Effect of Direct Fluorination on the Transport Properties and Swelling of Polymeric Materials: A Review
Abstract
1. Introduction
| Chemical Structure | Abbreviation | Cost 1, EUR | Tg, °C | Density, g/cm3 | He-CH4 Selectivity | N2-CH4 Selectivity | He-H2 Selectivity | Ref. |
|---|---|---|---|---|---|---|---|---|
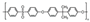 | Polysulfone | 12 | 190 | 1.24 | 50 | 0.96 | 0.93 | [29] |
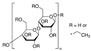 | Ethyl cellulose | 0.7 | 110–130 | 1.14 | 5.7 | 0.46 | 0.71 | This work |
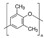 | Polyphenyleneoxide | 9.5 | 211 | 1.06 | 8.7 | 0.64 | 0.62 | This work |
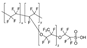 | Nafion | 486 | - | 2.0 | 401 | 2.5 | 4.4 | [30,31] |
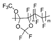 m = 40 mol %, n = 60 mol % | Hyflon AD60 | 180 | 125 | - | 167 | 3.4 | 2.9 | [32] |
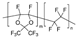 m = 65 mol %, n = 35 mol % | AF1600 | 384 | 160 | 1.78 | 20 | 1.3 | 1.7 | [33] |
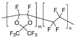 m = 87 mol %, n = 13 mol % | AF2400 | 400 | 240 | 1.67 | 8 | 1.4 | 1.2 | [34] |
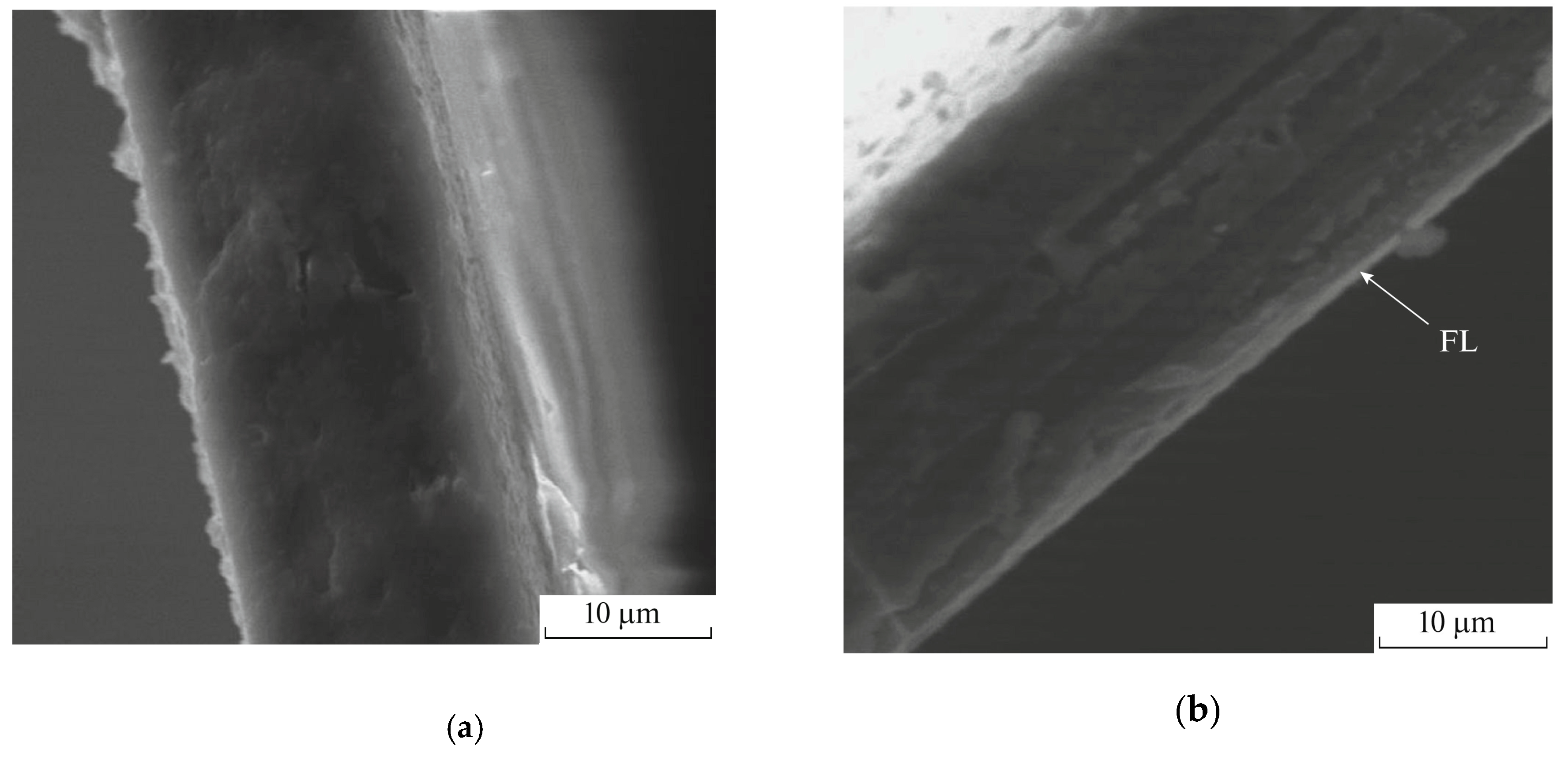
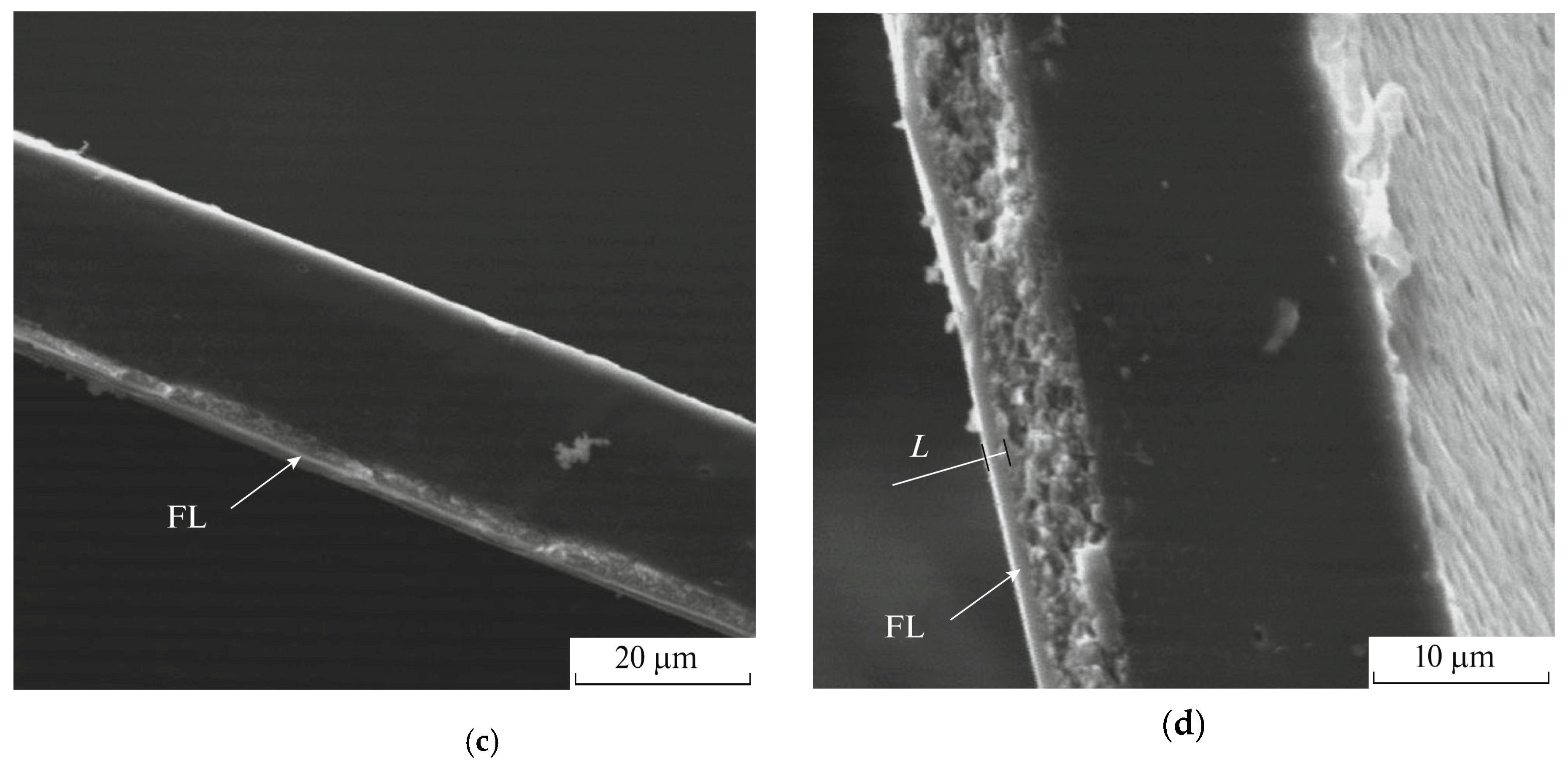
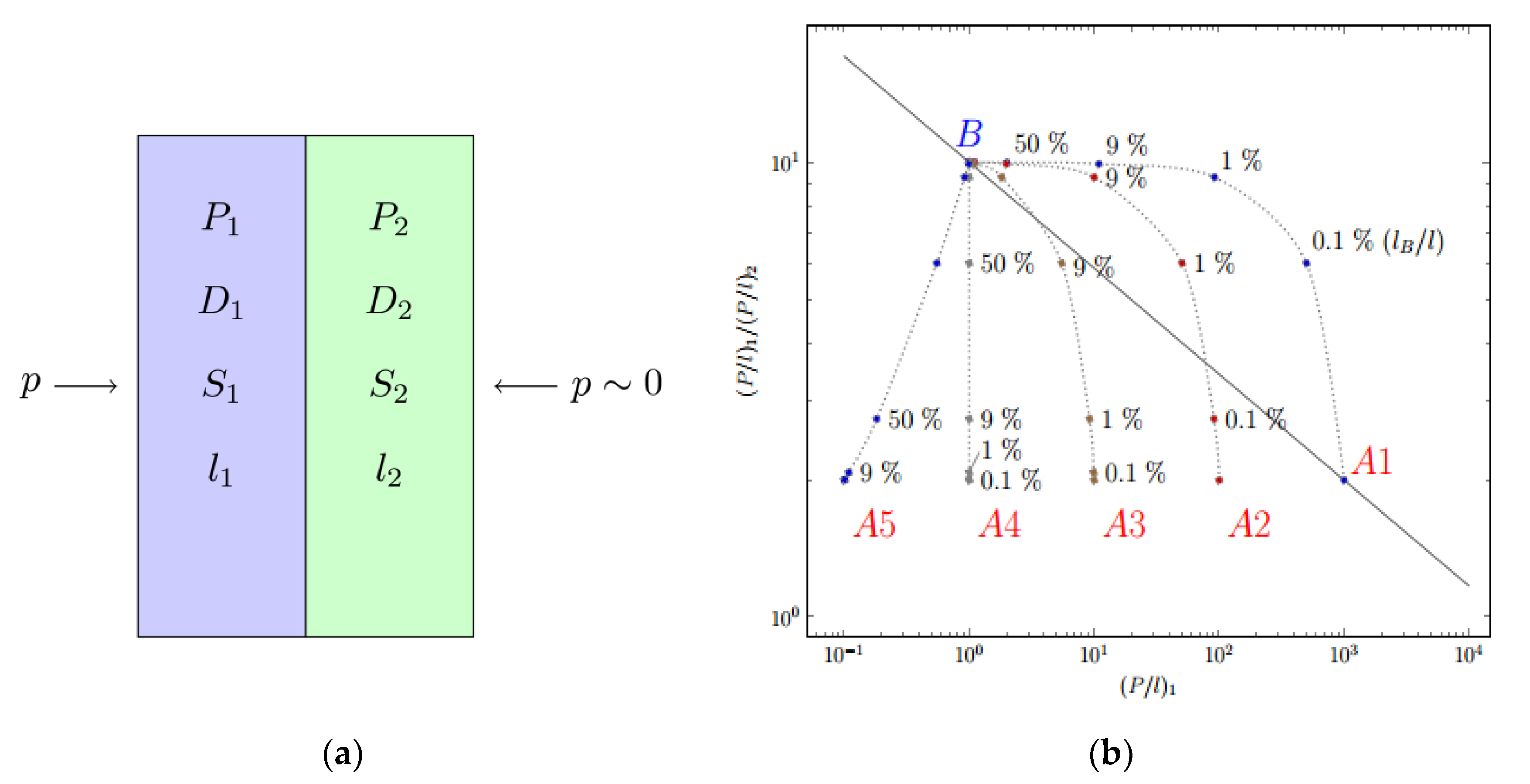
2. Chemical Composition and Laminate Structure of Fluorinated Membranes
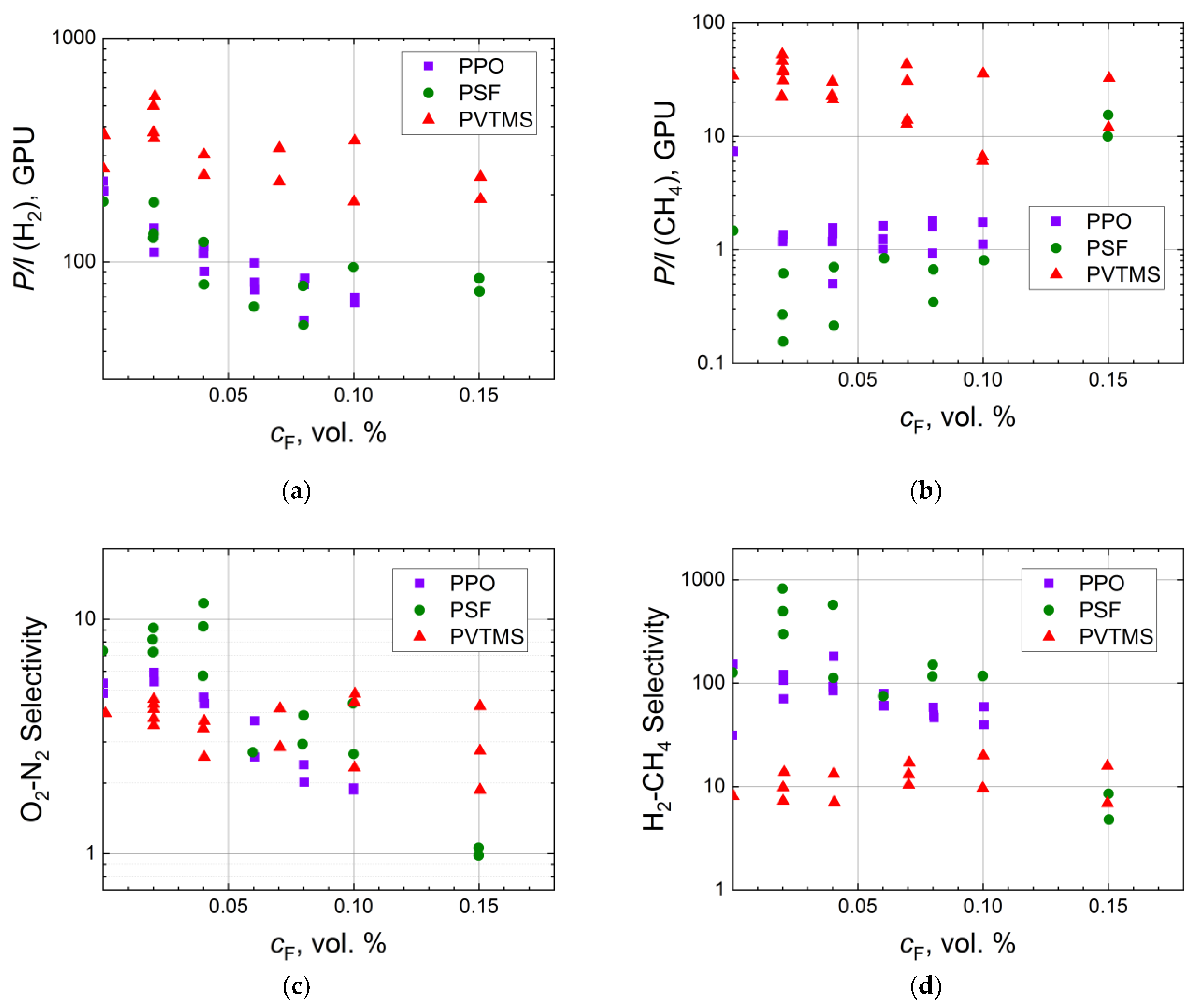
3. Gas Separation Properties of Surface Fluorinated Membranes
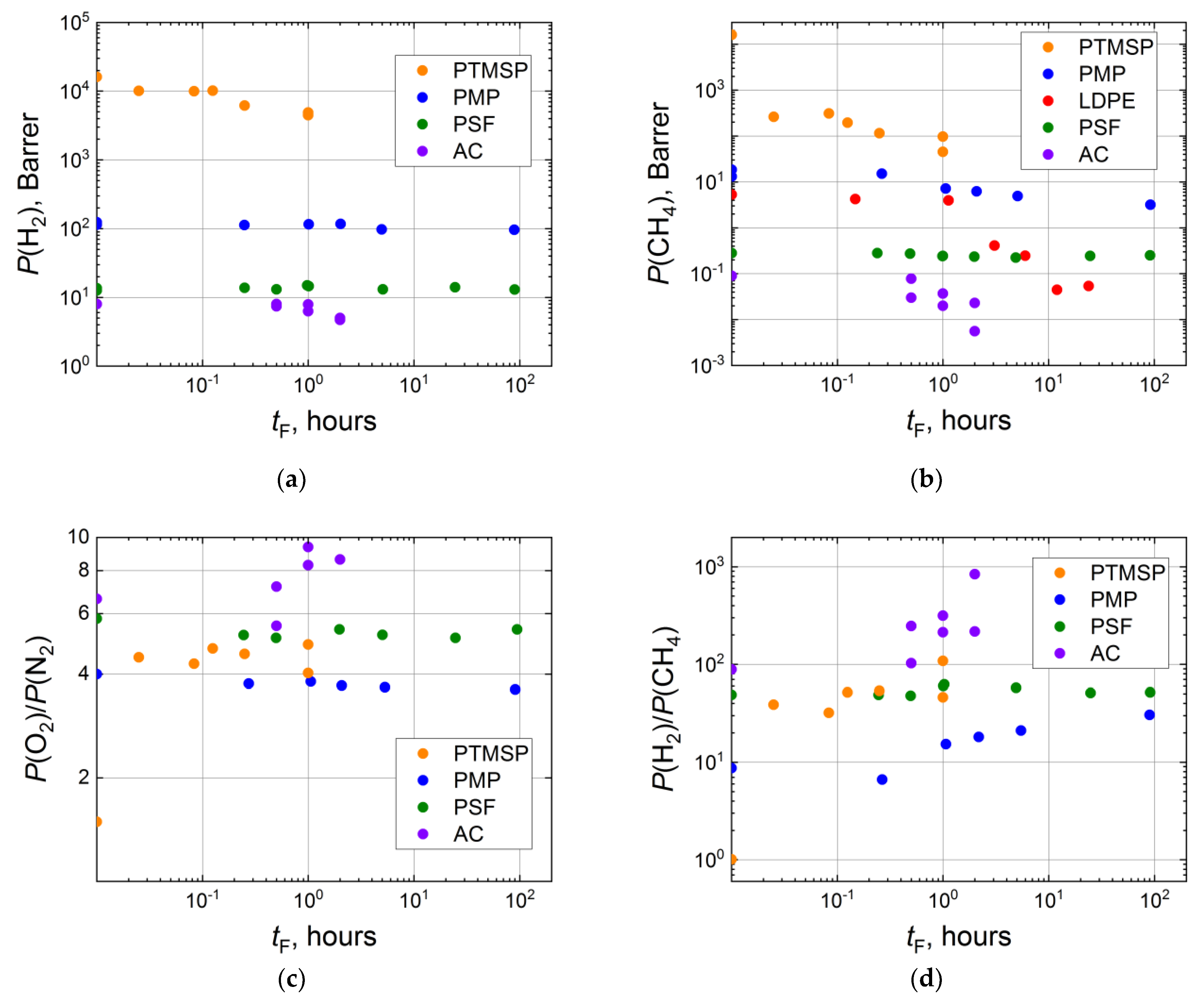
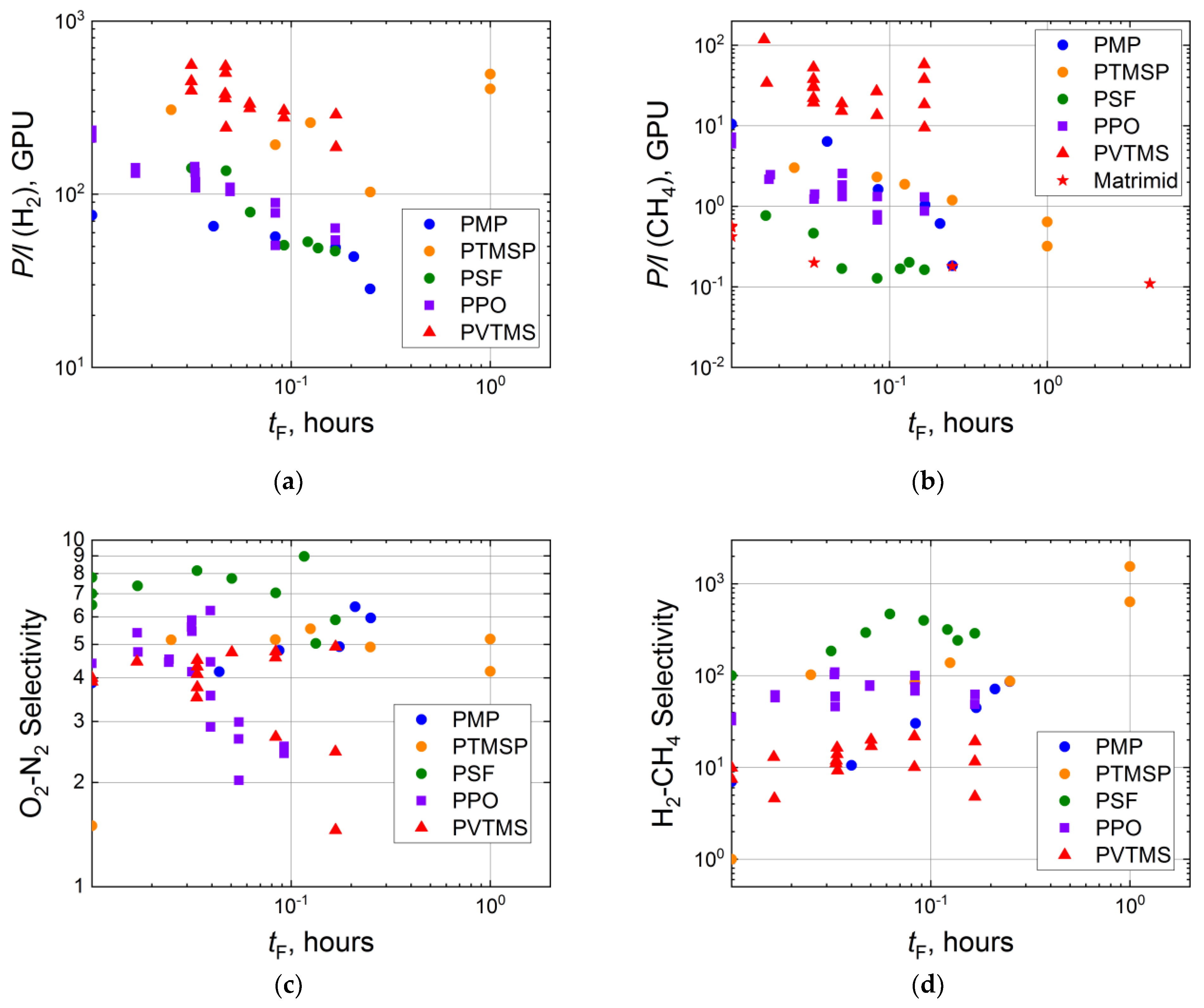
3.1. Influence of Fluorination Regimes on Gas Permeability
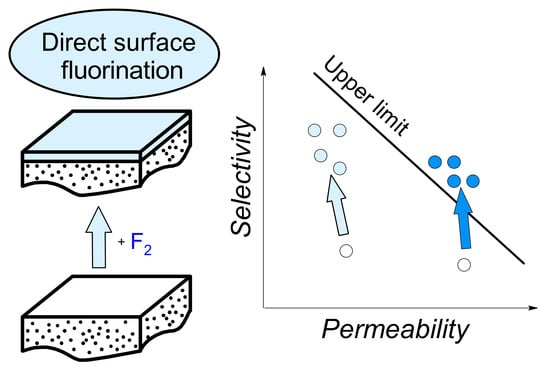
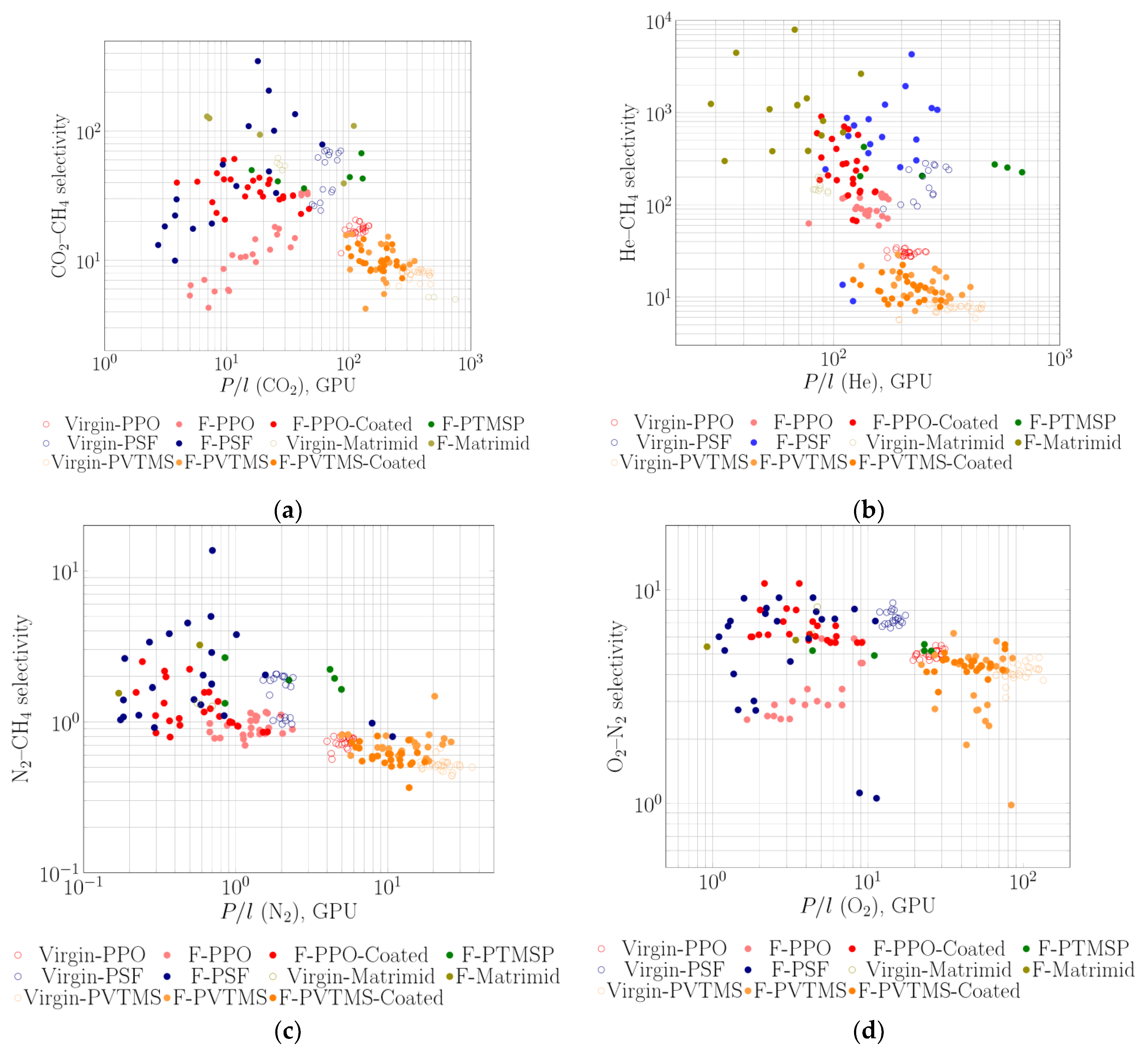
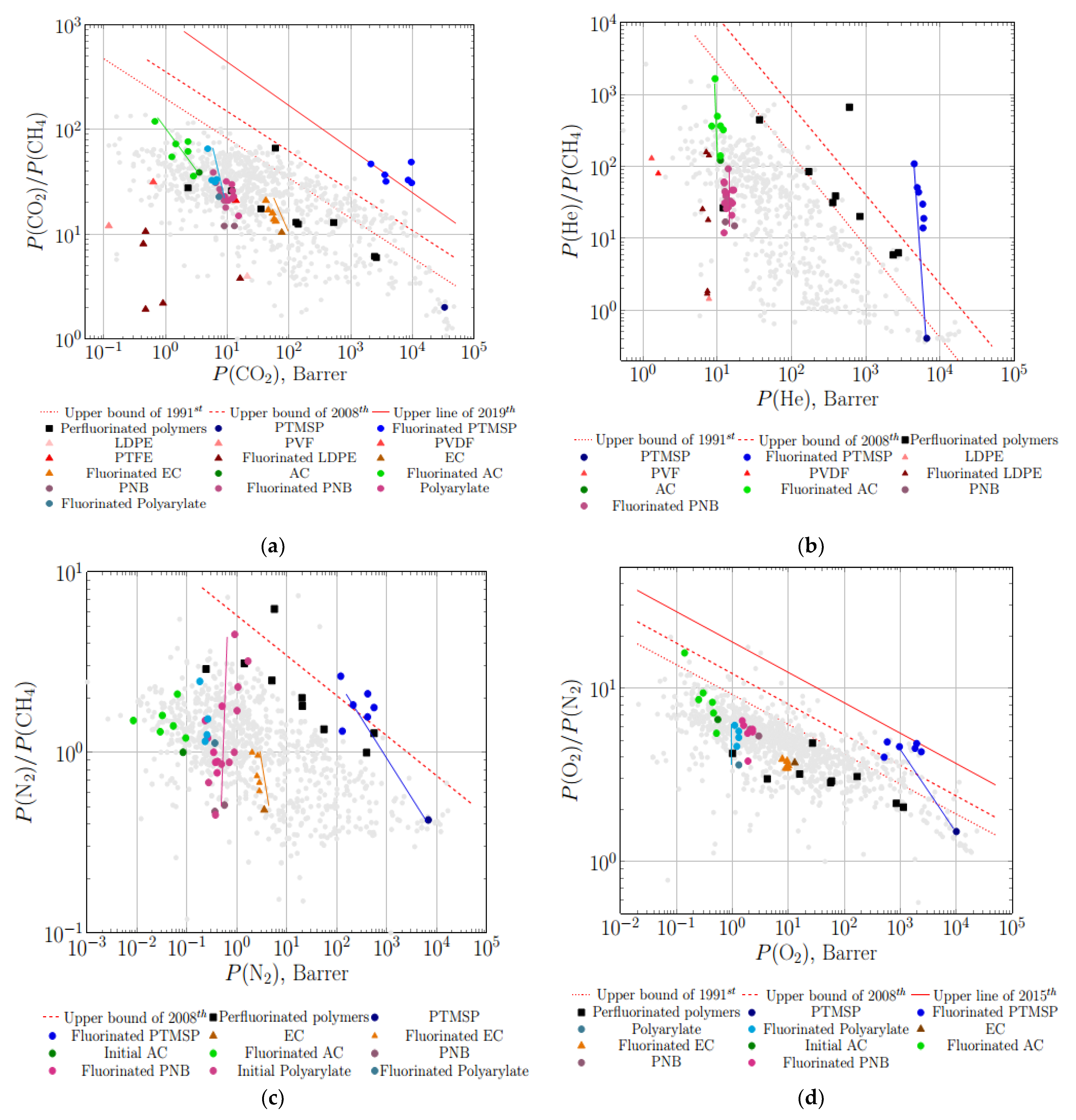
3.2. Gas Separation Properties on Robeson Diagrams
4. Vapor and Liquid Permeation and Related Processes
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| ABS rubber | copolymer of acrylonitrile with butadiene and styrene |
| AC | acetyl cellulose |
| Co-S-AN | copolymer of styrene and acrylonitrile |
| EC | ethyl cellulose |
| ESR | electron spin resonance |
| HDPE | high density polyethylene |
| LDPE | low density polyethylene |
| Matrimid | polyimide based on 3,3′,4,4′-benzophenonetetracarboxylic dianhydride and diaminophenylindane |
| PDMS | polydimethylsiloxane |
| PE | polyethylene |
| PETP | polyethylene terephthalate |
| PIM | polymer wit intrinsic microporosity (polybenzodioxanes) |
| PPO | poly(2,6-dimethyl-1,4-phenylene oxide) |
| PMP | poly(4-methylpentene-1) |
| PS | polystyrene |
| PSF | polysulfone |
| PTFE | polytetrafluoroethylene |
| PTMSP | polytrimethylsilyl propyne |
| PVF | polyvinyl fluoride |
| PVDF | polyvinylidene fluoride |
| PVTMS | poly(vinyl trimethylsilane) |
| SEM | scanning electron microscopy |
| XPS | X-ray photoelectron spectroscopy |
References
- Plunkett, R.J. Tetrafluoroethylene Polymers. U.S. Patent 2230654, 4 February 1941. [Google Scholar]
- Putnam, R.E. Development of Thermoplastic Fluoropolymers. In High Performance Polymers: Their Origin and Development; Seymour, R.B., Kirshenbaum, G.S., Eds.; Springer: Dordrecht, The Netherlands, 1986; pp. 279–286. ISBN 978-94-011-7075-8. [Google Scholar]
- Ameduri, B. Fluorinated (Co)Polymers: Synthesis, Properties, and Applications. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; p. pst575. ISBN 978-0-471-44026-0. [Google Scholar]
- Gardiner, J.; Gardiner, J. Fluoropolymers: Origin, Production, and Industrial and Commercial Applications. Aust. J. Chem. 2014, 68, 13–22. [Google Scholar] [CrossRef]
- Smith, D.W.; Iacono, S.T.; Iyer, S.S. (Eds.) Handbook of Fluoropolymer Science and Technology; Wiley: Hoboken, NJ, USA, 2014; ISBN 978-1-118-85008-4. [Google Scholar]
- Ebnesajjad, S. Introduction to Fluoropolymers Materials, Technology and Applications; Elsevier Science & Technology Books: San Diego, CA, USA, 2013; ISBN 978-1-4557-7551-4. [Google Scholar]
- Banerjee, S. Handbook of Specialty Fluorinated Polymers: Preparation, Properties, and Applications; Plastics Design Library; Elsevier William Andrew: Waltham, MA, USA, 2015; ISBN 978-0-323-35792-0. [Google Scholar]
