Synthesis of Polyurethane Membranes Derived from Red Seaweed Biomass for Ammonia Filtration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Samples
2.2. Equipment
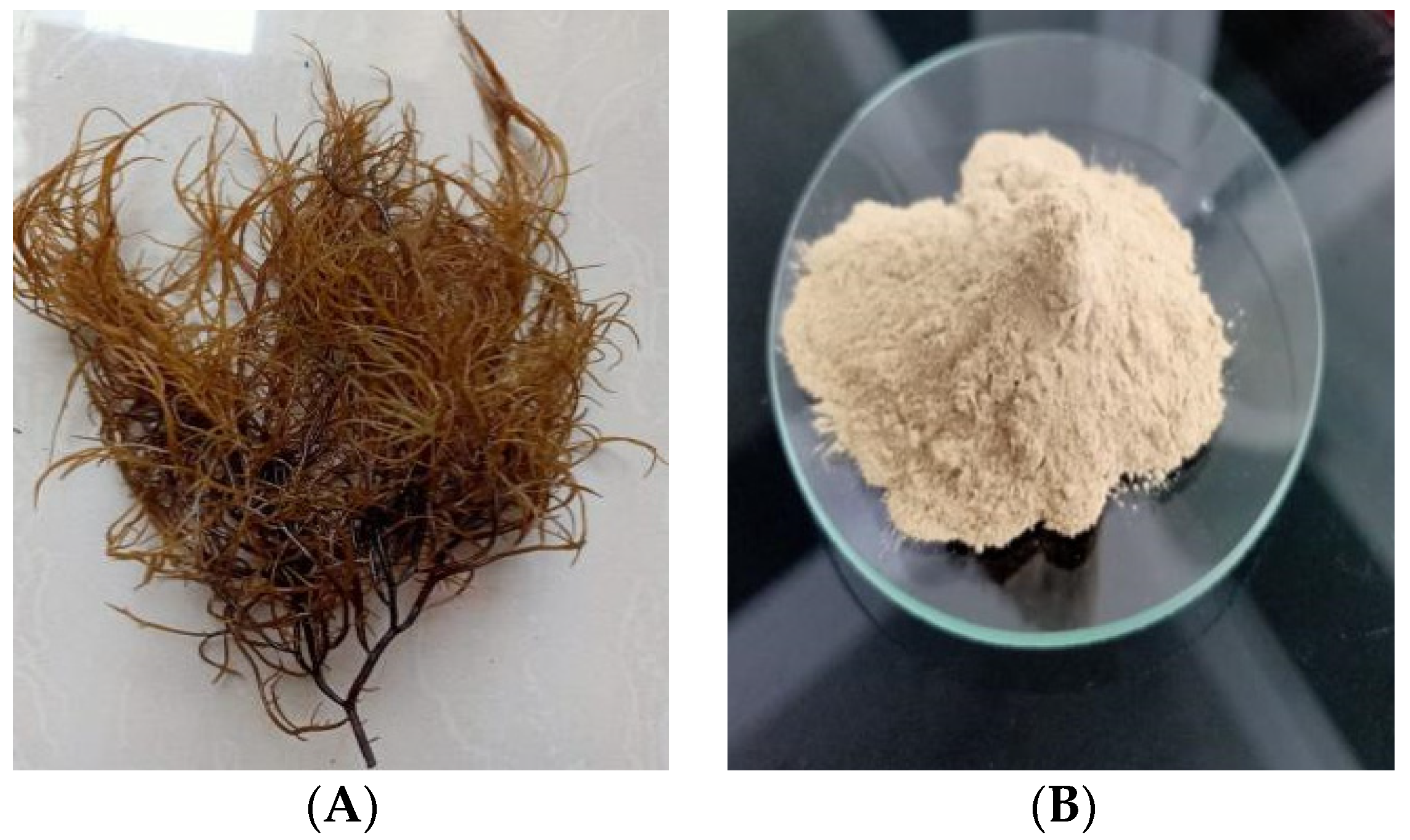
2.3. Red Seaweed Biomass Preparation
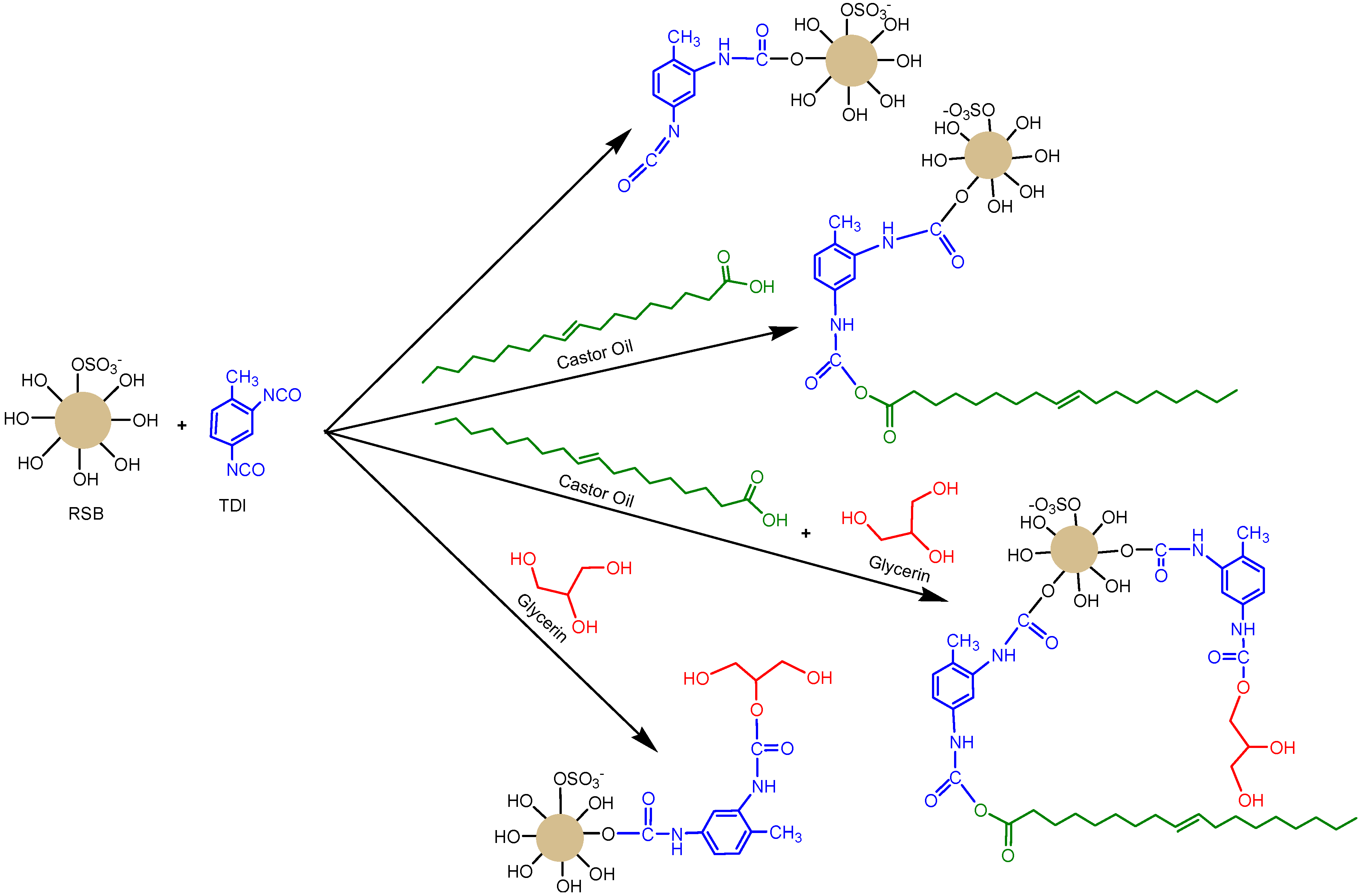
2.4. Polyurethane Membrane Preparation
2.5. Polyurethane Membrane Characterization
2.6. Filtration Experiment
- J: Flux (mL/cm2·min·bar)
- V: Permeate volume (mL)
- A: Surface area (cm2)
- t: Time (min)
- P: Pressure (bar)
- R: Rejection factor (%)
- C1: Feed concentration (ppm)
- C2: Permeate concentration (ppm)
3. Results and Discussion
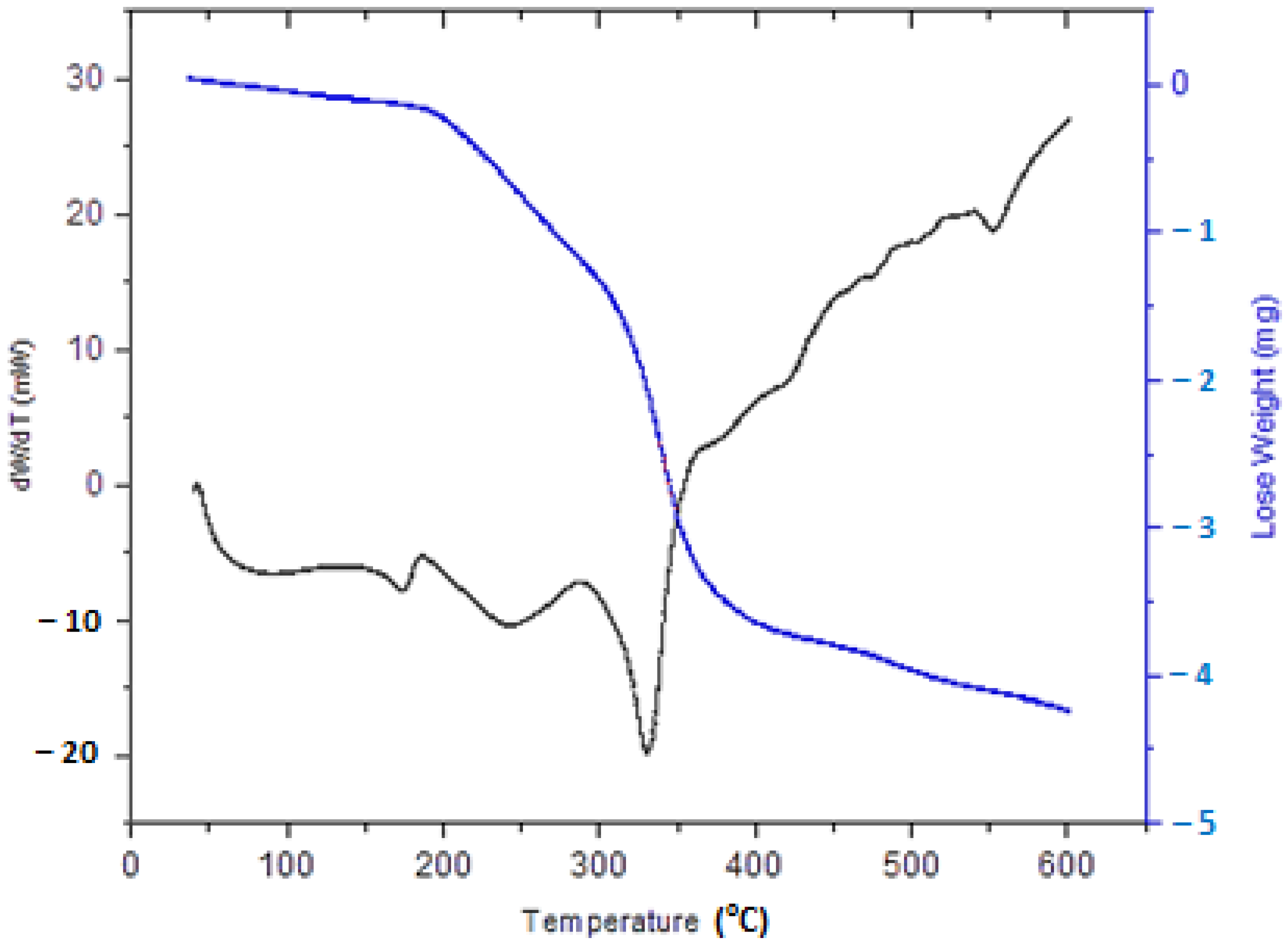
3.1. Red Seaweed Biomass
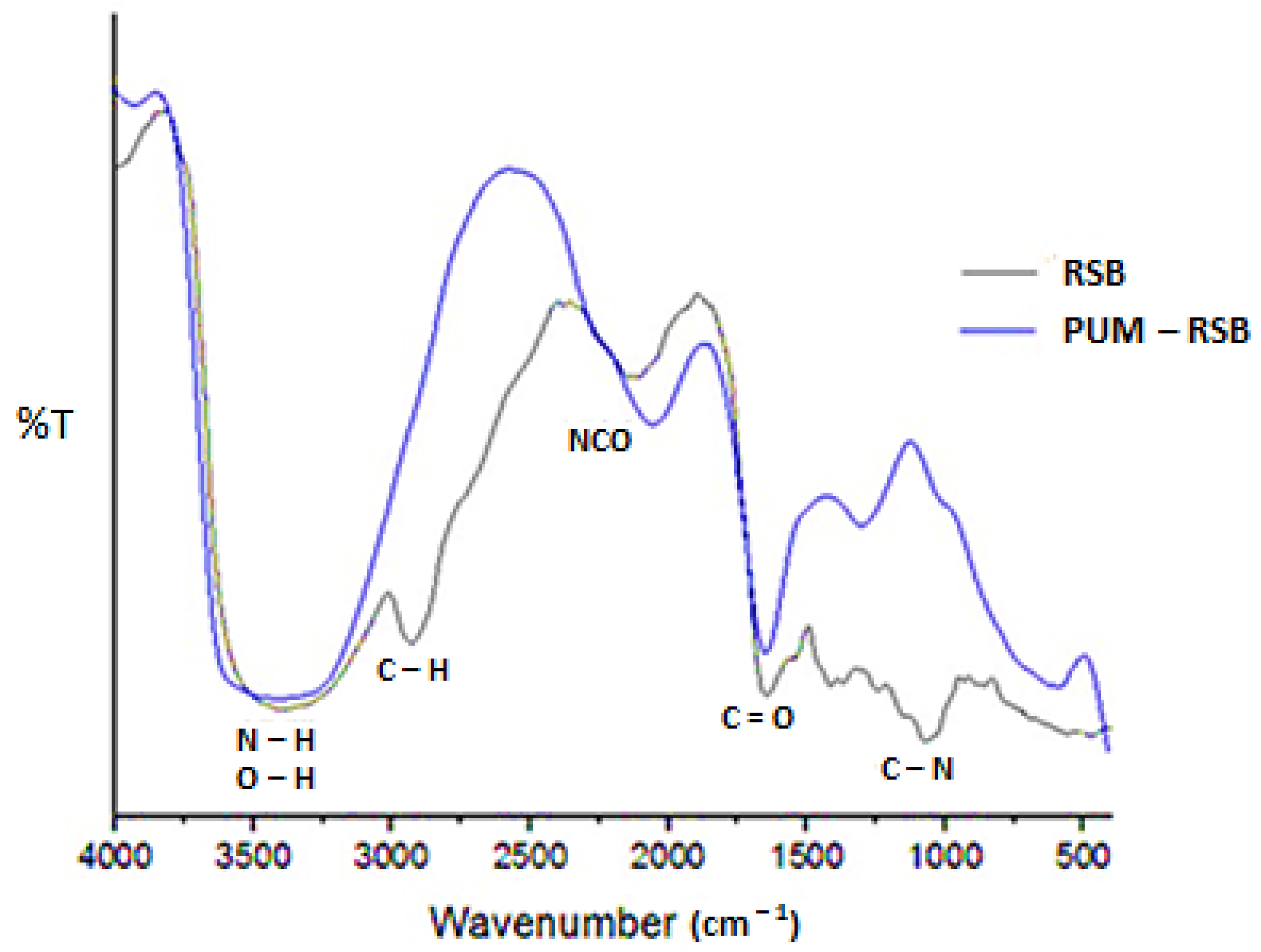
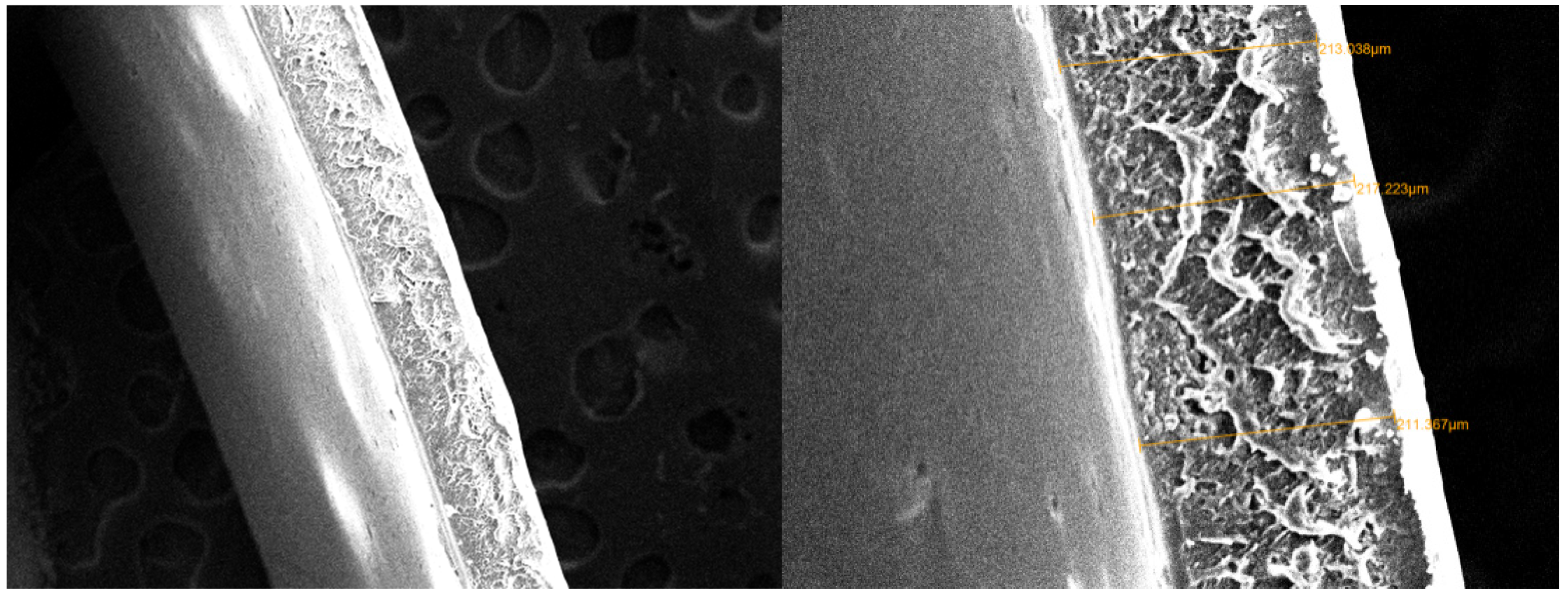
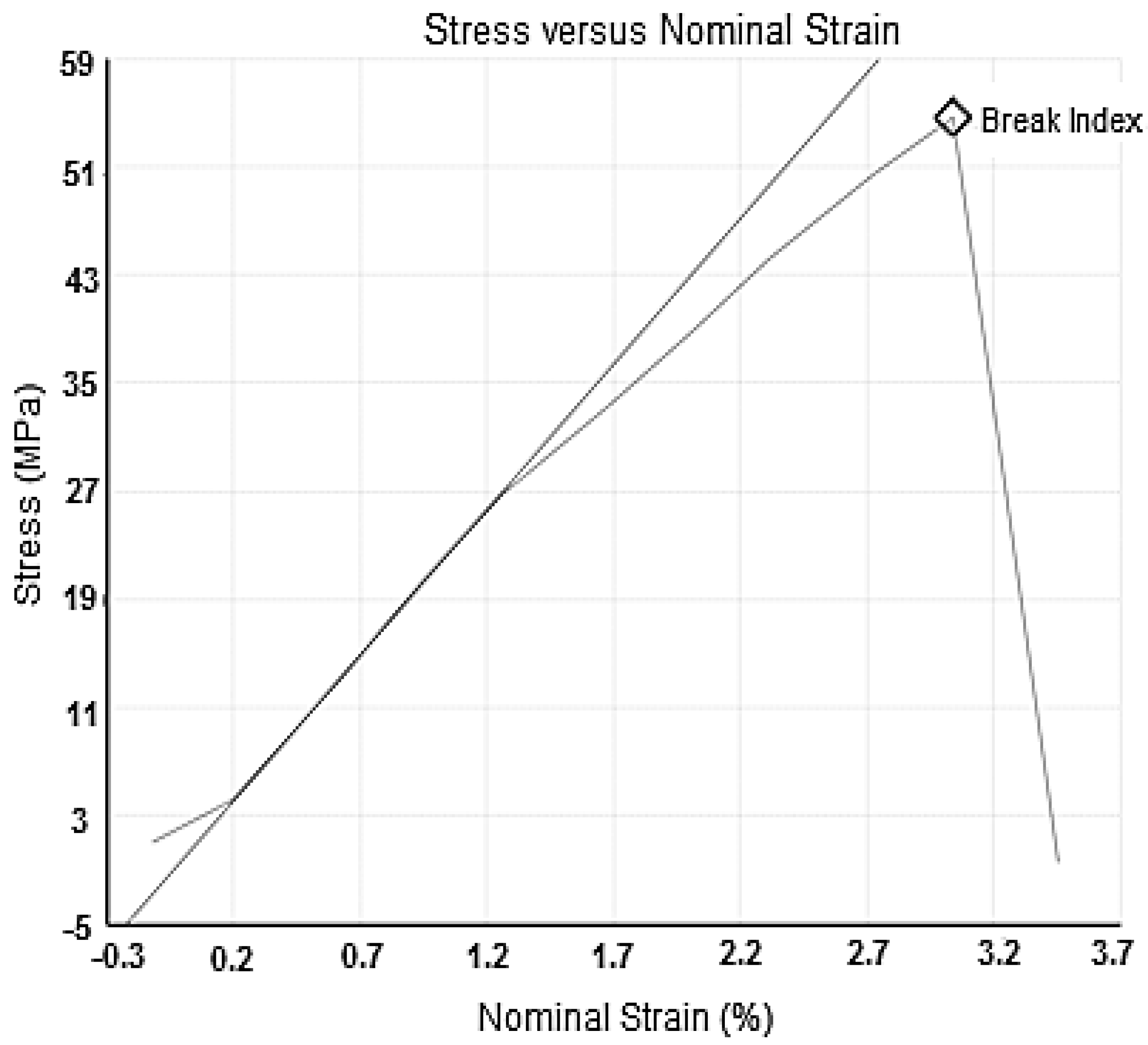
3.2. Polyurethane Membrane
3.3. Ammonia Filtration
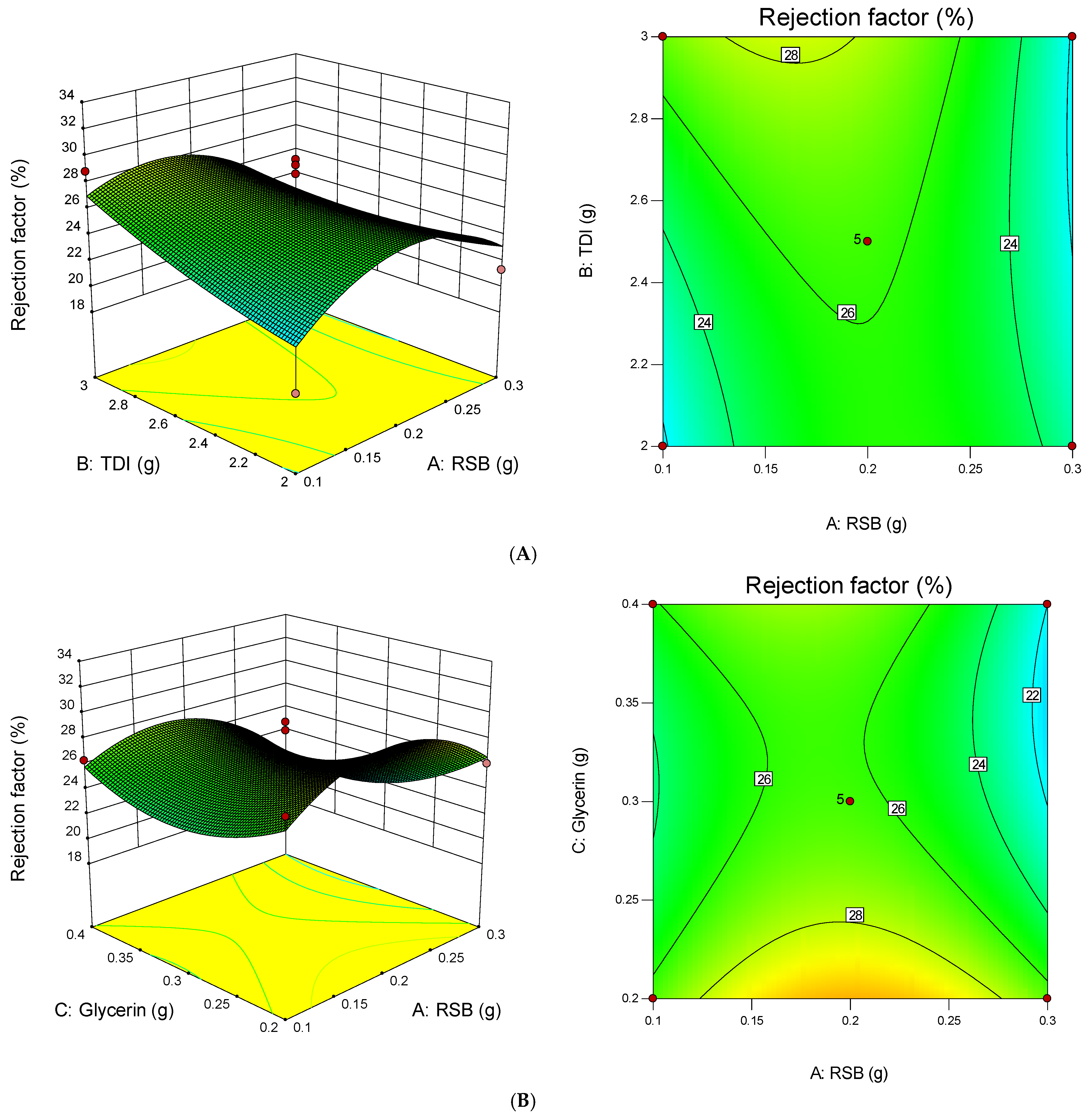
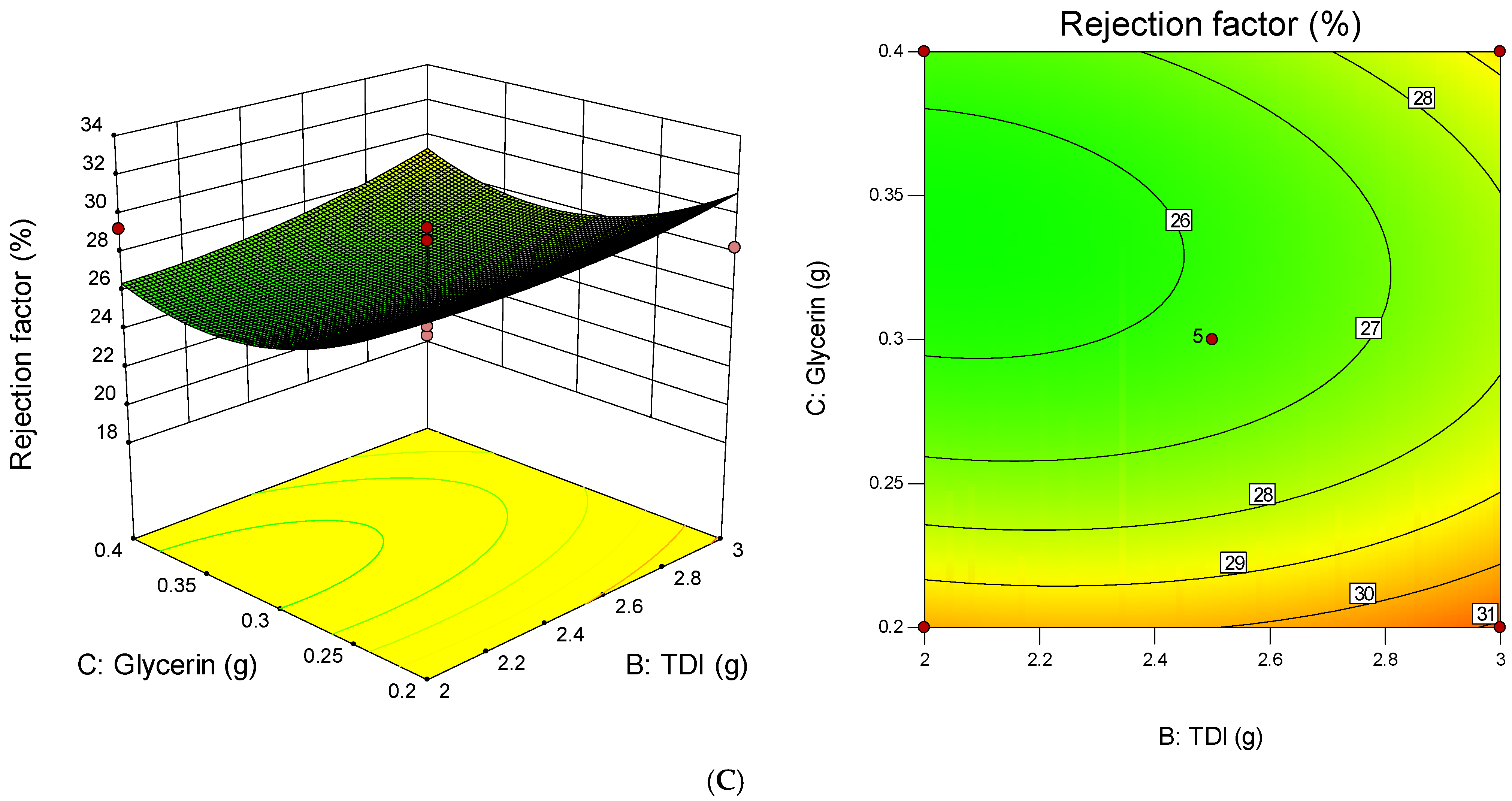
3.4. Statistical Design Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanagaraj, P.; Mohamed, I.M.; Huang, W.; Liu, C. Membrane fouling mitigation for enhanced water flux and high separation of humic acid and copper ion using hydrophilic polyurethane modified cellulose acetate ultrafiltration membranes. React. Funct. Polym. 2020, 150, 104538. [Google Scholar] [CrossRef]
- Adam, M.R. Impact of sintering temperature and pH of feed solution on adsorptive removal of ammonia from wastewater using clinoptilolite based hollow fibre ceramic membrane. J. Water Process. Eng. 2020, 33, 1–10. [Google Scholar] [CrossRef]
- Júnior, J.H.S.A.; Bertuol, D.A.; Meneguzzi, A.; Ferreira, C.A.; Amado, F.D.R. Castor oil and commercial thermoplastic polyurethane membranes modified with polyaniline: A comparative study. Mater. Res. 2013, 16, 860–866. [Google Scholar] [CrossRef]
