Porous Medium Equation in Graphene Oxide Membrane: Nonlinear Dependence of Permeability on Pressure Gradient Explained
Abstract
:1. Introduction
2. Materials and Methods
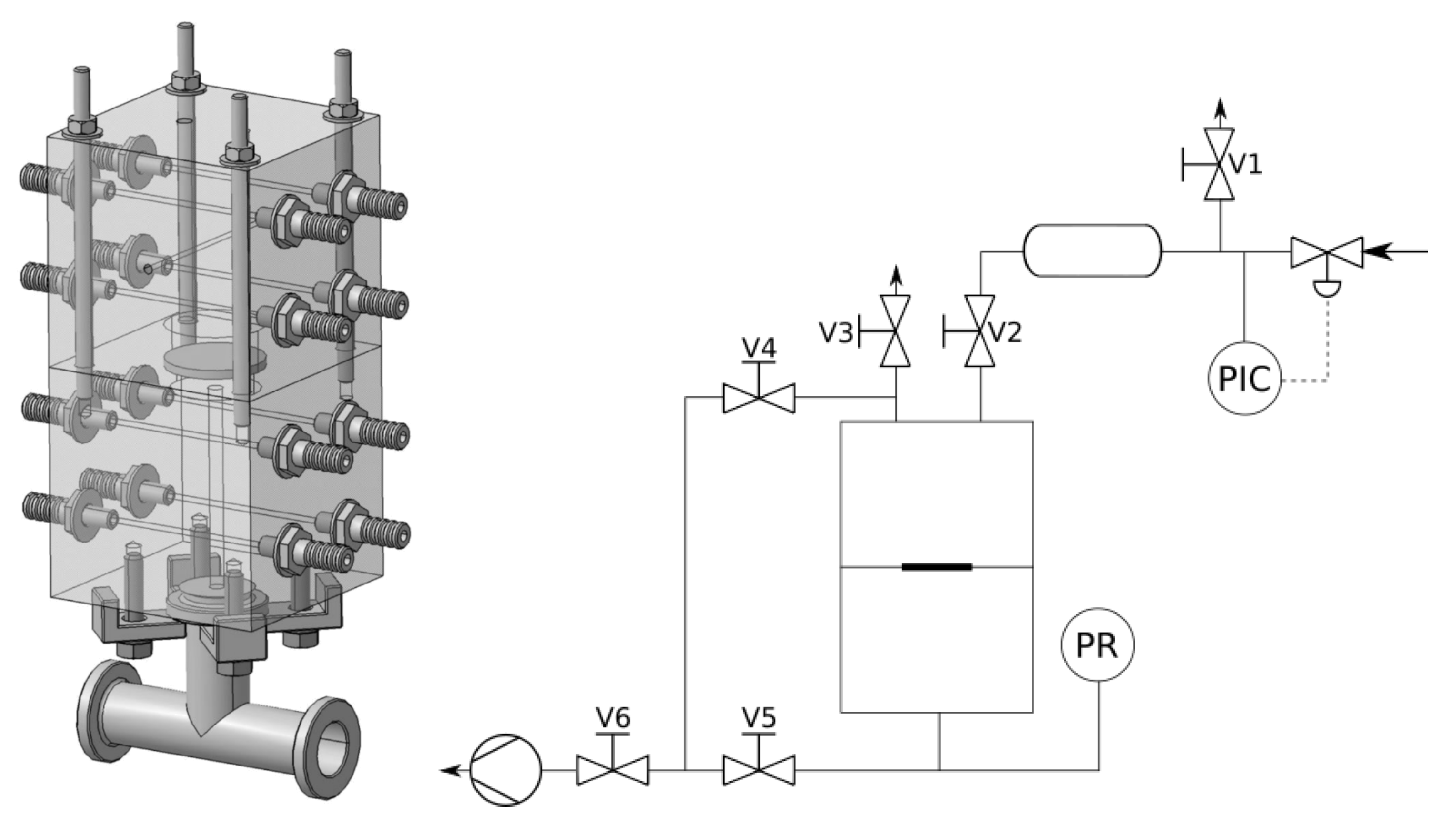
2.1. Manometric Integral Permeameter
2.2. Membrane
A volume corresponding to 82 mg of GO was taken from the stock suspension with a GO concentration of 20 mg/mL. The GO suspension was diluted with distilled water to a volume of 25 mL and sonicated in an ultrasonic tray (37 kHz, 300 W) for 15 min. The diluted GO suspension was placed in a pressurized filter cell equipped with a polyamide filter membrane. Filtration was performed using compressed air at a pressure of 3–5 bar. After filtration, the still wet GO membrane was moved (still with the polyamide support) to a desiccator with a constant 30% relative humidity (provided by saturated solution at 20 °C). In this environment the membrane was stored for at least 24 h in order to balance the moisture in the membrane.
