Optimization of a MOF Blended with Modified Polyimide Membrane for High-Performance Gas Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of UiO-66-NH2
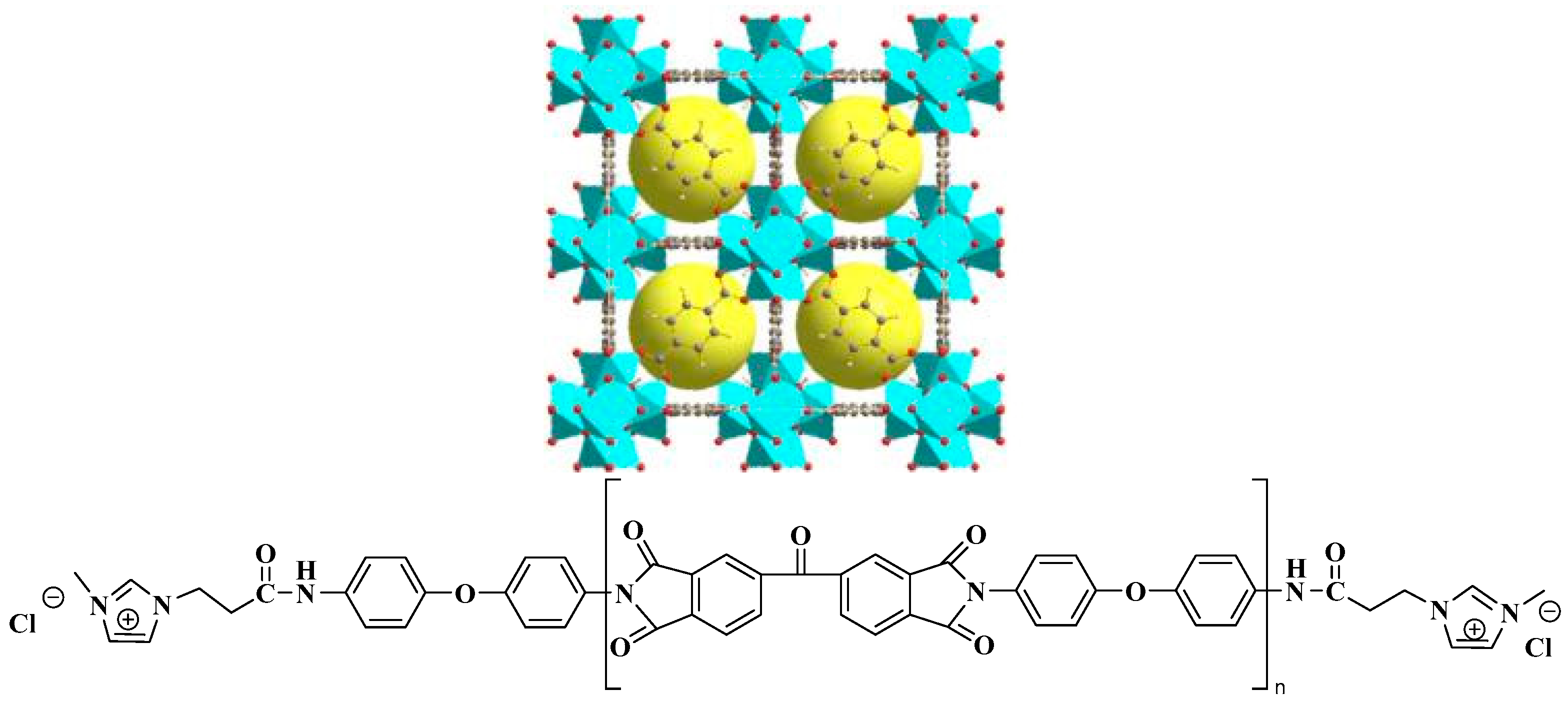
2.3. Synthesis of PI-IL/UiO-66-NH2 Membranes
2.4. Characterization of the Materials
2.5. Gas Permeation Measurements of Membranes
3. Results
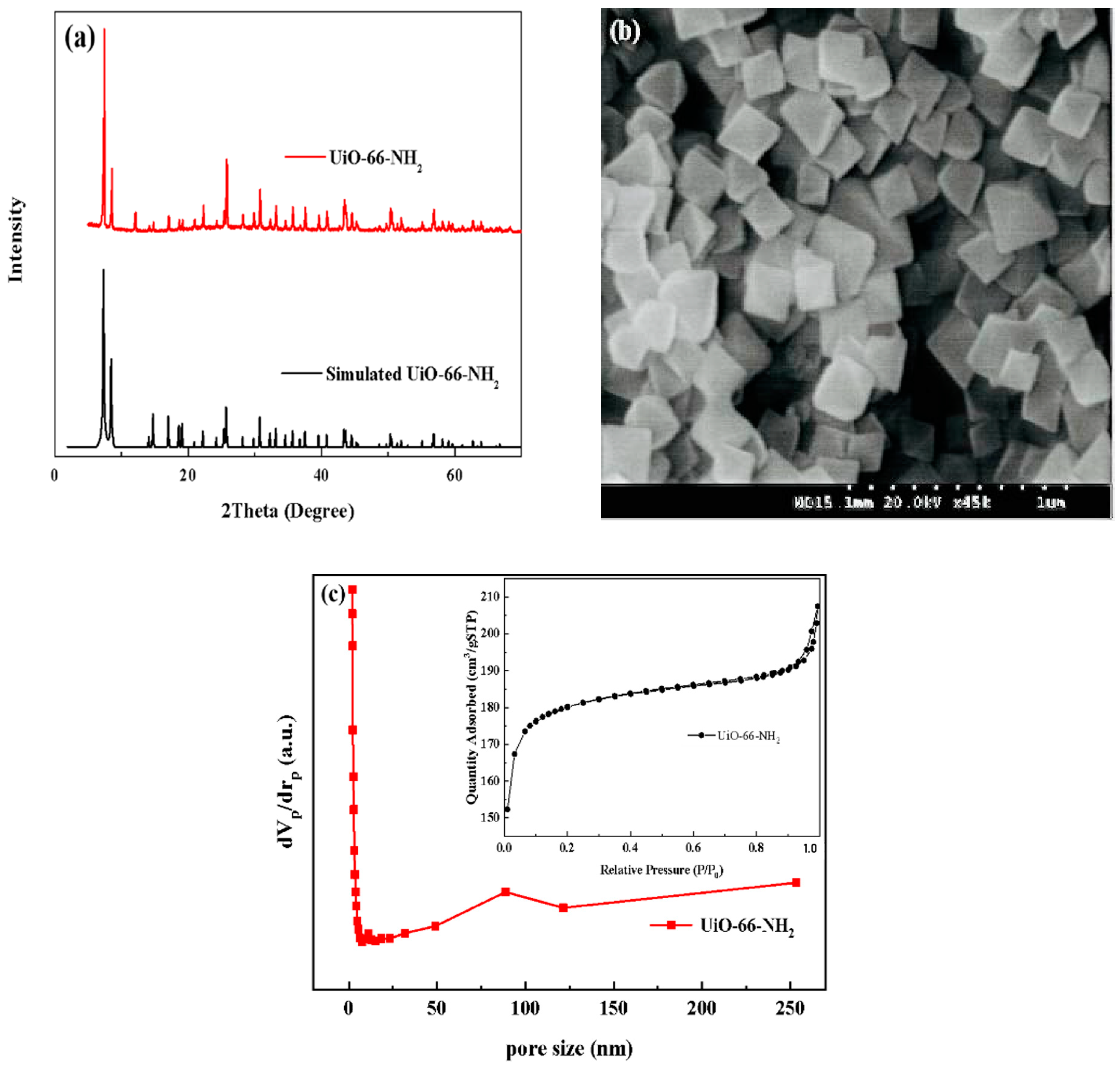
3.1. Fabrication and Characterization of UiO-66-NH2
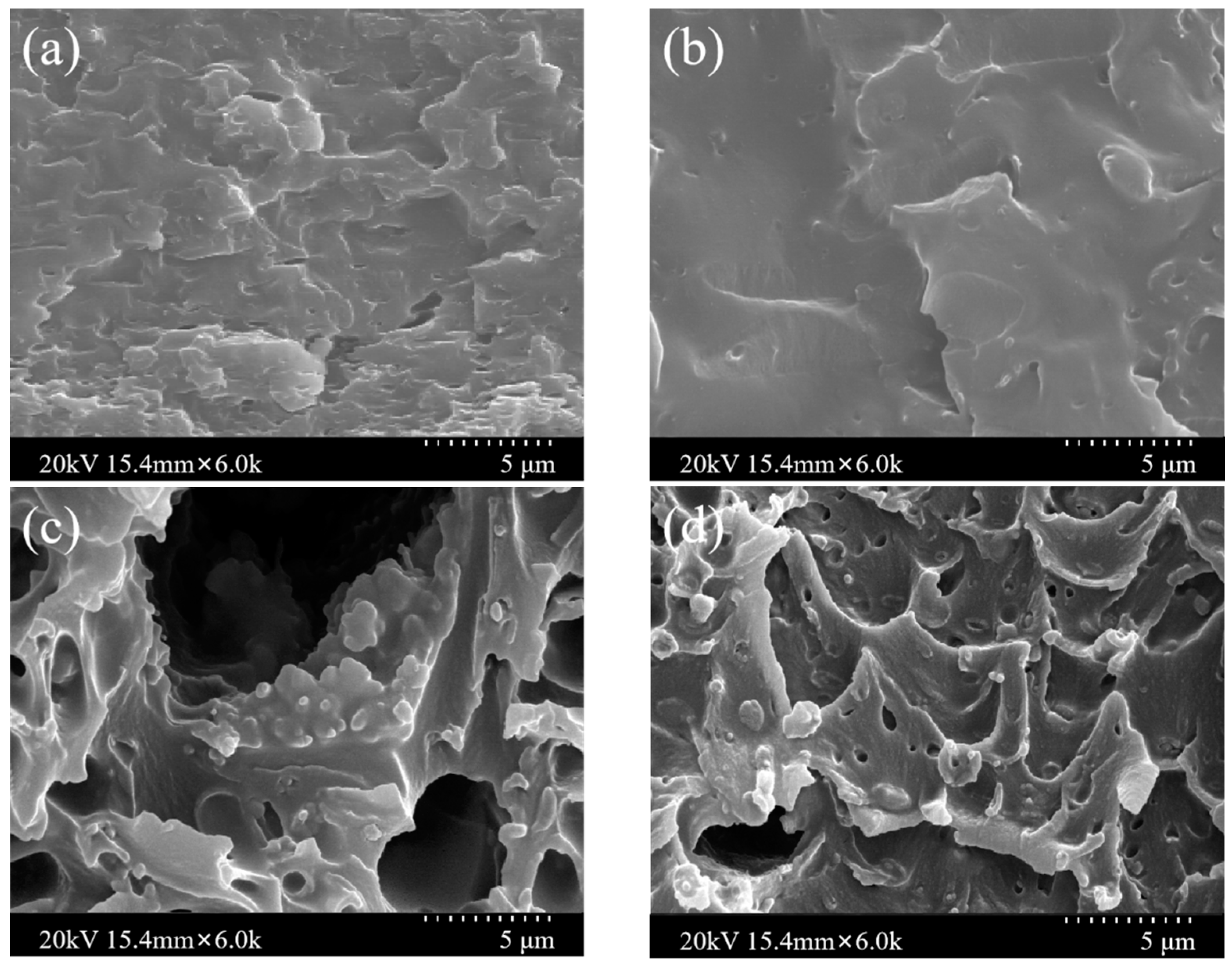
3.2. Characterization of MMMs
3.3. Mechanical Properties of Membranes
3.4. Thermal Properties of the Membranes
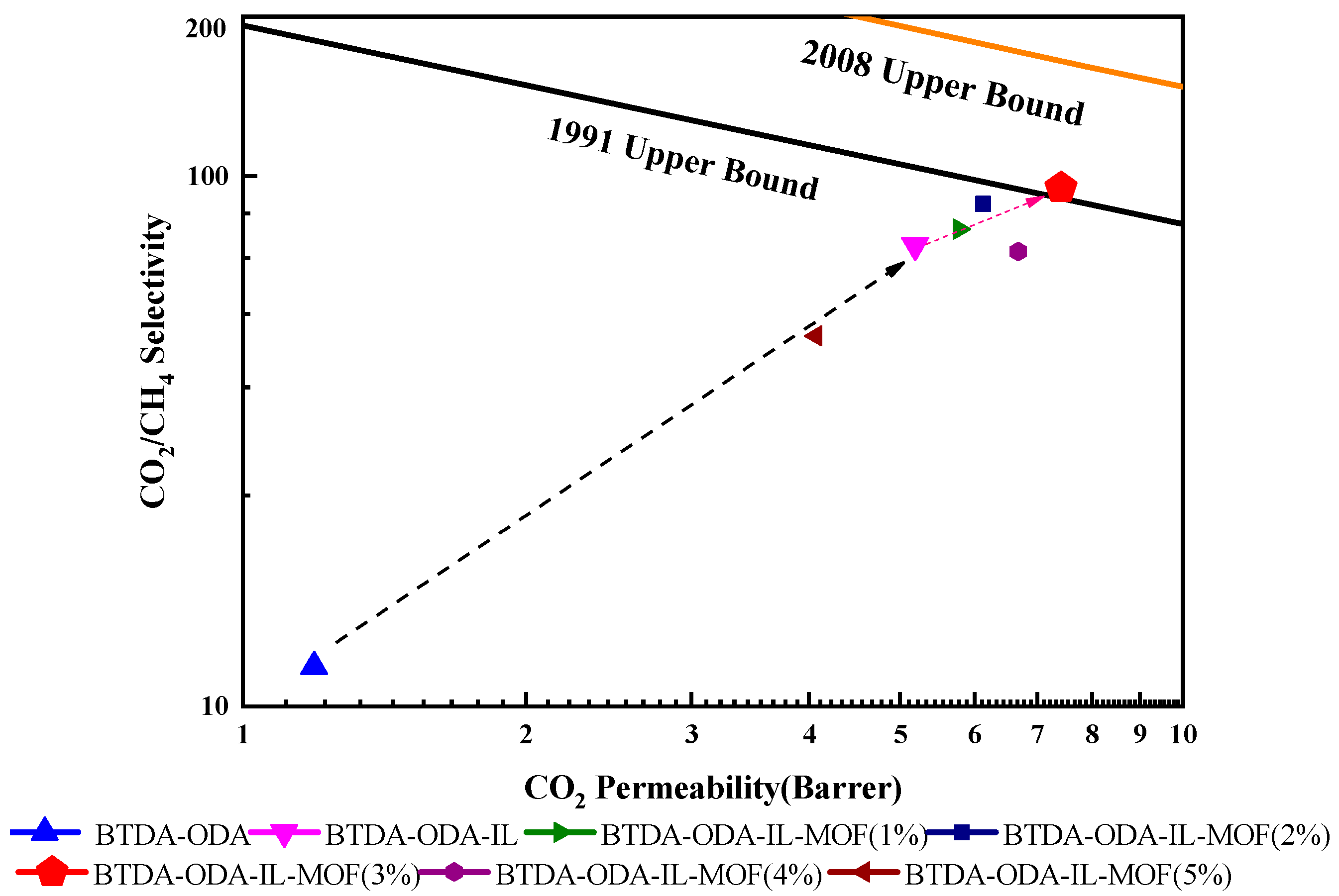
3.5. Gas Permeation Performance of the MMMs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michael, T. Publikationen. Energy Technology Perspectives 2010: Scenarios and Strategies to 2050; International Energy Agency: Paris, France, 2010. [Google Scholar]
- Pratibha, P.; Chauhan, R.S. Memranes for gas separation. Prog. Polym. Sci. 2001, 26, 853–893. [Google Scholar] [CrossRef]
- Galizia, M.; Chi, W.S.; Smith, Z.P.; Merkel, T.C.; Baker, R.W.; Freeman, B.D. 50th anniversary perspective: Polymers and mixed matrix membranes for gas and vapor separation: A review and prospective opportunities. Macromolecules 2017, 50, 7809–7843. [Google Scholar] [CrossRef]
- Basu, S.; Khan, A.L.; Angels, C.O.; Liu, C.; Vankelecom, I.F.J. Membrane-based technologies for biogas separations. Chem. Soc. Rev. 2010, 39, 750–768. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ma, W.; Zhou, H.; Zhang, Y.; Jia, H.; Xu, J.; Jiang, P.; Wang, X.; Zhao, W. Preparation of butadiene-bridged polymethylsiloxane (BBPMS)/ethyl cellulose(EC) hybrid membranes for gas separation. Eur. Polym. J. 2021, 157, 110679. [Google Scholar] [CrossRef]
