Tailoring the Stabilization and Pyrolysis Processes of Carbon Molecular Sieve Membrane Derived from Polyacrylonitrile for Ethylene/Ethane Separation
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of PAN-Based CMS Membranes
2.3. Ethylene/Ethane Mixed Gas Permeation Test
2.4. Characterizations
3. Results and Discussion
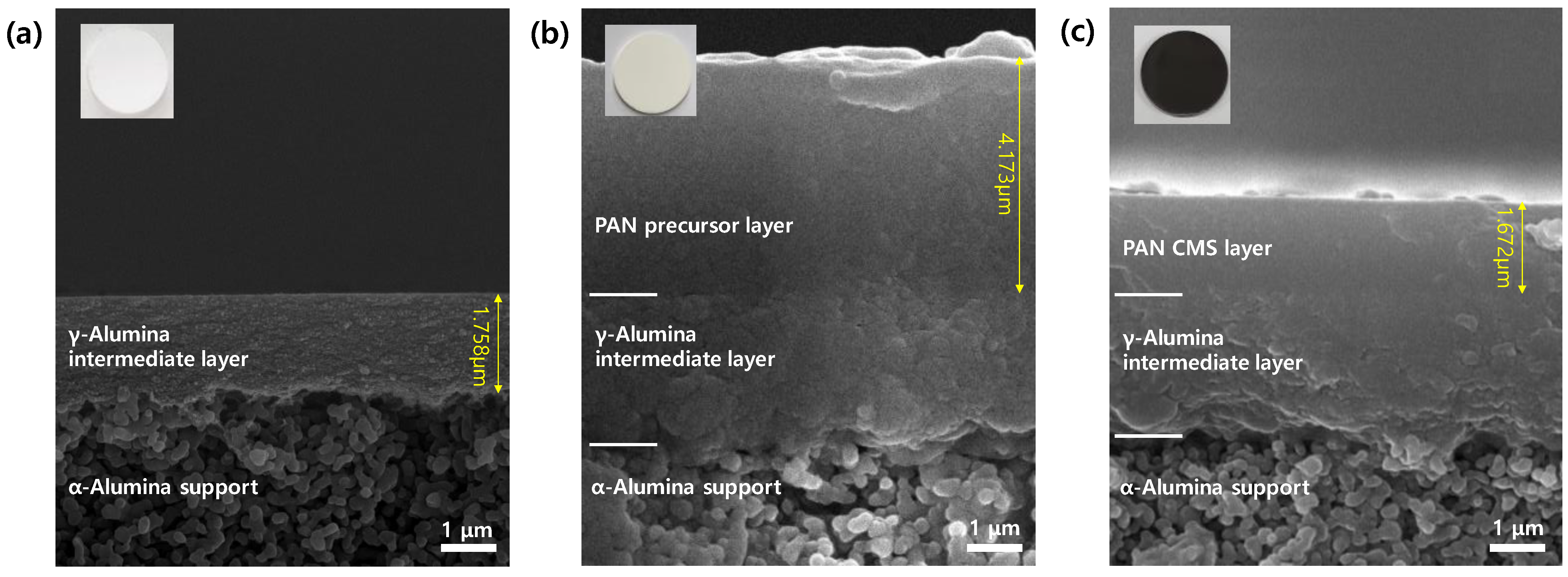
3.1. Structure of CMS Composite Membranes
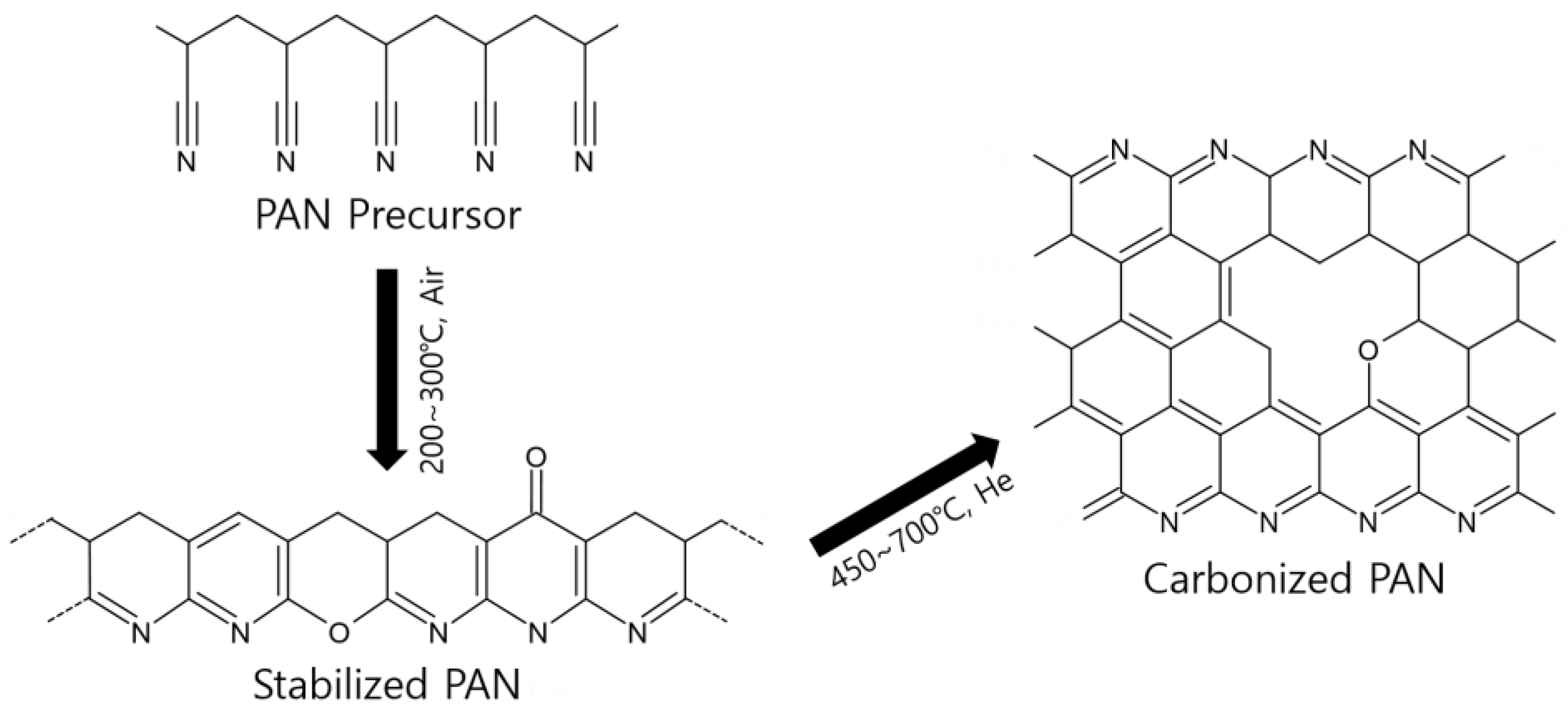
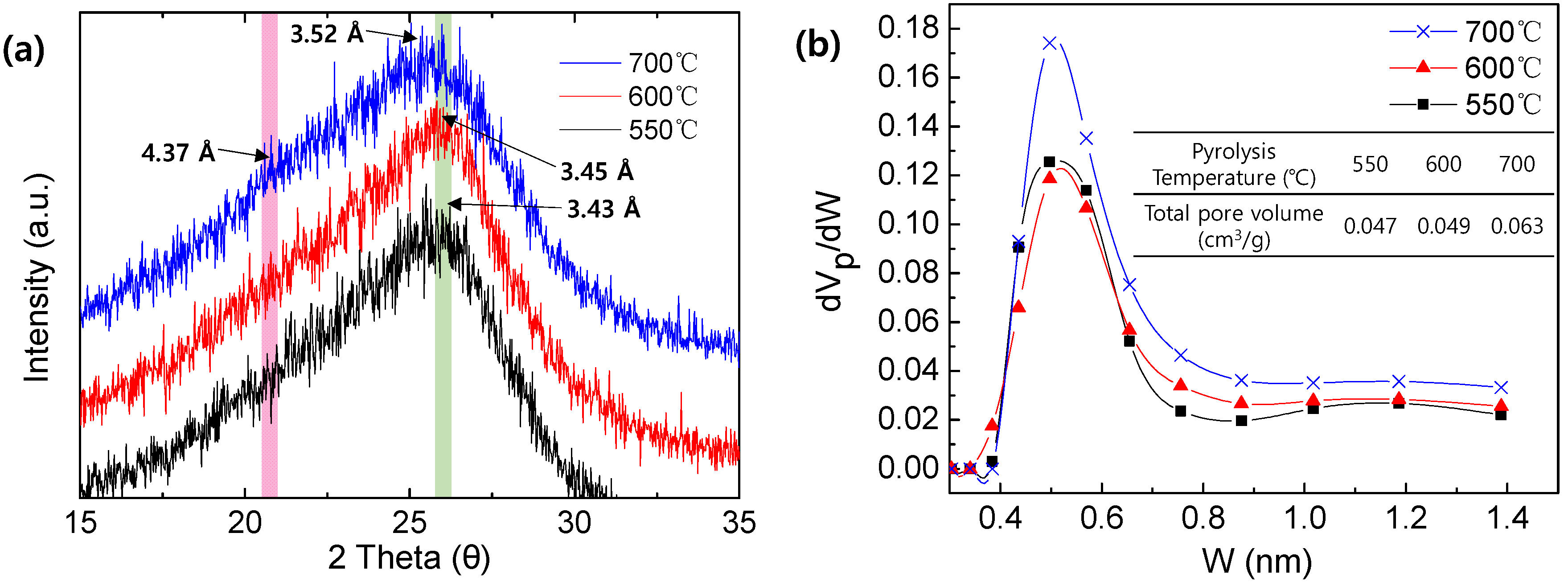
3.2. Characterization of the Pristine, Stabilized, and Carbonized PAN
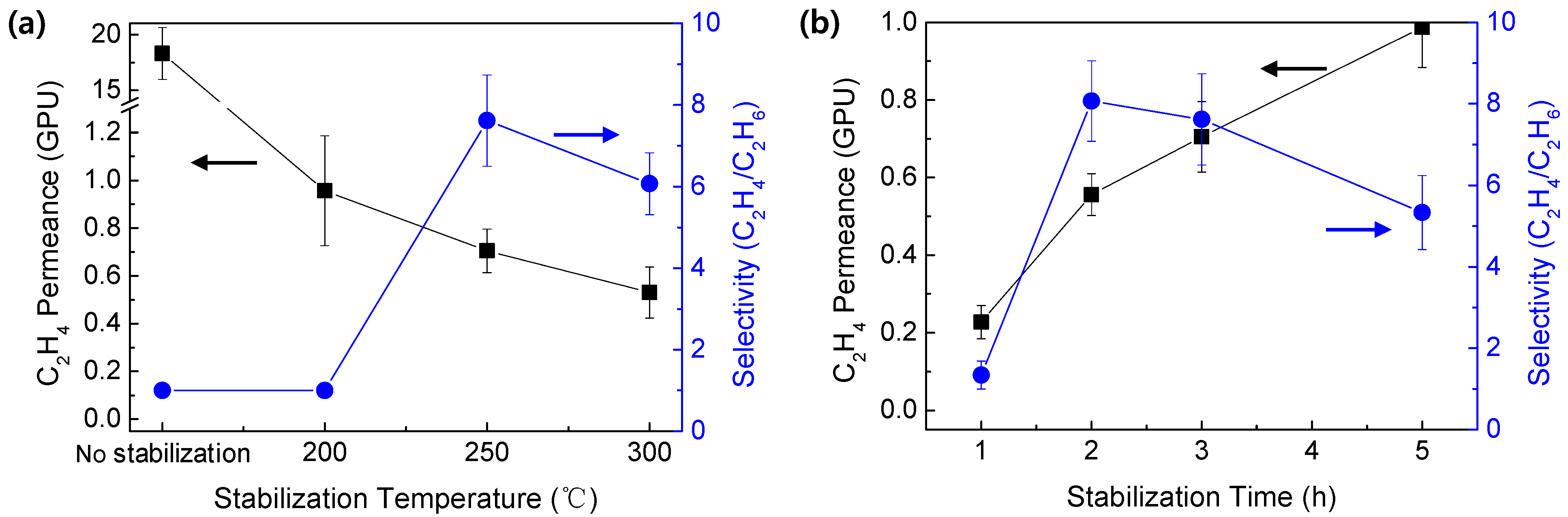
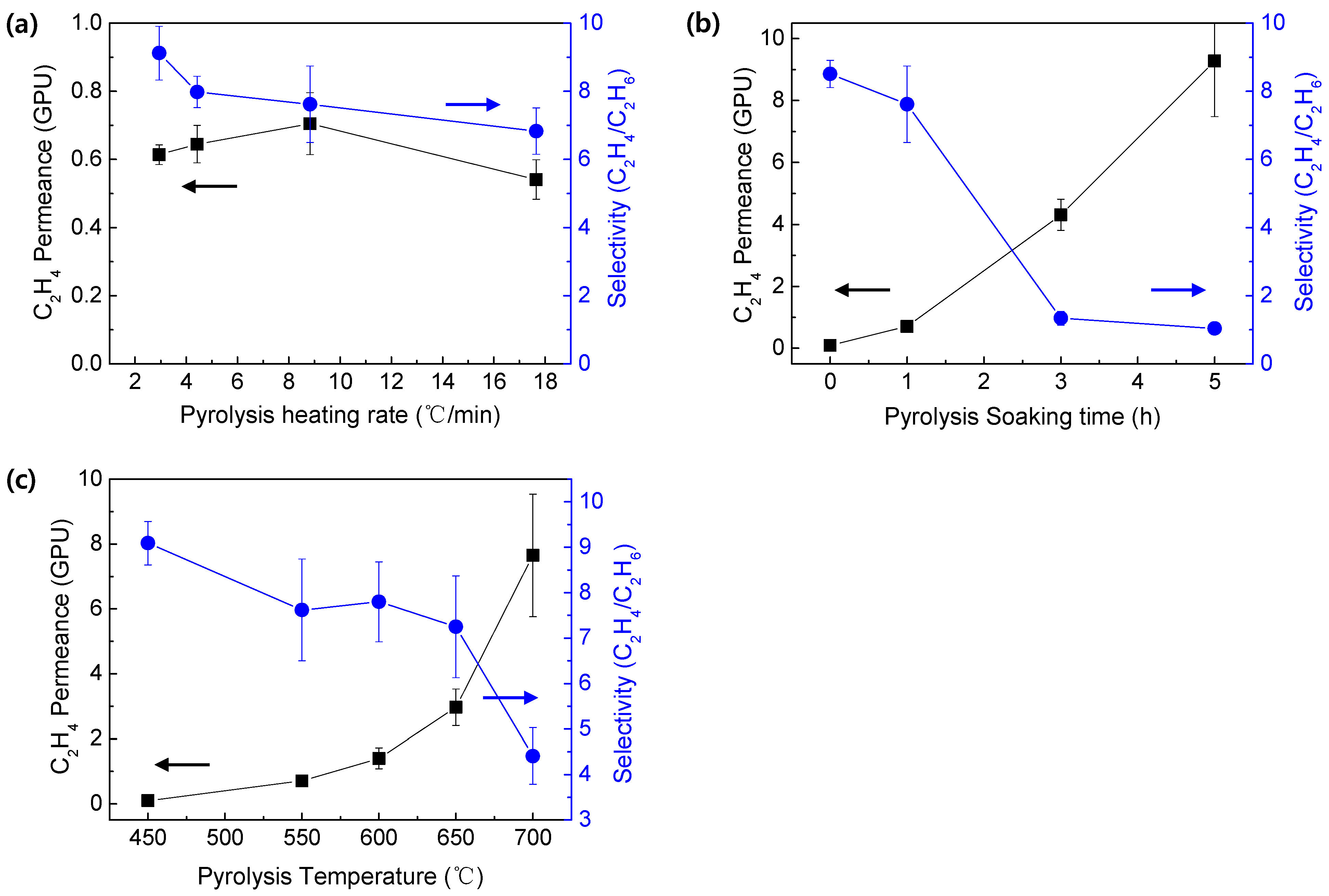
3.3. Ethylene/Ethane Mixed Gas Separation Performance of CMS Membranes
3.4. Comparison of Ethylene/Ethane Separation Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Amedi, H.R.; Aghajani, M. Economic Estimation of Various Membranes and Distillation for Propylene and Propane Separation. Ind. Eng. Chem. Res. 2018, 57, 4366–4376. [Google Scholar] [CrossRef]
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef]
- Kim, S.-J.; Kwon, Y.; Kim, D.; Park, H.; Cho, Y.H.; Nam, S.-E.; Park, Y.-I. A Review on Polymer Precursors of Carbon Molecular Sieve Membranes for Olefin/Paraffin Separation. Membranes 2021, 11, 482. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Liu, P.; Jiang, M.; Yu, L.; Li, L.; Tang, Z. Olefin/paraffin separation through membranes: From mechanisms to critical materials. J. Mater. Chem. A 2019, 7, 23489–23511. [Google Scholar] [CrossRef]
- Faiz, R.; Fallanza, M.; Ortiz, I.; Li, K. Separation of Olefin/Paraffin Gas Mixtures Using Ceramic Hollow Fiber Membrane Contactors. Ind. Eng. Chem. Res. 2013, 52, 7918–7929. [Google Scholar] [CrossRef]
- Kang, S.W.; Char, K.; Kang, Y.S. Novel Application of Partially Positively Charged Silver Nanoparticles for Facilitated Transport in Olefin/Paraffin Separation Membranes. Chem. Mater. 2008, 20, 1308–1311. [Google Scholar] [CrossRef]