3. Mathematical Formulation of the Experiment
3.1. Linearity of Single Measurement in the Integral Cell
3.2. Nearly-Stationary Behavior within the Membrane
3.3. Conservation of Mass on the Membrane/Integral Cell Interface
3.4. Boundary Conditions for Pressure Profile in the Membrane
3.5. Representation of the Nonlinearity
3.6. Numerical Simulation Support for the Assumptions
4. Stationary Solutions of Porous Medium Equation
Nonlinearity Predicted
5. Results
5.1. General Setting
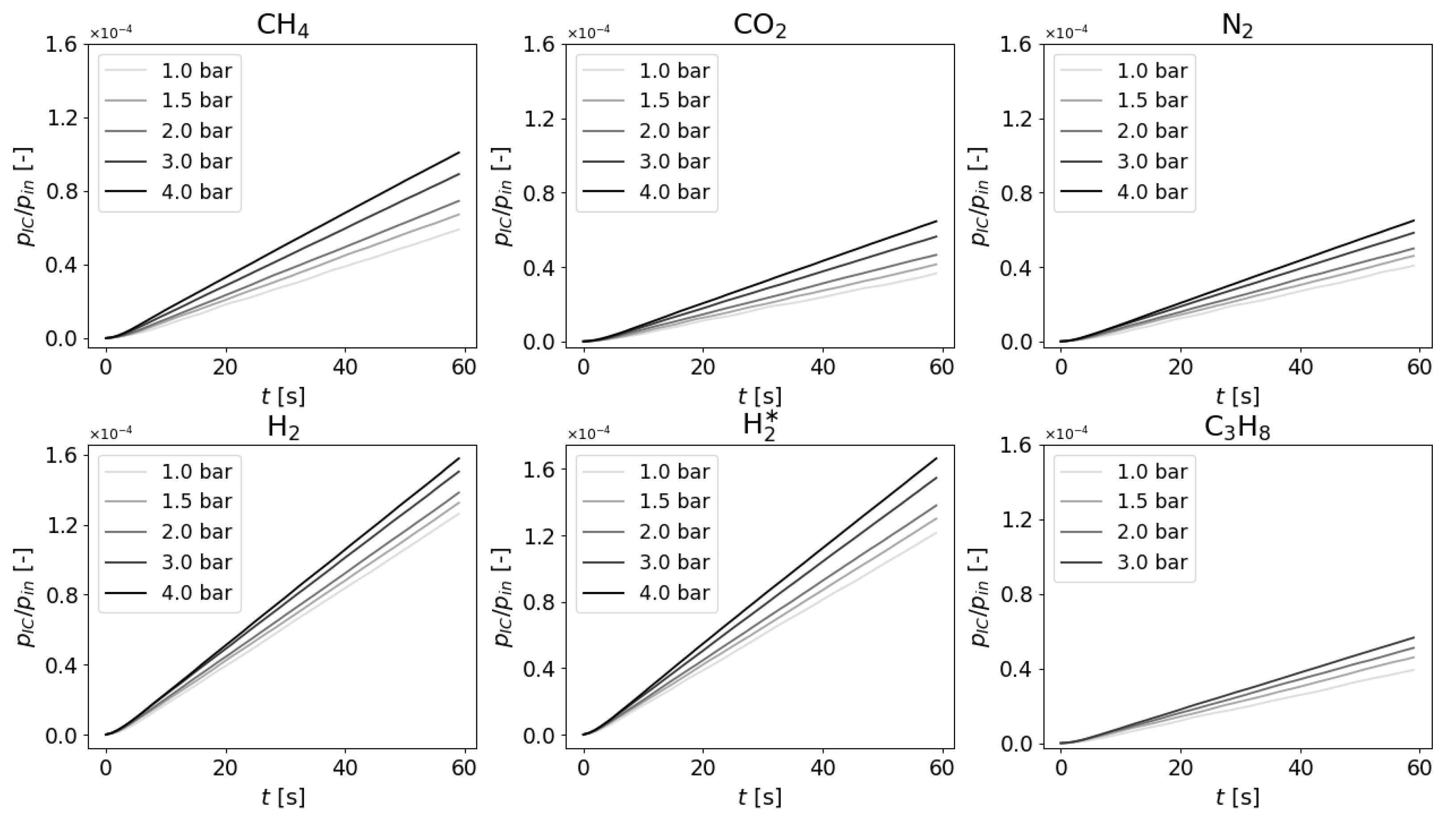
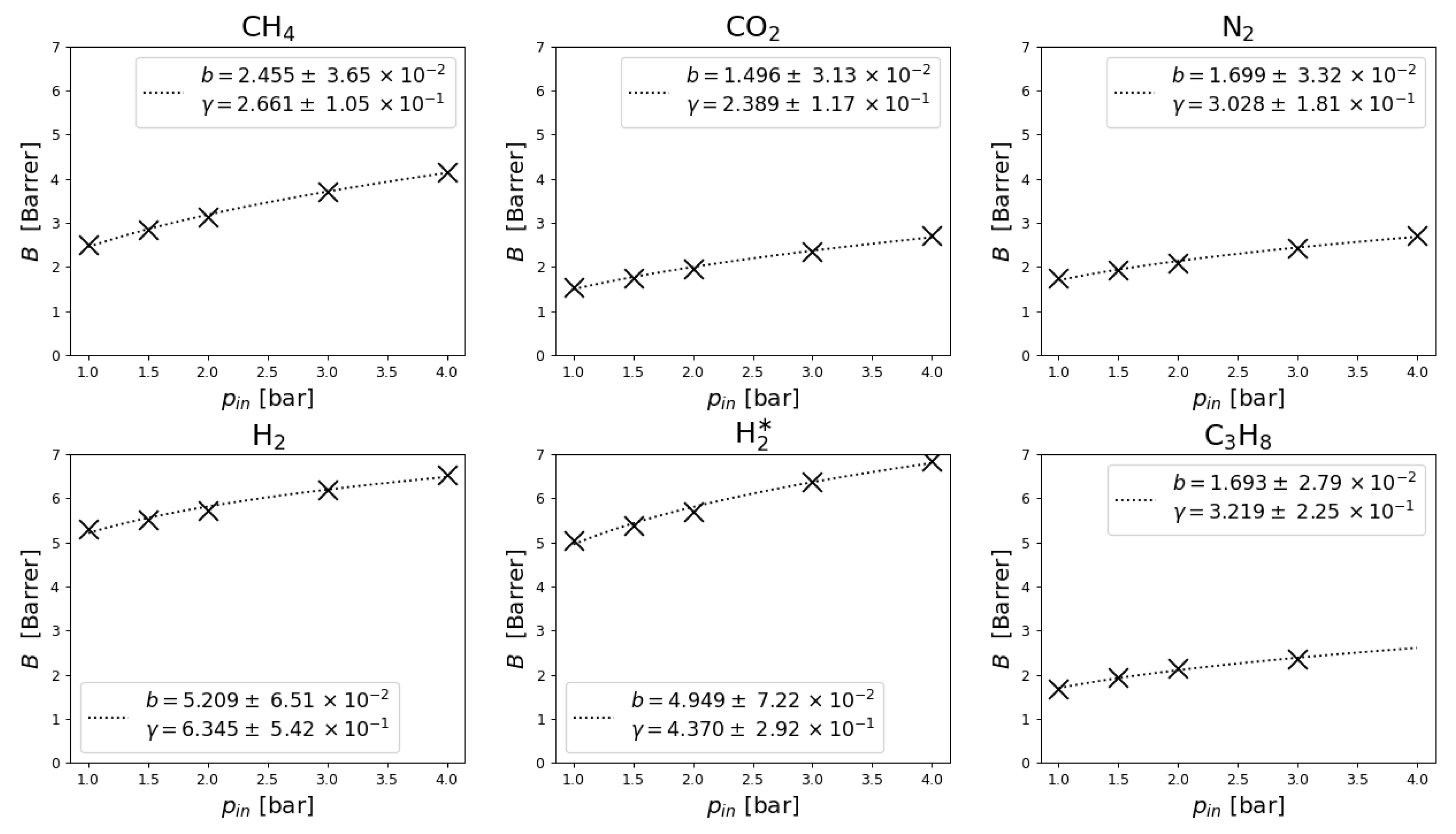
5.2. Measurements and Data Regression
5.3. Polytropic Exponent Interpretation
6. Discussion
6.1. Porous Medium Equation Capability of Describing Observed Nonlinearity
6.2. Nonlinearity Implications for Selectivity
6.3. Physical Interpretation of Polytropic Exponent
6.4. Pressure Range of the Measurement
6.5. Future Research Outline
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Symbol | Unit | Description |
| Membrane area | ||
| b | Power-law set-point | |
| B | Membrane gas permeability | |
| C | Heat capacity | |
| Heat capacity, isobaric | ||
| Heat capacity, isochoric | ||
| D | Diffusion coefficient | |
| Specific energy | ||
| k | Darcy’s permeability | |
| m | Mass | |
| M | Molar mass | |
| n | Molar amount | |
| p | Pressure | |
| Volume flux | ||
| Q | Heat | |
| R | Universal gas constant | |
| T | Temperature | |
| U | Internal energy | |
| Velocity vector field | ||
| W | Work | |
| – | Selectivity | |
| – | Polytropic exponent/index | |
| – | Porosity | |
| Dynamic viscosity | ||
| Density |
Appendix A. Derivation of Porous Medium Equation
Appendix A.1. Prerequisites
- Laplacian form, where the Laplace operator acts on nonlinear expression of our quantity—in this form we can easily compare PME to linear heat equation;
- Divergence form, which is numerically treatable with Finite Volume Method;
- Linearized form, where both differential operators (Laplacian and gradient) act on linear expression of our quantity—in this form the equation can be treated with Finite Differences Method.
Appendix A.2. Porous Medium Equation for Pressure
