Permeability and Stability of Hydrophobic Tubular Ceramic Membrane Contactor for CO2 Desorption from MEA Solution
Abstract
:1. Introduction
2. Experiment
2.1. Materials
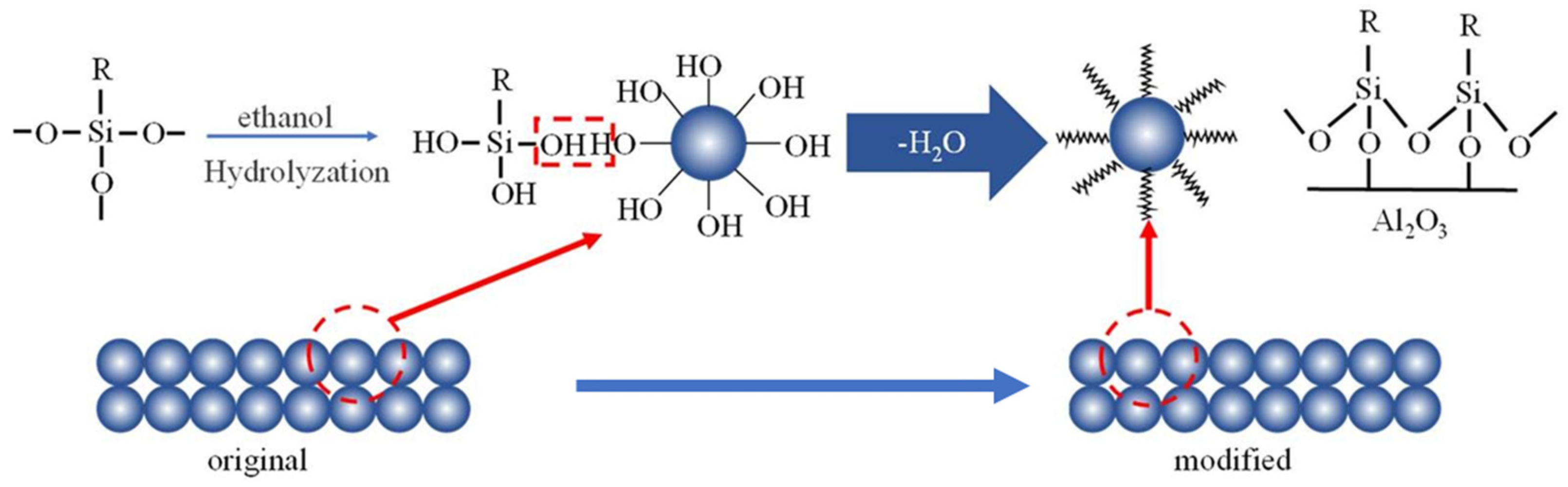
2.2. Preparation and Characterization of the Hydrophobic Membrane
2.3. Sample Analysis
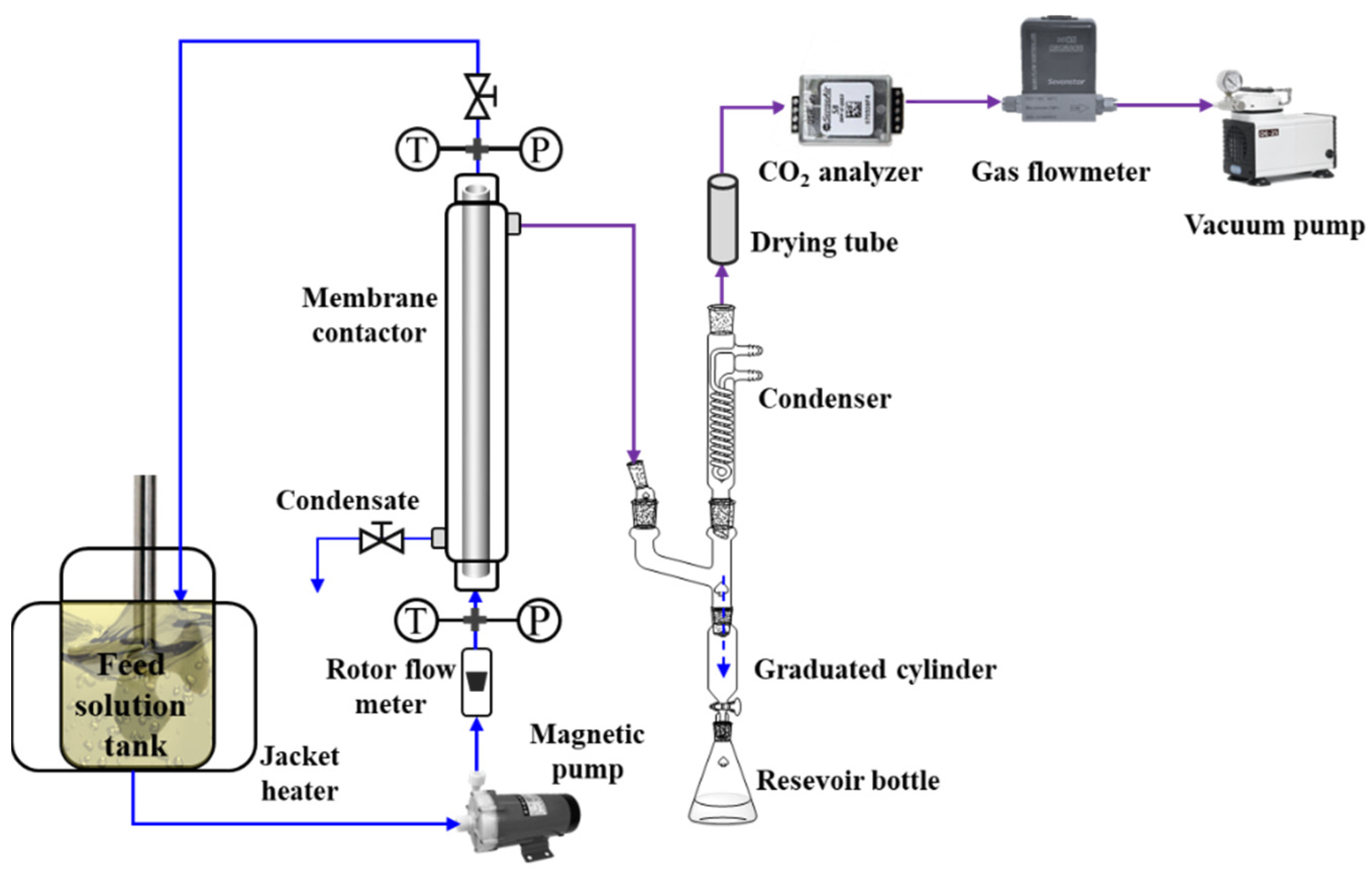
2.4. Experimental Apparatus and Procedure for Membrane CO2 Desorption
2.5. Stability Study of the α-Al2O3 Membrane
3. Results and Discussion
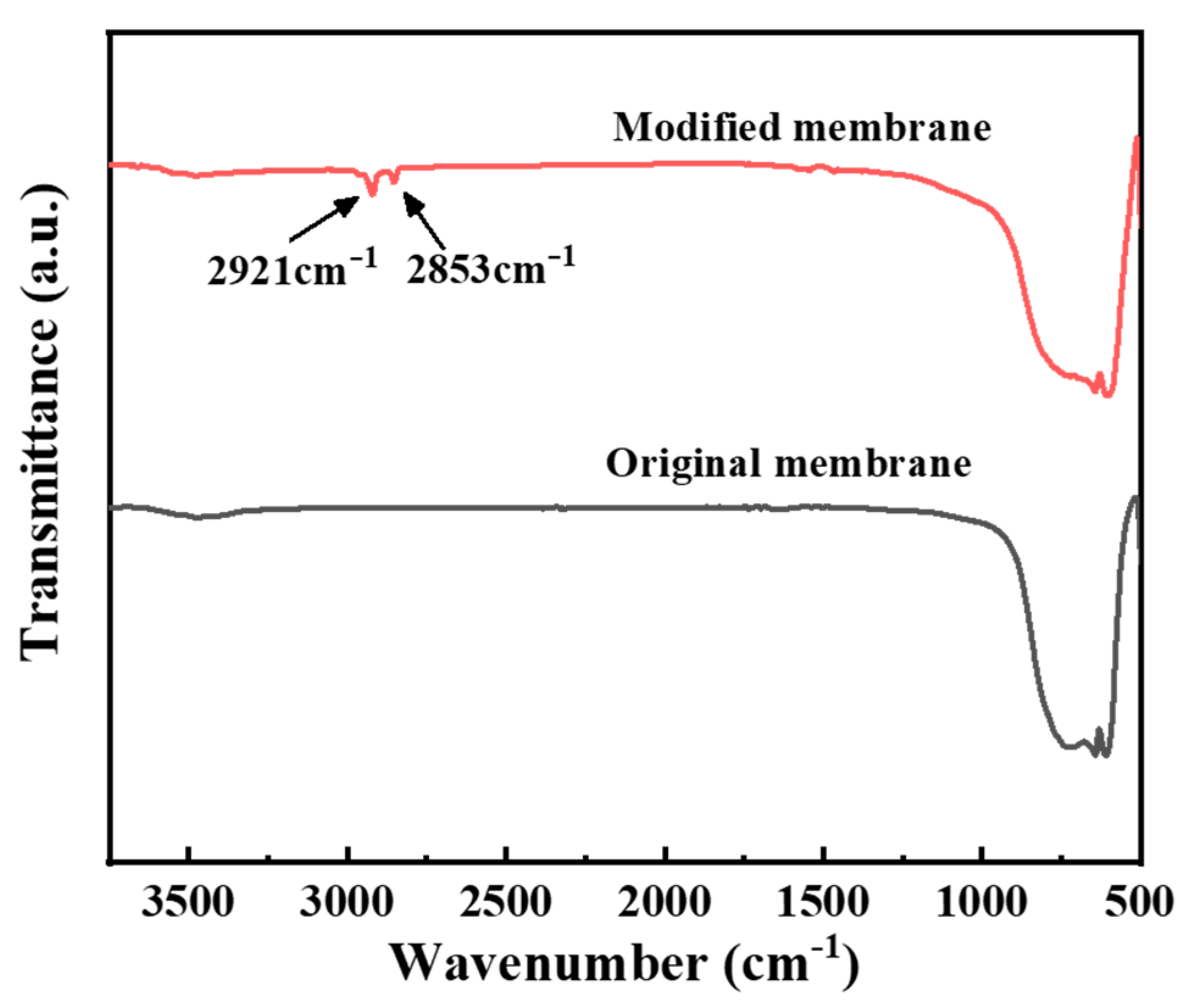
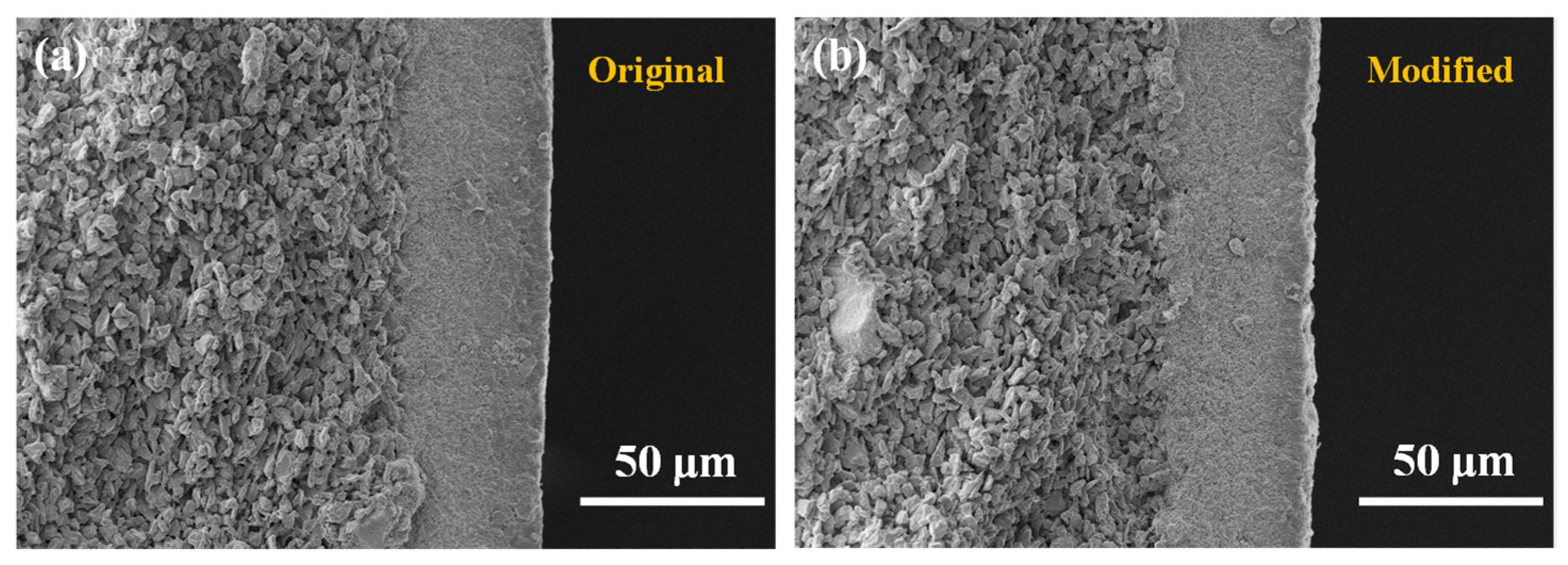
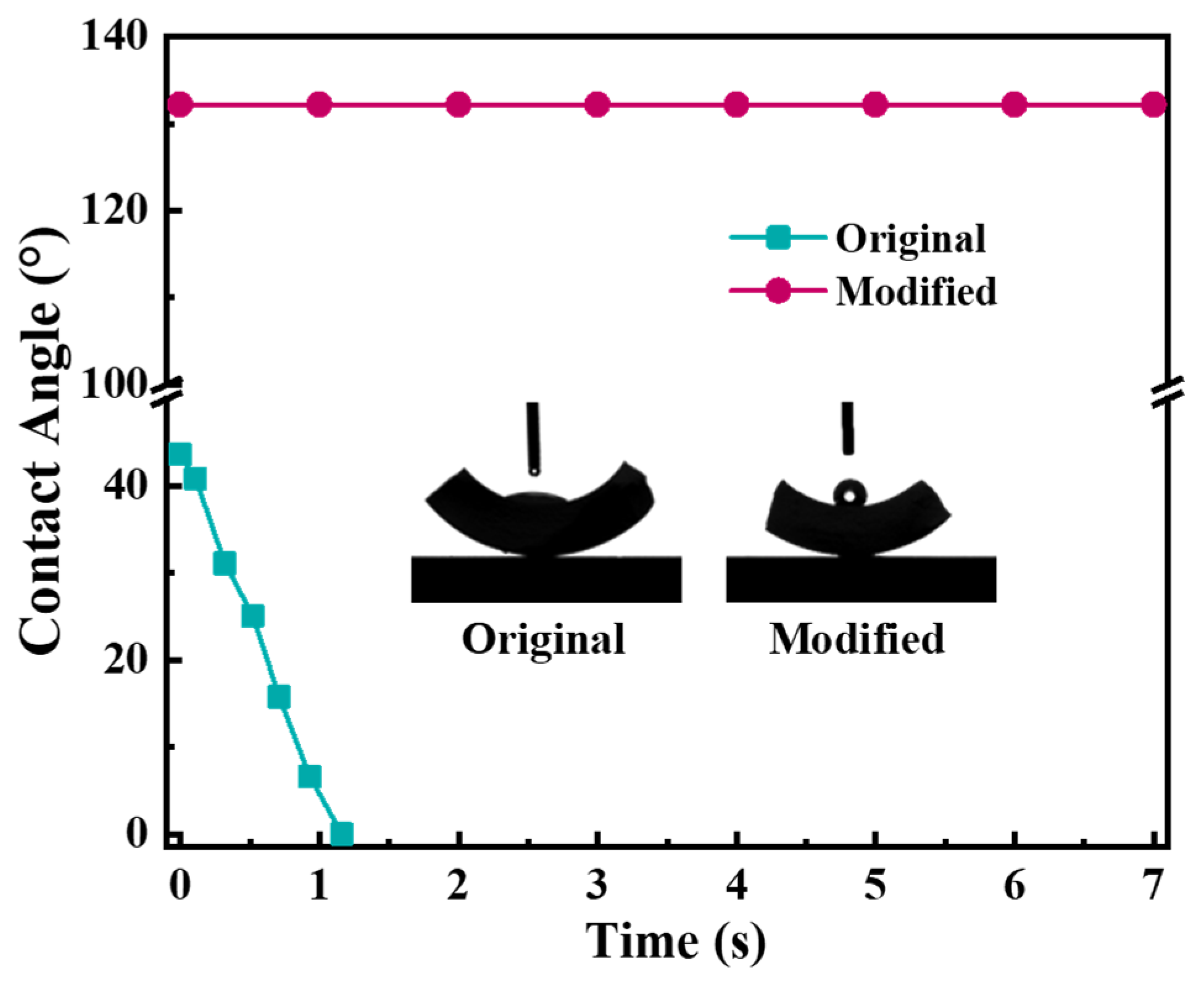
3.1. Characterization Results of the Hydrophobic Ceramic Membrane
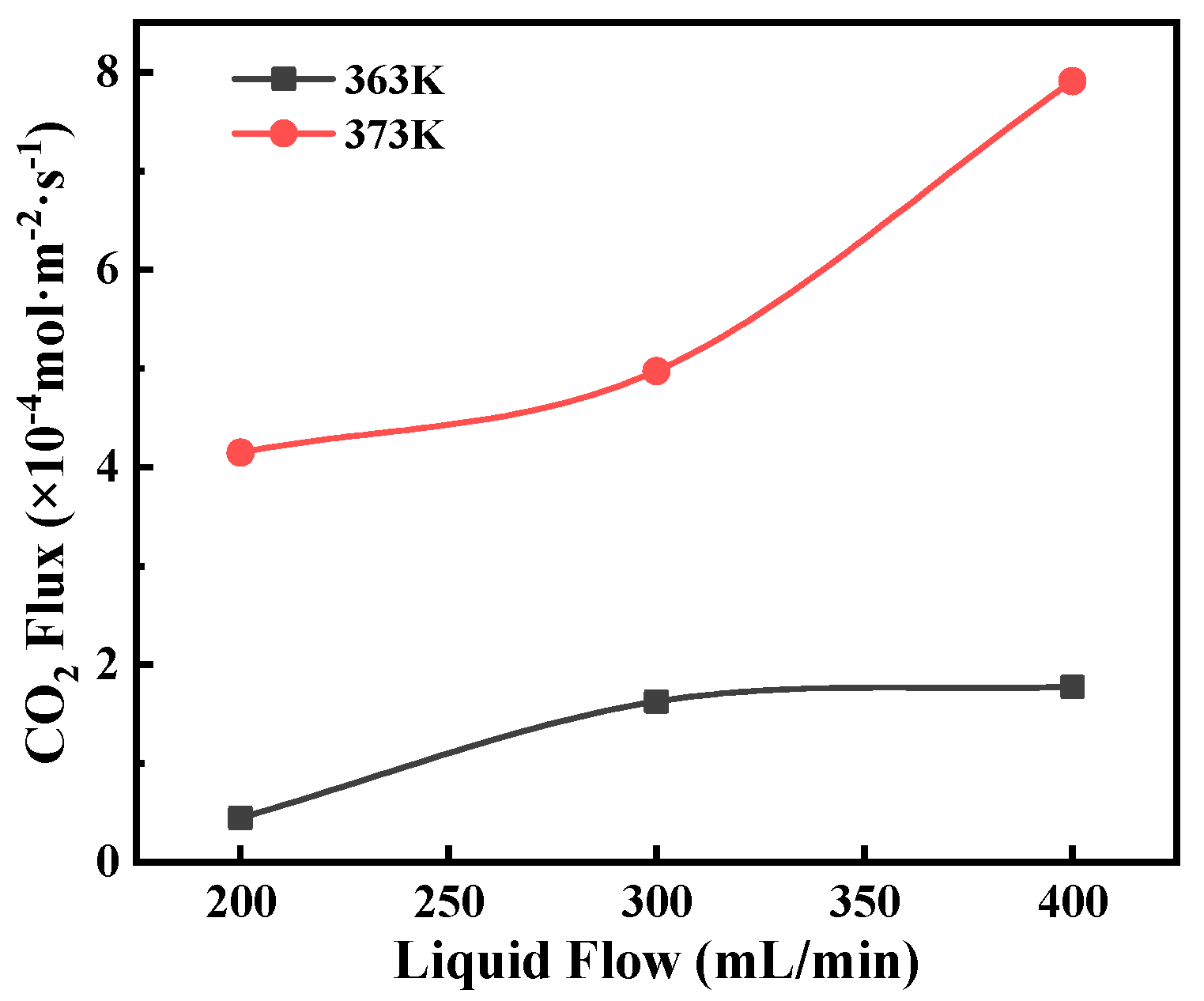
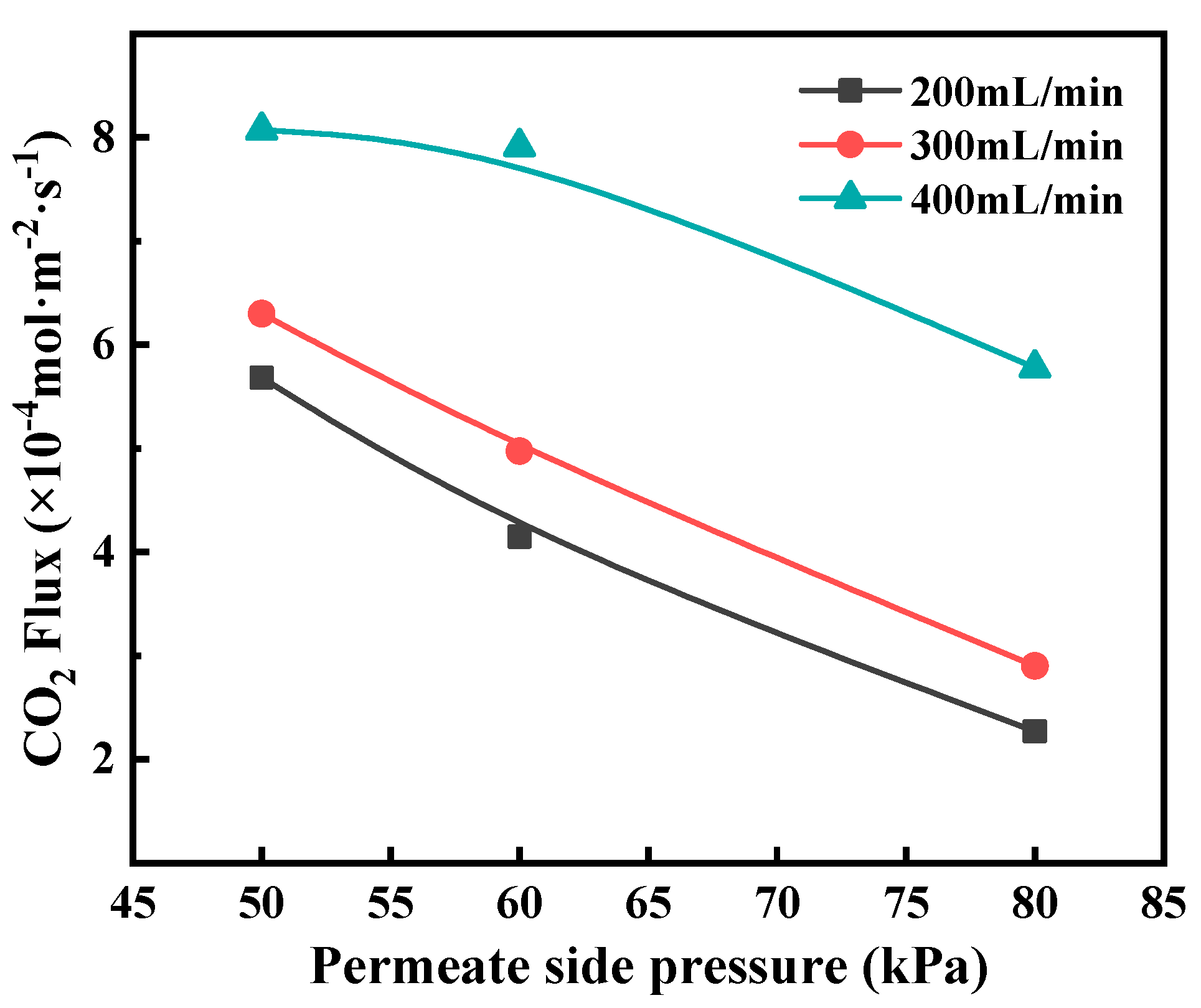
3.2. Effects of Key Operating Conditions
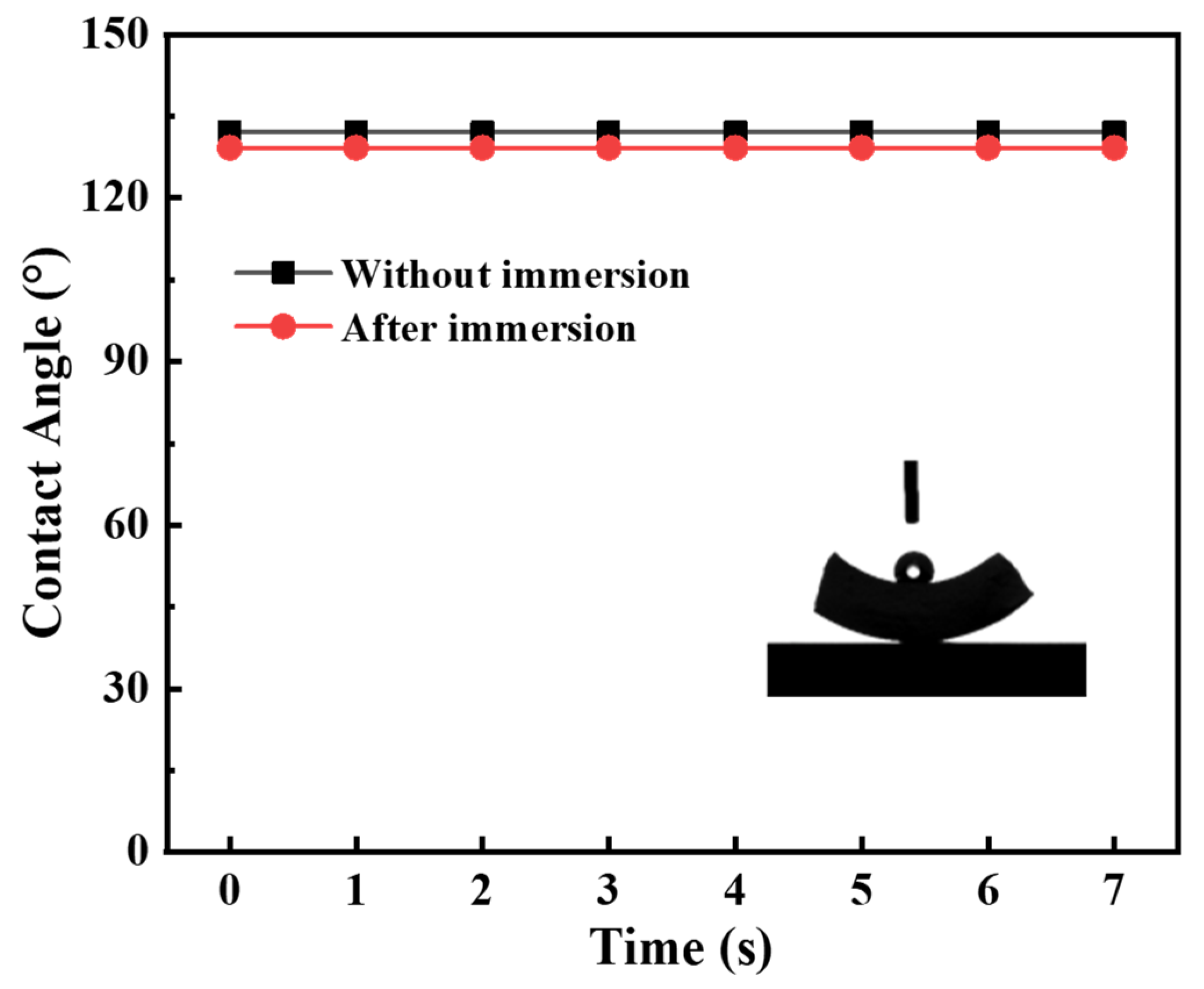
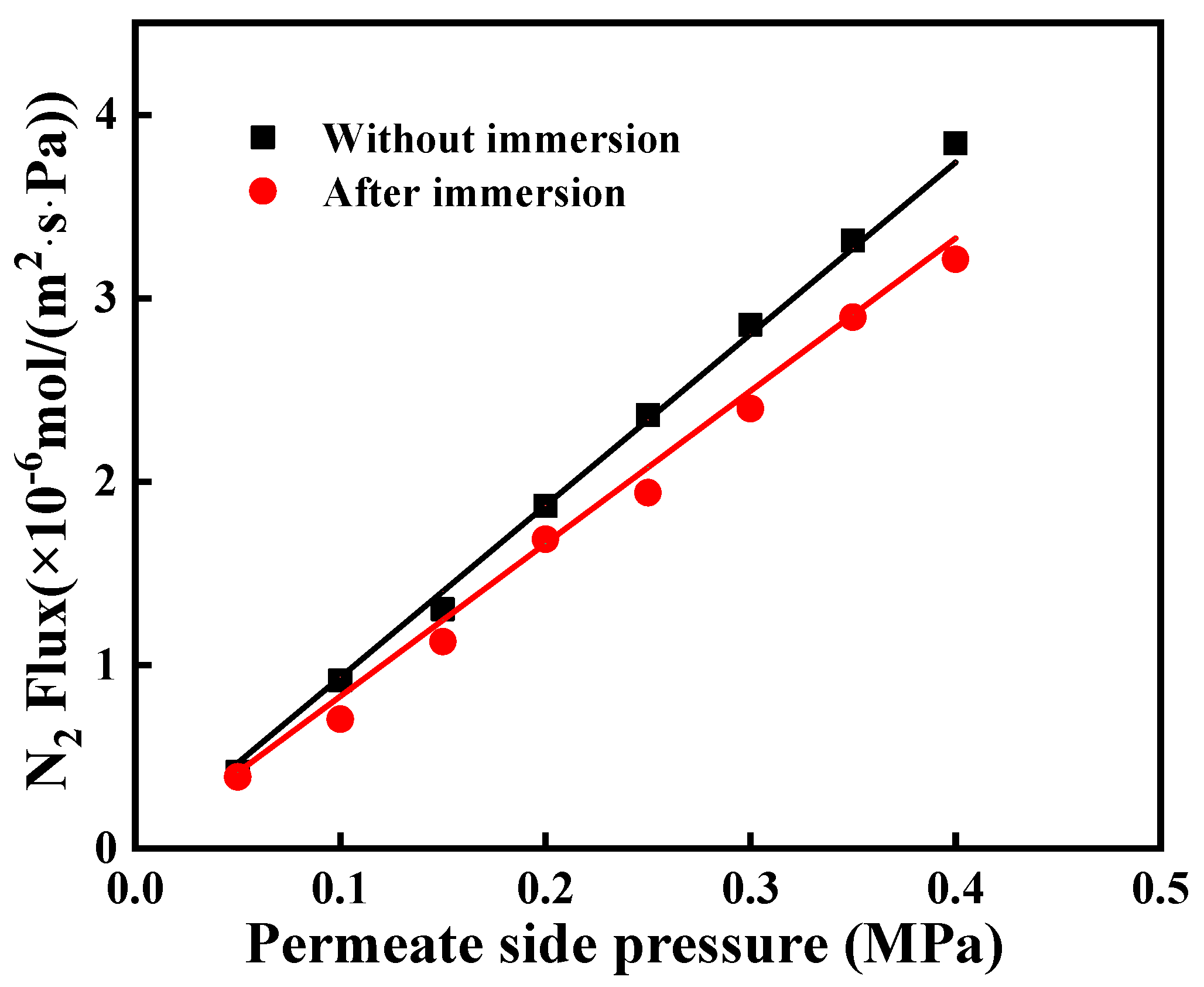
3.3. The Stability of the Modified Ceramic Membrane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vega, F.; Baena-Moreno, F.M.; Gallego Fernández, L.M.; Portillo, E.; Navarrete, B.; Zhang, Z. Current status of CO2 chemical absorption research applied to CCS: Towards full deployment at industrial scale. Appl. Energy 2020, 260, 114313. