Adipose-Derived Stromal Cells and Mineralized Extracellular Matrix Delivery by a Human Decellularized Amniotic Membrane in Periodontal Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Ethics
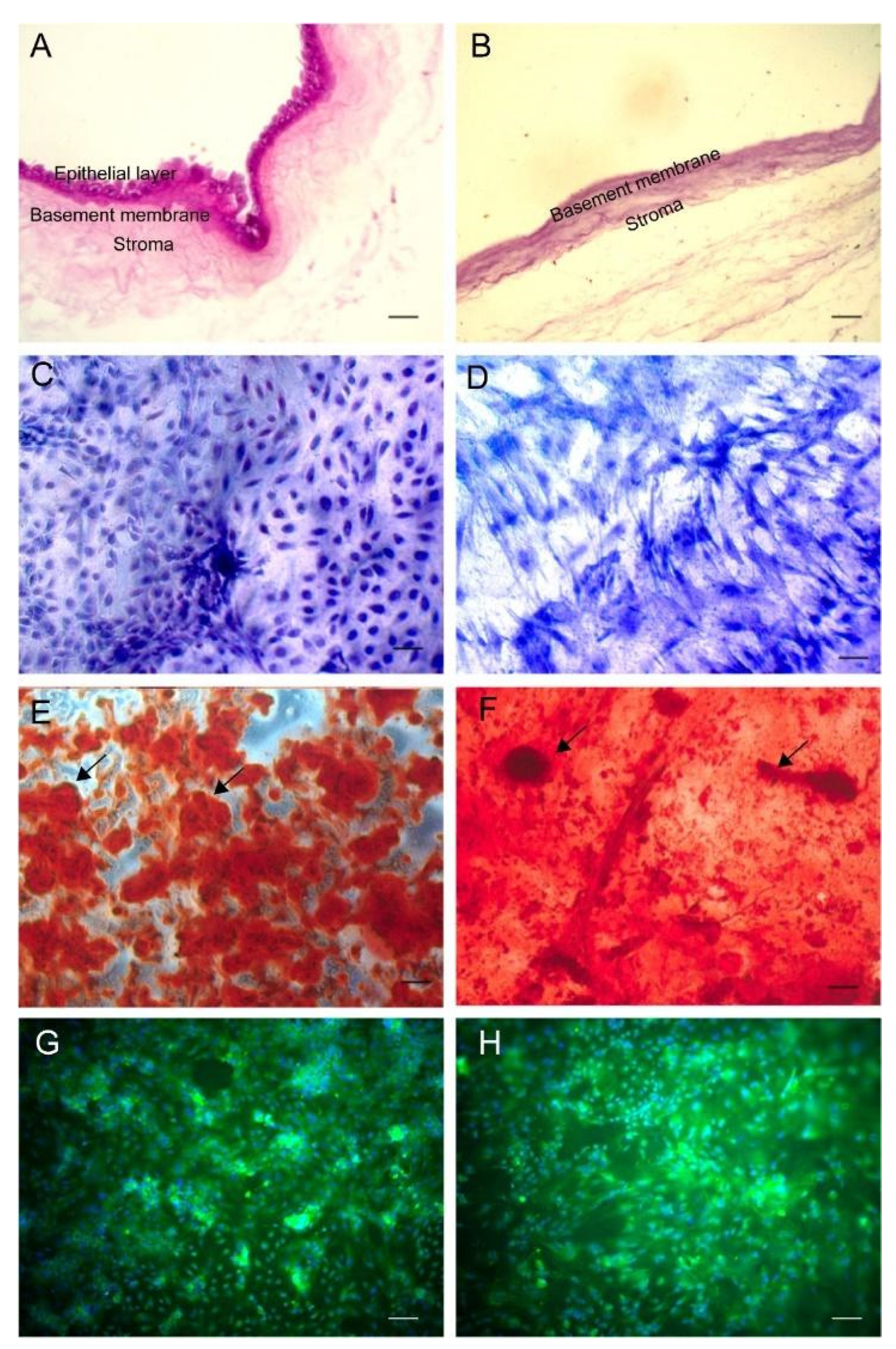
2.2. Amniotic Membrane
2.3. Adipose-Derived Stromal Cells
2.4. Transplantation Materials
2.5. Surgical Procedure
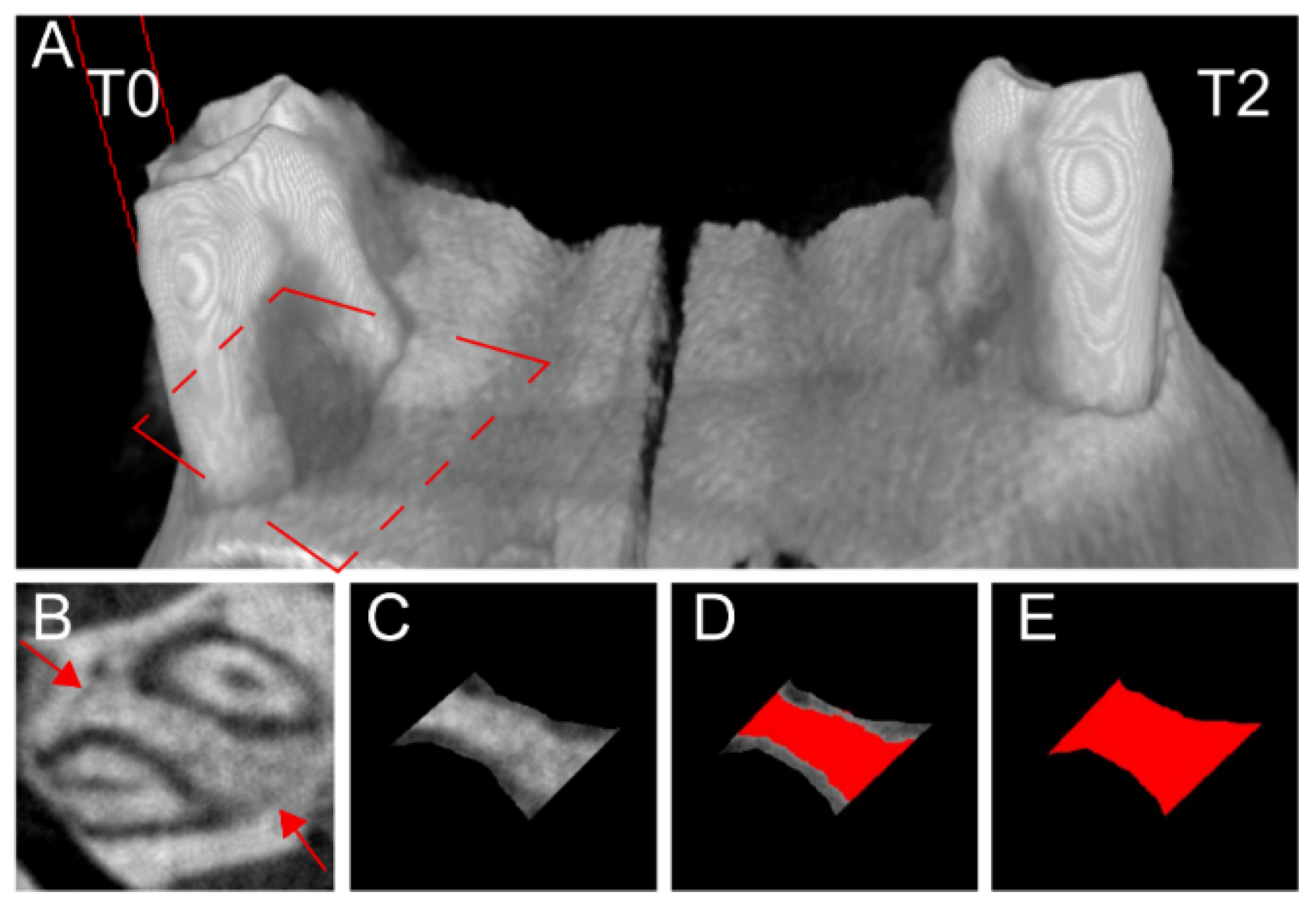
2.6. Micro-Computed Tomography
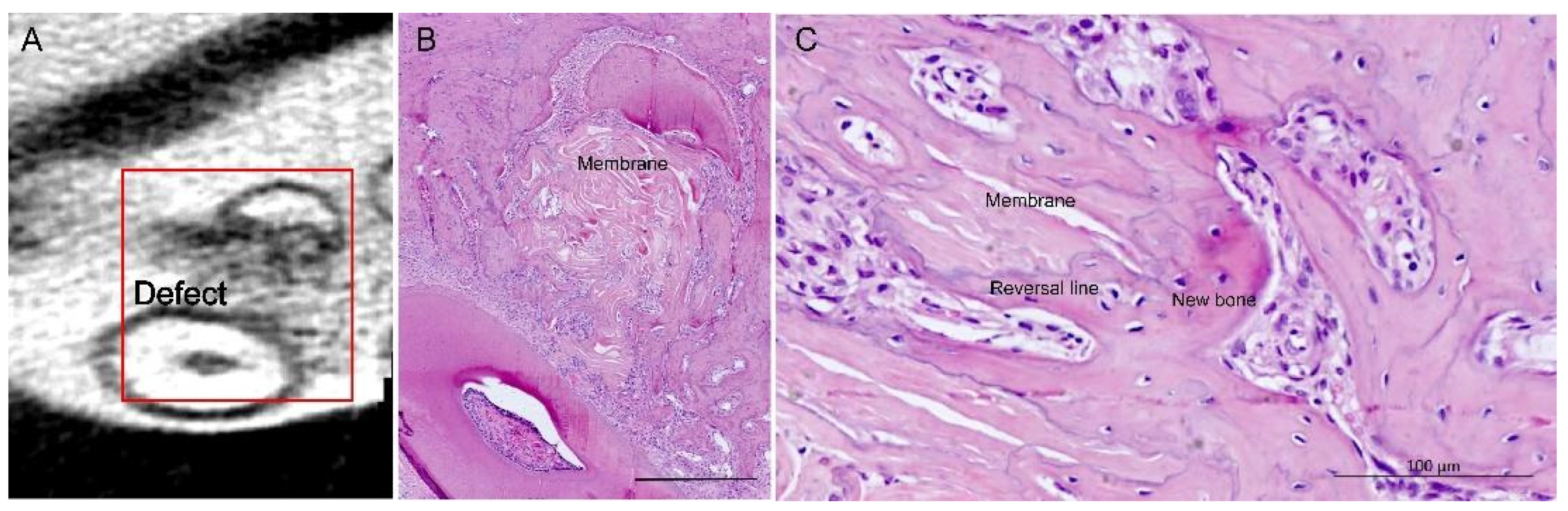
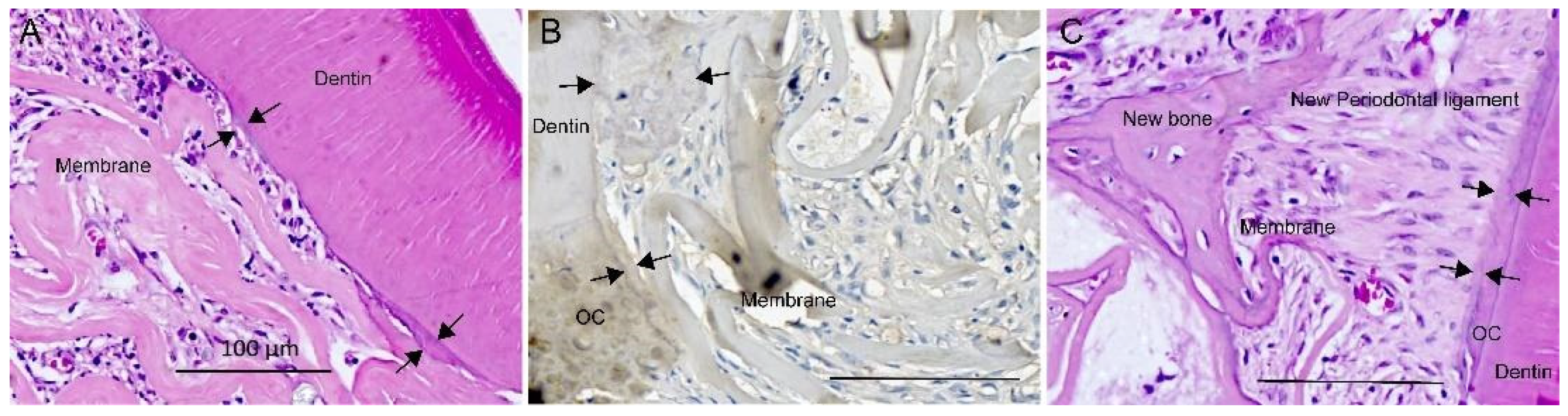
2.7. Histology
2.8. Statistical Analysis
3. Results
3.1. Transplantation Materials
3.2. Bone Healing
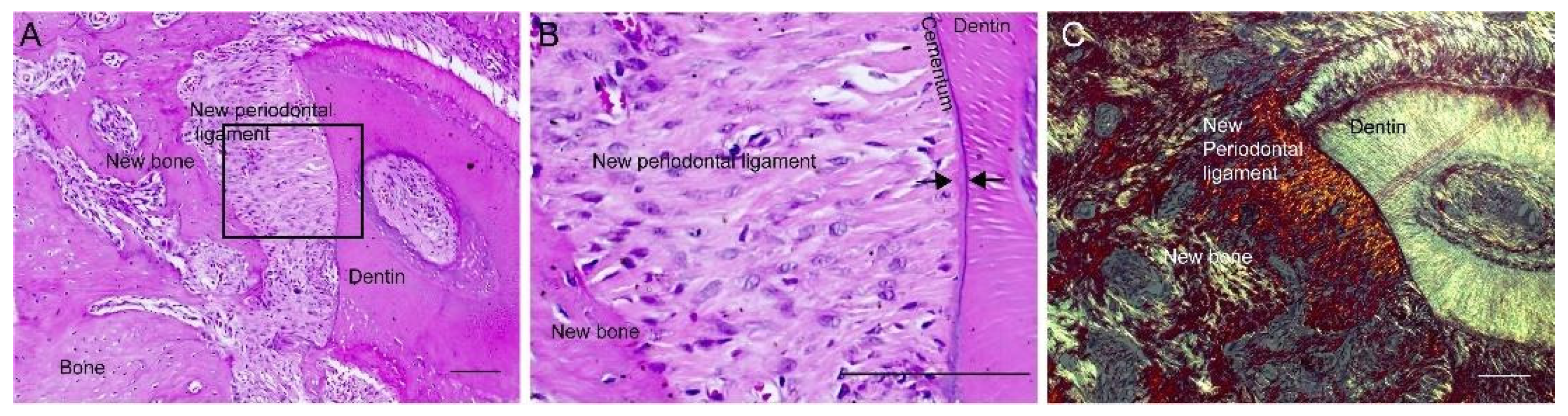
3.3. Periodontal Healing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hughes, F.J.; Ghuman, M.; Talal, A. Periodontal regeneration: A challenge for the tissue engineer? Proc. Inst. Mech. Eng. H J. Eng. Med. 2010, 224, 1345–1358. [Google Scholar] [CrossRef]
