Abstract
It is of great significance to search for efficient, renewable, biodegradable and economical membrane materials. Herein, we developed an organic-inorganic hybrid regenerated cellulose membrane (ZrO2/BCM) with excellent hydrophilic and anti-fouling properties. The membrane was prepared by introducing ZrO2 particles into an N-Methylmorpholine-N-oxide(NMMO)/bamboo cellulose(BC) solution system by the phase inversion method. The physi-chemical structure of the membranes were characterized based on thermal gravimetric analysis (TGA), Fourier transform infrared spectroscopy (ATR-FTIR), field emission scanning electron microscopy (FE-SEM), and X-ray diffraction (XRD). The modified regenerated cellulose membrane has the excellent rejection of bovine serum albumin (BSA) and anti-fouling performance. The membrane flux of ZrO2/BCM is 321.49 (L/m2·h), and the rejection rate of BSA is 91.2%. Moreover, the membrane flux recovery rate after cleaning with deionized water was 90.6%. This new type of separation membrane prepared with green materials holds broad application potential in water purification and wastewater treatment.
1. Introduction
With the continuous development of society, the problem of water pollution has attracted more and more attention [1]. Ultrafiltration technology plays a crucial role in water purification and wastewater treatment [2,3]. Ultrafiltration membranes materials mainly include polyethersulfone (PES) [4,5,6], polysulfone (PSF) [7,8] and polyvinylidene fluoride (PVDF) [9,10,11]. However, in the process of polymer membrane synthesis, non-biodegradable organic materials will cause environmental pollution and energy waste, so it is of great research significance to seek environmentally friendly and economical membrane raw materials [12].
Cellulose is one of the most abundant renewable and biodegradable organic materials [13,14]. Regenerated cellulose (poly(1,4)-d-glucose), obtained by dissolving cellulose, has the advantages of good chemical stability, high hydrophilicity and biodegradability, and has gradually become a research hotspot for membrane materials [15,16]. However, cellulose membranes suffer from low mechanical strength and poor anti-fouling property [17,18].
Membrane fouling is an crucial issue in ultrafiltration(UF) separation applications [19,20]. Contaminants such as proteins are easily adsorbed and deposited on the membrane surface and in the pores, which leads to a decrease in permeation flux and shortens the service life of the membrane [21]. In addition, the membrane structure with low mechanic strength would be damaged during the long-term operation of the membrane, resulting in reduced flux and poor flux recovery after cleaning. Therefore, improving the anti-fouling performance and stable performance of the membrane is still a challenge for membrane applications [22,23].
In recent years, the introduction of nano inorganic oxides, such as SiO2, Al2O3, TiO2, and ZrO2 into polymer membranes for improving the antifouling performance of the membrane has become one of the research hotspots [24,25,26]. The blending of these fillers would modulate the surface properties of the membrane and improves the antifouling. ZrO2 enjoys the merits of pleasurable thermal stability, corrosion resistance and good biocompatibility, which can improve the physical and chemical properties of the membrane [27,28,29]. Meanwhile, when ZrO2 is fixed on the surface of the membrane, the polarity of the Zr-O bond is easy to form a hydroxyl group (-OH) with the hydrolysis of the particle surface to improve the hydrophilicity of the membrane. Pang et al. [30] blended ZrO2 with PES to prepare an anti-fouling composite ultrafiltration membrane which has a higher porosity and a larger average pore size. Shen et al. [31] used a two-step method to introduce functionalized ZrO2 into the PVDF ultrafiltration membrane to improve the separation efficiency of the membrane for oil-water mixtures. Wen et al. [32] prepared a dense ZrO2 ultrafiltration membrane with nanocrystals as the precursor, which effectively reduced the pollution of ceramic membranes. It can be seen that the introduction of nano-ZrO2 into the membrane material improves the permeability and antifouling performance of the membrane.
However, the modification of cellulose membrane by nano-ZrO2 has been rarely reported. In this work, the ZrO2/BCM was prepared by blending nano-ZrO2 with natural bamboo cellulose (BC) using a convenient phase inversion method. This study focuses on the systematic analysis of the effect of ZrO2 on the surface microstructure, composition, crystal structure, and thermal stability of regenerated cellulose. In addition, the protein retention performance of ZrO2/cellulose membrane and the stability during the filtration process were also studied.
2. Materials and Methods
2.1. Materials
Cellulose with a polymerization degree of 650 was kindly provided by Sichuan Tianzhu Bamboo Resources Development Co., Ltd. (Chengdu, China). N-Methylmorpholine- N-oxide (NMMO) (Analytical reagent > 97%) was obtained from Tianjin Hainachuan Science and Technology Development Co., Ltd. (Tianjin, China). Gallic acid (PG, Sinopharm Group Chemical Reagent Co., Ltd., Shanghai, China). Zirconium Dioxide (ZrO2) was obtained from Macklin Co., Ltd. (Shanghai, China). Bovine serum albumin (BSA) was purchased from Aladdin Chemical Reagent Co., Ltd., Shanghai, China. The water used in this work was deionized.
2.2. Preparation of the Membrane
The preparation of the membrane was schemed in Figure 1. Firstly, an appropriate amount of BC was dried at 60 °C under vacuum for 12–24 h before use. Secondly, the nano-ZrO2 particles (0.5–2.5 wt.%, based on BC) were added into the 85 wt.% NMMO aqueous solution and ultrasonically dispersed for 30 min [33,34]. The above mixture was then heated to 90 °C, followed by the addition of 2~3 wt.‰ of antioxidant (n-propyl gallate). The dried BC (3 wt.%, based on NMMO) was further dissolved in the mixture with continuously stirring for 2~3 h at a raised temperature of 110 °C. The resulting mixture was defoamed for 4~6 h at 90 °C to obtain a uniform brown-yellow casting membrane liquid.