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Oyenekan, B.A.; Rochelle, G.T. Alternative stripper configurations for CO2 capture by aqueous amines. AlChE J. 2007, 53, 3144–3154. [Google Scholar] [CrossRef]
- Jassim, M.S.; Rochelle, G.T. Innovative absorber/stripper configurations for CO2 capture by aqueous monoethanolamine. Ind. Eng. Chem. Res. 2006, 45, 2465–2472. [Google Scholar] [CrossRef]
- Rahim, N.A.; Ghasem, N.; Al-Marzouqi, M. Stripping of CO2 from different aqueous solvents using PVDF hollow fiber membrane contacting process. J. Nat. Gas Sci. Eng. 2014, 21, 886–893. [Google Scholar] [CrossRef]
- Vadillo, J.M.; Gomez-Coma, L.; Garea, A.; Irabien, A. Hollow fiber membrane contactors in CO2 desorption: A review. Energy Fuel 2021, 35, 111–136. [Google Scholar] [CrossRef]
- Qi, G.; Liu, K.; Frimpong, R.A.; House, A.; Salmon, S.; Liu, K. Integrated bench-scale parametric study on CO2 capture using a carbonic anhydrase promoted K2CO3 solvent with low temperature vacuum stripping. Ind. Eng. Chem. Res. 2016, 55, 12452–12459. [Google Scholar] [CrossRef]
- Ahn, H.; Kim, J.; Kim, J.H. Low-temperature vacuum stripping of CO2 from aqueous amine solutions using thin-film silicalite-filled PDMS composite membranes. Int. J. Greenh. Gas Control 2013, 18, 165–172. [Google Scholar] [CrossRef]