- Di Noto, V.; Bettiol, M.; Bassetto, F.; Boaretto, N.; Negro, E.; Lavina, S.; Bertasi, F. Hybrid Inorganic-Organic Nanocomposite Polymer Electrolytes Based on Nafion and Fluorinated TiO2 for PEMFCs. Int. J. Hydrogen Energy 2012, 37, 6169–6181. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, H. Investigation of Nafion Series Membranes on the Performance of Iron-chromium Redox Flow Battery. Int. J. Energy Res. 2019, 43, 8739–8752. [Google Scholar] [CrossRef]
- Yao, M.; Tijing, L.D.; Naidu, G.; Kim, S.-H.; Matsuyama, H.; Fane, A.G.; Shon, H.K. A Review of Membrane Wettability for the Treatment of Saline Water Deploying Membrane Distillation. Desalination 2020, 479, 114312. [Google Scholar] [CrossRef]
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B. Membrane Synthesis for Membrane Distillation: A Review. Sep. Purif. Technol. 2017, 182, 36–51. [Google Scholar] [CrossRef]
- Yampolskii, Y.P.; Belov, N.A.; Alentiev, A.Y. Fluorine in the Structure of Polymers: Influence on the Gas Separation Properties. Russ. Chem. Rev. 2019, 88, 387–405. [Google Scholar] [CrossRef]
- Belov, N.; Yampolskii, Y. Gas transport in fluorine-containing polymers. In Fascinating Fluoropolymers and Their Applications; Ameduri, B., Fomin, S., Eds.; Progress in Fluorine Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 157–203. ISBN 978-0-12-821873-0. [Google Scholar]
- Wu, A.X.; Drayton, J.A.; Rodriguez, K.M.; Qian, Q.; Lin, S.; Smith, Z.P. Influence of Aliphatic and Aromatic Fluorine Groups on Gas Permeability and Morphology of Fluorinated Polyimide Films. Macromolecules 2020, 53, 5085–5095. [Google Scholar] [CrossRef]
- Okamoto, Y.; Chiang, H.-C.; Merkel, T. Perfluoropolymers for gas separation membrane applications. In Fascinating Fluoropolymers and Their Applications; Ameduri, B., Fomin, S., Eds.; Progress in Fluorine Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 143–155. ISBN 978-0-12-821873-0. [Google Scholar]
- Robeson, L.M. The Upper Bound Revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Yampolskii, Y.; Belov, N.; Alentiev, A. Perfluorinated Polymers as Materials of Membranes for Gas and Vapor Separation. J. Membr. Sci. 2020, 598, 117779. [Google Scholar] [CrossRef]
- Shundrina, I.K.; Vaganova, T.A.; Kusov, S.Z.; Rodionov, V.I.; Karpova, E.V.; Malykhin, E.V. Synthesis and Properties of Organosoluble Polyimides Based on Novel Perfluorinated Monomer Hexafluoro-2,4-Toluenediamine. J. Fluor. Chem. 2011, 132, 207–215. [Google Scholar] [CrossRef]
- Okamoto, Y.; Zhang, H.; Mikes, F.; Koike, Y.; He, Z.; Merkel, T.C. New Perfluoro-Dioxolane-Based Membranes for Gas Separations. J. Membr. Sci. 2014, 471, 412–419. [Google Scholar] [CrossRef]
- Karpov, G.O.; Bermeshev, M.V.; Borisov, I.L.; Sterlin, S.R.; Tyutyunov, A.A.; Yevlampieva, N.P.; Bulgakov, B.A.; Volkov, V.V.; Finkelshtein, E.S. Metathesis-Type Poly-Exo-Tricyclononenes with Fluoroorganic Side Substituents: Synthesis and Gas-Transport Properties. Polymer 2018, 153, 626–636. [Google Scholar] [CrossRef]
- Reisinger, J.J.; Hillmyer, M.A. Synthesis of Fluorinated Polymers by Chemical Modification. Prog. Polym. Sci. 2002, 27, 971–1005. [Google Scholar] [CrossRef]
- Inagaki, N. Plasma Surface Modification and Plasma Polymerization; CRC Press: Boca Raton, FL, USA, 2014; ISBN 978-0-429-15685-4. [Google Scholar]
- Kharitonov, A.P.; Simbirtseva, G.V.; Tressaud, A.; Durand, E.; Labrugère, C.; Dubois, M. Comparison of the Surface Modifications of Polymers Induced by Direct Fluorination and Rf-Plasma Using Fluorinated Gases. J. Fluor. Chem. 2014, 165, 49–60. [Google Scholar] [CrossRef]
- Lagow, R.J.; Margrave, J.L. Direct Fluorination: A “New” Approach to Fluorine Chemistry. In Progress in Inorganic Chemistry; Lippard, S.J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 161–210. ISBN 978-0-470-16627-7. [Google Scholar]
- Kharitonov, A.P. Direct Fluorination of Polymers—From Fundamental Research to Industrial Applications. Prog. Org. Coat. 2008, 61, 192–204. [Google Scholar] [CrossRef]