- Gradinaru, L.M.; Vlad, S.; Spiridon, I.; Petrescu, M. Durability of polyurethane membranes in artificial weathering environment. Polym. Test. 2019, 80, 106144. [Google Scholar] [CrossRef]
- Marlina; Iqhrammullah, M.; Saleha, S.; Fathurrahmi; Maulina, F.P.; Idroes, R. Polyurethane film prepared from ball-milled algal polyol particle and activated carbon filler for NH3–N removal. Heliyon 2020, 6, e04590. [Google Scholar] [CrossRef]
- Karimi, M.B.; Khanbabaei, G.; Sadeghi, G.M.M. Vegetable oil-based polyurethane membrane for gas separation. J. Membr. Sci. 2017, 527, 198–206. [Google Scholar] [CrossRef]
- Saha, K.; Dutta, K.; Basu, A.; Adhikari, A.; Chattopadhyay, D.; Sarkar, P. Controlled delivery of tetracycline hydrochloride intercalated into smectite clay using polyurethane nanofibrous membrane for wound healing application. Nano-Struct. Nano-Objects 2020, 21, 100418. [Google Scholar] [CrossRef]
- Marlina, M. Sintesis Membran Poliuretan Berbasis Bahan Alam; Syiah Kuala University Press: Banda Aceh, Indonesia, 2017. [Google Scholar]
- Marlina, M. Pemanfaatan asam lemak bebas teroksidasi dari minyak jarak untuk sintesis membran poliuretan. J. Rekayasa Kim. Dan Lingkung. 2007, 6, 67–70. [Google Scholar]
- Nurman, S.; Marlina, M.; Saiful, S.; Saleha, S. Sintesis dan Karakterisasi Membran Poliuretan dari Minyak Biji Karet dan Heksametilen-1,6-diisosianat. J. Rekayasa Kim. Lingkung. 2015, 10, 188. [Google Scholar] [CrossRef][Green Version]
- Fitriani, F.; Khairan, K.; Marlina, M. Pemanfaatan Minyak Biji Alpukat (Avocado seed oil) untuk sintesis membran poliuretan. J. Has. Penelit. Ind. 2016, 2, 19–27. [Google Scholar]
- Marlina, M.; Farida, M.; Yahya, M. Synthesis and Characterization of Polyurethane Membrane from Nyamplung Seed Oils (Calophyllum inophillum). Asian J. Chem. 2017, 29, 1912–1916. [Google Scholar] [CrossRef]