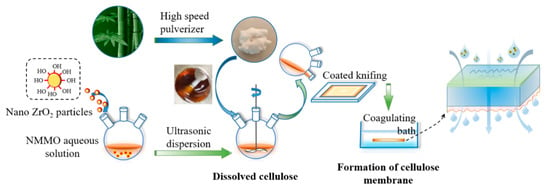
Figure 1.
The scheme of the preparation of ZrO2/BCM.
The casting membrane liquid was poured onto the non-woven fabric on the coating machine. The scraper was heated to 60~90 °C and the scraping speed was controlled to 20 cm/min. The as-scraped membrane was placed in air for 10~15 s and then soaked in deionized water for another 24~48 h [35]. Finally, the membrane was taken out and dried in the air to obtain a regenerated cellulose membrane with a thickness of 300 μm. The obtained modified cellulose regenerated membrane was recorded as ZrO2/BCM. The unmodified regenerated cellulose membrane was prepared as the same procedure except for the addition of ZrO2, and recorded as BCM.
2.3. Membrane Characterization
Cellulose regenerated membranes were observed on a scanning electron microscope (SEM, Zeiss Sigma 300, Carl Zeiss AG, Jena, Germany). The crystallinity of the BCM and ZrO2/BCM were determined by an X-ray diffraction system (Ultima IV, Rigaku, Tokyo, Japan). The test angle was set from 5° to 90° at the speed of 2.4 °/min. Infrared spectra in the 4000~600 cm−1 range were recorded with an FT-IR instrument (Bruker VERTEX 70 & ALPHA, ) at room temperature. The mass change of the membrane material as a function of temperature was recorded with a TG-DTA Instruments (TG209F3, NETZSCH, Selb, Germany) at a nitrogen flow rate of 20 mL/min. Approximately 2–3 mg of sample was weighed and heated from 25 to 900 °C.
2.4. Performance of Regenerated Cellulose Membranes
(1)Pure Water Flux of the Membrane.
The permeation performance of the membranes was tested as shown in Figure 2. The membrane was pretreated in the system under testing conditions for 30 min at a pressure of 0.1 MPa until the pressure and water output were stable. After that, the volume of water passing through the membrane was recorded every three minutes. The water flux was calculated by Equation (1):
where is the permeate flux (L/m2∙h), V is the volume of permeate (L), A is the effective area of the membrane (m2), and t is the permeate collection time (h).
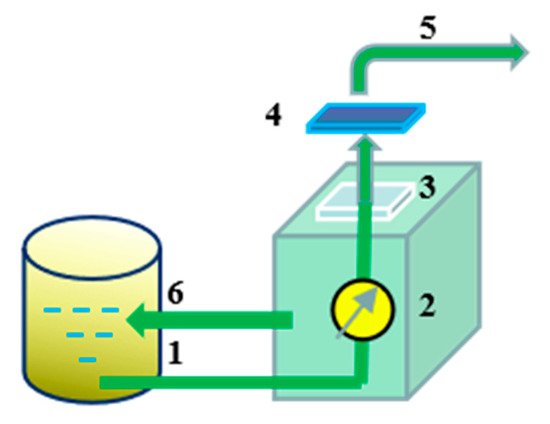
Figure 2.
Membrane filtration system: (1) Feed inlet; (2) Pump assembly; (3) Membrane carrier; (4) Membrane; (5) Effluent; (6) Circulating effluent.
(2) Separation performance of membrane.
Ultrafiltration of membranes was carried out with bovine serum albumin (BSA) as contaminant with an initial concentration of 1000 mg/L. The concentration of BSA solution in the filtrate was measured at the 278.5 nm wavelength by a UV spectrophotometer. The rejection rate of the membrane was defined by Equation (2):
where R is the membrane rejection rate (%), is initial concentration (mg/L), is filtrate concentration (mg/L).
(3) Acid and alkali resistance of regenerated cellulose membrane.
The solutions with pH of 2, 4, 8, and 10 were prepared by dilution of 1 mol/L HCl or 1 mol/L NaOH. The membrane was immersed in acid and alkali solution for five days, and the change of membrane water flux was measured to analyze the acid and alkali resistance of the regenerated cellulose membrane.
(4) Anti-fouling of regenerated cellulose membrane.
The used regenerated cellulose membrane was placed in the membrane filtration system (Figure 2) and treated with deionized water, 0.01 mol/L HCl, or 0.01 mol/L NaOH for 0.5 h, respectively. Then, the membrane was taken out and cleaned with deionized water on both sides several times. The membrane flux recovery rate (r) was obtained by comparing the water flux before and after the cleaning procedure, which was used to evaluate the anti-fouling performance of the regenerated cellulose membrane. Where r is the membrane flux recovery rate (%), is the initial water flux of the membrane (L/m2∙h), is the water flux after membrane cleaning (L/m2∙h).
2.5. Characterization of Porosity and Average Pore Size
(1) The porosity of regenerated cellulose membrane was calculated by Equation (3):
where is membrane porosity (%), is wet membrane weight (g), is dry membrane weight (g), is the density of pure water (0.998 g/cm3) at 25 °C, - is membrane area (cm2), is membrane thickness (cm).
(2) The average pore size was calculated by Caout-erfurt Ferry equation [36], Equation (4):
where is average membrane pore size (nm), is membrane porosity (%), is deionized water viscosity at 25 °C (8.9 × 10−4 Pa·s), is membrane thickness (cm), is deionized water flux (L/m2∙h), is membrane pressure at operation (MPa).
