High-Efficiency Separation of Mg2+/Sr2+ through a NF Membrane under Electric Field
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
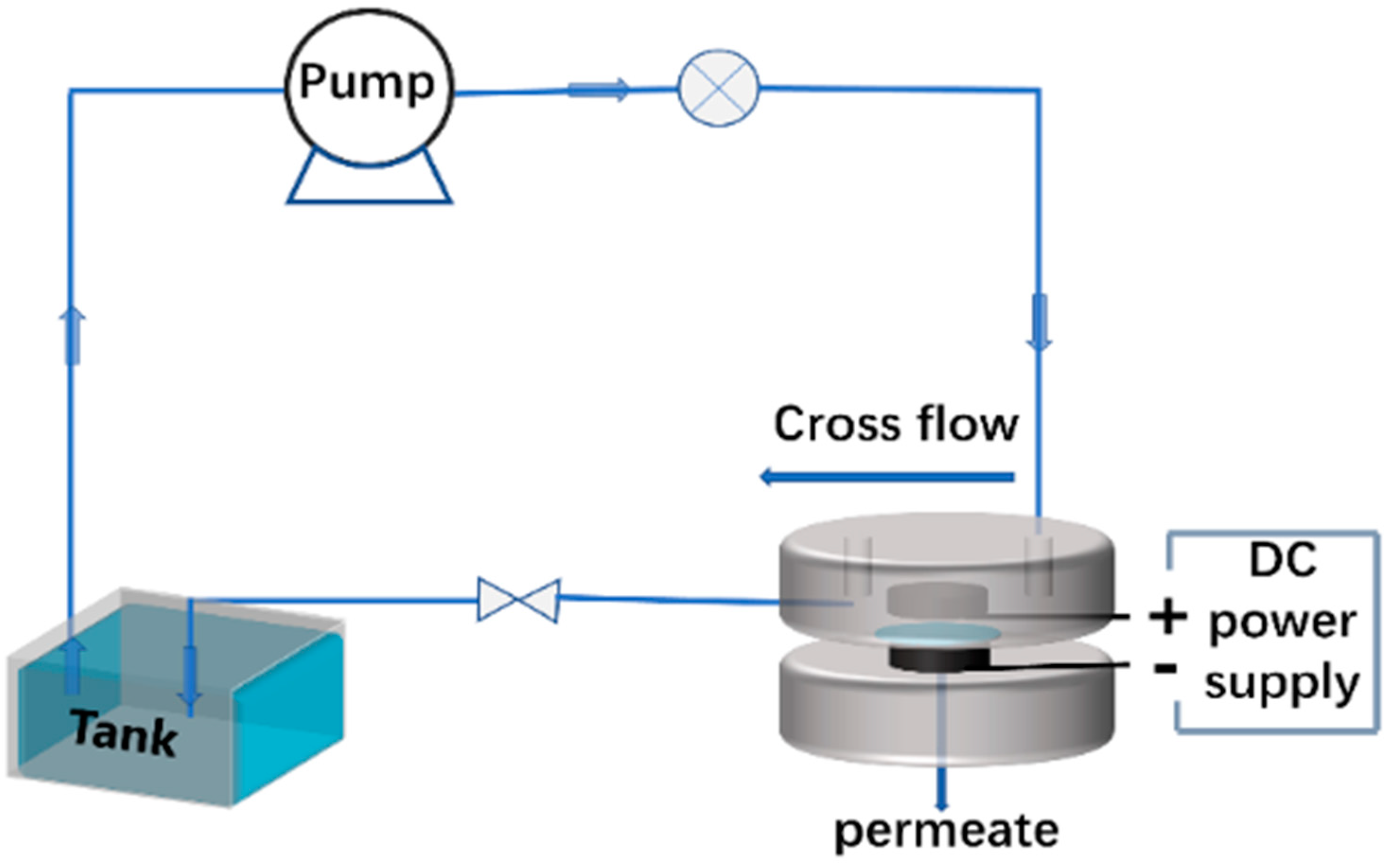
2.2. Separation of Mg2+ and Sr2+ Ions
2.3. Molecular Dynamics (MD) Simulation of the Effect of Electric Field on Hydrated Mg2+ and Sr2+
3. Results and Discussion
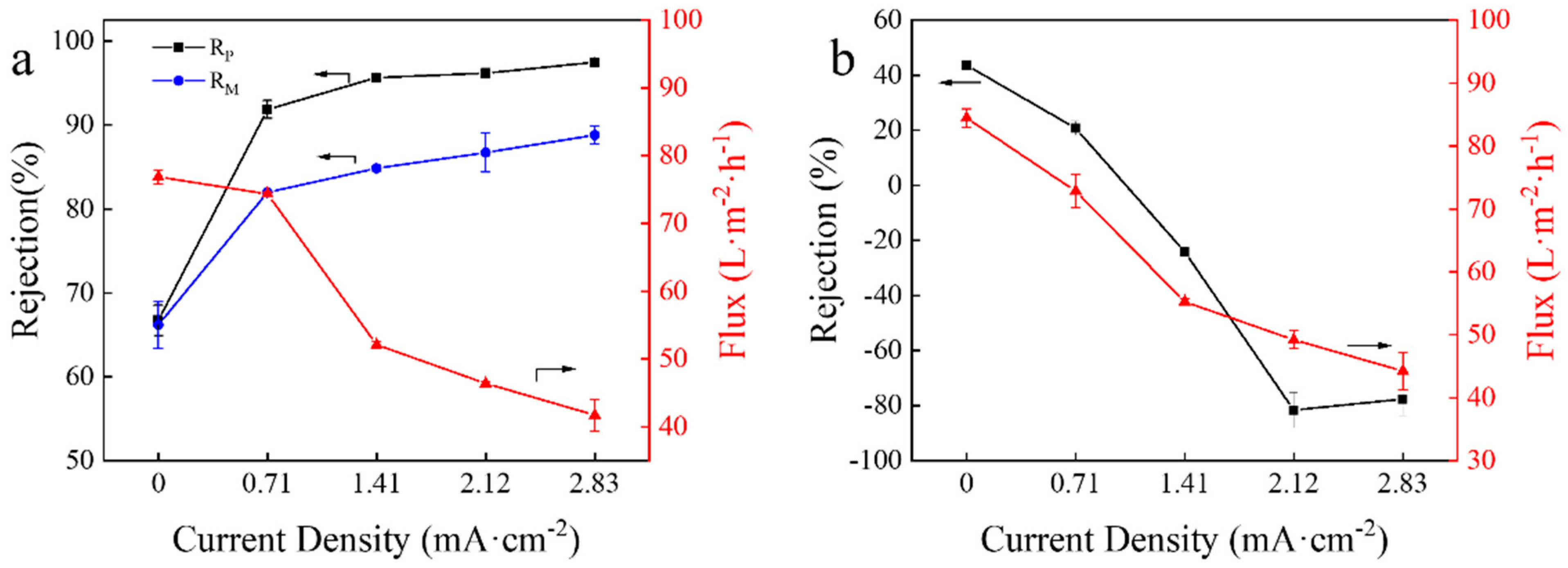
3.1. Effect of Current Density on Rejection of Mg2+ or Sr2+ in Single Salt System
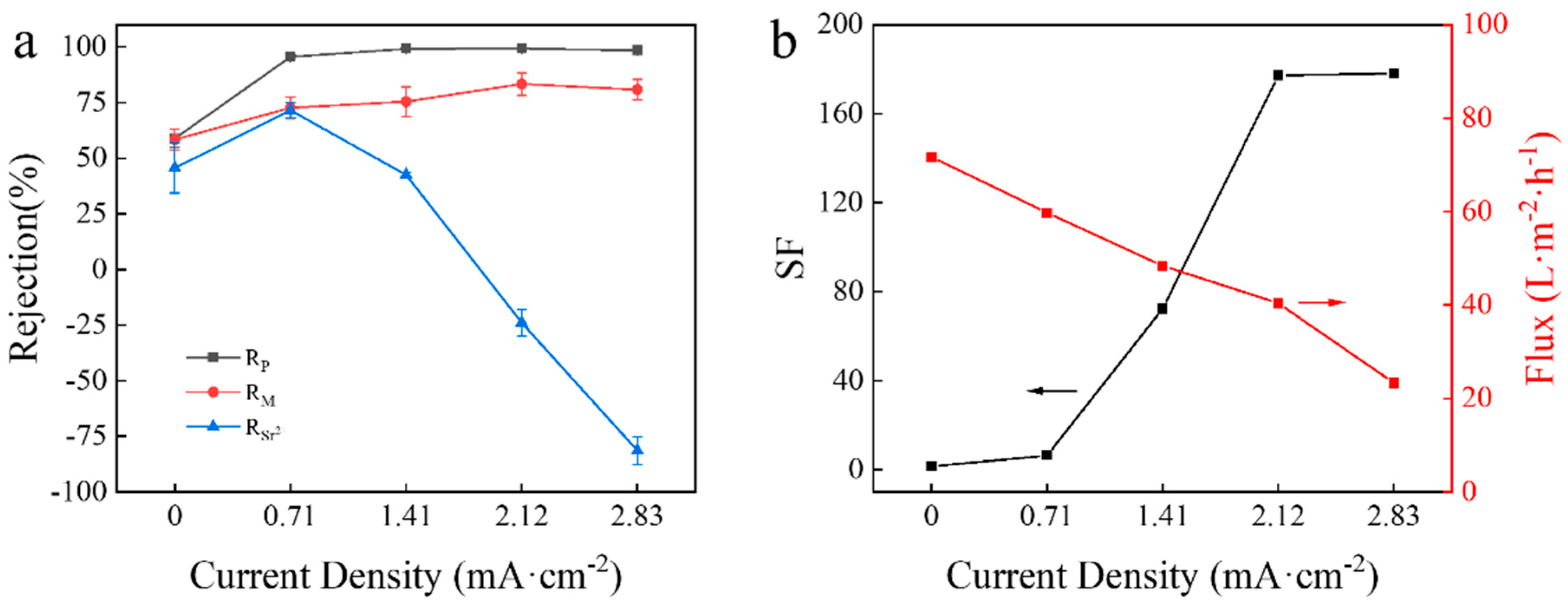
3.2. Effect of Current Density on Separation of Mg2+/Sr2+ in Mixed Salt System
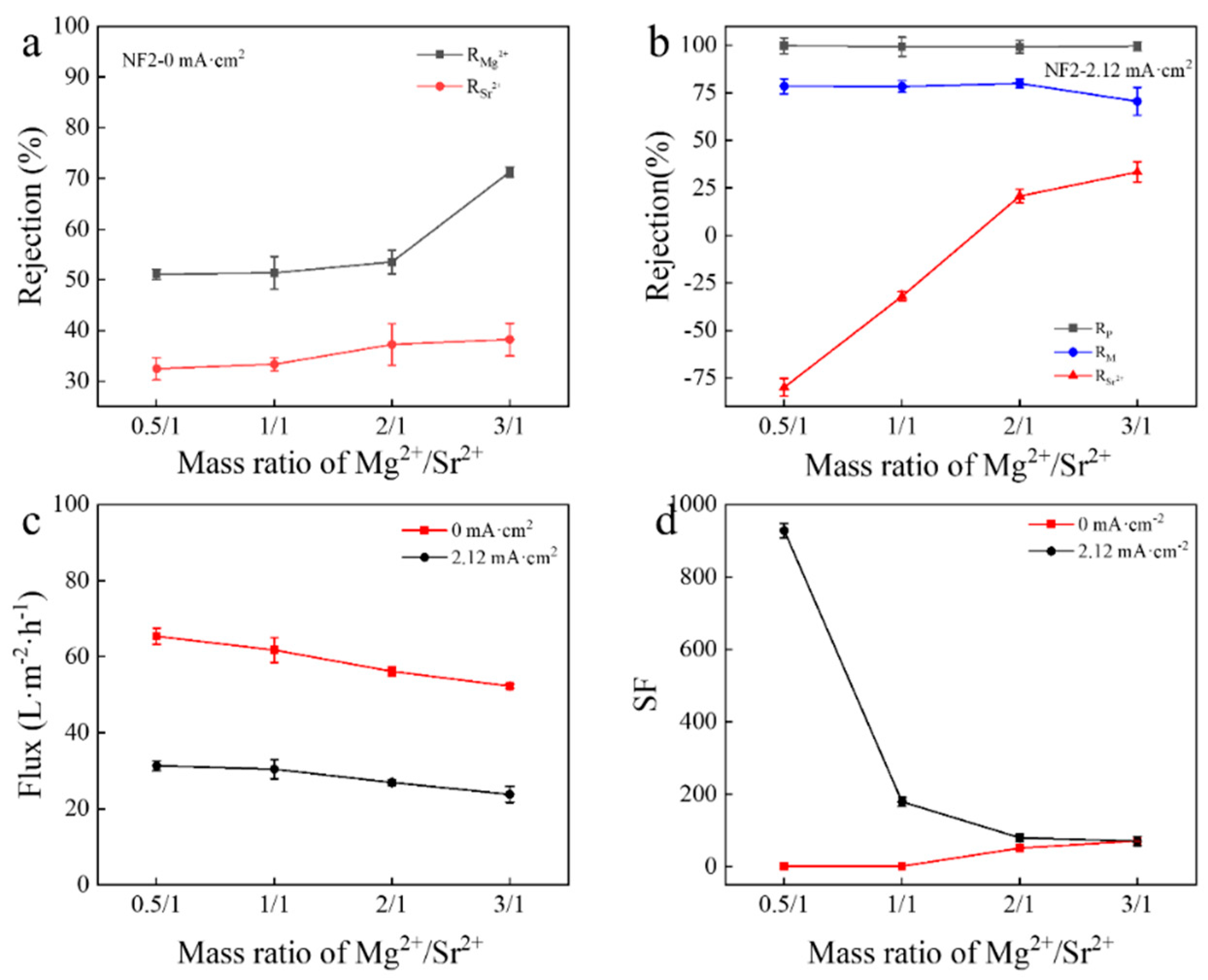
3.3. Effect of Mg2+/Sr2+ Mass Ratio on Separation of Mg2+/Sr2+ in Mixed Salt System
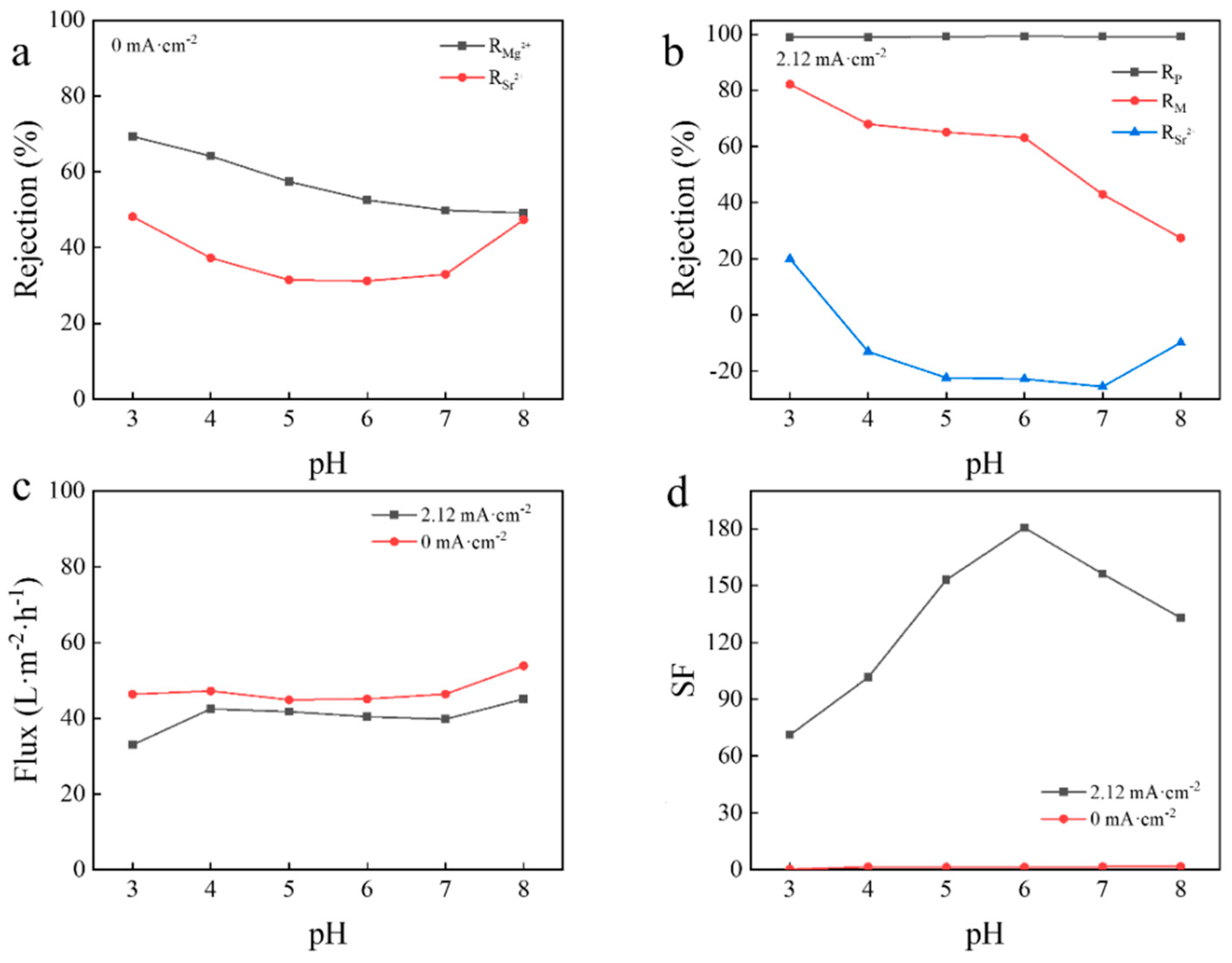
3.4. Effect of pH of Feed on Separation of Mg2+/Sr2+ in Mixed Salt System
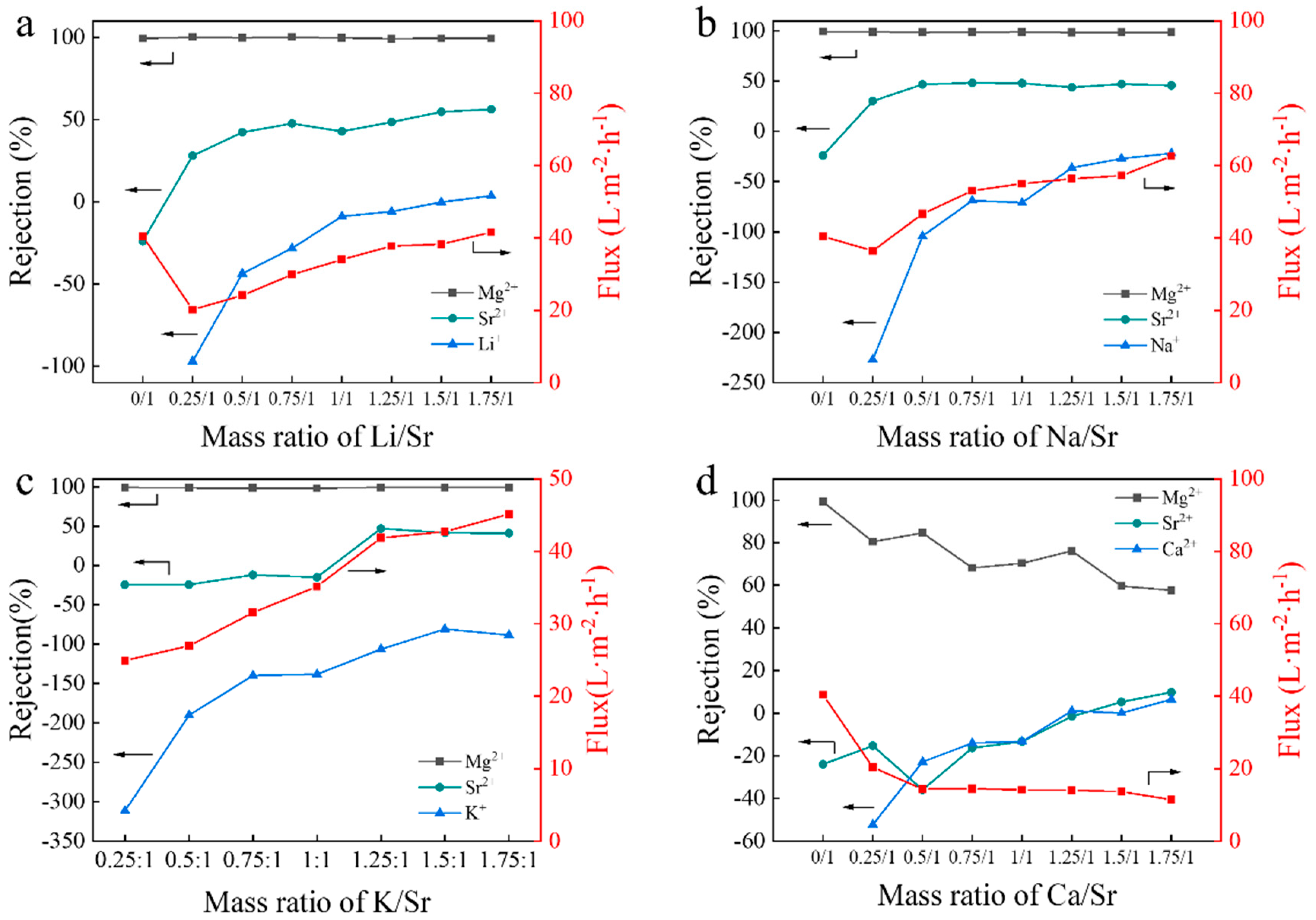
3.5. Effect of Other Coexisting Ions on Separation of Mg2+/Sr2+
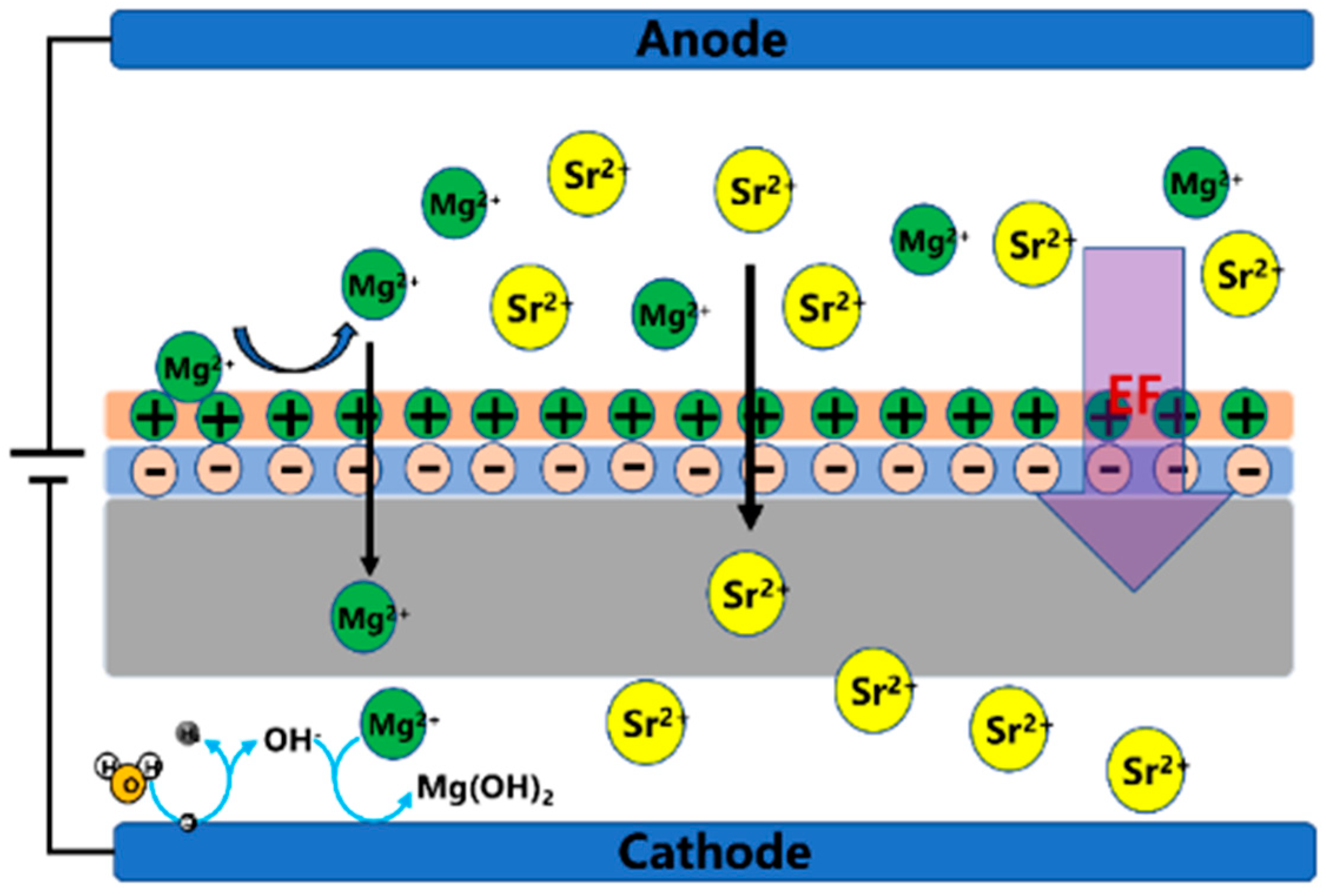
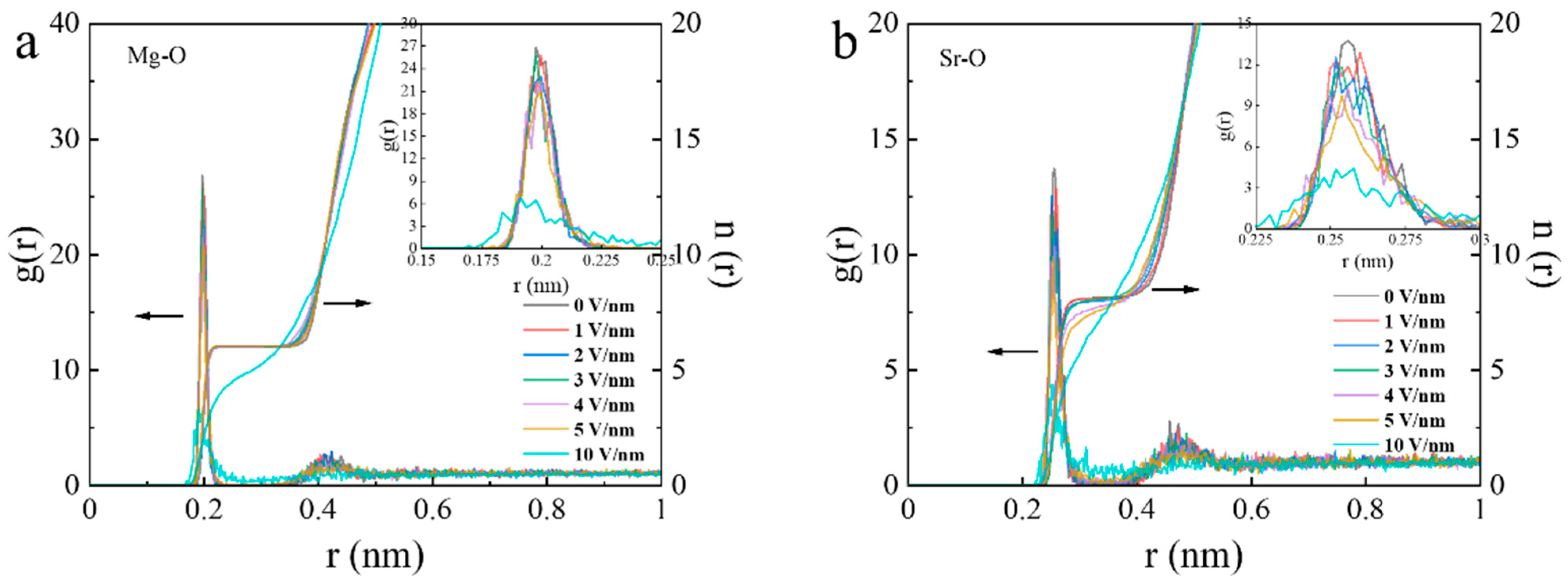
3.6. The Effect of Electric Field (EF) on Dehydration of Mg2+ and Sr2+ Ions by Molecular Dynamics (MD) Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Liu, S.; Zhou, Y.; Luo, J.; Shi, J.; Zhou, Z. Extraction of Sr2+ from aqueous solutions using an asymmetric pulsed current-assisted electrochemical method. Sep. Purif. Technol. 2021, 271, 119235. [Google Scholar] [CrossRef]