Appendix A.3. Porous Medium Equation for Density
References
- TechNavio. Global Gas Separation Membrane Market 2021–2025; Technical Report; ReportLinker: Lyon, France, 2021. [Google Scholar]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Bouša, D.; Friess, K.; Pilnáček, K.; Vopička, O.; Lanč, M.; Fónod, K.; Pumera, M.; Sedmidubský, D.; Luxa, J.; Sofer, Z. Thin, High-Flux, Self-Standing, Graphene Oxide Membranes for Efficient Hydrogen Separation from Gas Mixtures. Chem. Eur. J. 2017, 23, 11416–11422. [Google Scholar] [CrossRef]
- Guan, K.; Shen, J.; Liu, G.; Zhao, J.; Zhou, H.; Jin, W. Spray-evaporation assembled graphene oxide membranes for selective hydrogen transport. Sep. Purif. Technol. 2017, 174, 126–135. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Gao, J.; Chung, T.S. Layer-by-layer construction of graphene oxide (GO) framework composite membranes for highly efficient heavy metal removal. J. Membr. Sci. 2016, 515, 230–237. [Google Scholar] [CrossRef]
- Shen, J.; Liu, G.; Huang, K.; Jin, W.; Lee, K.R.; Xu, N. Membranes with Fast and Selective Gas-Transport Channels of Laminar Graphene Oxide for Efficient CO2 Capture. Angew. Chem. Int. Ed. 2015, 54, 578–582. [Google Scholar] [CrossRef]
- Huang, H.; Ying, Y.; Peng, X. Graphene oxide nanosheet: An emerging star material for novel separation membranes. J. Mater. Chem. A 2014, 2, 13772–13782. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerapandian, M.; Yun, K.; Kim, S.J. The chemical and structural analysis of graphene oxide with different degrees of oxidation. Carbon 2013, 53, 38–49. [Google Scholar] [CrossRef]
- Compton, O.C.; Nguyen, S.T. Graphene Oxide, Highly Reduced Graphene Oxide, and Graphene: Versatile Building Blocks for Carbon-Based Materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.B.; Evmenenko, G.; Bguyen, S.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–560. [Google Scholar] [CrossRef] [PubMed]
- Freeman, B.D. Basis of Permeability/Selectivity Tradeoff Relations in Polymeric Gas Separation Membranes. Macromolecules 1999, 32, 375–380. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, eaab0530. [Google Scholar] [CrossRef] [Green Version]