- Zulhairun, A.K.; Fachrurrazi, Z.G.; Izwanne, M.N.; Ismail, A.F. Asymmetric hollow fiber membrane coated with polydimethylsiloxane-metal organic framework hybrid layer for gas separation. Sep. Purif. Technol. 2015, 146, 85–93. [Google Scholar] [CrossRef]
- Zulhairun, A.K.; Ng, B.C.; Ismail, A.F.; Murali, R.S.; Abdullah, M.S. Production of Mixed Matrix Hollow Fiber Membrane for CO2/CH4 separation. Sep. Purif. Technol. 2014, 137, 1–12. [Google Scholar] [CrossRef]
- Zulhairun, A.K.; Ismail, A.F.; Matsuura, T.; Abdullah, M.S.; Mustafa, A. Asymmetric mixed matrix membrane incorporating organically modified clay particle for gas separation. Chem. Eng. J. 2014, 241, 495–503. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, N.Y.; Caro, J. In situ formation of LDH membranes of different microstructures with molecular sieve gas selectivity. J. Mater. Chem. A 2014, 2, 5716–5723. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Pan, J.; Wang, N.; Steinbach, F.; Liu, X.; Caro, J. Remarkably Enhanced Gas Separation by Partial Self-Conversion of a Laminated Membrane to Metal–Organic Frameworks. Angew. Chem. 2015, 127, 3071–3075. [Google Scholar] [CrossRef]
- Nafisi, V.; Hagg, M.B. Gas separation properties of ZIF-8/6FDA-durene diamine mixed matrix membrane. Sep. Purif. Technol. 2014, 128, 31–38. [Google Scholar] [CrossRef]
- Ghanem, A.S.; Shammakh, M.B.; Usman, M.; Khan, M.F.; Dafallah, H.; Mohamed, A.M. High gas permselectivity in ZIF-302/polyimide self-consistent mixed matrix membrane. J. Appl. Polym. Sci. 2019, 137, 48513. [Google Scholar] [CrossRef]