- Liu, C.; Yu, X.P.; Ma, C.; Guo, Y.F.; Deng, T.L. Selective recovery of strontium from oilfield water by ion-imprinted alginate microspheres modified with thioglycollic acid. Chem. Eng. J. 2021, 410, 128267. [Google Scholar] [CrossRef]
- Shan, J.J.; Wang, J.P.; Shan, F.S.; Teng, X.M.; Fan, Q.S.; Li, Q.K.; Qin, Z.J.; Zhang, X.R. Origin and circulation of saline springs in the Kuqa Basin of the Tarim Basin, Northwest China. J. Arid Land 2020, 12, 331–348. [Google Scholar] [CrossRef]
- Amesh, P.; Suneesh, A.S.; Venkatesan, K.A.; Maheswari, R.U.; Vijayalakshmi, S. Preparation and ion exchange studies of cesium and strontium on sodium iron titanate. Sep. Purif. Technol. 2020, 238, 116393. [Google Scholar] [CrossRef]
- Ryu, J.; Kim, S.; Hong, H.J.; Hong, J.; Kim, M.; Ryu, T.; Park, I.S.; Chung, K.S.; Jang, J.S.; Kim, B.G. Strontium ion (Sr2+) separation from seawater by hydrothermally structured titanate nanotubes: Removal vs. recovery. Chem. Eng. J. 2016, 304, 503–510. [Google Scholar] [CrossRef]
- Su, T.; Han, Z.J.; Qu, Z.; Chen, Y.; Lin, X.; Zhu, S.Y.; Bian, R.; Xie, X.F. Effective recycling of Co and Sr from Co/Sr-bearing wastewater via an integreated Fe coagulation and hematite precipitation approach. Environ. Res. 2020, 187, 109654. [Google Scholar] [CrossRef] [PubMed]