- Khaisri, S.; de Montigny, D.; Tontiwachwuthikul, P.; Jiraratananon, R. CO2 stripping from monoethanolamine using a membrane contactor. J. Membr. Sci. 2011, 376, 110–118. [Google Scholar] [CrossRef]
- Kosaraju, P.; Kovvali, A.S.; Korikov, A.; Sirkar, K.K. Hollow fiber membrane contactor based CO2 absorption-stripping using novel solvents and membranes. Ind. Eng. Chem. Res. 2005, 44, 1250–1258. [Google Scholar] [CrossRef]
- Chen, X.; Chen, T.; Li, J.; Qiu, M.; Fu, K.; Cui, Z.; Fan, Y.; Drioli, E. Ceramic nanofiltration and membrane distillation hybrid membrane processes for the purification and recycling of boric acid from simulative radioactive waste water. J. Membr. Sci. 2019, 579, 294–301. [Google Scholar] [CrossRef]
- Smart, S.; Liu, S.; Serra, J.M.; Diniz da Costa, J.C.; Iulianelli, A.; Basile, A. 8-Porous ceramic membranes for membrane reactors. In Handbook of Membrane Reactors; Basile, A., Ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 298–336. [Google Scholar]
- Hussain, A.; Seidel-Morgenstern, A.; Tsotsas, E. Heat and mass transfer in tubular ceramic membranes for membrane reactors. Int. J. Heat Mass Transf. 2006, 49, 2239–2253. [Google Scholar] [CrossRef]