- Yu, G.; Zou, X.; Sun, L.; Liu, B.; Wang, Z.; Zhang, P.; Zhu, G. Constructing Connected Paths between UiO-66 and PIM-1 to Improve Membrane CO2 Separation with Crystal-Like Gas Selectivity. Adv. Mater. 2019, 31, 1806853. [Google Scholar] [CrossRef]
- Kandian, M.; Nilsen, M.H.; Usseglio, S.; Jakobsen, S.; Olsbye, U.; Tilset, M.; Larabi, C.; Quadrelli, E.A.; Bonino, F.; Lillerud, K.P. Synthesis and Stability of Tagged UiO-66 Zr-MOFs. Chem. Mat. 2010, 22, 6632–6640. [Google Scholar] [CrossRef]
- Wang, H.; He, S.; Qin, X.; Li, C.; Li, T. Interfacial engineering in metal-organic framework-based mixed matrix membranes using covalently grafted polyimide brushes. J. Am. Chem. Soc. 2018, 140, 17203–17210. [Google Scholar] [CrossRef] [PubMed]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Calle, M.; Jo, H.J.; Doherty, C.M.; Hill, A.J.; Lee, Y.M. Cross-linked thermally rearranged poly(benzoxazole-co-imide) membranes prepared from ortho-hydroxycopolyimides containing pendant carboxyl groups and gas separation properties. Macromolecules 2015, 48, 2603–2613. [Google Scholar] [CrossRef]
- Zhuang, Y.B.; Seong, J.G.; Lee, W.H.; Do, Y.S.; Lee, M.J.; Wang, G.; Guiver, M.D.; Lee, Y.M. Mechanically tough, thermally rearranged (TR) random/block poly(benzoxazole-co-imide) gas separation membrane. Macromolecules 2015, 48, 5286–5299. [Google Scholar] [CrossRef]
- Xu, S.P.; Zhou, H.L.; Jia, H.G.; Xu, J.Y.; Ma, L.Q.; Zang, Y.; Jiang, P.F.; Ma, W.Q.; Zhang, Y.S.; Zhao, W.W.; et al. Preparation and high performance of cellulose acetate films by grafting with imidazole ionic liquid. ACS Omega 2020, 6, 12500–12506. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; Li, G.; Li, H. Selective binding and removal of guests in a microporous metal-organic framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Denny, J.M.S.; Moreton, J.C.; Benz, L.; Cohen, S.M. Metal-organic frameworks for membrane-based separations. Nat. Rev. Mater. 2016, 1, 16078. [Google Scholar] [CrossRef]