- Khan, P.N.; Bhattacharyya, A.; Sharma, J.N.; Manohar, S. The recovery of strontium from acidic medium using novel strontium selective extractant: An experimental and DFT study. J. Hazard. Mater. 2020, 397, 122476. [Google Scholar] [CrossRef]
- Xu, C.; Wang, J.C.; Chen, J. Solvent extraction of strontium and cesium: A review of recent progress. Solvent Extr. Ion. Exch. 2012, 30, 623–650. [Google Scholar] [CrossRef]
- Kim, H.; Kang, Y.G.; Lee, Y.J.; Choi, S.D.; Lim, J.M.; Lee, J.H. Automated extraction chromatographic radionuclide separation system for analysis of Sr-90 in seawater. Talanta 2020, 217, 121055. [Google Scholar] [CrossRef]
- Hong, H.-J.; Jeong, H.S.; Kim, B.-G.; Hong, J.; Park, I.-S.; Ryu, T.; Chung, K.-S.; Kim, H.; Ryu, J. Highly stable and magnetically separable alginate/Fe3O4 composite for the removal of strontium (Sr) from seawater. Chemosphere 2016, 165, 231–238. [Google Scholar] [CrossRef]
- Luo, J.Q.; Wan, Y.H. Effects of pH and salt on nanofiltration-a critical review. J. Membr. Sci. 2013, 438, 18–28. [Google Scholar] [CrossRef]
- Wang, R.Y.; Lin, S.H. Pore model for nanofiltration: History, theoretical framework, key predictions, limitations, and prospects. J. Membr. Sci. 2021, 620, 118809. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, C.H.; Tong, T.Z.; Epsztein, R.; Sun, M.; Verduzco, R.; Ma, J.; Elimelech, M. Selective removal of divalent cations by polyelectrolyte multilayer nanofiltration membrane: Role of polyelectrolyte charge, ion size, and ionic