- Chen, X.F.; Gao, X.Y.; Fu, K.Y.; Qiu, M.H.; Xiong, F.; Ding, D.; Cui, Z.L.; Wang, Z.H.; Fan, Y.Q.; Drioli, E. Tubular hydrophobic ceramic membrane with asymmetric structure for water desalination via vacuum membrane distillation process. Desalination 2018, 443, 212–220. [Google Scholar] [CrossRef]
- Pagliero, M.; Bottino, A.; Comite, A.; Costa, C. Silanization of tubular ceramic membranes for application in membrane distillation. J. Membr. Sci 2020, 601, 117911. [Google Scholar] [CrossRef]
- Cui, Q.; Liu, S.; Xu, L.; Tu, T.; He, Q.; Yan, S. Modification of rich-split carbon capture process using ceramic membrane for reducing the reboiler duty: Effect of membrane arrangements. Sep. Purif. Technol. 2020, 235, 116148. [Google Scholar] [CrossRef]
- Tu, T.; Cui, Q.F.; Liang, F.H.; Xu, L.Q.; He, Q.Y.; Yan, S.P. Water recovery from stripping gas overhead CO2 desorber through air cooling enhanced by transport membrane condensation. Sep. Purif. Technol. 2019, 215, 625–633. [Google Scholar] [CrossRef]
- Gude, U.; Baumann, S.; Meulenberg, W.A.; Muller, M. Towards the development of materials for chemically stable carbonate- ceramic membranes to be used for CO2 separation in water-gas-shift reactors. Sep. Purif. Technol. 2019, 215, 378–383. [Google Scholar] [CrossRef]
