. J. Hazard. Mater. 2009, 171, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Gueccia, R.; Randazzo, S.; Chillura Martino, D.; Cipollina, A.; Micale, G. Experimental investigation and modeling of diffusion dialysis for hcl recovery from waste pickling solution. J. Environ. Manag. 2019, 235, 202–212. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, F.; Ma, X.; Zhang, G. Recovery of nitric and acetic acids from etching waste solutions using a synergistic system consisting of n235 and trpo in cyclohexane. Hydrometallurgy 2019, 185, 23–29. [Google Scholar] [CrossRef]
- Chen, F.; Wang, X.; Liu, W.; Liang, B.; Yue, H.; Li, C. Selective extraction of nitric and acetic acids from etching waste acid using n235 and mibk mixtures. Sep. Purif. Technol. 2016, 169, 50–58. [Google Scholar] [CrossRef]
- Narěbska, A.; Staniszewski, M. Separation of fermentation products by membrane techniques. I. Separation of lactic acid/lactates by diffusion dialysis. Sep. Sci. Technol. 1997, 32, 1669–1682. [Google Scholar] [CrossRef]
- Palatý, Z.; Stoček, P.; Bendová, H.; Prchal, P. Continuous dialysis of carboxylic acids: Solubility and diffusivity in neosepta-amh membranes. Desalination 2009, 243, 65–73. [Google Scholar] [CrossRef]
- Luo, J.; Wu, C.; Xu, T.; Wu, Y. Diffusion dialysis-concept, principle and applications. J. Membr. Sci. 2011, 366, 1–16. [Google Scholar] [CrossRef]
- Lie, H.; Ming, C.; Xiong-jiao, Z. Acid waste water comprehensive treatment rebuilding engineering practice. Metal Products 2009, 1, 020. [Google Scholar]
- Lei, Q.; He, D.; Zhou, K.; Zhang, X.; Peng, C.; Chen, W. Separation and recovery of scandium and titanium from red mud leaching liquor through a neutralization precipitation-acid leaching approach. J. Rare Earths 2020, 39, 1126–1132. [Google Scholar] [CrossRef]
- Ni, C.; Wu, X.; Dan, J.; Du, D. Facile recovery of acetic acid from waste acids of electronic industry via a partial neutralization pretreatment (pnp)–distillation strategy. Sep. Purif. Technol. 2014, 132, 23–26. [Google Scholar] [CrossRef]
- Khan, M.I.; Shanableh, A.; Elboughdiri, N.; Kriaa, K.; Ghernaout, D.; Ghareba, S.; Khraisheh, M.; Lashari, M.H. Higher acid recovery efficiency of novel functionalized inorganic/organic composite anion exchange membranes from acidic wastewater. Membranes 2021, 11, 133. [Google Scholar] [CrossRef]
- Shin, C.-H.; Kim, J.-Y.; Kim, J.-Y.; Kim, H.-S.; Lee, H.-S.; Mohapatra, D.; Ahn, J.-W.; Ahn, J.-G.; Bae, W. Recovery of nitric acid from waste etching solution using solvent extraction. J. Hazard. Mater. 2009, 163, 729–734. [Google Scholar] [CrossRef]
- Ahmad, A.; Othman, I.; Taher, H.; Banat, F. Lactic acid recovery from date pulp waste fermentation broth by ions exchange resins. Environ. Technol. Innov. 2021, 22, 101438. [Google Scholar] [CrossRef]