3. Results and Discussion
3.1. The Influence of ZrO2 Content on the Physical Properties of the Membrane
Membrane filtration performance is a key index to judge the function of a membrane. In this study, the effect of the addition of nano-ZrO2 particles on the filtration performance of the regenerated cellulose membrane was investigated under a test pressure of 0.1 MPa. Ultrafiltration of the membranes was carried out with bovine serum albumin (BSA) as contamination.
The influence of ZrO2 dosage on the properties of regenerated cellulose membrane is shown in Figure 3. It can be observed that the addition of ZrO2 particles increases the porosity of the regenerated cellulose membrane. The greater the porosity ensures a high membrane flux. The mass fraction of ZrO2 particles increases to 1 wt.%, the water flux of the regenerated cellulose membrane continuously increases and reaches the maximum value. Its water flux is 321.49 (L/m2∙h), indicating that the addition of ZrO2 particles improved the hydrophilicity of the regenerated cellulose membrane to a certain extent. However, with the excessive content of ZrO2 in the casting membrane liquid system, this resulted in a decrease in the pore size of the membrane, which will lead to a sharp drop in the water flux of the cellulose hybrid membrane [32,37].
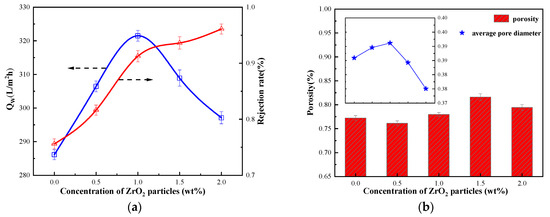
Figure 3.
(a) Filtration performance of cellulose membrane with nanoparticles; (b) Porosity of cellulose membranes with nanoparticles.
The addition of excessive nanoparticles leads to the reduction of membrane pore size and porosity. Similar experimental results were obtained previously by Shen and coworkers for the PVDF/ZrO2-g-PACMO hybrid membrane fabricated with the phase inversion method [31]. The decrease in pore size makes the membrane compact, resulting in a decreasing trend in the average pore size of the regenerated cellulose membrane. As shown in Figure 3, the decrease in pore size leads to an increase in the resistance of water to pass through the membrane, and the rejection rate of BSA continues to increase.
3.2. The Influence of ZrO2 Content on the Antifouling Performance of Membrane
Through the rejection experiment of BSA under continuous operation time, the antifouling performance of ZrO2/BCM was evaluated. The fouling of the membrane could be originated from the effects of adsorption fouling, membrane pore blockage, steric hindrance, and/or concentration polarization [24]. The inorganic nanoparticles were dispersed uniformly in the polymer, so that the interaction between the nanoparticles and the membrane matrix made the membrane have a stable antifouling performance [38]. As shown in Figure 4, ZrO2 modifies the surface of the regenerated cellulose membrane, effectively resisting the deposition of contaminants on the surface and reducing the interaction force between the contaminants and the surface [32].
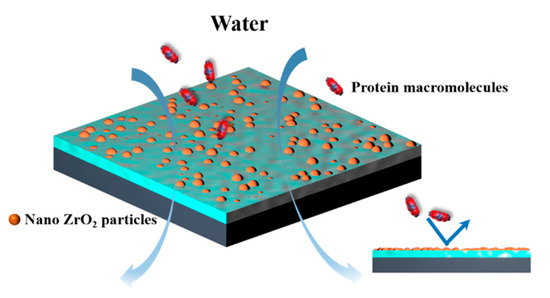
Figure 4.
Schematic diagram of ZrO2/BCM resistance to contaminant.
It can be seen intuitively from Figure 5 that before operation, the separation efficiency of BSA by the membrane is improved as ZrO2 particles are embedded into the membrane structure. It can be obtained from Table 1 that the rejection rate of BSA for the regenerated cellulose membrane with 1 wt.% of ZrO2 is 91.3%, which was decreased over time. When operating for 180 min, the BSA rejection rate of the BCM decreased by 40% to 45.3%. In the same period, the rejection rate of 1 wt.% ZrO2/BCM to BSA was 60% higher than that of BCM. ZrO2/BCM shows excellent antifouling performance. The ZrO2 ultrafiltration membrane prepared by Wen et al. also showed excellent resistance to BSA [32].
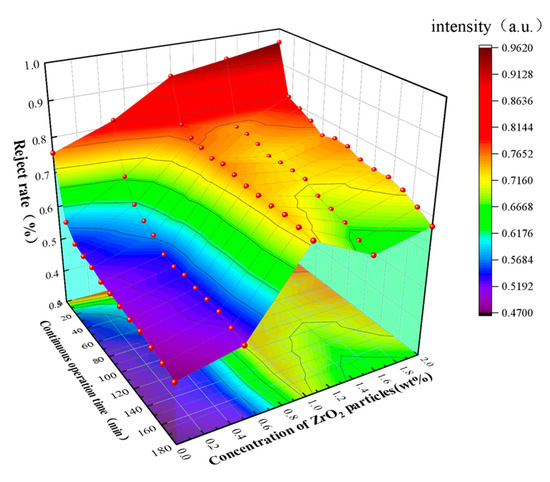
Figure 5.
The impact of running time on membrane rejection performance.

Table 1.
Changes of BSA separation efficiency under different ZrO2 content.
The change of membrane flux of BSA solution during continuous operation time is shown in Figure 6. At the beginning of the operation, BSA rapidly accumulates on the membrane surface and in the pores, resulting in a significant decrease in membrane flux. When the contaminants on the surface and inside of the membrane accumulate to a certain extent, the shear force generated by the cross-flow of the filtrate on the membrane surface will gradually reach equilibrium. The interaction between pollutants becomes the dominant factor in changing the membrane flux, and the resistance on the membrane surface tends to be flat. Therefore, the membrane flux changes smoothly in the later stage of the experiment, and the membrane flux change of ZrO2/BCM was below 4%. In summary, when the mass fraction of ZrO2 particles is 1 wt.%, ZrO2/BCM has good stability and rejection of BSA. Table 2 shows BCM and 1 wt.% ZrO2/BCM membrane performance parameters.