- Roy, A.; Venna, S.R.; Rogers, G.; Tang, L.; Fitzgibbons, T.C.; Liu, J.; McCurry, H.; Vickery, D.J.; Flick, D.; Fish, B. Membranes for olefin–paraffin separation: An industrial perspective. Proc. Natl. Acad. Sci. USA 2021, 118, e2022194118. [Google Scholar] [CrossRef]
- Rungta, M.; Xu, L.; Koros, W.J. Carbon molecular sieve dense film membranes derived from Matrimid® for ethylene/ethane separation. Carbon 2012, 50, 1488–1502. [Google Scholar] [CrossRef]
- Okamoto, K.-I.; Kawamura, S.; Yoshino, M.; Kita, H.; Hirayama, Y.; Tanihara, N.; Kusuki, Y. Olefin/Paraffin Separation through Carbonized Membranes Derived from an Asymmetric Polyimide Hollow Fiber Membrane. Ind. Eng. Chem. Res. 1999, 38, 4424–4432. [Google Scholar] [CrossRef]
- Lagorsse, S.; Magalhaes, F.D.; Mendes, A. Carbon molecular sieve membranes: Sorption, kinetic and structural characterization. J. Membr. Sci. 2004, 241, 275–287. [Google Scholar] [CrossRef]
- Rodrigues, S.C.; Whitley, R.; Mendes, A. Preparation and characterization of carbon molecular sieve membranes based on resorcinol–formaldehyde resin. J. Membr. Sci. 2014, 459, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.-J.; Liao, K.-S.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Development and characterization of micropores in carbon molecular sieve membrane for gas separation. Microporous Mesoporous Mater. 2011, 143, 78–86. [Google Scholar] [CrossRef]
- Fu, S.; Wenz, G.B.; Sanders, E.S.; Kulkarni, S.S.; Qiu, W.; Ma, C.; Koros, W.J. Effects of pyrolysis conditions on gas separation properties of 6FDA/DETDA:DABA(3:2) derived carbon molecular sieve membranes. J. Membr. Sci. 2016, 520, 699–711. [Google Scholar] [CrossRef] [Green Version]
- Tanco, M.A.L.; Tanaka, D.A.P. Recent Advances on Carbon Molecular Sieve Membranes (CMSMs) and Reactors. Processes 2016, 4, 29. [Google Scholar] [CrossRef] [Green Version]