- Yan, R.; Luo, D.; Fu, C.; Wang, Y.; Zhang, H.; Wu, P.; Jiang, W. Harmless treatment and selective recovery of acidic Cu(II)-Cr(VI) hybrid wastewater via coupled photo-reduction and ion exchange. Sep. Purif. Technol. 2020, 234, 116130. [Google Scholar] [CrossRef]
- Chen, K.; Hao, S.; Lyu, H.; Luo, G.; Zhang, S.; Chen, J. Ion exchange separation for recovery of monosaccharides, organic acids and phenolic compounds from hydrolysates of lignocellulosic biomass. Sep. Purif. Technol. 2017, 172, 100–106. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, S.-T. In situ recovery of fumaric acid by intermittent adsorption with ira-900 ion exchange resin for enhanced fumaric acid production by rhizopus oryzae. Biochem. Eng. J. 2015, 96, 38–45. [Google Scholar] [CrossRef]
- Lin, S.H.; Kiang, C.D. Chromic acid recovery from waste acid solution by an ion exchange process: Equilibrium and column ion exchange modeling. Chem. Eng. J. 2003, 92, 193–199. [Google Scholar] [CrossRef]
- Choi, J.-H.; Kim, S.-H.; Moon, S.-H. Recovery of lactic acid from sodium lactate by ion substitution using ion-exchange membrane. Sep. Purif. Technol. 2002, 28, 69–79. [Google Scholar] [CrossRef]
- Khan, M.I.; Khraisheh, M. Synthesis and characterization of stable anion exchange membranes for desalination applications. Desalin. Water Treat. 2018, 113, 36–44. [Google Scholar] [CrossRef]
- Xu, T. Ion exchange membranes: State of their development and perspective. J. Membr. Sci. 2005, 263, 1–29. [Google Scholar] [CrossRef]
- Afsar, N.U.; Erigene, B.; Irfan, M.; Wu, B.; Xu, T.; Ji, W.; Emmanuel, K.; Ge, L.; Xu, T. High performance anion exchange membrane with proton transport pathways for diffusion dialysis. Sep. Purif. Technol. 2018, 193, 11–20. [Google Scholar] [CrossRef]
- Lin, J.; Huang, J.; Wang, J.; Yu, J.; You, X.; Lin, X.; Van der Bruggen, B.; Zhao, S. High-performance porous anion exchange membranes for efficient acid recovery from acidic wastewater by diffusion dialysis. J. Membr. Sci. 2021, 624, 119116. [Google Scholar] [CrossRef]
- Merkel, A.; Čopák, L.; Dvořák, L.; Golubenko, D.; Šeda, L. Recovery of spent sulphuric acid by diffusion dialysis using a spiral wound module. Int. J. Mol. Sci. 2021, 22, 11819. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Li, X.; Deng, Z.; Fan, G.; Li, M.; Li, C. Recovery of H2SO4 from an acid leach solution by diffusion dialysis. J. Hazard. Mater. 2010, 176, 226–230. [Google Scholar] [CrossRef]
- Emmanuel, K.; Cheng, C.; Mondal, A.N.; Erigene, B.; Hossain, M.M.; Afsar, N.U.; Khan, M.I.; Wu, L.; Xu, T. Covalently cross-linked pyridinium based aems with aromatic pendant groups for acid recovery via diffusion dialysis. Sep. Purif. Technol. 2016, 164, 125–131. [Google Scholar] [CrossRef]