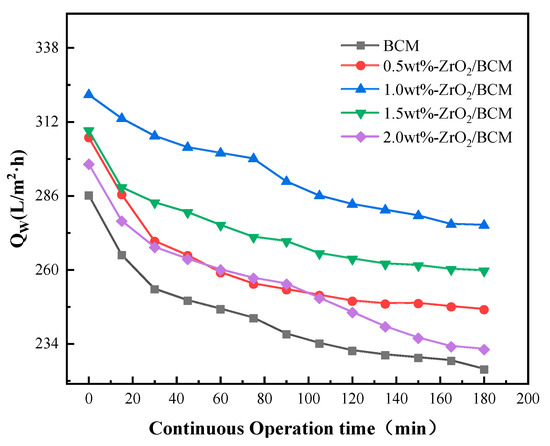
Figure 6.
The influence of operation time on the change of membrane flux of BSA solution.

Table 2.
Membrane performance parameters of BCM and ZrO2/BCM.
Hydrophilicity is an important attribute of the membrane, which directly affects the permeability of the membrane. As shown in Figure 7. The contact angle of the BCM is 43.9° ± 2.2°. Cellulose itself is highly hydrophilic. The contact angle of the 1 wt.% ZrO2/BCM is 33.6° ± 3.7°. It indicates that during the membrane formation process, nano-ZrO2 is distributed on the surface and throughout the bulk of the membrane, and the hydroxyl groups carried by them enrich the hydrophilicity of the membrane [30].
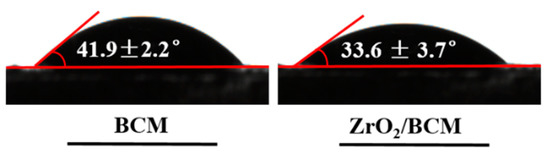
Figure 7.
Contact angle images of BCM and ZrO2/BCM.
3.3. Characterization of Regenerated Cellulose Membrane
3.3.1. SEM Observations
The microstructures of the regenerated cellulose membrane were observed by SEM scanning electron microscope. As shown in Figure 8a, the surface of the BCM membrane presents a large number of pore structures. Regenerated cellulose membranes are extremely hydrophilic, and will quickly undergo liquid-liquid stratification with water in the water coagulation bath, thereby forming larger pores [39]. Nanoparticles will spontaneously form agglomerates [25,40]. As shown in Figure 8c, after adding 1 wt.% of ZrO2 particles, the surface of the membrane became dense without agglomeration, indicating that the nanoparticles were uniformly dispersed in the regenerated cellulose membrane. However, with the addition of excessive ZrO2 particles (2 wt.%), agglomeration of nanoparticles appears on the surface of the membrane. It indicates a decrease in the average pore size of the membrane and an improvement in the structure of the membrane surface by adding nanoparticles [41].
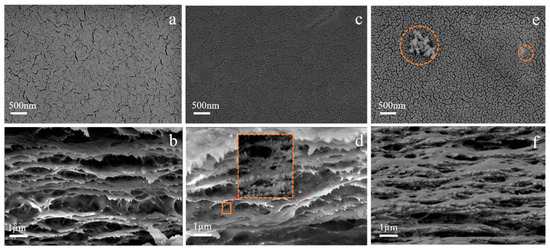
Figure 8.
(a,c,e) SEM of the surface of BCM, 1 wt.% ZrO2/BCM and 2 wt.% ZrO2/BCM; (b,d,f) SEM of the cross section of BCM, 1 wt.% ZrO2/BCM and 2 wt.% ZrO2/BCM.
It can be seen from Figure 8b that the BCM membrane structure has a clear layered and porous structure. It will reduce the stability of the membrane. In Figure 8d there are a large number of dispersed white spots in the regenerated cellulose membrane structure when 1.0 wt% ZrO2 particles are added. Under high magnification, it can be seen that the ZrO2 particles are uniformly attached to the surface of the membrane. The addition of ZrO2 reduces the macropores of the membrane structure and improves the membrane structure. However, the excessive amount of ZrO2 caused the blockage of the membrane structure.
The elemental composition of ZrO2/BCM was investigated by EDSs detection from two randomly selected points from ZrO2/BCM [42]. As shown in Figure 9, the C and O elements were detected, proving the chemical composition of cellulose. Meanwhile, the presence of Zr elements was also detected. Combined with the SEM images of the surface and cross-section of ZrO2/BCM, it is shown that ZrO2 nanoparticles have been uniformly dispersed in the casting membrane liquid and embedded in the regenerated cellulose membrane to improve the structure of the membrane [27,37].
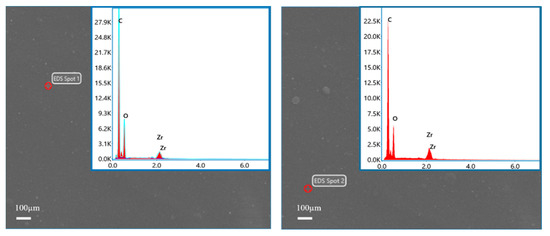
Figure 9.
EDs spectrum of 1 wt.% ZrO2/BCM.