- Malgras, V.; Ji, Q.; Kamachi, Y.; Mori, T.; Shieh, F.K.; Wu, K.C.W.; Ariga, K.; Yamauchi, Y. Templated synthesis for nanoarchitectured porous materials. Bull. Chem. Soc. Jpn. 2015, 88, 1171–1200. [Google Scholar] [CrossRef]
- Li, W.; Samarasinghe, S.; Bae, T.H. Enhancing CO2/CH4 separation performance and mechanical strength of mixed-matrix membrane via combined use of graphene oxide and ZIF-8. J. Ind. Eng. Chem. 2018, 67, 156–163. [Google Scholar] [CrossRef]
- Zhu, J.; Meng, X.; Zhao, J.; Jin, Y.; Yang, N.; Zhang, S.; Sunarso, J.; Liu, S. Facile hydrogen/nitrogen separation through graphene oxide membranes supported on YSZ ceramic hollow fibers. J. Membr. Sci. 2017, 535, 143–150. [Google Scholar] [CrossRef]
- Jia, M.; Feng, Y.; Liu, S.; Qiu, J.; Yao, J. Graphene oxide gas separation membranes intercalated by UiO-66-NH2 with enhanced hydrogen separation performance. J. Membr. Sci. 2017, 539, 172–177. [Google Scholar] [CrossRef]
- Yoo, B.M.; Shin, J.E.; Lee, H.D.; Park, H.B. Graphene and graphene oxide membranes for gas separation applications. Curr. Opin. Chem. Eng. 2017, 16, 39–47. [Google Scholar] [CrossRef]
- Meng, N.; Priestley, R.C.E.; Zhang, Y.; Wang, H.; Zhang, X. The effect of reduction degree of GO nanosheets on microstructure and performance of PVDF/GO hybrid membranes. J. Membr. Sci. 2016, 501, 169–178. [Google Scholar] [CrossRef]
- Zahid, M.; Akram, S.; Rashid, A.; Rehan, Z.A.; Javed, T.; Shabbir, R.; Hessien, M.M.; El-Sayed, M.E. Investigating the Antibacterial Activity of Polymeric Membranes Fabricated with Aminated Graphene Oxide. Membranes 2021, 11, 510. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, W.; Liu, Y.; Qin, C.; Meng, M.; Jiang, Y.; Qiu, J.; Peng, J. A mussel inspired highly stable graphene oxide membrane for efficient oil-in-water emulsions separation. Sep. Purif. Technol. 2018, 199, 37–46. [Google Scholar] [CrossRef]