- Sharma, J.; Misra, S.K.; Kulshrestha, V. Internally cross-linked poly(2,6-dimethyl-1,4-phenylene ether) based anion exchange membrane for recovery of different acids by diffusion dialysis. Chem. Eng. J. 2021, 414, 128776. [Google Scholar] [CrossRef]
- Yadav, V.; Raj, S.K.; Rathod, N.H.; Kulshrestha, V. Polysulfone/graphene quantum dots composite anion exchange membrane for acid recovery by diffusion dialysis. J. Membr. Sci. 2020, 611, 118331. [Google Scholar] [CrossRef]
- Irfan, M.; Afsar, N.U.; Wang, Y.; Xu, T. Investigation of key process parameters in acid recovery for diffusion dialysis using novel (MDMH-QPPO) anion exchange membranes. J. Taiwan Inst. Chem. Eng. 2018, 93, 405–413. [Google Scholar] [CrossRef]
- Irfan, M.; Bakangura, E.; Afsar, N.U.; Xu, T. Augmenting acid recovery from different systems by novel q-dan anion exchange membranes via diffusion dialysis. Sep. Purif. Technol. 2018, 201, 336–345. [Google Scholar] [CrossRef]
- Mondal, A.N.; Cheng, C.; Khan, M.I.; Hossain, M.M.; Emmanuel, K.; Ge, L.; Wu, B.; He, Y.; Ran, J.; Ge, X.; et al. Improved acid recovery performance by novel poly(dmaem-co-γ-mps) anion exchange membrane via diffusion dialysis. J. Membr. Sci. 2017, 525, 163–174. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, W.; Wang, Y. Diffusion dialysis for acid recovery from acidic waste solutions: Anion exchange membranes and technology integration. Membranes 2020, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Wu, C.; Wu, Y.; Zhang, C. An optimized process for treating sodium acetate waste residue: Coupling of diffusion dialysis or electrodialysis with bipolar membrane electrodialysis. Chem. Eng. Res. Des. 2018, 129, 237–247. [Google Scholar] [CrossRef]
- Ruiz-Aguirre, A.; Lopez, J.; Gueccia, R.; Randazzo, S.; Cipollina, A.; Cortina, J.L.; Micale, G. Diffusion dialysis for the treatment of H2SO4-CuSO4 solutions from electroplating plants: Ions membrane transport characterization and modelling. Sep. Purif. Technol. 2021, 266, 118215. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, F.; Li, Z.; Liao, J.; Huang, Y.; Lei, Y.; Li, N. Mixed-charge poly(2,6-dimethyl-phenylene oxide) anion exchange membrane for diffusion dialysis in acid recovery. J. Membr. Sci. 2018, 549, 543–549. [Google Scholar] [CrossRef]
- Lin, X.; Shamsaei, E.; Kong, B.; Liu, J.Z.; Hu, Y.; Xu, T.; Wang, H. Porous diffusion dialysis membranes for rapid acid recovery. J. Membr. Sci. 2016, 502, 76–83. [Google Scholar] [CrossRef]
- Stachera, D.M.; Childs, R.F. Tuning the acid recovery performance of poly(4-vinylpyridine)-filled membranes by the introduction of hydrophobic groups. J. Membr. Sci. 2001, 187, 213–225. [Google Scholar] [CrossRef]
- Pandey, A.K.; Childs, R.F.; West, M.; Lott, J.N.; McCarry, B.E.; Dickson, J.M. Formation of pore-filled ion-exchange membranes with in situ crosslinking: Poly (vinylbenzyl ammonium salt)-filled membranes. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 807–820. [Google Scholar] [CrossRef]