- David, L.I.B.; Ismail, A.F. Influence of the thermastabilization process and soak time during pyrolysis process on the polyacrylonitrile carbon membranes for O2/N2 separation. J. Membr. Sci. 2003, 213, 285–291. [Google Scholar] [CrossRef]
- Saufi, S.M.; Ismail, A.F. Development and characterization of polyacrylonitrile (PAN) based carbon hollow fiber membrane. Songklanakarin J. Sci. Technol. 2002, 24, 843–854. [Google Scholar]
- Lafyatis, D.S.; Tung, J.; Foley, H.C. Poly(furfuryl alcohol)-derived carbon molecular sieves: Dependence of adsorptive properties on carbonization temperature, time, and poly(ethylene glycol) additives. Ind. Eng. Chem. Res. 1991, 30, 865–873. [Google Scholar] [CrossRef]
- Song, C.; Wang, T.; Jiang, H.; Wang, X.; Cao, Y.; Qiu, J. Gas separation performance of C/CMS membranes derived from poly(furfuryl alcohol) (PFA) with different chemical structure. J. Membr. Sci. 2010, 361, 22–27. [Google Scholar] [CrossRef]
- Geiszler, V.C.; Koros, W.J. Effects of polyimide pyrolysis conditions on carbon molecular sieve membrane properties. Ind. Eng. Chem. Res. 1996, 35, 2999–3003. [Google Scholar] [CrossRef]
- Vu, D.Q.; Koros, W.J.; Miller, S.J. Mixed matrix membranes using carbon molecular sieves: I. Preparation and experimental results. J. Membr. Sci. 2003, 211, 311–334. [Google Scholar] [CrossRef]
- Centeno, T.A.; Fuertes, A.B. Supported carbon molecular sieve membranes based on a phenolic resin. J. Membr. Sci. 1999, 160, 201–211. [Google Scholar] [CrossRef]
- Teixeira, M.; Campo, M.C.; Tanaka, D.A.P.; Tanco, M.A.L.; Magen, C.; Mendes, A. Composite phenolic resin-based carbon molecular sieve membranes for gas separation. Carbon 2011, 49, 4348–4358. [Google Scholar] [CrossRef]
- Yoshimune, M.; Fujiwara, I.; Haraya, K. Carbon molecular sieve membranes derived from trimethylsilyl substituted poly(phenylene oxide) for gas separation. Carbon 2007, 45, 553–560. [Google Scholar] [CrossRef]