- Hou, J.M.; Wei, Y.Y.; Zhou, S.; Wang, Y.J.; Wang, H.H. Highly efficient H2/CO2 separation via an ultrathin metal-organic framework membrane. Chem. Eng. Sci. 2018, 182, 180–188. [Google Scholar] [CrossRef]
- Bernabe, D.P.; Caparanga, A.R.; Hu, C.C.; You, S.J.; Lee, K.R.; Lai, J.Y. Microporous Aluminum Fumarate (A520) Metal-Organic Framework as modifier to free-standing mixed matrix membrane. Mater. Sci. Forum 2018, 934, 170–175. [Google Scholar] [CrossRef]
- Bernabe, D.P.; Caparanga, A.R.; Hu, C.C.; You, S.J.; Lee, K.R.; Lai, J.Y. MOF-modified high permeability polyimide membrane for gas separation. Key Eng. Mater. 2019, 801, 313–318. [Google Scholar] [CrossRef]
- Liu, B.; Li, D.; Yao, J.; Sun, H. Improved CO2 separation performance and interfacial affinity of mixed matrix membrane by incorporating UiO-66-PEI@[bmim][Tf2N] particles. Sep. Purif. Technol. 2020, 239, 116519. [Google Scholar] [CrossRef]
- Erucar, I.; Keskin, S. Screening Metal-organic framework-based mixed-matrix membranes for CO2/CH4 separations. Ind. Eng. Chem. Res. 2011, 50, 12606–12616. [Google Scholar] [CrossRef] [Green Version]
- Baker, R.W. Future directions of membrane gas separation technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
- Zhang, X.M.; Xiong, W.J.; Tu, Z.H.; Peng, L.L.; Hu, X.B. Supported ionic liquid membranes with dual-site interaction mechanism for efficient separation of CO2. ACS Sustain. Chem. Eng. 2019, 7, 10792–10799. [Google Scholar] [CrossRef]