- Kharitonov, A.P.; Kharitonova, L.N. Surface Modification of Polymers by Direct Fluorination: A Convenient Approach to Improve Commercial Properties of Polymeric Articles. Pure Appl. Chem. 2009, 81, 451–471. [Google Scholar] [CrossRef]
- Okazoe, T. Development of the “PERFECT” Direct Fluorination Method and Its Industrial Applications. J. Fluor. Chem. 2015, 174, 120–131. [Google Scholar] [CrossRef]
- Belov, N.A.; Alentiev, A.Y.; Bogdanova, Y.G.; Vdovichenko, A.Y.; Pashkevich, D.S. Direct Fluorination as Method of Improvement of Operational Properties of Polymeric Materials. Polymers 2020, 12, 2836. [Google Scholar] [CrossRef]
- McHattie, J.S.; Koros, W.J.; Paul, D.R. Gas Transport Properties of Polysulphones: 1. Role of Symmetry of Methyl Group Placement on Bisphenol Rings. Polymer 1991, 32, 840–850. [Google Scholar] [CrossRef]
- Chiou, J.S.; Paul, D.R. Gas Permeation in a Dry Nafion Membrane. Ind. Eng. Chem. Res. 1988, 27, 2161–2164. [Google Scholar] [CrossRef]
- Mukaddam, M.; Litwiller, E.; Pinnau, I. Gas Sorption, Diffusion, and Permeation in Nafion. Macromolecules 2016, 49, 280–286. [Google Scholar] [CrossRef]
- Jansen, J.; Macchione, M.; Drioli, E. On the Unusual Solvent Retention and the Effect on the Gas Transport in Perfluorinated Hyflon AD® Membranes. J. Membr. Sci. 2007, 287, 132–137. [Google Scholar] [CrossRef]
- Alentiev, A.Y.; Shantarovich, V.P.; Merkel, T.C.; Bondar, V.I.; Freeman, B.D.; Yampolskii, Y.P. Gas and Vapor Sorption, Permeation, and Diffusion in Glassy Amorphous Teflon AF1600. Macromolecules 2002, 35, 9513–9522. [Google Scholar] [CrossRef]
- Alentiev, A.Y.; Yampolskii, Y.P.; Shantarovich, V.P.; Nemser, S.M.; Platé, N.A. High Transport Parameters and Free Volume of Perfluorodioxole Copolymers. J. Membr. Sci. 1997, 126, 123–132. [Google Scholar] [CrossRef]
- Nazarov, V.G. Surface Modification of Polymers; Ministry of Education and Science of Russian Federation, Moscow State University of Printing Arts: Moscow, Russia, 2008; p. 471. ISBN 978-5-8122-0934-6. [Google Scholar]
- Liang, C.Z.; Chung, T.-S.; Lai, J.-Y. A Review of Polymeric Composite Membranes for Gas Separation and Energy Production. Prog. Polym. Sci. 2019, 97, 101141. [Google Scholar] [CrossRef]
- Quartarone, E.; Angioni, S.; Mustarelli, P. Polymer and Composite Membranes for Proton-Conducting, High-Temperature Fuel Cells: A Critical Review. Materials 2017, 10, 687. [Google Scholar] [CrossRef] [PubMed]
- Vazirinasab, E.; Jafari, R.; Momen, G. Application of Superhydrophobic Coatings as a Corrosion Barrier: A Review. Surf. Coat. Technol. 2018, 341, 40–56. [Google Scholar] [CrossRef]
- Manin, V.N.; Nazarov, V.G.; Gukov, A.M. Diffusion of liquids through surface modified polyethylene. Vysokkmmolek. Soed. Seriya B 1980, 22, 141–144. [Google Scholar]
- Koros, W.J.; Stannett, V.T.; Hopfenberg, H.B. Estimation of the Effective Permeability of Thin Surface Layers Created by Exposure of Polyethylene to Fluorine. Polym. Eng. Sci. 1982, 22, 738–746. [Google Scholar] [CrossRef]
- Kamenskaya, L.A.; Doronin, P.A.; Stolyarov, V.P.; Nazarov, V.G. Influence of fluorination on stability of printing roller rubbers to agressive media. Izv. Vyss. Uchebnikh Zaved. Probl. Polygrafii I Izd. Dela 2014, 1, 8–18. [Google Scholar]
- Kamenskaya, L.A.; Doronin, P.A.; Stolyarov, V.P.; Uvarova, N.V.; Nazarov, V.G. Influence of surface modification on characteristics of rubbers utilezed for fabrication of roller moisturizing apparatus. Izv. Vyss. Uchebnikh Zaved. Probl. Polygrafii I Izd. Dela 2014, 4, 16–24. [Google Scholar]
- Kamenskaya, L.A.; Doronin, P.A.; Stolyarov, V.P.; Evdokimov, A.G.; Nazarov, V.G. Influence of fluorination of rubbery rollers on thier wear in ink-distribution system. Izv. Vyss. Uchebnikh Zaved. Probl. Polygrafii I Izd. Dela 2015, 2, 13–20. [Google Scholar]
- Kiplinger, C.L.; Persico, D.F.; Lagow, R.J.; Paul, D.R. Gas Transport in Partially Fluorinated Low-Density Polyethylene. J. Appl. Polym. Sci. 1986, 31, 2617–2626. [Google Scholar] [CrossRef]
- Gentilcore, J.; Trialo, M.; Waytek, A. Single-Step Barrier Coating for Containers. Plast. Engng. 1978, 34, 40–43. [Google Scholar]