- Yoshimune, M.; Fujiwara, I.; Suda, H.; Haraya, K. Novel Carbon Molecular Sieve Membranes Derived from Poly(phenylene oxide) and Its Derivatives for Gas Separation. Chem. Lett. 2005, 34, 958–959. [Google Scholar] [CrossRef]
- Araújo, T.; Bernardo, G.; Mendes, A. Cellulose-Based Carbon Molecular Sieve Membranes for Gas Separation: A Review. Molecules 2020, 25, 3532. [Google Scholar] [CrossRef]
- Song, Q.; Cao, S.; Pritchard, R.H.; Ghalei, B.; Al-Muhtaseb, S.A.; Terentjev, E.M.; Cheetham, A.K.; Sivaniah, E. Controlled thermal oxidative crosslinking of polymers of intrinsic microporosity towards tunable molecular sieve membranes. Nat. Commun. 2014, 5, 4813. [Google Scholar] [CrossRef] [Green Version]
- Swaidan, R.; Ma, X.; Litwiller, E.; Pinnau, I. High pressure pure-and mixed-gas separation of CO2/CH4 by thermally-rearranged and carbon molecular sieve membranes derived from a polyimide of intrinsic microporosity. J. Membr. Sci. 2013, 447, 387–394. [Google Scholar] [CrossRef]
- Rungta, M.; Xu, L.; Koros, W.J. Structure–performance characterization for carbon molecular sieve membranes using molecular scale gas probes. Carbon 2015, 85, 429–442. [Google Scholar] [CrossRef]
- Rungta, M.; Wenz, G.B.; Zhang, C.; Xu, L.; Qiu, W.; Adams, J.S.; Koros, W.J. Carbon molecular sieve structure development and membrane performance relationships. Carbon 2017, 115, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Chu, Y.-H.; Yancey, D.; Xu, L.; Martinez, M.; Brayden, M.; Koros, W. Iron-containing carbon molecular sieve membranes for advanced olefin/paraffin separations. J. Membr. Sci. 2018, 548, 609–620. [Google Scholar] [CrossRef]
- Xu, L.; Rungta, M.; Hessler, J.; Qiu, W.; Brayden, M.; Martinez, M.; Barbay, G.; Koros, W.J. Physical aging in carbon molecular sieve membranes. Carbon 2014, 80, 155–166. [Google Scholar] [CrossRef]