- Nikolaeva, D.; Azcune, I.; Tanczyk, M.; Warmuzinski, K.; Jaschik, M.; Sandru, M.; Dahl, P.I.; Genua, A.; Los, S.; Sheridan, E. The performance of affordable and stable cellulose-based poly-ionic membranes in CO2/N2 and CO2/CH4 gas separation. J. Membr. Sci. 2018, 564, 552–561. [Google Scholar] [CrossRef]
- Nik, O.G.; Chen, X.Y.; Kaliaguine, S. Functionalized metal organic framework-polyimide mixed matrix membranes for CO2/CH4 separation. J. Membr. Sci. 2012, 413, 48–61. [Google Scholar] [CrossRef]
- Sarmadi, R.; Salimi, M.; Pirouzfar, V. The assessment of honeycomb structure UiO-66 and amino functionalized UiO-66 metal–organic frameworks to modify the morphology and performance of Pebax®1657-based gas separation membranes for CO2 capture applications. Environ. Sci. Pollut. Res. 2020, 27, 40618–40632. [Google Scholar] [CrossRef]
- Azizi, N.; Mohammadi, T.; Behbahani, R.Z. Comparison of permeability performance of PEBAX-1074/TiO2, PEBAX-1074/SiO2 and PEBAX-1074/Al2O3 nanocomposite membranes for CO2/CH4 Separation. Chem. Eng. Res. Des. 2017, 117, 177–189. [Google Scholar] [CrossRef]
- Fragaa, S.; Monteleoneb, M.; Lanc, M.; Esposito, E.; Fuoco, A.; Giorno, L.; Pilnacek, K.; Friess, K.; Carta, M.; McKeown, N.C.; et al. A novel time lag method for the analysis of mixed gas diffffusion in polymeric membranes by on-line mass spectrometry: Method development and validation. J. Membr. Sci. 2018, 561, 39–58. [Google Scholar] [CrossRef]
- Taddei, M.; Dau, P.V.; Cohen, S.M.; Ranocchiari, M.; Bokhoven, J.A.V.; Costantino, F.; Sabatini, S.; Vivani, R. Efficient microwave assisted synthesis of metal-organic framework UiO-66: Optimization and scale up. Dalton Trans. 2015, 44, 14019–14026. [Google Scholar] [CrossRef]