- Marlina, M. Sintesis membran Poliuretan dari Karagenan dan 2,4 Toylulene diisosianat. J. Rekayasa Kim. Dan Lingkung. 2010, 7, 138–148. [Google Scholar]
- Matavos-Aramyan, S.; Jazebizadeh, M.H.; Babaei, S. Investigating CO2, O2 and N2 permeation properties of two new types of nanocomposite membranes: Polyurethane/silica and polyesterurethane/silica. Nano-Struct. Nano-Objects 2020, 21, 100414. [Google Scholar] [CrossRef]
- Li, R.; Shan, Z. Study on structure-induced heat transfer capabilities of waterborne polyurethane membranes. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 598, 124879. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet. Med. 2013, 58, 187–205. [Google Scholar] [CrossRef]
- Moreno, J.; Arregui, F.J.; Matías, I.R. Fiber optic ammonia sensing employing novel thermoplastic polyurethane membranes. Sensors Actuators B Chem. 2005, 105, 419–424. [Google Scholar] [CrossRef]
- Ramírez, M.; Gómez, J.M.; Aroca, G.; Cantero, D. Removal of ammonia by immobilized Nitrosomonas europaea in a biotrickling filter packed with polyurethane foam. Chemosphere 2009, 74, 1385–1390. [Google Scholar] [CrossRef]
- Wu, Y.-J.; Liu, Y.-W.; Cheng, H.-H.; Ke, C.-W.; Lin, T.-F.; Whang, L.-M. Biological pre-treatment system for ammonia removal from slightly contaminated river used as a drinking water source. Process. Saf. Environ. Prot. 2021, 147, 385–391. [Google Scholar] [CrossRef]
- Adam, M.R.; Mustafa, A. Current trends and future prospects of ammonia removal in wastewater: A comprehensive review on adsorptive membrane development. Sep. Purif. Technol. 2019, 213, 114–132. [Google Scholar] [CrossRef]
- Song, W. Performance of a novel hybrid membrane bioreactor for treating saline wastewater from mariculture: Assessment of pollutants removal and membrane filtration performance. Chem. Eng. J. 2018, 331, 695–703. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Shirazian, S.; Ashrafizadeh, S.N. Simulation of ammonia removal from industrial wastewater streams by means of a hollow-fiber membrane contactor. Desalination 2012, 285, 383–392. [Google Scholar] [CrossRef]