- Xu, L.; Rungta, M.; Brayden, M.K.; Martinez, M.V.; Stears, B.A.; Barbay, G.A.; Koros, W.J. Olefins-selective asymmetric carbon molecular sieve hollow fiber membranes for hybrid membrane-distillation processes for olefin/paraffin separations. J. Membr. Sci. 2012, 423–424, 314–323. [Google Scholar] [CrossRef]
- Salinas, O.; Ma, X.; Litwiller, E.; Pinnau, I. Ethylene/ethane permeation, diffusion and gas sorption properties of carbon molecular sieve membranes derived from the prototype ladder polymer of intrinsic microporosity (PIM-1). J. Membr. Sci. 2016, 504, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Salinas, O.; Ma, X.; Litwiller, E.; Pinnau, I. High-performance carbon molecular sieve membranes for ethylene/ethane separation derived from an intrinsically microporous polyimide. J. Membr. Sci. 2016, 500, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Liao, K.-S.; Japip, S.; Lai, J.-Y.; Chung, T.-S. Boron-embedded hydrolyzed PIM-1 carbon membranes for synergistic ethylene/ethane purification. J. Membr. Sci. 2017, 534, 92–99. [Google Scholar] [CrossRef]
- Salleh, W.N.W.; Ismail, A.F.; Matsuura, T.; Abdullah, M.S. Precursor Selection and Process Conditions in the Preparation of Carbon Membrane for Gas Separation: A Review. Sep. Purif. Rev. 2011, 40, 261–311. [Google Scholar] [CrossRef]
- Saufi, S.M.; Ismail, A.F. Fabrication of carbon membranes for gas separation––A review. Carbon 2004, 42, 241–259. [Google Scholar] [CrossRef]
- Jones, C.W.; Koros, W.J. Carbon molecular sieve gas separation membranes-I. Preparation and characterization based on polyimide precursors. Carbon 1994, 32, 1419–1425. [Google Scholar] [CrossRef]
- Chang, C.-H.; Gopalan, R.; Lin, Y. A comparative study on thermal and hydrothermal stability of alumina, titania and zirconia membranes. J. Membr. Sci. 1994, 91, 27–45. [Google Scholar] [CrossRef]