- Xu, P.; Huang, Y.; Kong, X.; Gong, D.; Fu, K.; Chen, X.; Qiu, M.; Fan, Y. Hydrophilic membrane contactor for improving selective removal of SO2 by NaOH solution. Sep. Purif. Technol. 2020, 250, 117134. [Google Scholar] [CrossRef]
- Qiu, M.; Chen, X.; Fan, Y.; Xing, W. 1.11 Ceramic Membranes. In Comprehensive Membrane Science and Engineering, 2nd ed.; Drioli, E., Giorno, L., Fontananova, E., Eds.; Elsevier: Oxford, UK, 2017; pp. 270–297. [Google Scholar]
- Qiu, M.H.; Kong, X.L.; Fu, K.Y.; Han, S.X.; Gao, X.Y.; Chen, X.F.; Fan, Y.Q. Optimization of microstructure and geometry of hydrophobic ceramic membrane for SO2 absorption from ship exhaust. AlChE J. 2019, 65, 409–420. [Google Scholar] [CrossRef] [Green Version]
- Gao, N.; Li, M.; Jing, W.; Fan, Y.; Xu, N. Improving the filtration performance of ZrO2 membrane in non-polar organic solvents by surface hydrophobic modification. J. Membr. Sci. 2011, 375, 276–283. [Google Scholar] [CrossRef]
- Horwitz, W. Association of Official analytical Chemists (AOAC) Methods, 12th ed.; George Banta Company: Menasha, WI, USA, 1975. [Google Scholar]