3.3.2. ATR-FTIR Analysis
Figure 10a shows the FT-IR spectra of BC, BCM, 1 wt.% ZrO2/BCM. Since intramolecular and intermolecular hydrogen bonds are generated during the dissolution of cellulose, -OH and -NH stretching vibration intensity peaks appear at 3378.03 cm−1 and 3354.17 cm−1. In the figure, there is C-H stretching at 2900 cm−1 and 2898.64 cm−1 [12]. In addition, there ais C=O stretching at 1630 cm−1 and C-O stretching vibration peaks at 1060 cm−1 in all three, which prove the presence of cellulose components [15,43]. The position of the ZrO2/BCM absorption peak is the same as that of BCM. There is no new absorption peak in the FT-IR spectrum.
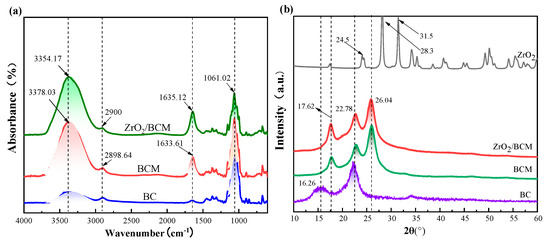
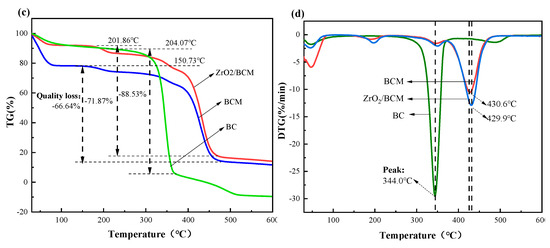
Figure 10.
(a) FT-IR spectra of regenerated cellulose membrane; (b) XRD of BC, BCM, 1 wt.% ZrO2/BCM; (c,d) TG and DTG patterns for BC, BCM, 1 wt.% ZrO2/BCM.
3.3.3. XRD of Regenerated Cellulose Membrane
Figure 10b shows that there are strong diffraction peaks at the 2θ of 16.26°, 22.78° and 26.04°, corresponding to the three crystal planes (101), (002), and (040) of cellulose [44]. As the intramolecular and intermolecular hydrogen bonds of cellulose are opened during the dissolution process, the crystalline structure of cellulose is destroyed, and the crystallinity of cellulose gradually decreases during the process of dissolution and regeneration [45]. The XRD spectra showed strong diffraction peaks consistent with the characteristic peak positions of ZrO2 particles at 2θ of 24.5°, 28.3°, 31.5°, and 34.5° [37]. Observing the XRD spectrum of 1 wt.% ZrO2/BCM, no other new diffraction peaks were found. Combined with the absence of new characteristic peaks in FT-IR, it can be inferred that the ZrO2 particles and cellulose are combined in a blended form. In addition, the addition of ZrO2 particles gradually weakened the degree of crystallinity of the regenerated cellulose membrane [32,46]. It can be inferred that there is a certain force between the ZrO2 particles and the cellulose polymer, which changes the stress distribution of the regenerated cellulose membrane. This shows the compatibility of ZrO2 particles and good affinity with the membrane matrix.
3.3.4. TGA Analysis
TG shows that there is evaporation of water on the surface of all three of them below 100 °C as shown in Figure 10c [47]. The initial decomposition temperatures of the three are 204.07 °C, 150.73 °C, and 201.86 °C, respectively. The results show that compared with the original bamboo cellulose (BC), the thermal stability of BCM was slightly lower, which might be caused by cellulose degradation in the dissolution and regeneration processes [48]. In addition, the residual amount of ZrO2/BCM after thermal decomposition is also higher than that of BCM, which proves the existence of ZrO2 in the membrane matrix. The addition of ZrO2 particles increased the initial decomposition temperature of the membrane, ZrO2/BCM has better thermal stability.
3.4. Acid and Alkali Resistance of Regenerated Cellulose Membrane
The acid and alkali resistance of membranes was further explored to expand the application of membranes in different conditions. As shown in Figure 11, the membrane flux of regenerated cellulose membrane under acid/base conditions is higher than the initial membrane flux. It may be due to the corrosion in a strong acid/base. After soaking for five days in pH 10 solution, the membrane flux of the regenerated cellulose membrane changed the most, in which the flux of BCM and 1wt.% ZrO2/BCM reached 447.13 L/m2 h and 412.7 L/m2 h, respectively. Compared with the initial membrane flux, the membrane flux of 1 wt%ZrO2/BCM has a smaller change compared with BCM. It indicates that the addition of ZrO2 particles improves the acid and alkali resistance of the regenerated cellulose membrane.
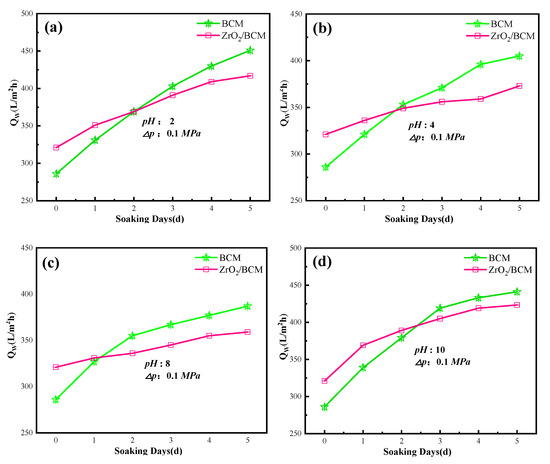
Figure 11.
The influence of pH on the membrane fluxes of BCM and 1 wt.% ZrO2/BCM. The test conditions (a–d) of the membrane are respectively: pH 2, 4, 8, 10.
3.5. Anti-Fouling of Regenerated Cellulose Membrane
It is necessary to clean the contaminated membrane to improve the ultrafiltration performance of the membrane.