- Pandey, R.P.; Shukla, G.; Manohar, M.; Shahi, V.K. Graphene oxide based nanohybrid proton exchange membranes for fuel cell applications: An overview. Adv. Colloid Interface Sci. 2017, 240, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Liu, G.; Lou, Y.; Dong, Z.; Shen, J.; Jin, W. A Graphene Oxide Membrane with Highly Selective Molecular Separation of Aqueous Organic Solution. Angew. Chem. Int. Ed. 2014, 53, 6929–6932. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xu, Z.; Gao, C. Ultrathin Graphene Nanofiltration Membrane for Water Purification. Adv. Funct. Mater. 2013, 23, 3693–3700. [Google Scholar] [CrossRef]
- Hu, M.; Mi, B. Enabling Graphene Oxide Nanosheets as Water Separation Membranes. Environ. Sci. Technol. 2013, 47, 3715–3723. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wang, D.; Kim, S.; Kim, S.Y.; Hu, Y.; Yakes, M.K.; Laracuente, A.R.; Dai, Z.; Marder, S.R.; Berger, C.; et al. Nanoscale Tunable Reduction of Graphene Oxide for Graphene Electronics. Science 2010, 328, 1373–1376. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, S.A.; Nasir, A.; Aziz, F.; Kumar, G.; Sallehhudin, W.; Jaafar, J.; Lau, W.; Yusof, N.; Salleh, W.; Ismail, A. CO2/N2 selectivity enhancement of PEBAX MH 1657/Aminated partially reduced graphene oxide mixed matrix composite membrane. Sep. Purif. Technol. 2019, 223, 142–153. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, J.; Bai, Y.; Wen, Y.; Zhao, N.; Zhang, X.; Zhang, Y.; Li, Q.; Wei, L. The Preparation and Properties of Nanocomposite from Bio-Based Polyurethane and Graphene Oxide for Gas Separation. Nanomaterials 2019, 9, 15. [Google Scholar] [CrossRef] [Green Version]
- Zomorodkia, A.A.; Bazgir, S.; Zaarei, D.; Gorji, M.; Ardjmand, M. Permeation of water, ammonia and dichloromethane through graphene oxide/polymeric matrix composite membranes. New Carbon Mater. 2020, 35, 739–751. [Google Scholar] [CrossRef]
- Cheng, L.; Yang, H.; Chen, X.; Liu, G.; Guo, Y.; Liu, G.; Jin, W. MIL-101(Cr) microporous nanocrystals intercalating graphene oxide membrane for efficient hydrogen purification. Chem. Asian J. 2021. [Google Scholar] [CrossRef]
- Qu, L.; Wang, N.; Xu, H.; Wang, W.; Liu, Y.; Kuo, L.; Yadav, T.P.; Wu, J.; Joyner, J.; Song, Y.; et al. Gold Nanoparticles and g-C3N4-Intercalated Graphene Oxide Membrane for Recyclable Surface Enhanced Raman Scattering. Adv. Funct. Mater. 2017, 27, 1701714. [Google Scholar] [CrossRef]