- Cafiero, C.; Matarasso, S. Predictive, preventive, personalised and participatory periodontology: ‘The 5Ps age’ has already started. EPMA J. 2013, 4, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosshardt, D.D.; Stadlinger, B.; Terheyden, H. Cell-to-cell communication--periodontal regeneration. Clin. Oral Implants Res. 2015, 26, 229–239. [Google Scholar] [CrossRef]
- de Jong, T.; Bakker, A.D.; Everts, V.; Smit, T.H. The intricate anatomy of the periodontal ligament and its development: Lessons for periodontal regeneration. J. Periodontal Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Sun, H.H.; Lu, H.; Yu, Q. Stem cell-delivery therapeutics for periodontal tissue regeneration. Biomaterials 2012, 33, 6320–6344. [Google Scholar] [CrossRef]
- Chen, F.M.; Gao, L.N.; Tian, B.M.; Zhang, X.Y.; Zhang, Y.J.; Dong, G.Y.; Lu, H.; Chu, Q.; Xu, J.; Yu, Y.; et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: A randomized clinical trial. Stem Cell Res. Ther. 2016, 7, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwata, T.; Yamato, M.; Washio, K.; Yoshida, T.; Tsumanuma, Y.; Yamada, A.; Onizuka, S.; Izumi, Y.; Ando, T.; Okano, T.; et al. Periodontal