- Beckman, I.N.; Buntseva, I.M. Method of Radionuclides Sorption Defectoscopy in Diagnostics of Near-Surface Layers of Fluorinated Polyethylene. Vestn. Mosk. Univ. Seriya 2 Khimiya 1999, 40, 414–418. [Google Scholar]
- Wang, Z.; Li, S.; Li, B.; Lai, W.; Liu, Y.; Cheng, Z.; Wang, X.; Liu, X. The Preparation of Surface Fluorinated Polyethylene Films with Excellent Properties Similar to That of Fluoropolymers. J. Fluor. Chem. 2017, 200, 169–178. [Google Scholar] [CrossRef]
- Yang, H.W.; Yang, S.L.; Wu, C.; Fei, Y.W.; Wei, X.Y. The Applications of Direct Fluorinated HDPE in Oil & Gas Storage and Transportation. Adv. Mater. Res. 2011, 328–330, 2436–2439. [Google Scholar] [CrossRef]
- Novikova, S.M.; Stolyarov, V.P.; Nazarov, V.G. Modification of surface of packaging materials by fluorination and study of their properties. Izv. Vyss. Uchebnikh Zaved. Probl. Polygrafii I Izd. Dela 2010, 6, 20–30. [Google Scholar]
- Nazarov, V.G.; Stolyarov, V.P.; Novikova, S.M.; Mukhaleva, L.A.; Bablyuk, E.B.; Benda, E.F. Influence of surface modification on characteristics of polymeric materials. Izv. Vyss. Uchebnikh Zaved. Probl. Polygrafii I Izd. Dela 2011, 2, 118–127. [Google Scholar]
- Zazhivikhina, N.A.; Kolbina, E.L. Development of flexible polymeric packaging with improved barrier properties. Din. Sist. Mekhanizmov I Mashin 2012, 2, 361–365. [Google Scholar]
- Prorokova, N.P.; Istratkin, V.A.; Kharitonov, A.P. Technology of direct gas-phase fluorination of polypropylene non-woven material. Justification of optimal regime choice. Dizajn. Mater. Tekhnol. 2015, 40, 28–34. [Google Scholar]
- Prorokova, N.P.; Istratkin, V.A.; Kumeeva, T.Y.; Vavilova, S.Y.; Kharitonov, A.P.; Bouznik, V.M. Improvement of Polypropylene Nonwoven Fabric Antibacterial Properties by the Direct Fluorination. RSC Adv. 2015, 5, 44545–44549. [Google Scholar] [CrossRef]
- Mohr, J.M.; Paul, D.R.; Mlsna, T.E.; Lagow, R.J. Surface Fluorination of Composite Membranes. Part I. Transport Properties. J. Membr. Sci. 1991, 55, 131–148. [Google Scholar] [CrossRef]
- Mohr, J.M.; Paul, D.R.; Tam, Y.; Mlsna, T.E.; Lagow, R.J. Surface Fluorination of Composite Membranes. Part II. Characterization of the Fluorinated Layer. J. Membr. Sci. 1991, 55, 149–171. [Google Scholar] [CrossRef]
- Mohr, J.M.; Paul, D.R.; Taru, Y.; Mlsna, T.E.; Lagow, R.J. XPS Characterization of Surface Fluorinated Poly(4-Methyl-1-Pentene). J. Appl. Polym. Sci. 1991, 42, 2509–2516. [Google Scholar] [CrossRef]
- Langsam, M. Fluorinated Polymeric Membranes for Gas Separation Processes. U.S. Patent 4657564, 14 April 1987. [Google Scholar]
- Langsam, M.; Anand, M.; Karwacki, E.J. Substituted Propyne Polymers: I. Chemical Surface Modification of Poly[1-(Trimethylsilyl] Propyne] for Gas Separation Membranes. Gas Sep. Purif. 1988, 2, 162–170. [Google Scholar] [CrossRef]
- Langsam, M.; Robeson, L.M. Substituted Propyne Polymers—Part II. Effects of Aging on the Gas Permeability Properties of Poly[1-(Trimethylsilyl)Propyne] for Gas Separation Membranes. Polym. Eng. Sci. 1989, 29, 44–54. [Google Scholar] [CrossRef]
- Le Roux, J.D.; Paul, D.R.; Kampa, J.; Lagow, R.J. Surface Fluorination of Poly (Phenylene Oxide) Composite Membranes Part I. Transport Properties. J. Membr. Sci. 1994, 90, 21–35. [Google Scholar] [CrossRef]
- Le Roux, J.D.; Paul, D.R.; Arendt, M.F.; Yuan, Y.; Cabasso, I. Surface Fluorination of Poly (Phenylene Oxide) Composite Membranes: Part II. Characterization of the Fluorinated Layer. J. Membr. Sci. 1994, 90, 37–53. [Google Scholar] [CrossRef]
- Kharitonov, A.P.; Teplyakov, V.V.; Syrtsova, D.; Badyal, J.P.; Strathmann, H.; Pud, A.A. Direct Fluorination Of Polymers: Fundamental Features And Possible Industrial Applications. Polym. Prepr. 1998, 39, 918–919. [Google Scholar]
- Okazoe, T.; Shirakawa, D.; Murata, K. Application of Liquid-Phase Direct Fluorination: Novel Synthetic Methods for a Polyfluorinated Coating Material and a Monomer of a Perfluorinated Polymer Electrolyte Membrane. Appl. Sci. 2012, 2, 327–341. [Google Scholar] [CrossRef]