- Stachera, D.M.; Childs, R.F.; Mika, A.M.; Dickson, J.M. Acid recovery using diffusion dialysis with poly(4-vinylpyridine)-filled microporous membranes. J. Membr. Sci. 1998, 148, 119–127. [Google Scholar] [CrossRef]
- Kim, D.-H.; Park, J.-H.; Seo, S.-J.; Park, J.-S.; Jung, S.; Kang, Y.S.; Choi, J.-H.; Kang, M.-S. Development of thin anion-exchange pore-filled membranes for high diffusion dialysis performance. J. Membr. Sci. 2013, 447, 80–86. [Google Scholar] [CrossRef]
- Khan, M.I.; Mondal, A.N.; Cheng, C.; Pan, C.; Emmanuel, K.; Wu, L.; Xu, T. Porous BPPO-based membranes modified by aromatic amine for acid recovery. Sep. Purif. Technol. 2016, 157, 27–34. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, J.; Wu, C.; Xu, T.; Fu, Y. Bionic multisilicon copolymers used as novel cross-linking agents for preparing anion exchange hybrid membranes. J. Phys. Chem. B 2011, 115, 6474–6483. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wu, Y.; Luo, J.; Xu, T.; Fu, Y. Anion exchange hybrid membranes from pva and multi-alkoxy silicon copolymer tailored for diffusion dialysis process. J. Membr. Sci. 2010, 356, 96–104. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, C.; Li, Y.; Xu, T.; Fu, Y. PVA–silica anion-exchange hybrid membranes prepared through a copolymer crosslinking agent. J. Membr. Sci. 2010, 350, 322–332. [Google Scholar] [CrossRef]
- Klaysom, C.; Moon, S.-H.; Ladewig, B.P.; Lu, G.Q.M.; Wang, L. Preparation of porous ion-exchange membranes (iems) and their characterizations. J. Membr. Sci. 2011, 371, 37–44. [Google Scholar] [CrossRef]
- Klaysom, C.; Marschall, R.; Moon, S.-H.; Ladewig, B.P.; Lu, G.Q.M.; Wang, L. Preparation of porous composite ion-exchange membranes for desalination application. J. Mater. Chem. 2011, 21, 7401–7409. [Google Scholar] [CrossRef]
- Sun, F.; Wu, C.; Wu, Y.; Xu, T. Porous BPPO-based membranes modified by multisilicon copolymer for application in diffusion dialysis. J. Membr. Sci. 2014, 450, 103–110. [Google Scholar] [CrossRef]
- Khan, M.I.; Wu, L.; Hossain, M.M.; Pan, J.; Ran, J.; Mondal, A.N.; Xu, T. Preparation of diffusion dialysis membrane for acid recovery via a phase-inversion method. Membr. Water Treat. 2015, 6, 365–378. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, L.; Varcoe, J.; Li, C.; Ong, A.L.; Poynton, S.; Xu, T. Aromatic polyelectrolytes via polyacylation of pre-quaternized monomers for alkaline fuel cells. J. Mater. Chem. A 2013, 1, 2595–2601. [Google Scholar] [CrossRef] [Green Version]
- Yue, X.; Wu, W.; Chen, G.; Yang, C.; Liao, S.; Li, X. Influence of 2,2′,6,6′-tetramethyl biphenol-based anion-exchange membranes on the diffusion dialysis of hydrochloride acid. J. Appl. Polym. Sci. 2017, 134, 45333. [Google Scholar] [CrossRef]