- Agrahari, G.K.; Shukla, S.K.; Verma, N.; Bhattacharya, P.K. Model prediction and experimental studies on the removal of dissolved NH3 from water applying hollow fiber membrane contactor. J. Membr. Sci. 2012, 390–391, 164–174. [Google Scholar] [CrossRef]
- Cheng, H.; Zhu, Q.; Xing, Z. Adsorption of ammonia nitrogen in low temperature domestic wastewater by modification bentonite. J. Clean. Prod. 2019, 233, 720–730. [Google Scholar] [CrossRef]
- Tekindal, M.A.; Bayrak, H.; Ozkaya, B.; Genc, Y. Box-Behnken Experimental Design in Factorial Experiments: The Importance of Bread For Nutrition and Health. Turkish J. F. Crop. 2012, 17, 115–123. [Google Scholar]
- Khajeh, M.; Gharan, M. Separation of organic acid compounds from biological samples by zinc oxide nanoparticles-chitosan using genetic algorithm based on response surface methodology and artificial neural network. J. Chemom. 2014, 28, 539–547. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C. Response Surface Methodology, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Khajeh, M.; Kaykhaii, M.; Sharafi, A. Application of PSO-artificial neural network and response surface methodology for removal of methylene blue using silver nanoparticles from water samples. J. Ind. Eng. Chem. 2013, 19, 1624–1630. [Google Scholar] [CrossRef]
- Khajeh, M.; Moghaddam, M.G.; Danesh, A.Z.; Khajeh, B. Response surface modeling of betulinic acid pre-concentration from medicinal plant samples by miniaturized homogenous liquid–liquid extraction and its determination by high performance liquid chromatography. Arab. J. Chem. 2015, 8, 400–406. [Google Scholar] [CrossRef]