- Chen, D.; Hickner, M.A.; Wang, S.; Pan, J.; Xiao, M.; Meng, Y. Directly Fluorinated Polyaromatic Composite Membranes for Vanadium Redox Flow Batteries. J. Membr. Sci. 2012, 415–416, 139–144. [Google Scholar] [CrossRef]
- Belov, N.A.; Blinov, I.A.; Suvorov, A.V.; Nikiforov, R.Y.; Chirkov, S.V.; Alentiev, A.Y.; Kambur, M.P.; Kostina, Y.V.; Levin, I.S.; Shapagin, A.V.; et al. Gas Permeability of Cellulose Acetate Films Treated with Fluorine in Perfluorodecalin. Membr. Membr. Technol. 2021, 3, 114–123. [Google Scholar] [CrossRef]
- Prorokova, N.P.; Kumeeva, T.Y.; Vavilova, S.Y. Improving the Wettability of Polyester Fabric with Using Direct Fluorination. J. Fluor. Chem. 2019, 219, 115–122. [Google Scholar] [CrossRef]
- Doronin, P.A.; Evdokimov, V.G.; Kartasheva, O.A.; Dedov, A.V.; Nazarov, B.G. Influence of surface fluorination on properties of flexographic printing forms. Izvestia vysshikh uchebnikh zavedenij. Probl. Polygrafii I Izd. Dela 2015, 6, 27–35. [Google Scholar]
- Hu, H.; Xiao, M.; Wang, S.J.; Shen, P.K.; Meng, Y.Z. Surface Fluorination of Poly(Fluorenyl Ether Ketone) Ionomers as Proton Exchange Membranes for Fuel Cell Application. Fuel Cells 2011, 11, 353–360. [Google Scholar] [CrossRef]
- Mohr, J.M.; Paul, D.R.; Pinnau, I.; Koros, W.J. Surface Fluorination of Polysulfone Asymmetric Membranes and Films. J. Membr. Sci. 1991, 56, 77–98. [Google Scholar] [CrossRef]
- Suryamurali, R.; Sankarshana, T.; Sridhar, S.; Kharitonov, A. Fluorinated Polymer Membranes for Separation of Industrial Gas Mixtures. J. Polym. Mater. 2012, 29, 317–330. [Google Scholar]
- Sedath, R.H.; Taylor, D.R.; Li, N.N. Reduced Fouling of Ultrafiltration Membranes Via Surface Fluorination. Sep. Sci. Technol. 1993, 28, 255–269. [Google Scholar] [CrossRef][Green Version]
- Syrtsova, D.A.; Kharitonov, A.P.; Teplyakov, V.V.; Koops, G.-H. Improving Gas Separation Properties of Polymeric Membranes Based on Glassy Polymers by Gas Phase Fluorination. Desalination 2004, 163, 273–279. [Google Scholar] [CrossRef]
- Syrtsova, D.A.; Shalygin, M.G.; Teplyakov, V.V. Fluorinated Hollow Fiber Membranes Based on Matrimid 5218 and Their Application in the Process of Helium Recovery from Natural Gas. Pet. Chem. 2018, 58, 760–769. [Google Scholar] [CrossRef]
- Kharitonov, A.P.; Moskvin, Y.L.; Syrtsova, D.A.; Starov, V.M.; Teplyakov, V.V. Direct Fluorination of the Polyimide Matrimid® 5218: The Formation Kinetics and Physicochemical Properties of the Fluorinated Layers. J. Appl. Polym. Sci. 2004, 92, 6–17. [Google Scholar] [CrossRef]
- Kim, C.S.; Kang, S.; Rhim, J.W.; Park, S.-G. Synthesis of Aminated Poly(Ether Imide) for the Preparation of Bi-Polar Membranes and Their Application to Hypochlorite Production through the Surface Direct Fluorination. Polym. Korea 2015, 39, 338–345. [Google Scholar] [CrossRef][Green Version]
- Tressaud, A.; Durand, E.; Labrugčre, C.; Kharitonov, A.P.; Simbirtseva, G.V.; Kharitonova, L.N.; Dubois, M. Surface Modification of Polymers Treated by Various Fluorinating Media. Acta Chim. Slov. 2013, 60, 495–504. [Google Scholar]
- Fan, C.; Li, B.; Ren, M.; Wu, P.; Liu, Y.; Chen, T.; Cheng, Z.; Qin, J.; Liu, X. The Reaction Kinetics and Mechanism of Crude Fluoroelastomer Vulcanized by Direct Fluorination with Fluorine/Nitrogen Gas. RSC Adv. 2015, 5, 18932–18938. [Google Scholar] [CrossRef]
- Lagow, R.J.; Wei, H.-C. Direct Fluorination of Polymers. In Fluoropolymers 1: Synthesis; Hougham, G., Cassidy, P.E., Johns, K., Davidson, T., Eds.; Topics in Applied Chemistry; Springer: Boston, MA, USA, 2002; pp. 209–221. ISBN 978-0-306-46918-3. [Google Scholar]
- Kharitonov, A.P.; Moskvin, Y.L. Direct Fluorination of Polystyrene Films. J. Fluor. Chem. 1998, 91, 87–93. [Google Scholar] [CrossRef]
- Blinov, I.A.; Mukhortov, D.A.; Yampolskii, Y.P.; Belov, N.A.; Alentiev, A.Y.; Chirkov, S.V.; Bondarenko, G.N.; Kostina, Y.V.; Legkov, S.A.; Perepuchov, A.M.; et al. Direct Fluorination of Poly-2,6-Dimethyl-1,4-Phenylene Oxide in Perfluorinated Liquid Medium. J. Fluor. Chem. 2020, 234, 109526. [Google Scholar] [CrossRef]