- Garibay, S.J.; Cohen, S.M. Isoreticular synthesis and modifification of frameworks with the UiO-66 topology. Chem. Commun. 2010, 46, 7700–7702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, P.; Jia, H.; Xu, J.; Zhou, H.; Zhang, M.; Xu, S.; Zang, Y.; Zhang, X.; Zhang, Y. Preparation of high-strength polyimide membranes capped by ionic liquids. High Perform. Polym. 2020, 33, 568–575. [Google Scholar] [CrossRef]
- Lang, L.Z.; Gan, Q.; Nancarrow, P. Composite ionic liquid and polymer membranes for gas separation at elevated temperatures. J. Membr. Sci. 2014, 450, 407–417. [Google Scholar] [CrossRef]
- Zhang, M.X.; Yu, A.; Wu, X.Y.; Shao, P.P.; Huang, X.; Ma, D.; Han, X.H.; Xie, J.; Feng, X.; Wang, B. Sealing functional ionic liquids in conjugated microporous polymer membrane by solvent-assisted micropore tightening. Nano Res. 2021, 7, 1–6. [Google Scholar] [CrossRef]
- Wang, C.; Luo, X.; Luo, H.; Jiang, D.E.; Li, H.; Dai, S. Tuning the Basicity of Ionic Liquids for Equimolar CO2 Capture. Angew. Chem. Int. Ed. 2011, 50, 4918–4922. [Google Scholar] [CrossRef]
- Shi, Y.P.; Wu, S.S.; Wang, Z.G.; Bi, X.Y.; Huang, M.H.; Zhang, Y.T.; Jina, J. Mixed matrix membranes with highly dispersed MOF nanoparticles for improved gas separation. Sep. Purif. Technol. 2021, 277, 119449. [Google Scholar] [CrossRef]
- Li, Y.; Chuang, T.; Cao, C.; Kulprathipanja, S. The effects of polymer chain rigidifification, zeolite pore size and pore blockage on polyethersulfone (PES)-zeolite A mixed matrix membranes. J. Membr. Sci. 2005, 260, 45–55. [Google Scholar] [CrossRef]