Figure 12 shows the membrane flux recovery rate after cleaning the used regenerated cellulose membrane under different conditions. As can be seen, the membrane flux recovery rate of the regenerated cellulose membrane basically reached 90% by washing with HCl and NaOH. The membrane flux of BCM could hardly be recovered with water as the washing agent, and the recovery was about 65%. On the other hand, the membrane flux of 1 wt.% ZrO2/BCM after cleaning with deionized water reaches 290.8 (L/m2h). It also has a recovery rate of 90.6%, 24.6% higher than that of BCM. It was rationalized that the addition of ZrO2 effectively reduces the contact of pollutants with the membrane surface, facilitating the removal of the pollutants adsorbed on the modified regenerated cellulose membrane by shearing force.
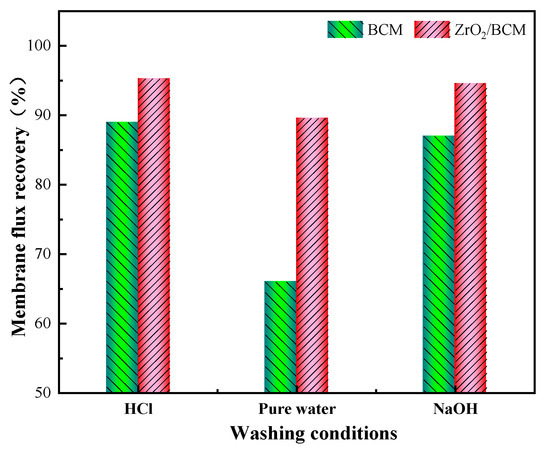
Figure 12.
The flux recovery rate of BCM and 1 wt.%-ZrO2/BCM.
4. Conclusions
Membrane fouling and membrane materials are the main challenges in membrane applications. In this study, an ultrafiltration membrane (ZrO2/BCM) was prepared by blending ZrO2 particles with natural bamboo cellulose by the phase inversion method. Compared with the original regenerated cellulose membrane, ZrO2/BCM has better stability and anti-fouling properties. The water flux of the ZrO2/BCM membrane reaches 321.49 (L/m2∙h). In the dynamic ultrafiltration experiment, ZrO2/BCM showed long-term performance stability, and the membrane flux recovery rate after cleaning with deionized water was 90.6%. In summary, the ZrO2/BCM membrane has broader application prospects in the field of water treatment. The membrane prepared in this study can improve the application of degradable membrane material in the green, clean membrane separation process, and promote the sustainable development of communities of human destiny.
Author Contributions
X.H.: Methodology, Investigation, Writing-Original draft, Data curation, Visualization, Supervision. F.T. and G.C.: Investigation, Data curation. F.W.: Writing-Reviewing and Editing, Supervision. R.W.: Supervision, Writing-Reviewing and Editing, Project administration, Funding acquisition. B.X.: Supervision, Writing-Reviewing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China [Grant NO.2019YFC1904101], the Research Development Foundation of Fujian University of Technology [GY-Z18186], the Initial Scientific Research Foundation of Fujian University of Technology [GY-Z18063,GY-Z20014].
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alexandratos, S.D.; Barak, N.; Bauer, D.; Davidson, F.T.; Gibney, B.R.; Hubbard, S.S.; Taft, H.L.; Westerhof, P. Sustaining Water Resources: Environmental and Economic Impact. ACS Sustain. Chem. Eng. 2019, 7, 2879–2888. [Google Scholar] [CrossRef] [Green Version]
- Cheryan, M. Ultrafiltration and Microfiltration Handbook; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Pendergast, M.M.; Hoek, E.M. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef] [Green Version]
- Okoro, H.K.; Ndlwana, L.; Ikhile, M.I.; Barnard, T.G.; Ngila, J.C. Hyperbranched polyethylenimine-modified polyethersulfone (HPEI/PES) and nAg@HPEI/PES membranes with enhanced ultrafiltration, antibacterial, and antifouling properties. Heliyon 2021, 7, e07961. [Google Scholar] [CrossRef]
- Ndlwana, L.; Motsa, M.M.; Mamba, B.B. A New Method for a Polyethersulfone-Based Dopamine-Graphene (xGnP-DA/PES) Nanocomposite Membrane in Low/Ultra-Low Pressure Reverse Osmosis (L/ULPRO) Desalination. Membranes 2020, 10, 439. [Google Scholar] [CrossRef]
- Omidvar, M.; Hejri, Z.; Moarefian, A. The effect of Merpol surfactant on the morphology and performance of PES/PVP membranes: Antibiotic separation. Int. J. Ind. Chem. 2019, 10, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Cheshomi, N.; Pakizeh, M.; Namvar-Mahboub, M. Preparation and characterization of TiO2/Pebax/(PSf-PES) thin film nanocomposite membrane for humic acid removal from water. Polym. Adv. Technol. 2018, 29, 1303–1312. [Google Scholar] [CrossRef]
- Shaari, N.Z.K.; Basri, M.F.; Yazid, R.R.M.; Sulaiman, N.A.; Ramlee, S. Thin Film Composite Membrane from PSF/Chitosan/PEG: Effect of PVA Concentration on the Removal of Mercury and Antifouling Properties. Int. J. Recent Technol. Eng. IJRTE 2019, 8, 6924–6928. [Google Scholar]
- Fangshu, Q.; Akun, C.; Yang, Y.; Sakil, M.; Peiyang, S.; Jingxin, Y.; Zijun, H.; Qiaoyun, L.; Lijing, Z.; Zhipeng, T.; et al. Hierarchically superhydrophilic poly(vinylidene fluoride) membrane with self-cleaning fabricated by surface mineralization for stable separation of oily wastewater. J. Membr. Sci. 2021, 640, 119864. [Google Scholar]