regeneration with autologous periodontal ligament-derived cell—A safety and efficacy study in ten patients. Regen. Ther. 2018, 9, 38–44. [Google Scholar] [CrossRef]
- Tassi, S.A.; Sergio, N.Z.; Misawa, M.Y.O.; Villar, C.C. Efficacy of stem cells on periodontal regeneration: Systematic review of pre-clinical studies. J. Periodontal Res. 2017, 52, 793–812. [Google Scholar] [CrossRef]
- Ern, C.; Berger, T.; Frasheri, I.; Heym, R.; Hickel, R.; Folwaczny, M. Differentiation of hMSC and hPDLSC induced by PGE2 or BMP-7 in 3D models. Prostaglandins Leukot. Essent. Fatty Acids 2017, 122, 30–37. [Google Scholar] [CrossRef]
- Ripamonti, U.; Parak, R.; Klar, R.M.; Dickens, C.; Dix-Peek, T.; Duarte, R. Cementogenesis and osteogenesis in periodontal tissue regeneration by recombinant human transforming growth factor-beta3: A pilot study in Papio ursinus. J. Clin. Periodontol. 2017, 44, 83–95. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafieian, R.; Matin, M.M.; Rahpeyma, A.; Fazel, A.; Sedigh, H.S.; Sadr-Nabavi, A.; Hassanzadeh, H.; Ebrahimzadeh-Bideskan, A. The effect of platelet-rich plasma on human mesenchymal stem cell-induced bone regeneration of canine alveolar defects with calcium phosphate-based scaffolds. Iran. J. Basic Med. Sci. 2017, 20, 1131–1140. [Google Scholar] [CrossRef]
- Tobita, M.; Uysal, C.A.; Guo, X.; Hyakusoku, H.; Mizuno, H. Periodontal tissue regeneration by combined implantation of adipose tissue-derived stem cells and platelet-rich plasma in a canine model. Cytotherapy 2013. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, M.; Monsarrat, P.; Blasco-Baque, V.; Loubieres, P.; Burcelin, R.; Casteilla, L.; Planat-Benard, V.; Kemoun, P. Periodontal Tissue Regeneration Using Syngeneic Adipose-Derived Stromal Cells in a Mouse Model. Stem Cells Transl. Med. 2017, 6, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, A.; Sato, S. Application of dedifferentiated fat cells for periodontal tissue regeneration. Hum. Cell 2014, 27, 12–21. [Google Scholar] [CrossRef]