- Agusman, A.; Apriani, S.N.K.; Murdinah, M. Penggunaan Tepung Rumput Laut Eucheuma cottonii pada Pembuatan Beras Analog dari Tepung Modified Cassava Flour (MOCAF). J. Pascapanen Dan Bioteknol. Kelaut. Dan Perikan. 2014, 9, 1–10. [Google Scholar] [CrossRef][Green Version]
- Nurman, S.; Saiful, S.; Ginting, B.; Rahmi, R.; Marlina, M. Optimization of Polyurethane Membrane Physical Characteristics of Red Seaweed Biomass Using a Box-Behnken Design. Indones. J. Chem. 2021, 21, 932–941. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Prasetyo, Y.A.; Abdassah, M. Preparation and Characterization of Glucosamine Nanoparticle by Ionic Gelation Method Using Chitosan and Alginate. Indones. J. Pharm. Sci. Technol. 2018, 1, 1–10. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Farsi, M.; Honarvar, B.; Heydarinasab, A.; Arjmand, M. Experimental survey of temperature, time and cross-linking agent effects on polydimethylsiloxane composite membranes performances in sulfur removal. Int. J. Ind. Chem. 2018, 9, 177–183. [Google Scholar] [CrossRef]
- Marlina, M.; Jabar, S.; Rahmi, R.; Saleha, S.; Nurman, S. Synthesis and Characterization New Polyurethane Membrane From Hydroxylated Rubber Seed Oil. Orient. J. Chem. 2017, 33, 199–206. [Google Scholar] [CrossRef]
- Tian, H.; Li, Z.; Lu, P.; Wang, Y.; Jia, C.; Wang, H.; Liu, Z.; Zhang, M. Starch and castor oil mutually modified, cross-linked polyurethane for improving the controlled release of urea. Carbohydr. Polym. 2021, 251, 117060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, W. Applications of hydrophobic α,ω-bis(amino)-terminated polydimethylsiloxane-graphene oxide in enhancement of anti-corrosion ability of waterborne polyurethane. Colloids Surfaces A: Physicochem. Eng. Asp. 2020, 600, 124981. [Google Scholar] [CrossRef]