- Sivasankaran, A.; Rajesh, P.; YoungHo, A. Antibacterial and Adsorption Properties of Sulfonated GO-PVDF Nanocomposite Ultrafiltration Membranes for Environmental Applications. J. Environ. Eng. 2021, 147, 04021042. [Google Scholar]
- Xinyan, G.; Tao, L.; Fuchun, J.; Xue, Z. Impacts on characteristics and effluent safety of PVDF ultrafiltration membranes aged by different chemical cleaning types. J. Membr. Sci. 2021, 640, 119770, (prepublish). [Google Scholar]
- Weng, R.; Huang, X.; Liao, D.; Xu, S.; Peng, L.; Liu, X. A novel cellulose/chitosan composite nanofiltration membrane prepared with piperazine and trimesoyl chloride by interfacial polymerization. RSC Adv. 2020, 10, 1309–1318. [Google Scholar] [CrossRef] [Green Version]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Polym. Sci. 2005, 44, 3358–3393. [Google Scholar]
- Feng, N.L.; Malingam, S.D.; Jenal, R.; Mustafa, Z.; Subramonian, S. A review of the tensile and fatigue responses of cellulosic fibre-reinforced polymer composites. Mech. Adv. Mater. Struct. 2018, 27, 645–660. [Google Scholar] [CrossRef]
- Guo, H.; Peng, Y.; Liu, Y.; Wang, Z.; Hu, J.; Liu, J.; Ding, Q.; Gu, J. Development and investigation of novel antifouling cellulose acetate ultrafiltration membrane based on dopamine modification. Int. J. Biol. Macromol. 2020, 160, 652–659. [Google Scholar] [CrossRef]
- Yang, S.; Wang, T.; Tang, R.; Yan, Q.; Tian, W.; Zhang, L. Enhanced permeability, mechanical and antibacterial properties of cellulose acetate ultrafiltration membranes incorporated with lignocellulose nanofibrils. Int. J. Biol. Macromol. 2020, 151, 159–167. [Google Scholar] [CrossRef]
- Zhang, J.; Kitayama, H.; Gotoh, Y.; Potthast, A.; Rosenau, T. Non-woven fabrics of fine regenerated cellulose fibers prepared from ionic-liquid solution via wet type solution blow spinning. Carbohydr. Polym. 2019, 226, 115258. [Google Scholar] [CrossRef]
- Moriam, K.; Sawada, D.; Nieminen, K.; Hummel, M.; Ma, Y.; Rissanen, M.; Sixta, H. Towards regenerated cellulose fibers with high toughness. Cellulose 2021, 28, 9547–9566. [Google Scholar] [CrossRef]
- Kanagaraj, P.; Mohamed, I.M.A.; Huang, W.; Liu, C. Membrane fouling mitigation for enhanced water flux and high separation of humic acid and copper ion using hydrophilic polyurethane modified cellulose acetate ultrafiltration membranes. React. Funct. Polym. 2020, 150, 9547–9566. [Google Scholar] [CrossRef]
- Weng, R.; Tian, F.; Huang, X.; Ni, L.; Xi, B. Preparation of cellulose nanofiltration membranes and their removal of typical pollutants from drinking water. Water Suppl. 2021, 21, 4355–4368. [Google Scholar] [CrossRef]
- Jurchevsky, E.B.; Pervov, A.G. Potentialities of Membrane Water Treatment for Removing Organic Pollutants from Natural Water. Therm. Eng. 2020, 67, 484–491. [Google Scholar] [CrossRef]
- Hou, S.; Xing, J.; Dong, X.; Zheng, J.; Li, S. Integrated antimicrobial and antifouling ultrafiltration membrane by surface grafting PEO and N-chloramine functional groups. J. Colloid Interface Sci. 2017, 500, 333–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Zhang, B.; Liu, Z.; Deng, B.; Yu, M.; Li, L.; Jiang, H.; Li, J. Preparation of the antifouling microfiltration membranes from poly(N,N-dimethylacrylamide) grafted poly(vinylidene fluoride) (PVDF) powder. J. Mater. Chem. 2011, 21, 11908–11915. [Google Scholar] [CrossRef]
- Yin, J.; Zhou, J. Novel polyethersulfone hybrid ultrafiltration membrane prepared with SiO2-g-(PDMAEMA- co -PDMAPS) and its antifouling performances in oil-in-water emulsion application. Desalination 2015, 365, 46–56. [Google Scholar] [CrossRef]
- Shi, F.; Ma, Y.; Ma, J.; Wang, P.; Sun, W. Preparation and characterization of PVDF/TiO2 hybrid membranes with different dosage of nano-TiO2. J. Membr. Sci. 2012, 389, 522–531. [Google Scholar] [CrossRef]
- Bindes, M.M.M.; Terra, N.M.; Patience, G.S.; Boffito, D.C.; Cardoso, V.L.; Reis, M.H.M. Asymmetric Al2O3 and PES/Al2O3 hollow fiber membranes for green tea extract clarification. J. Food Eng. 2020, 277, 109889. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, Y.; Guan, K.; Peng, C.; Wu, J. Preparation of ZrO2 fiber modified Al2O3 membrane supports with enhanced strength and permeability. J. Eur. Ceram. Soc. 2019, 39, 1712–1716. [Google Scholar] [CrossRef]
- Maximous, N.; Nakhla, G.; Wan, W.; Wong, K. Performance of a novel ZrO2/PES membrane for wastewater filtration. J. Membr. Sci. 2010, 352, 222–230. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Thanikaivelan, P. Fabrication of cellulose acetate–zirconia hybrid membranes for ultrafiltration applications: Performance, structure and fouling analysis. Sep. Purif. Technol. 2010, 74, 230–235. [Google Scholar] [CrossRef]