- Hudiono, Y.C.; Carlisle, T.K.; LaFrate, A.L.; Gin, D.L.; Noble, R.D. Novel mixed matrix membranes based on polymerizable room-temperature ionic liquids and SAPO-34 particles to improve CO2 separation. J. Membr. Sci. 2011, 370, 141–148. [Google Scholar] [CrossRef]




| Membrane | Composition |
|---|---|
| Pure PI | BTDA + ODA |
| PI-IL | BTDA + ODA + IL |
| PI-IL/1% MOF | 1% UiO-66-NH2 + 99% PI-IL |
| PI-IL/2% MOF | 2% UiO-66-NH2 + 98% PI-IL |
| PI-IL/3% MOF | 3% UiO-66-NH2 + 97% PI-IL |
| PI-IL/4% MOF | 4% UiO-66-NH2 + 96% PI-IL |
| PI-IL/5% MOF | 5% UiO-66-NH2 + 95% PI-IL |
| Sample | Yield Strength (MPa) | Elongation at Break (%) | Tensile Strength (MPa) | Modulus of Elasticity (MPa) |
|---|---|---|---|---|
| PI | 0.61 ± 0.03 | 8.96 ± 0.09 | 53.6 ± 0.25 | 59.2 ± 0.21 |
| PI-IL | 54.4 ± 0.23 | 37.3 ± 0.16 | 6203 ± 2.13 | 177 ± 0.67 |
| PI-IL-1% MOF | 55.6 ± 0.27 | 37.4 ± 0.21 | 6434 ± 2.34 | 183 ± 0.78 |
| PI-IL-2% MOF | 52.4 ± 0.56 | 33.7 ± 0.12 | 6031 ± 2.54 | 174 ± 0.63 |
| PI-IL-3% MOF | 49.4 ± 0.41 | 29.4 ± 0.14 | 5724 ± 2.16 | 143 ± 0.58 |
| PI-IL-4% MOF | 46.5 ± 0.45 | 18.7 ± 0.15 | 5352 ± 2.11 | 105 ± 0.46 |
| PI-IL-5% MOF | 34.5 ± 0.48 | 9.04 ± 0.08 | 3042 ± 1.51 | 98.4 ± 0.33 |
| No. | Sample | PCO2/Bar a | PCH4/Bar a | PCO2/PCH4 |
|---|---|---|---|---|
| 1 | PI | 1.19 | 0.10 | 11.93 |
| 2 | PI-IL | 5.19 | 0.07 | 74.15 |
| 3 | PI-IL/1% MOF | 5.57 | 0.07 | 79.53 |
| 4 | PI-IL/2% MOF | 6.21 | 0.07 | 88.74 |
| 5 | PI-IL/3% MOF | 7.61 | 0.08 | 95.10 |
| 6 | PI-IL/4% MOF | 6.49 | 0.09 | 72.13 |
| 7 | PI-IL/5% MOF | 4.01 | 0.08 | 50.08 |
| No. | Sample | DCO2 (cm2 s−1) | SCO2 (cm3 (STP) cm2·cmHg) | DCH4 (cm2 s−1) | SCH4 (cm3 (STP) cm2·cmHg) |
|---|---|---|---|---|---|