- Mojerlou, F.; Lakouraj, M.M.; Barikani, M.; Mohammadi, A. Highly efficient polyurethane membrane based on nanocomposite of sulfonated thiacalix[4]arene-sodium alginate for desalination. Carbohydr. Polym. 2019, 205, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, H.; Razmi, F.; Shahalizade, T. Polyurethane TFC nanofiltration membranes based on interfacial polymerization of poly(bis-MPA) and MDI on the polyethersulfone support. Sep. Purif. Technol. 2016, 162, 37–44. [Google Scholar] [CrossRef]
- Melnig, V.; Apostu, M.; Tura, V.; Ciobanu, C. Optimization of polyurethane membranes: Morphology and structure studies. J. Membr. Sci. 2005, 267, 58–67. [Google Scholar] [CrossRef]
- Ghadimi, A.; Gharibi, R.; Yeganeh, H.; Sadatnia, B. Ionic liquid tethered PEG-based polyurethane-siloxane membranes for efficient CO2/CH4 separation. Mater. Sci. Eng. C 2019, 102, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, C.; Xiao, Y.; Mu, C.; Lin, W. Fabrication of water-resistance and durable antimicrobial adhesion polyurethane coating containing weakly amphiphilic poly(isobornyl acrylate) Side chains. Prog. Org. Coatings 2020, 147, 105812. [Google Scholar] [CrossRef]
- Ray, H.; Perreault, F.; Boyer, T.H. Rejection of nitrogen species in real fresh and hydrolyzed human urine by reverse osmosis and nanofiltration. J. Environ. Chem. Eng. 2020, 8, 103993. [Google Scholar] [CrossRef]
- Chelladurai, S.J.S. Optimization of process parameters using response surface methodology: A review. Mater. Today Proc. 2021, 37, 1301–1304. [Google Scholar] [CrossRef]
- Zhao, Z.; Bermudez, S.C.; Ilyas, A.; Muylaert, K.; Vankelecom, I.F. Optimization of negatively charged polysulfone membranes for concentration and purification of extracellular polysaccharides from Arthrospira platensis using the response surface methodology. Sep. Purif. Technol. 2020, 252, 117385. [Google Scholar] [CrossRef]