- Pang, R.; Li, X.; Li, J.; Lu, Z.; Sun, X.; Wang, L. Preparation and characterization of ZrO2/PES hybrid ultrafiltration membrane with uniform ZrO2 nanoparticles. Desalination 2014, 332, 60–66. [Google Scholar] [CrossRef]
- Shen, X.; Xie, T.; Wang, J.; Liu, P.; Wang, F. An anti-fouling poly(vinylidene fluoride) hybrid membrane blended with functionalized ZrO2 nanoparticles for efficient oil/water separation. RSC Adv. 2017, 7, 5262–5271. [Google Scholar] [CrossRef] [Green Version]
- Wen, J.; Yang, C.; Chen, X.; Qiu, M.; Fan, Y. Effective and efficient fabrication of high-flux tight ZrO2 ultrafiltration membranes using a nanocrystalline precursor. J. Membr. Sci. 2021, 634, 119378. [Google Scholar] [CrossRef]
- Nguyen, H.V.D.; De Vries, R.; Stoyanov, S.D. Natural Deep Eutectics as a “Green” Cellulose Cosolvent. ACS Sustain. Chem. Eng. 2020, 8, 14166–14178. [Google Scholar] [CrossRef]
- Protz, R.; Lehmann, A.; Ganster, J.; Fink, H.P. Solubility and spinnability of cellulose-lignin blends in aqueous NMMO. Carbohydr. Polym. 2021, 251, 117027. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.N.; Ruan, H.M.; Wu, L.G.; Gao, C.J. Preparation and characterization of PES–SiO2 organic–inorganic composite ultrafiltration membrane for raw water pretreatment. Chem. Eng. J. 2011, 168, 1272–1278. [Google Scholar] [CrossRef]
- Rezaee, R.; Nasseri, S.; Mahvi, A.H.; Nabizadeh, R.; Mousavi, S.A.; Rashidi, A.; Jafari, A.; Nazmara, S. Fabrication and characterization of a polysulfone-graphene oxide nanocomposite membrane for arsenate rejection from water(Article). J. Environ. Health Sci. Eng. 2015, 13, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Jin, Z.; Chen, X.; Fan, J.; Qiu, M.; Fu, K.; Fan, Y. Modified wet chemical method synthesis of nano-ZrO2 and its application in preparing membranes. Ceram. Int. 2021, 47, 13432–13439. [Google Scholar] [CrossRef]
- Li, J.F.; Xu, Z.L.; Hu, Y.; Yu, L.Y.; Min, L.J.A.S.S. Effect of TiO2 nanoparticles on the surface morphology and performance of microporous PES membrane. Appl. Surf. Sci. 2009, 255, 4725–4732. [Google Scholar] [CrossRef]
- Yuan, H.; Wu, J.; Wang, D.; Huang, L.; Chen, L.; Lin, S. Ultra-high-strength composite films prepared from NMMO solutions of bamboo-derived dissolving pulp and chitosan. Ind. Crops Prod. 2021, 170, 113747. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, H.-Z.; Xu, Z.-F.; Sun, S.-W.; Liu, Y.-H. Study on characterization of core-shell nano-Al2O3/PS composite particles and toughening polystyrene prepared by SLS. J. Mater. Eng. 2007, 3, 24–27. [Google Scholar] [CrossRef]
- Razmjou, A.; Mansouri, J.; Chen, V. The effects of mechanical and chemical modification of TiO2 nanoparticles on the surface chemistry, structure and fouling performance of PES ultrafiltration membranes. J. Membr. Sci. 2011, 378, 73–84. [Google Scholar] [CrossRef]
- Mosayebi, A.; Esfahani, H.; Hoor, M. Influence of zeta potential of ZrO2 and Al2O3 nanoparticles on removal of metal ions by hybrid electrospun polyamide 6 membrane: Kinetics of adsorption and fouling mechanisms. Can. J. Chem. Eng. 2021, 99, S654–S667. [Google Scholar] [CrossRef]
- Fink, H.-P.; Weigel, P.; Purz, H.J. Structure formation of regenerated cellulose materials from NMMO-solutions. Prog. Polym. Sci. 2001, 26, 1473–1524. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, Y.; Chen, Q.; Yu, Z.; Wang, C.; Jin, S.; Ding, Y.; Wu, G. Dissolution of cellulose with ionic liquids and its application: A mini-review. Green Chem. 2006, 8, 325–327. [Google Scholar] [CrossRef]
- Li, S.; Liu, S.; Huang, F.; Lin, S.; Zhang, H.; Cao, S.; Chen, L.; He, Z.; Lutes, R.; Yang, J.; et al. Preparation and Characterization of Cellulose-Based Nanofiltration Membranes by Interfacial Polymerization with Piperazine and Trimesoyl Chloride. ACS Sustain. Chem. Eng. 2018, 6, 13168–13176. [Google Scholar] [CrossRef]
- Weng, R.; Chen, L.; Lin, S.; Zhang, H.; Wu, H.; Liu, K.; Cao, S.; Huang, L. Preparation and Characterization of Antibacterial Cellulose/Chitosan Nanofiltration Membranes. Polymers 2017, 9, 116. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Fan, M.; Chen, L.; Zhuang, J. Lignocellulosic fibre mediated rubber composites: An overview. Compos. Part B Eng. 2015, 76, 180–191. [Google Scholar] [CrossRef]
- Lin, S.; Chen, L.; Huang, L.; Cao, S.; Luo, X.; Liu, K.; Huang, Z. Preparation and characterization of chitosan/cellulose blend films using ZnCl2·3H2O as a solvent. BioResources 2013, 7, 5488–5499. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).