- Dubois, M.; Guérin, K.; Giraudet, J.; Pilichowski, J.-F.; Thomas, P.; Delbé, K.; Mansot, J.-L.; Hamwi, A. Direct Fluorination of Poly(p-Phenylene). Polymer 2005, 46, 6736–6745. [Google Scholar] [CrossRef]
- Du, B.X.; Li, Z.L.; Li, J. Surface Charge Accumulation and Decay of Direct-Fluorinated RTV Silicone Rubber. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 2338–2342. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, P.; Wang, X.; Yang, J.; Liu, X. Effect of Direct Fluorination on the Bonding Property of Ultra-High Molecular Weight Polyethylene. Acta Polym. Sin. 2011, 5, 543–547. [Google Scholar] [CrossRef]
- Peyroux, J.; Dubois, M.; Tomasella, E.; Frézet, L.; Kharitonov, A.P.; Flahaut, D. Enhancement of Surface Properties on Low Density Polyethylene Packaging Films Using Various Fluorination Routes. Eur. Polym. J. 2015, 66, 18–32. [Google Scholar] [CrossRef]
- Kharitonov, A.P.; Simbirtseva, G.V.; Bouznik, V.M.; Chepezubov, M.G.; Dubois, M.; Guérin, K.; Hamwi, A.; Kharbache, H.; Masin, F. Modification of Ultra-High-Molecular Weight Polyethylene by Various Fluorinating Routes. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3559–3573. [Google Scholar] [CrossRef]
- Kuzina, S.I.; Kharitonov, A.P.; Moskvin, Y.L.; Mikhailov, A.I. Formation of Free Radicals in the Low-Temperature Fluorination of Polymers. Russ. Chem. Bull. 1996, 45, 1623–1627. [Google Scholar] [CrossRef]
- Peyroux, J.; Dubois, M.; Tomasella, E.; Batisse, N.; Kharitonov, A.P.; Flahaut, D.; Romana, L.; Thomas, P. Surface Modification of Low-Density Polyethylene Packaging Film via Direct Fluorination. Surf. Coat. Technol. 2016, 292, 144–154. [Google Scholar] [CrossRef]
- Gao, J.; Dai, Y.; Wang, X.; Huang, J.; Yao, J.; Yang, J.; Liu, X. Effects of Different Fluorination Routes on Aramid Fiber Surface Structures and Interlaminar Shear Strength of Its Composites. Appl. Surf. Sci. 2013, 270, 627–633. [Google Scholar] [CrossRef]
- Luo, L.; Wu, P.; Cheng, Z.; Hong, D.; Li, B.; Wang, X.; Liu, X. Direct Fluorination of Para-Aramid Fibers 1: Fluorination Reaction Process of PPTA Fiber. J. Fluor. Chem. 2016, 186, 12–18. [Google Scholar] [CrossRef]
- Nazarov, V.G.; Stolyarov, V.P.; Gagarin, M.V. Simulation of Chemical Modification of Polymer Surface. J. Fluor. Chem. 2014, 161, 120–127. [Google Scholar] [CrossRef]
- Kharitonov, A.P.; Loginov, B.A. Direct Fluorination of Polymer Final Products: From Fundamental Study to Practical Application. Russ. J. Gen. Chem. 2009, 79, 635–641. [Google Scholar] [CrossRef]
- Chambers, R.D.; Joel, A.K.; Rees, A.J. Elemental Fluorine: Part 11 [1]. Fluorination of Modified Ethers and Polyethers. J. Fluor. Chem. 2000, 101, 97–105. [Google Scholar] [CrossRef]
- Le Roux, J.D.; Paul, D.R.; Kampa, J.; Lagow, R.J. Modification of Asymmetric Polysulfone Membranes by Mild Surface Fluorination Part I. Transport Properties. J. Membr. Sci. 1994, 94, 121–141. [Google Scholar] [CrossRef]
- Le Roux, J.D.; Teplyakov, V.V.; Paul, D.R. Gas Transport Properties of Surface Fluorinated Poly (Vinyltrimethylsilane) Films and Composite Membranes. J. Membr. Sci. 1994, 90, 55–68. [Google Scholar] [CrossRef]
- Allayarov, S.R.; Konovalova, T.A.; Waterfield, A.; Focsan, A.L.; Jackson, V.; Craciun, R.; Kispert, L.D.; Thrasher, J.S.; Dixon, D.A. Low-Temperature Fluorination of Fluoro-Containing Polymers: EPR Studies of Polyvinylidenefluoride and the Copolymer of Tetrafluoroethylene with Ethylene. J. Fluor. Chem. 2006, 127, 1294–1301. [Google Scholar] [CrossRef]
- Low, Z.-X.; Budd, P.M.; McKeown, N.B.; Patterson, D.A. Gas Permeation Properties, Physical Aging, and Its Mitigation in High Free Volume Glassy Polymers. Chem. Rev. 2018, 118, 5871–5911. [Google Scholar] [CrossRef] [PubMed]
- Pfromm, P.H. The Impact of Physical Aging of Amorphous Glassy Polymers on Gas Separation Membranes. In Materials Science of Membranes for Gas and Vapor Separation; Yampolskii, Y., Freeman, B., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 293–306. ISBN 978-0-470-02903-9. [Google Scholar]