| RSB 1 (g) | TDI 2 (g) | Castor Oil (g) | Glycerin (g) | Visual Description PUM-RSB 3 | |
|---|---|---|---|---|---|
| 0.2 | 2.5 | 0 | 0 |  | dry, brittle, crumbles easily |
| 0.2 | 2.5 | 0.5 | 0 |  | less dry, less elastic, not brittle |
| 0.2 | 2.5 | 1 | 0 |  | less dry, less elastic |
| 0.2 | 2.5 | 0.5 | 0.3 |  | dry, elastic, and not easily torn |
| 0.2 | 2.5 | 0 | 0.3 |  | dry, stiff, less elastic |
| 0 | 2.5 | 0.5 | 0.3 | 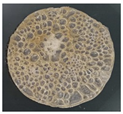 | dry, hard as glass |
| 0 | 2.5 | 0 | 0.3 |  | dry, hard foam |
| Run | Factor 1 A: RSB (g) | Factor 2 B: TDI (g) | Factor 3 C: Glycerin (g) | Flux (mL/cm2·min·bar) | Rejection Factor (%) |
|---|---|---|---|---|---|
| 1 | 0.2 | 3.0 | 0.4 | 0.952 | 26.944 |
| 2 | 0.2 | 2.5 | 0.3 | 0.700 | 24.231 |
| 3 | 0.3 | 2.5 | 0.4 | 0.731 | 21.037 |
| 4 | 0.2 | 2.0 | 0.2 | 1.660 | 32.369 |
| 5 | 0.2 | 3.0 | 0.2 | 0.935 | 28.330 |
| 6 | 0.1 | 2.5 | 0.4 | 0.930 | 26.342 |
| 7 | 0.2 | 2.5 | 0.3 | 1.322 | 28.692 |
| 8 | 0.1 | 2.0 | 0.3 | 0.754 | 18.505 |
| 9 | 0.3 | 2.0 | 0.3 | 0.732 | 21.339 |
| 10 | 0.1 | 2.5 | 0.2 | 1.156 | 27.547 |
| 11 | 0.3 | 2.5 | 0.2 | 0.928 | 26.100 |
| 12 | 0.2 | 2.5 | 0.3 | 1.141 | 29.355 |
| 13 | 0.2 | 2.0 | 0.4 | 1.662 | 29.295 |
| 14 | 0.3 | 3.0 | 0.3 | 0.701 | 25.196 |
| 15 | 0.2 | 2.5 | 0.3 | 0.840 | 25.437 |
| 16 | 0.2 | 2.5 | 0.3 | 1.058 | 23.749 |
| 17 | 0.1 | 3.0 | 0.3 | 0.992 | 28.873 |
| Source | Linear | 2FI | Quadratic |
|---|---|---|---|
| Std. Dev | 3.61 | 3.93 | 3.47 |
| R-Square | 0.1474 | 0.2231 | 0.5746 |
| Adj R-Square | −0.0494 | −0.2431 | 0.0277 |
| Pred R-Square | −0.6545 | −2.4505 | −3.8734 |
| Adeq Precisior | 2.651 | 2.702 | 3.495 |
| PRESS | 328.60 | 685.27 | 967.87 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F | Characterization |
|---|---|---|---|---|---|---|
| Model | 114.12 | 9 | 12.68 | 1.05 | 0.4853 | Not significant |