| 1 | PI | 2.33 × 10−6 | 0.51 × 10−4 | 2.78 × 10−5 | 3.6 × 10−7 |
| 2 | PI-IL | 3.02 × 10−6 | 1.72 × 10−4 | 3.62 × 10−5 | 1.93 × 10−7 |
| 3 | PI-IL/1% MOF | 2.72 × 10−6 | 2.05 × 10−4 | 3.79 × 10−5 | 1.85 × 10−7 |
| 4 | PI-IL/2% MOF | 2.74 × 10−6 | 2.27 × 10−4 | 3.90 × 10−5 | 1.79 × 10−7 |
| 5 | PI-IL/3% MOF | 3.02 × 10−6 | 2.52 × 10−4 | 4.09 × 10−5 | 1.96 × 10−7 |
| 6 | PI-IL/4% MOF | 2.82 × 10−6 | 2.30 × 10−4 | 3.95 × 10−5 | 2.28 × 10−7 |
| 7 | PI-IL/5% MOF | 2.24 × 10−6 | 1.79 × 10−4 | 3.52 × 10−5 | 2.27 × 10−7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Jia, H.; Wang, Q.; Ma, W.; Yang, G.; Xu, S.; Li, S.; Su, G.; Qu, Y.; Zhang, M.; et al. Optimization of a MOF Blended with Modified Polyimide Membrane for High-Performance Gas Separation. Membranes 2022, 12, 34. https://doi.org/10.3390/membranes12010034
Zhang Y, Jia H, Wang Q, Ma W, Yang G, Xu S, Li S, Su G, Qu Y, Zhang M, et al. Optimization of a MOF Blended with Modified Polyimide Membrane for High-Performance Gas Separation. Membranes. 2022; 12(1):34. https://doi.org/10.3390/membranes12010034
Chicago/Turabian StyleZhang, Yushu, Hongge Jia, Qingji Wang, Wenqiang Ma, Guoxing Yang, Shuangping Xu, Shaobin Li, Guiming Su, Yanqing Qu, Mingyu Zhang, and et al. 2022. "Optimization of a MOF Blended with Modified Polyimide Membrane for High-Performance Gas Separation" Membranes 12, no. 1: 34. https://doi.org/10.3390/membranes12010034
APA StyleZhang, Y., Jia, H., Wang, Q., Ma, W., Yang, G., Xu, S., Li, S., Su, G., Qu, Y., Zhang, M., & Jiang, P. (2022). Optimization of a MOF Blended with Modified Polyimide Membrane for High-Performance Gas Separation. Membranes, 12(1), 34. https://doi.org/10.3390/membranes12010034