- Chen, X.; Qiu, M.; Ding, H.; Fu, K.; Fan, Y. A reduced graphene oxide nanofiltration membrane intercalated by well-dispersed carbon nanotubes for drinking water purification. Nanoscale 2016, 8, 5696–5705. [Google Scholar] [CrossRef]
- Alen, S.K.; Nam, S.; Dastgheib, S.A. Recent Advances in Graphene Oxide Membranes for Gas Separation Applications. Int. J. Mol. Sci. 2019, 20, 5609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassani, N.; Rashidi, R.; Milošević, M.V.; Neek-Amal, M. Evaluating gas permeance through graphene nanopores and porous 2D-membranes: A generalized approach. Carbon Trends 2021, 5, 100086. [Google Scholar] [CrossRef]
- Tronci, G.; Raffone, F.; Cicero, G. Theoretical Study of Nanoporous Graphene Membranes for Natural Gas Purification. Appl. Sci. 2018, 8, 1547. [Google Scholar] [CrossRef] [Green Version]
- Esfandiarpoor, S.; Fazli, M.; Ganji, M.D. Reactive molecular dynamic simulations on the gas separation performance of porous graphene membrane. Sci. Rep. 2017, 7, 16561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Yang, X.; Liu, S. Molecular dynamics simulation of water transport through graphene-based nanopores: Flow behavior and structure characteristics. Chin. J. Chem. Eng. 2015, 23, 1587–1592. [Google Scholar] [CrossRef]
- Levdansky, V.; Šolcová, O.; Friess, K.; Izák, P. Mass Transfer Through Graphene-Based Membranes. Appl. Sci. 2020, 10, 455. [Google Scholar] [CrossRef] [Green Version]
- Koenig, S.P.; Wang, L.; Pellegrino, J.; Buch, J.S. Selective molecular sieving through porous graphene. Nat. Nanotechnol. 2012, 7, 728–732. [Google Scholar] [CrossRef] [Green Version]
- Otřísal, P.; Friess, K.; Fehorova, L.; Melicharix, Z.; Bungau, C.; Mosteanu, D. The Heat Stress Effects on the Gases Permeability of the Isolative Type Garment of the Czech Armed Forces Chemical Corps Specialist Body Surface Protection. Rev. Chim. Buchar. Orig. Ed. 2019, 70, 1597–1602. [Google Scholar] [CrossRef]
- Mrazík, L. Modelling of Gas Transport through a Membrane Using PDEs. Master’s Thesis, University of Chemistry and Technology Prague, Praha, Czech Republic, 2020. [Google Scholar]