- Tillman, N.; Ulman, A.; Penner, T.L.J.L. Formation of multilayers by self-assembly. Langmuir 1989, 5, 101–111. [Google Scholar] [CrossRef]
- Meth, S.; Sukenik, C.N. Siloxane-anchored thin films on silicon dioxide-modified stainless steel. Thin Solid Films 2003, 425, 49–58. [Google Scholar] [CrossRef]
- Weiland, R.H.; Rawal, M.; Rice, R.G. Stripping of carbon dioxide from monoethanolamine solutions in a packed column. AlChE J. 1982, 28, 963–973. [Google Scholar] [CrossRef]
- Versteeg, G.; Van Swaaij, W.P.M. Solubility and diffusivity of acid gases (carbon dioxide, nitrous oxide) in aqueous alkanolamine solutions. J. Chem. Eng. Data 1988, 33, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Ko, J.J.; Tsai, T.C.; Lin, C.Y.; Wang, H.M.; Li, M.H. Diffusivity of nitrous oxide in aqueous alkanolamine solutions. J. Chem. Eng. Data 2001, 46, 160–165. [Google Scholar] [CrossRef]
- Khaisri, S.; Demontigny, D.; Tontiwachwuthikul, P.; Jiraratananon, R. Comparing membrane resistance and absorption performance of three different membranes in a gas absorption membrane contactor. Sep. Purif. Technol. 2009, 65, 290–297. [Google Scholar] [CrossRef]