- Khayyam, H.; Jazar, R.N.; Nunna, S.; Golkarnarenji, G.; Badii, K.; Fakhrhoseini, S.M.; Kumar, S.; Naebe, M. PAN precursor fabrication, applications and thermal stabilization process in carbon fiber production: Experimental and mathematical modelling. Prog. Mater. Sci. 2020, 107, 100575. [Google Scholar] [CrossRef]
- Devasia, R.; Nair, C.P.R.; Sadhana, R.; Babu, N.S.; Ninan, K.N. Fourier transform infrared and wide-angle X-ray diffraction studies of the thermal cyclization reactions of high-molar-mass poly(acrylonitrile-co-itaconic acid). J. Appl. Polym. Sci. 2006, 100, 3055–3062. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, J.; Zhou, T.; Liu, X.; Yuan, Q.; Zhang, A. New understanding on the reaction pathways of the polyacrylonitrile copolymer fiber pre-oxidation: Online tracking by two-dimensional correlation FTIR spectroscopy. RSC Adv. 2016, 6, 4397–4409. [Google Scholar] [CrossRef]
- Lee, S.H.; Jeong, Y.G.; Yoon, Y.I.; Park, W.H. Hydrolysis of oxidized polyacrylonitrile nanofibrous webs and selective adsorption of harmful heavy metal ions. Polym. Degrad. Stab. 2017, 143, 207–213. [Google Scholar] [CrossRef]
- Kaniyoor, A.; Ramaprabhu, S. A Raman spectroscopic investigation of graphite oxide derived graphene. AIP Adv. 2012, 2, 032183. [Google Scholar] [CrossRef] [Green Version]
- Jiao, F.; Zhang, F.; Zang, Y.; Zou, Y.; Di, C.; Xu, W.; Zhu, D. An easily accessible carbon material derived from carbonization of polyacrylonitrile ultrathin films: Ambipolar transport properties and application in a CMOS-like inverter. Chem. Commun. 2014, 50, 2374–2376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.; Wang, R.; Hong, M. Structural Evolution from Metal–Organic Framework to Hybrids of Nitrogen-Doped Porous Carbon and Carbon Nanotubes for Enhanced Oxygen Reduction Activity. Chem. Mater. 2015, 27, 7610–7618. [Google Scholar] [CrossRef]