| A-RSB | 7.21 | 1 | 7.21 | 0.60 | 0.4649 | |
| B-TDI | 7.67 | 1 | 7.67 | 0.64 | 0.4514 | |
| C-Glycerin | 14.39 | 1 | 14.39 | 1.19 | 0.3111 | |
| AB | 10.60 | 1 | 10.60 | 0.88 | 0.3799 | |
| AC | 3.72 | 1 | 3.72 | 0.31 | 0.5960 | |
| BC | 0.71 | 1 | 0.71 | 0.059 | 0.8150 | |
| A2 | 48.57 | 1 | 48.57 | 4.02 | 0.0849 | |
| B2 | 1.42 | 1 | 1.42 | 0.12 | 0.7412 | |
| C2 | 23.45 | 1 | 23.45 | 1.94 | 0.2060 | |
| Residual | 84.48 | 7 | 12.07 | |||
| Lack of Fit | 57.90 | 3 | 19.30 | 2.90 | 0.1649 | Not significant |
| Pure Error | 26.59 | 4 | 6.65 | |||
| Cor Total | 198.60 | 16 |
| RSB (g) | TDI (g) | Glycerin (g) | Rejection Factor (Theory) (%) | Desirability | Rejection Factor (Experiment) (%) |
|---|---|---|---|---|---|
| 0.176 | 3.000 | 0.200 | 31.324 | 0.925 | 23.870 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurman, S.; Saiful, S.; Ginting, B.; Rahmi, R.; Marlina, M.; Wibisono, Y. Synthesis of Polyurethane Membranes Derived from Red Seaweed Biomass for Ammonia Filtration. Membranes 2021, 11, 668. https://doi.org/10.3390/membranes11090668
Nurman S, Saiful S, Ginting B, Rahmi R, Marlina M, Wibisono Y. Synthesis of Polyurethane Membranes Derived from Red Seaweed Biomass for Ammonia Filtration. Membranes. 2021; 11(9):668. https://doi.org/10.3390/membranes11090668
Chicago/Turabian StyleNurman, Salfauqi, Saiful Saiful, Binawati Ginting, Rahmi Rahmi, Marlina Marlina, and Yusuf Wibisono. 2021. "Synthesis of Polyurethane Membranes Derived from Red Seaweed Biomass for Ammonia Filtration" Membranes 11, no. 9: 668. https://doi.org/10.3390/membranes11090668
APA StyleNurman, S., Saiful, S., Ginting, B., Rahmi, R., Marlina, M., & Wibisono, Y. (2021). Synthesis of Polyurethane Membranes Derived from Red Seaweed Biomass for Ammonia Filtration. Membranes, 11(9), 668. https://doi.org/10.3390/membranes11090668