- Scholes, C.A.; Kentish, S.E.; Stevens, G.W.; Demontigny, D. Asymmetric composite PDMS membrane contactors for desorption of CO2 from monoethanolamine. Int. J. Greenh. Gas Control 2016, 55, 195–201. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, M.K.; Park, J.H. Decompression stripping of carbon dioxide from rich monoethanolamine through porous hydrophobic modified ceramic hollow fiber membrane contactor. Sep. Purif. Technol. 2020, 236, 116304. [Google Scholar] [CrossRef]
- Fang, M.; Wang, Z.; Yan, S.; Cen, Q.; Luo, Z. CO2 desorption from rich alkanolamine solution by using membrane vacuum regeneration technology. Int. J. Greenh. Gas Control 2012, 9, 507–521. [Google Scholar] [CrossRef]





| Membrane Properties | Values | |
|---|---|---|
| Membrane Layer | Support Layer | |
| Mean pore size | 0.1 (μm) | 1.0 (μm) |
| Thickness | 40 (μm) | 2.0 (mm) |
| Porosity | 0.4 | 0.4 |
| Tortuosity factor | 2.5 | 2.5 |
| Membrane tube (OD/ID) | 12/8 (mm) | |
| Module (ID) | 22 (mm) | |
| Length | 600 (mm) | |
| Material | CO2 Flux (mol·m−2·s−1) | Absorbent Concentration (mol/L) | CO2-Loading (mol CO2/mol Absorbent) | Feed Temperature (K) | Permeate Side Pressure (kPa) | Ref. |
|---|---|---|---|---|---|---|
| PVDF | 3 × 10−4 | 5.0 | 0.45 | 373 | 100 | [29] |
| PDMS + Psf | 1 × 10−3 | 5.0 | 0.49 | 373 | 120 | [30] |