- Bao, Y.; Tay, Y.S.; Lim, T.-T.; Wang, R.; Webster, R.D.; Hu, X. Polyacrylonitrile (PAN)-induced carbon membrane with in-situ encapsulated cobalt crystal for hybrid peroxymonosulfate oxidation-filtration process: Preparation, characterization and performance evaluation. Chem. Eng. J. 2019, 373, 425–436. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, N.Y.; Shin, D.Y.; Park, H.-Y.; Lee, S.-S.; Kwon, S.J.; Lim, D.-H.; Bong, K.W.; Son, J.G.; Kim, J.Y. Nitrogen-doped graphene-wrapped iron nanofragments for high-performance oxygen reduction electrocatalysts. J. Nanoparticle Res. 2017, 19, 98. [Google Scholar] [CrossRef]
- Centeno, T.A.; Vilas, J.L.; Fuertes, A.B. Effects of phenolic resin pyrolysis conditions on carbon membrane performance for gas separation. J. Membr. Sci. 2004, 228, 45–54. [Google Scholar] [CrossRef]
- Salleh, W.N.W.; Ismail, A.F. Effects of carbonization heating rate on CO2 separation of derived carbon membranes. Sep. Purif. Technol. 2012, 88, 174–183. [Google Scholar] [CrossRef]
- Steel, K.M.; Koros, W.J. An investigation of the effects of pyrolysis parameters on gas separation properties of carbon materials. Carbon 2005, 43, 1843–1856. [Google Scholar] [CrossRef]
- Xu, L.; Rungta, M.; Koros, W.J. Matrimid® derived carbon molecular sieve hollow fiber membranes for ethylene/ethane separation. J. Membr. Sci. 2011, 380, 138–147. [Google Scholar] [CrossRef]
- Kusuki, Y.; Shimazaki, H.; Tanihara, N.; Nakanishi, S.; Yoshinaga, T. Gas permeation properties and characterization of asymmetric carbon membranes prepared by pyrolyzing asymmetric polyimide hollow fiber membrane. J. Membr. Sci. 1997, 134, 245–253. [Google Scholar] [CrossRef]
- Fuertes, A.B. Adsorption-selective carbon membrane for gas separation. J. Membr. Sci. 2000, 177, 9–16. [Google Scholar] [CrossRef]
- Menendez, I.; Fuertes, A.B. Aging of carbon membranes under different environments. Carbon 2001, 39, 733–740. [Google Scholar] [CrossRef]
- Fuertes, A.B. Preparation and Characterization of Adsorption-Selective Carbon Membranes for Gas Separation. Adsorption 2001, 7, 117–129. [Google Scholar] [CrossRef]