strength. J. Membr. Sci. 2018, 559, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Nicod, L.; Chitry, F.; Gaubert, E.; Lemaire, M.; Barnier, H. Application of Water Soluble Resorcinarenes in Nanofiltration-Complexation with Caesium and Strontium as Targets. J. Incl. Phenom. Macrocycl. Chem. 1999, 34, 143–154. [Google Scholar] [CrossRef]
- Hwang, E.D.; Lee, K.W.; Choo, K.H.; Choi, S.J.; Lee, C.H. Effect of precipitation and complexation on nanofiltration of strontium-containing nuclear wastewater. Desalination 2002, 147, 289–294. [Google Scholar] [CrossRef]
- Sujish, D.; Mohanakrishnan, G.; Sharma, B.K.; Anand Babu, C.; Rajan, K.K. Application of nanofiltration membrane and ethyleneimine oligomer mixture for selective separation of Strontium from a simulated nuclear waste solution. Desalination Water Treat. 2014, 52, 401–406. [Google Scholar] [CrossRef]
- Tang, C.; Bruening, M.L. Ion separations with membranes. J. Membr. Sci. 2020, 58, 2831–2856. [Google Scholar] [CrossRef]
- Razmjou, A.; Asadnia, M.; Hosseini, E.; Habibnejad Korayem, A.; Chen, V. Design principles of ion selective nanostructured membranes for the extraction of lithium ions. Nat. Commun 2019, 10, 5793. [Google Scholar] [CrossRef] [Green Version]
- Epsztein, R.; DuChanois, R.M.; Ritt, C.L.; Noy, A.; Elimelech, M. Towards single-species selectivity of membranes with subnanometre pores. Nat. Nanotechnol. 2020, 15, 426–436. [Google Scholar] [CrossRef]