- Pinnau, I.; Casillas, C.G.; Morisato, A.; Freeman, B.D. Long-Term Permeation Properties of Poly(1-Trimethylsilyl-1-Propyne) Membranes in Hydrocarbon—Vapor Environment. J. Polym. Sci. Part B Polym. Phys. 1997, 35, 1483–1490. [Google Scholar] [CrossRef]
- Barrie, J.A.; Levine, J.D.; Michaels, A.S.; Wong, P. Diffusion and Solution of Gases in Composite Rubber Membranes. Trans. Faraday Soc. 1963, 59, 869–878. [Google Scholar] [CrossRef]
- Henis, J.M.S.; Tripodi, M.K. Composite Hollow Fiber Membranes for Gas Separation: The Resistance Model Approach. J. Membr. Sci. 1981, 8, 233–246. [Google Scholar] [CrossRef]
- Chiao, C.C. Surface Modified Gas Separation Membranes. U.S. Patent 4828585, 9 May 1989. [Google Scholar]
- Pasternak, R.A.; Christensen, M.V.; Heller, J. Diffusion and Permeation of Oxygen, Nitrogen, Carbon Dioxide, and Nitrogen Dioxide through Polytetrafluoroethylene. Macromolecules 1970, 3, 366–371. [Google Scholar] [CrossRef]
- Belov, N.; Nizhegorodova, Y.; Zharov, A.; Konovalova, I.; Shantarovich, V.; Yampolskii, Y. A New Polymer, Poly(Perfluoropropylvinyl Ether) and Its Comparison with Other Perfluorinated Membrane Materials. J. Membr. Sci. 2015, 495, 431–438. [Google Scholar] [CrossRef]
- Nikiforov, R.; Belov, N.; Zharov, A.; Konovalova, I.; Shklyaruk, B.; Yampolskii, Y. Gas Permeation and Diffusion in Copolymers of Tetrafluoroethylene and Hexafluoropropylene: Effect of Annealing. J. Membr. Sci. 2017, 540, 129–135. [Google Scholar] [CrossRef]
- Belov, N.; Nikiforov, R.; Polunin, E.; Pogodina, Y.; Zavarzin, I.; Shantarovich, V.; Yampolskii, Y. Gas Permeation, Diffusion, Sorption and Free Volume of Poly(2-Trifluoromethyl-2-Pentafluoroethyl-1,3-Perfluorodioxole). J. Membr. Sci. 2018, 565, 112–118. [Google Scholar] [CrossRef]
- Matteucci, S.; Yampolskii, Y.; Freeman, B.D.; Pinnau, I. Transport of Gases and Vapors in Glassy and Rubbery Polymers. In Materials Science of Membranes for Gas and Vapor Separation; Yampolskii, Y., Freeman, B., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 1–47. ISBN 978-0-470-02903-9. [Google Scholar]
- Elkin, A.K.; Akulinkin, A.A.; Dyachenko, L.L.; Chepezubov, M.G.; Makaseev, Y.N.; Makaseev, A.Y.; Damm, Y.P.; Bujnovskij, A. S.Method of Reducing Oxygen Penetrability of Films Made from Polyethyleneterephthalate Used for Storage of Different Food Products. Russia Patent RU 2608027 C2, 12 September 2017. [Google Scholar]
- El’kin, A.K.; Akulinkin, A.A.; Dyachenko, L.L.; Chepezubov, M.G.; Makaseev, J.N.; Makaseev, A.J.; Damm, J.P.; Bujnovskij, A.S. Method for Reduction Oxygen-Permeability of Biaxially Oriented Polypropylene Films. Russia Patent RU 2575269 C1, 20 February 2016. [Google Scholar]
- El’kin, A.K.; Akulinkin, A.A.; Dyachenko, L.L.; Chepezubov, M.G.; Makaseev, J.N.; Makaseev, A.J.; Damm, J.P.; Bujnovskij, A.S. Method for Reduction of Oxygen Permeability of Polyethylene Films. Russia Patent RU 2575281 C1, 20 February 2016. [Google Scholar]
- Nazarov, V.G.; Stoljarov, V.P.; Evlampieva, L.A.; Baranov, V.A. Method of Modifying Polymers. Russia Patent RU 2373232 C2, 20 November 2009. [Google Scholar]
- Peyroux, J.; Dubois, M.; Tomasella, E.; Batisse, N.; Frezet, L.; Petit, E.; Kharitonov, A.P.; Flahaut, D. Plasma and Fluorination Combination for Stable Multifunctionality of LDPE Packaging Films. Plasma Process. Polym. 2017, 14, 1600066. [Google Scholar] [CrossRef]
- Peyroux, J.; Dubois, M.; Tomasella, E.; Petit, E.; Flahaut, D. Enhancement of Surface Properties on Commercial Polymer Packaging Films Using Various Surface Treatment Processes (Fluorination and Plasma). Appl. Surf. Sci. 2014, 315, 426–431. [Google Scholar] [CrossRef]
- Yampolskii, Y.; Belov, N. Investigation of Polymers by Inverse Gas Chromatography. Macromolecules 2015, 48, 6751–6767. [Google Scholar] [CrossRef]
- Belov, N.A.; Safronov, A.P.; Yampolskii, Y.P. Inverse-Gas Chromatography and the Thermodynamics of Sorption in Polymers. Polym. Sci. Ser. A 2012, 54, 859–873. [Google Scholar] [CrossRef]
- Wu, A.X.; Drayton, J.A.; Smith, Z.P. The Perfluoropolymer Upper Bound. AIChE J. 2019, 65, e16700. [Google Scholar] [CrossRef]