- Fuertes, A.B.; Menendez, I. Separation of hydrocarbon gas mixtures using phenolic resin-based carbon membranes. Sep. Purif. Technol. 2002, 28, 29–41. [Google Scholar] [CrossRef]


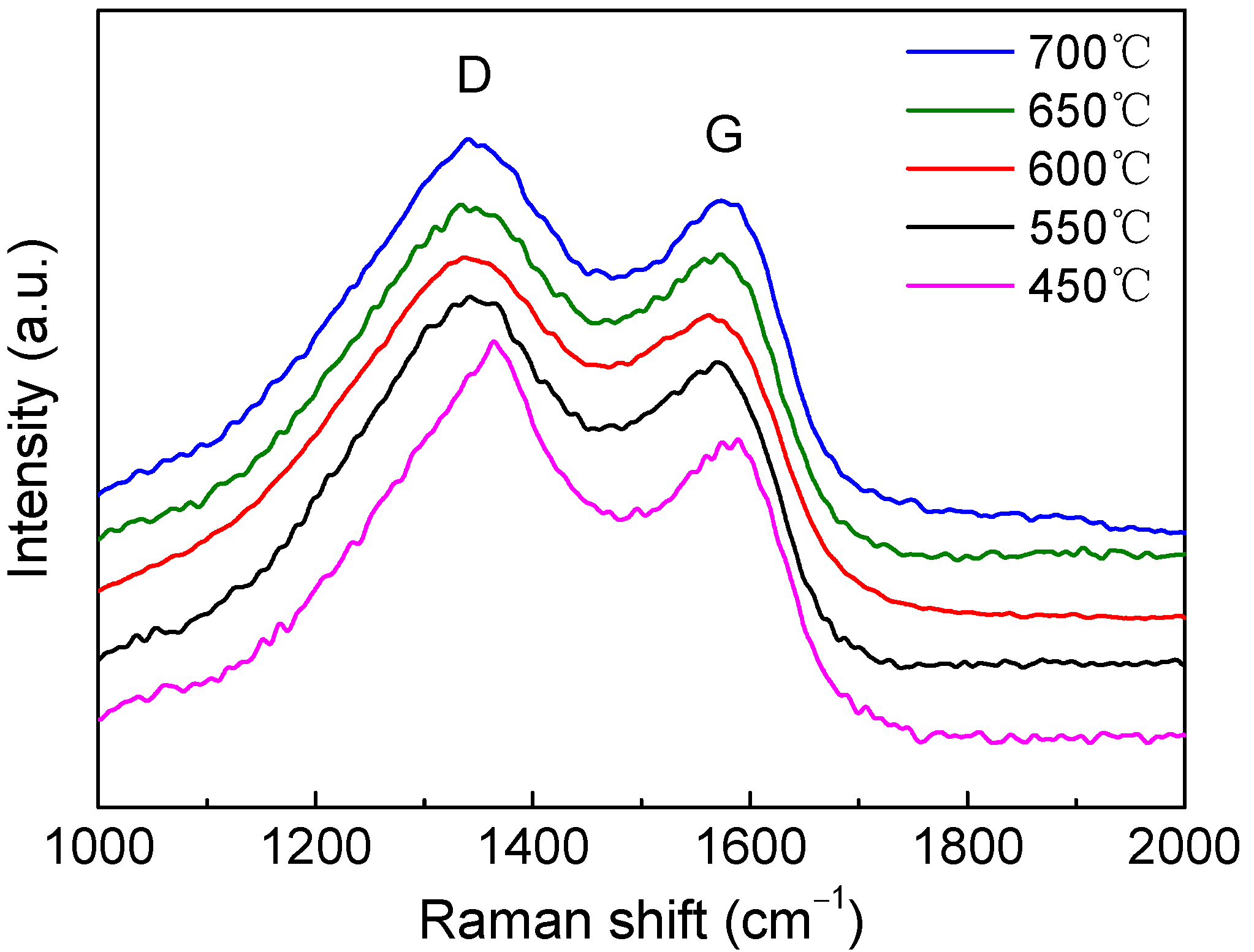
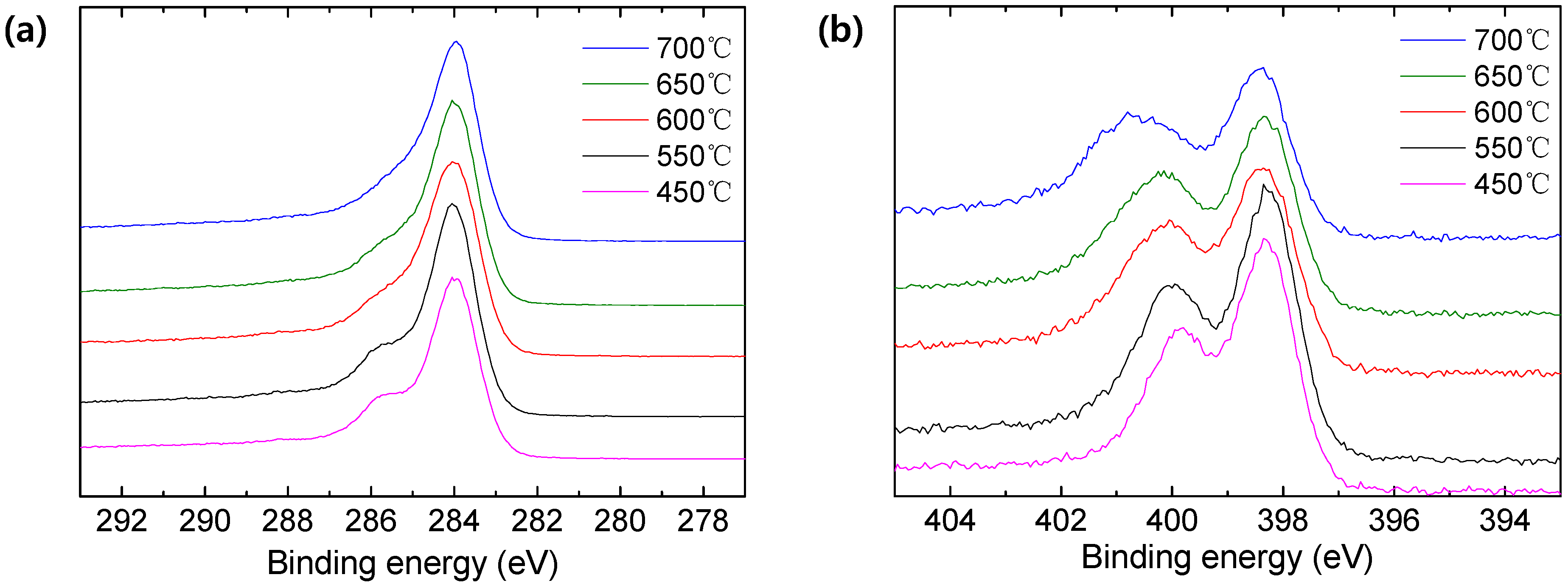
| Pyrolysis Temperature (°C) | 450 | 550 | 600 | 650 | 700 |
|---|---|---|---|---|---|
| ID/IG | 1.32 | 1.22 | 1.19 | 1.16 | 1.10 |
| Pyrolysis Temperature (°C) | Elemental (At. %) | ||
|---|---|---|---|
| C | N | O | |
| 450 | 80.79 | 13.73 | 5.48 |
| 550 | 81.99 | 13.10 | 4.91 |
| 600 | 81.65 | 12.61 | 5.74 |
| 650 | 83.02 | 12.29 | 4.67 |
| 700 | 85.49 | 11.25 | 3.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Kwon, Y.; Lee, J.-H.; Kim, S.-J.; Park, Y.-I. Tailoring the Stabilization and Pyrolysis Processes of Carbon Molecular Sieve Membrane Derived from Polyacrylonitrile for Ethylene/Ethane Separation. Membranes 2022, 12, 93. https://doi.org/10.3390/membranes12010093
Kim D, Kwon Y, Lee J-H, Kim S-J, Park Y-I. Tailoring the Stabilization and Pyrolysis Processes of Carbon Molecular Sieve Membrane Derived from Polyacrylonitrile for Ethylene/Ethane Separation. Membranes. 2022; 12(1):93. https://doi.org/10.3390/membranes12010093
Chicago/Turabian StyleKim, DaeHun, YongSung Kwon, Jung-Hyun Lee, Seong-Joong Kim, and You-In Park. 2022. "Tailoring the Stabilization and Pyrolysis Processes of Carbon Molecular Sieve Membrane Derived from Polyacrylonitrile for Ethylene/Ethane Separation" Membranes 12, no. 1: 93. https://doi.org/10.3390/membranes12010093
APA StyleKim, D., Kwon, Y., Lee, J.-H., Kim, S.-J., & Park, Y.-I. (2022). Tailoring the Stabilization and Pyrolysis Processes of Carbon Molecular Sieve Membrane Derived from Polyacrylonitrile for Ethylene/Ethane Separation. Membranes, 12(1), 93. https://doi.org/10.3390/membranes12010093