- Tang, C.; Yaroshchuk, A.; Bruening, M.L. Flow through negatively charged, nanoporous membranes separates Li(+)and K(+)due to induced electromigration. Chem. Commun. 2020, 56, 10954–10957. [Google Scholar] [CrossRef] [PubMed]
- Butylskii, D.Y.; Pismenskaya, N.D.; Apel, P.Y.; Sabbatovskiy, K.G.; Nikonenko, V.V. Highly selective separation of singly charged cations by countercurrent electromigration with a track-etched membrane. J. Membr. Sci. 2021, 635, 119449. [Google Scholar] [CrossRef]
- Huotari, H.M.; Tragardh, G.; Huisman, I.H. Crossflow membrane filtration enhanced by an external DC electric field: A review. Chem. Eng. Res. Des. 1999, 77, 461–468. [Google Scholar] [CrossRef]
- Pupunat, L.; Rios, G.M.; Joulie, R. Modeling of solvent and ionic fluxes in electronanofiltration. Sep. Sci. Technol. 1999, 34, 1947–1962. [Google Scholar] [CrossRef]
- Li, Q.; Liu, H.; He, B.; Shi, W.; Ji, Y.; Cui, Z.; Yan, F.; Mohammad, Y.; Li, J. Ultrahigh-efficient separation of Mg2+/Li+ using an in-situ reconstructed positively charged nanofiltration membrane under an electric field. J. Membr. Sci. 2022, 641, 119880. [Google Scholar] [CrossRef]
- He, Z.; Cui, H.; Hao, S.; Wang, L.; Zhou, J. Electric-Field Effects on Ionic Hydration: A Molecular Dynamics Study. J. Phys. Chem. B. 2018, 122, 5991–5998. [Google Scholar] [CrossRef]