- Vazquez, J.L. The Porous Medium Equation: Mathematical Theory; Oxford Mathematical Monographs; Clarendon Press: Oxford, UK, 2006. [Google Scholar]
- Stern, S.A. The “barrer” permeability unit. J. Polym. Sci. Part A-2 Polym. Phys. 1968, 6, 1933–1934. [Google Scholar] [CrossRef]
- Tepelná Kapacita Plynů. Available online: http://uchi-old.vscht.cz/uploads/etabulky/cpplyn.html (accessed on 9 August 2021).
- Whitaker, S. The Equations of Motion in Porous Media. Chem. Eng. Sci. 1966, 21, 291–300. [Google Scholar] [CrossRef]
- Neuman, S.P. Theoretical derivation of Darcy’s law. Acta Mech. 1977, 25, 153–170. [Google Scholar] [CrossRef]
- Whitaker, S. Flow in porous media I: A theoretical derivation of Darcy’s law. Transp. Porous Media 1986, 1, 3–25. [Google Scholar] [CrossRef]
| Form | Pressure | Density |
|---|---|---|
| Laplacian | ||
| Divergence | ||
| Linearized |
| Neumann Part | Dirichlet Part | ||
|---|---|---|---|
| u | |||
|---|---|---|---|
| p | 0 | ||
| 0 | |||
| p | 0 | ||
| 0 | |||
| p | |||
| p | |||
| u | |||
|---|---|---|---|
| p | |||
| p | |||
| p | |||
| p | |||
| Gas | ||||
|---|---|---|---|---|
| CH | 2.249 | |||
| CO | 1.371 | |||
| N | 1.556 | |||
| H | 4.772 | |||
| H | 4.534 | |||
| CH | 1.551 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrazík, L.; Kříž, P. Porous Medium Equation in Graphene Oxide Membrane: Nonlinear Dependence of Permeability on Pressure Gradient Explained. Membranes 2021, 11, 665. https://doi.org/10.3390/membranes11090665
Mrazík L, Kříž P. Porous Medium Equation in Graphene Oxide Membrane: Nonlinear Dependence of Permeability on Pressure Gradient Explained. Membranes. 2021; 11(9):665. https://doi.org/10.3390/membranes11090665
Chicago/Turabian StyleMrazík, Lukáš, and Pavel Kříž. 2021. "Porous Medium Equation in Graphene Oxide Membrane: Nonlinear Dependence of Permeability on Pressure Gradient Explained" Membranes 11, no. 9: 665. https://doi.org/10.3390/membranes11090665
APA StyleMrazík, L., & Kříž, P. (2021). Porous Medium Equation in Graphene Oxide Membrane: Nonlinear Dependence of Permeability on Pressure Gradient Explained. Membranes, 11(9), 665. https://doi.org/10.3390/membranes11090665