- Tressaud, A.; Labrugère, C.; Durand, E. Switchable Hydrophobic-Hydrophilic Fluorinated Layer for Offset Processing. In Functionalized Inorganic Fluorides: Synthesis, Characterization & Properties of Nanostructured Solids; Tressaud, A., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; pp. 571–582. [Google Scholar]
- Doronin, P. Influence of surface modification on stability of polygraphic rubbers to liquid agressive media. Vestn. Mosk. Gos. Univ. Pechati 2014, 3, 16–21. [Google Scholar]
- Ogungbemi, E.; Ijaodola, O.; Khatib, F.N.; Wilberforce, T.; El Hassan, Z.; Thompson, J.; Ramadan, M.; Olabi, A.G. Fuel Cell Membranes—Pros and Cons. Energy 2019, 172, 155–172. [Google Scholar] [CrossRef]
- Mai, Z.; Zhang, H.; Li, X.; Xiao, S.; Zhang, H. Nafion/Polyvinylidene Fluoride Blend Membranes with Improved Ion Selectivity for Vanadium Redox Flow Battery Application. J. Power Sources 2011, 196, 5737–5741. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Nale, A.; Pagot, G.; Vezzù, K.; Zawodzinski, T.A.; Meda, L.; Gambaro, C.; Di Noto, V. An Efficient Barrier toward Vanadium Crossover in Redox Flow Batteries: The Bilayer [Nafion/(WO3)x] Hybrid Inorganic-Organic Membrane. Electrochim. Acta 2021, 378, 138133. [Google Scholar] [CrossRef]
- Oldenburg, F.J.; Nilsson, E.; Schmidt, T.J.; Gubler, L. Tackling Capacity Fading in Vanadium Redox Flow Batteries with Amphoteric Polybenzimidazole/Nafion Bilayer Membranes. ChemSusChem 2019, 12, 2620–2627. [Google Scholar] [CrossRef]
- Esmaeili, N.; Gray, E.M.; Webb, C.J. Non-Fluorinated Polymer Composite Proton Exchange Membranes for Fuel Cell Applications—A Review. ChemPhysChem 2019, 20, 2016–2053. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lee, S.Y.; Lee, Y.M.; Lee, S.Y.; Rhim, J.W.; Lane, O.; McGrath, J.E. Surface-Fluorinated Proton-Exchange Membrane with High Electrochemical Durability for Direct Methanol Fuel Cells. ACS Appl. Mater. Interfaces 2009, 1, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Cho, H.I.; Kim, D.H.; Lee, B.S.; Lee, B.S.; Yoon, S.W.; Kim, Y.S.; Moon, G.Y.; Byun, H.; Rhim, J.W. Surface Fluorinated Poly(Vinyl Alcohol)/Poly(Styrene Sulfonic Acid-Co-Maleic Acid) Membrane for Polymer Electrolyte Membrane Fuel Cells. J. Membr. Sci. 2009, 342, 138–144. [Google Scholar] [CrossRef]
- Canghai, M.; Urban, J.J. Polymers of Intrinsic Microporosity (PIMs) Gas Separation Membranes: A Mini Review. Proc. Nat. Res. Soc. 2018, 2, 02002. [Google Scholar] [CrossRef]
- Zhou, H.; Jin, W. Membranes with Intrinsic Micro-Porosity: Structure, Solubility, and Applications. Membranes 2019, 9, 3. [Google Scholar] [CrossRef]
- Finkelshtein, E.; Gringolts, M.; Bermeshev, M.; Chapala, P.; Rogan, Y. Polynorbornenes. In Membrane Materials for Gas and Vapor Separation; Yampolskii, Y., Finkelshtein, E., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 143–221. ISBN 978-1-119-11274-7. [Google Scholar]
- Yampolskii, Y. A Current Position of Polyacetylenes Among Other Highly Permeable Membrane Materials. Polym. Rev. 2017, 57, 200–212. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belov, N.A.; Pashkevich, D.S.; Alentiev, A.Y.; Tressaud, A. Effect of Direct Fluorination on the Transport Properties and Swelling of Polymeric Materials: A Review. Membranes 2021, 11, 713. https://doi.org/10.3390/membranes11090713
Belov NA, Pashkevich DS, Alentiev AY, Tressaud A. Effect of Direct Fluorination on the Transport Properties and Swelling of Polymeric Materials: A Review. Membranes. 2021; 11(9):713. https://doi.org/10.3390/membranes11090713
Chicago/Turabian StyleBelov, Nikolay A., Dmitrii S. Pashkevich, Alexandre Yu Alentiev, and Alain Tressaud. 2021. "Effect of Direct Fluorination on the Transport Properties and Swelling of Polymeric Materials: A Review" Membranes 11, no. 9: 713. https://doi.org/10.3390/membranes11090713
APA StyleBelov, N. A., Pashkevich, D. S., Alentiev, A. Y., & Tressaud, A. (2021). Effect of Direct Fluorination on the Transport Properties and Swelling of Polymeric Materials: A Review. Membranes, 11(9), 713. https://doi.org/10.3390/membranes11090713