- Barger, J.P.; Dillon, P.F. Near-membrane electric field calcium ion dehydration. Cell Calcium 2016, 60, 415–422. [Google Scholar] [CrossRef]
- Richards, L.A.; Schafer, A.I.; Richards, B.S.; Corry, B. The importance of dehydration in determining ion transport in narrow pores. Small 2012, 8, 1701–1709. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Lu, L.; Zhu, Y.; Zhang, Y.; Cao, W.; Lu, X. Ionic hydration of Na+ inside carbon nanotubes, under electric fields. Fluid Phase Equilibria 2013, 353, 1–6. [Google Scholar] [CrossRef]
- Luo, J.; Ding, L.; Su, Y.; Wei, S.; Wan, Y. Concentration polarization in concentrated saline solution during desalination of iron dextran by nanofiltration. J. Membr. Sci. 2010, 363, 170–179. [Google Scholar] [CrossRef]
- Bowen, W.R.; Yousef, H.N.S. Effect of salts on water viscosity in narrow membrane pores. J. Colloid Interface Sci. 2003, 264, 452–457. [Google Scholar] [CrossRef]
- Sun, S.-Y.; Cai, L.-J.; Nie, X.-Y.; Song, X.; Yu, J.-G. Separation of magnesium and lithium from brine using a Desal nanofiltration membrane. J. Water Process Eng. 2015, 7, 210–217. [Google Scholar] [CrossRef]
- Luo, J.Q.; Wan, Y.H. Effect of highly concentrated salt on retention of organic solutes by nanofiltration polymeric membranes. J. Membr. Sci. 2011, 372, 145–153. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y.; Wang, J.; You, G.; Cai, C.; Chen, B.H. Modeling the permeate flux and rejection of nanofiltration membrane separation with high concentration uncharged aqueous solutions. Desalination 2012, 299, 44–49. [Google Scholar] [CrossRef]