| PTFE | 5 × 10−4 | 5.0 | 0.45 | 373 | 100 | [9] |
| Al2O3 | 2.56 × 10−3 | 5.0 | 0.45 | 353 | 61 | [31] |
| PP | 2.2 × 10−4 | 3.4 | 0.53 | 343 | 52 | [32] |
| α-Al2O3 | 1.17 × 10−3 | 5.0 | 0.41 | 373 | 60 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Qi, W.; Fu, K.; Chen, X.; Qiu, M.; Fan, Y. Permeability and Stability of Hydrophobic Tubular Ceramic Membrane Contactor for CO2 Desorption from MEA Solution. Membranes 2022, 12, 8. https://doi.org/10.3390/membranes12010008
Guo Y, Qi W, Fu K, Chen X, Qiu M, Fan Y. Permeability and Stability of Hydrophobic Tubular Ceramic Membrane Contactor for CO2 Desorption from MEA Solution. Membranes. 2022; 12(1):8. https://doi.org/10.3390/membranes12010008
Chicago/Turabian StyleGuo, Yunzhao, Wenbo Qi, Kaiyun Fu, Xianfu Chen, Minghui Qiu, and Yiqun Fan. 2022. "Permeability and Stability of Hydrophobic Tubular Ceramic Membrane Contactor for CO2 Desorption from MEA Solution" Membranes 12, no. 1: 8. https://doi.org/10.3390/membranes12010008
APA StyleGuo, Y., Qi, W., Fu, K., Chen, X., Qiu, M., & Fan, Y. (2022). Permeability and Stability of Hydrophobic Tubular Ceramic Membrane Contactor for CO2 Desorption from MEA Solution. Membranes, 12(1), 8. https://doi.org/10.3390/membranes12010008