- Khan, M.I.; Li, X.; Fernandez-Garcia, J.; Lashari, M.H.; Rehman, A.; Elboughdiri, N.; Kolsi, L.; Ghernaout, D. Effect of different quaternary ammonium groups on the hydroxide conductivity and stability of anion exchange membranes. ACS Omega 2021, 6, 7994–8001. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Mondal, A.N.; Tong, B.; Jiang, C.; Emmanuel, K.; Yang, Z.; Wu, L.; Xu, T. Development of BPPO-based anion exchange membranes for electrodialysis desalination applications. Desalination 2016, 391, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.; Su, J.; Guo, L. Preparation and characterization of high-performance anion exchange membranes for acid recovery. Desal. Water Treat. 2021, 209, 144–154. [Google Scholar] [CrossRef]
- Khan, M.I.; Zheng, C.; Mondal, A.N.; Hossain, M.M.; Wu, B.; Emmanuel, K.; Wu, L.; Xu, T. Preparation of anion exchange membranes from bppo and dimethylethanolamine for electrodialysis. Desalination 2017, 402, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.; Luque, R.; Prinsen, P.; Rehman, A.; Anjum, S.; Nawaz, M.; Shaheen, A.; Zafar, S.; Mustaqeem, M. BPPO-based anion exchange membranes for acid recovery via diffusion dialysis. Materials 2017, 10, 266. [Google Scholar] [CrossRef]
- Khan, M.I.; Su, J.; Lichtfouse, E.; Guo, L. Higher efficiency of triethanolamine-grafted anion exchange membranes for acidic wastewater treatment. Desal. Water Treat. 2020, 197, 41–51. [Google Scholar] [CrossRef]
- Khan, M.I.; Mondal, A.N.; Emmanuel, K.; Hossain, M.M.; Afsar, N.U.; Wu, L.; Xu, T. Preparation of pyrrolidinium-based anion-exchange membranes for acid recovery via diffusion dialysis. Sep. Sci. Technol. 2016, 51, 1881–1890. [Google Scholar] [CrossRef]
- Khan, M.I.; Khraisheh, M.; Almomani, F. Fabrication and characterization of pyridinium functionalized anion exchange membranes for acid recovery. Sci. Total Environ. 2019, 686, 90–96. [Google Scholar] [CrossRef]
- Yang, W.; Wu, C.; Gong, M.; Xu, T. New anion exchanger organic–inorganic hybrids and membranes from a copolymer of glycidylmethacrylate and -methacryloxypropyl trimethoxy silane. J. Appl. Polym. Sci. 2006, 102, 3580–3589. [Google Scholar]
- Arges, C.G.; Wang, L.; Jung, M.-S.; Ramani, V. Mechanically stable poly(arylene ether) anion exchange membranes prepared from commercially available polymers for alkaline electrochemical devices. J. Electrochem. Soc. 2015, 162, F686–F693. [Google Scholar] [CrossRef] [Green Version]
- Tang, B.; Xu, T.; Gong, M.; Yang, W. A novel positively charged asymmetry membranes from poly(2,6-dimethyl-1,4-phenylene oxide) by benzyl bromination and in situ amination: Membrane preparation and characterization. J. Membr. Sci. 2005, 248, 119–125. [Google Scholar] [CrossRef]