- Mazzoni, C.; Bruni, L.; Bandini, S. Nanofiltration: Role of the electrolyte and pH on desal DK performances. Ind. Eng. Chem. Res. 2007, 46, 2254–2262. [Google Scholar] [CrossRef]
- Mendret, J.; Hatat-Fraile, M.; Rivallin, M.; Brosillon, S. Influence of solution pH on the performance of photocatalytic membranes during dead-end filtration. Sep. Purif. Technol. 2013, 118, 406–414. [Google Scholar] [CrossRef]
- Szoke, S.; Patzay, G.; Weiser, L. Characteristics of thin-film nanofiltration membranes at various pH-values. Desalination 2003, 151, 123–129. [Google Scholar] [CrossRef]
- Wu, C.; Liu, S.; Wang, Z.; Zhang, J.; Wang, X.; Lu, X.; Jia, Y.; Hung, W.S.; Lee, K.R. Nanofiltration membranes with dually charged composite layer exhibiting super-high multivalent-salt rejection. J. Membr. Sci. 2016, 517, 64–72. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Wadekar, S.S.; Lokare, R.O.; Vidic, D.R. Comparison of calcium scaling in direct contact membrane distillation (DCMD) and nanofiltration (NF). J. Membr. Sci. 2021, 638, 119647. [Google Scholar] [CrossRef]
- Lin, D.C.; Bai, L.M.; Xu, D.L.; Wang, H.R.; Zhang, H.; Li, G.B.; Liang, H. Nanofiltration scaling influenced by coexisting pollutants considering the interaction between ferric coagulant and natural organic macromolecules. Chem. Eng. J. 2021, 413, 127403. [Google Scholar] [CrossRef]
- Zhou, K.; Xu, Z.P. Renormalization of Ionic Solvation Shells in Nanochannels. ACS Appl. Mater. Interfaces 2018, 10, 27801–27809. [Google Scholar] [CrossRef] [PubMed]
- Devanathan, R.; Venkatnathan, A.; Dupuis, M. Atomistic simulation of nafion membrane: I. Effect of hydration on membrane nanostructure. J. Phys. Chem. B 2007, 111, 8069–8079. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Li, Q.; He, B.; Sun, Z.; Yan, F.; Cui, Z.; Li, J. High-Efficiency Separation of Mg2+/Sr2+ through a NF Membrane under Electric Field. Membranes 2022, 12, 57. https://doi.org/10.3390/membranes12010057
Liu H, Li Q, He B, Sun Z, Yan F, Cui Z, Li J. High-Efficiency Separation of Mg2+/Sr2+ through a NF Membrane under Electric Field. Membranes. 2022; 12(1):57. https://doi.org/10.3390/membranes12010057
Chicago/Turabian StyleLiu, Huan, Quan Li, Benqiao He, Zhengguang Sun, Feng Yan, Zhenyu Cui, and Jianxin Li. 2022. "High-Efficiency Separation of Mg2+/Sr2+ through a NF Membrane under Electric Field" Membranes 12, no. 1: 57. https://doi.org/10.3390/membranes12010057
APA StyleLiu, H., Li, Q., He, B., Sun, Z., Yan, F., Cui, Z., & Li, J. (2022). High-Efficiency Separation of Mg2+/Sr2+ through a NF Membrane under Electric Field. Membranes, 12(1), 57. https://doi.org/10.3390/membranes12010057