- Spry, D.B.; Goun, A.; Glusac, K.; Moilanen, D.E.; Fayer, M.D. Proton transport and the water environment in nafion fuel cell membranes and aot reverse micelles. J. Am. Chem. Soc. 2007, 129, 8122–8130. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, C.; Wu, Y.; Gu, J.; Xu, T. Polyelectrolyte complex/PVA membranes for diffusion dialysis. J. Hazard. Mater. 2013, 261, 114–122. [Google Scholar] [CrossRef]
- Chakrabarty, T.; Singh, A.K.; Shahi, V.K. Zwitterionic silica copolymer based crosslinked organic-inorganic hybrid polymer electrolyte membranes for fuel cell applications. RSC Adv. 2012, 2, 1949–1961. [Google Scholar] [CrossRef]
- Mangiagli, P.; Ewing, C.; Xu, K.; Wang, Q.; Hickner, M. Dynamic water uptake of flexible ion-containing polymer networks. Fuel Cells 2009, 9, 432–438. [Google Scholar] [CrossRef]
- Naik, N.S.; Padaki, M.; Déon, S.; Murthy, D.H. Novel poly(ionic liquid)-based anion exchange membranes for efficient and rapid acid recovery from industrial waste. Chem. Eng. J. 2020, 401, 126148. [Google Scholar] [CrossRef]
- Mondal, R.; Pal, S.; Chatterjee, U. Alkylated imidazole moieties in a cross-linked anion exchange membrane facilitate acid recovery with high purity. ACS Appl. Polym. Mater. 2021, 3, 1544–1554. [Google Scholar] [CrossRef]
- Palatý, Z.; Bendová, H. Separation of HCl+FeCl2 mixture by anion-exchange membrane. Sep. Purif. Technol. 2009, 66, 45–50. [Google Scholar] [CrossRef]
- Cheng, C.; Yang, Z.; Pan, J.; Tong, B.; Xu, T. Facile and cost effective pva based hybrid membrane fabrication for acid recovery. Sep. Purif. Technol. 2014, 136, 250–257. [Google Scholar] [CrossRef]


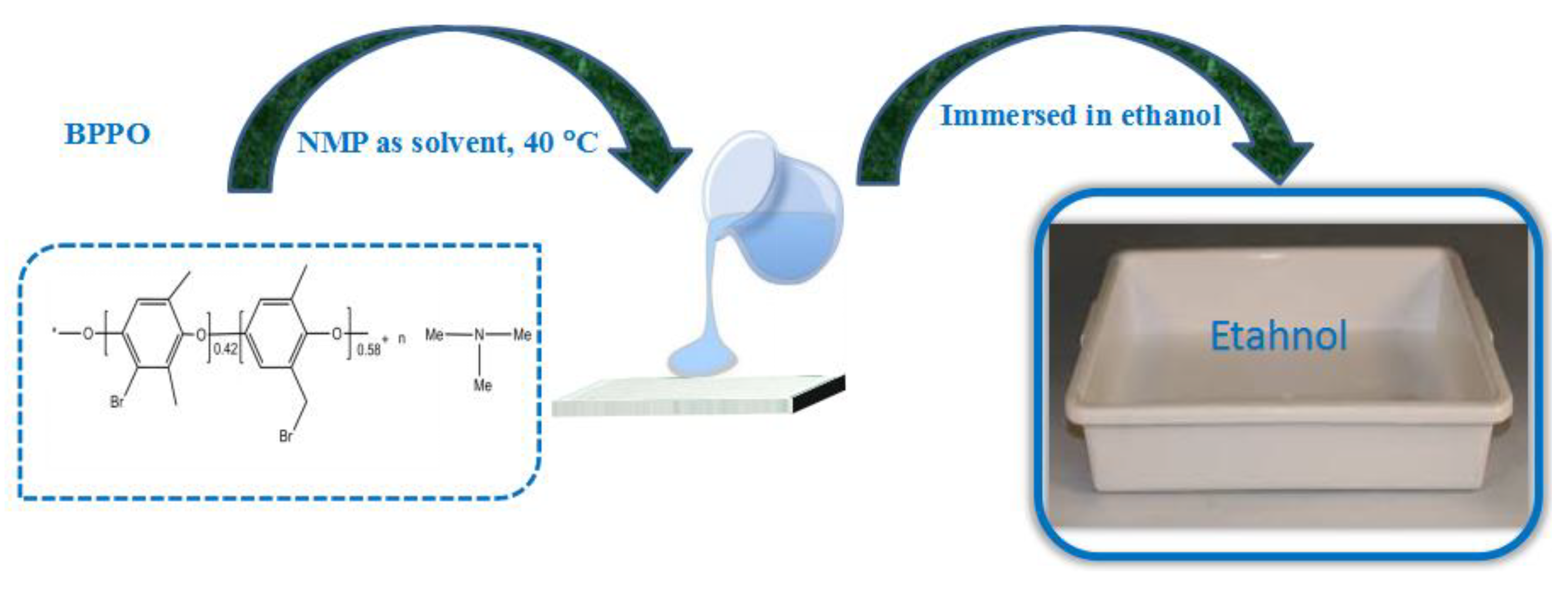

| Sr. No | BPPO (g) | TMA (g) | IEC (mmol/g) | WR (%) | LER (%) | C × 10−3 (mol/L) |
|---|---|---|---|---|---|---|
| M1 | 3 | 0.15 | 0.71 | 149.60 | 3.88 | 4.74 |
| M2 | 3 | 0.20 | 0.98 | 170.45 | 5.0 | 5.75 |
| M3 | 3 | 0.25 | 1.20 | 196.42 | 5.98 | 6.10 |
| M4 | 3 | 0.30 | 1.43 | 233.80 | 9.23 | 6.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.I.; Shanableh, A.; Khraisheh, M.; AlMomani, F. Synthesis of Porous BPPO-Based Anion Exchange Membranes for Acid Recovery via Diffusion Dialysis. Membranes 2022, 12, 95. https://doi.org/10.3390/membranes12010095
Khan MI, Shanableh A, Khraisheh M, AlMomani F. Synthesis of Porous BPPO-Based Anion Exchange Membranes for Acid Recovery via Diffusion Dialysis. Membranes. 2022; 12(1):95. https://doi.org/10.3390/membranes12010095
Chicago/Turabian StyleKhan, Muhammad Imran, Abdallah Shanableh, Majeda Khraisheh, and Fares AlMomani. 2022. "Synthesis of Porous BPPO-Based Anion Exchange Membranes for Acid Recovery via Diffusion Dialysis" Membranes 12, no. 1: 95. https://doi.org/10.3390/membranes12010095
APA StyleKhan, M. I., Shanableh, A., Khraisheh, M., & AlMomani, F. (2022). Synthesis of Porous BPPO-Based Anion Exchange Membranes for Acid Recovery via Diffusion Dialysis. Membranes, 12(1), 95. https://doi.org/10.3390/membranes12010095