- Alvira-Gonzalez, J.; Sanchez-Garces, M.A.; Cairo, J.R.; Del Pozo, M.R.; Sanchez, C.M.; Gay-Escoda, C. Assessment of Bone Regeneration Using Adipose-Derived Stem Cells in Critical-Size Alveolar Ridge Defects: An Experimental Study in a Dog Model. Int. J. Oral Maxillofac. Implants 2016, 31, 196–203. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Garces, M.A.; Alvira-Gonzalez, J.; Sanchez, C.M.; Barbany Cairo, J.R.; Del Pozo, M.R.; Gay-Escoda, C. Bone Regeneration Using Adipose-Derived Stem Cells with Fibronectin in Dehiscence-Type Defects Associated with Dental Implants: An Experimental Study in a Dog Model. Int. J. Oral Maxillofac. Implants 2017, 32, e97–e106. [Google Scholar] [CrossRef] [Green Version]
- Akita, D.; Kano, K.; Saito-Tamura, Y.; Mashimo, T.; Sato-Shionome, M.; Tsurumachi, N.; Yamanaka, K.; Kaneko, T.; Toriumi, T.; Arai, Y.; et al. Use of Rat Mature Adipocyte-Derived Dedifferentiated Fat Cells as a Cell Source for Periodontal Tissue Regeneration. Front. Physiol. 2016, 7, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akita, D.; Morokuma, M.; Saito, Y.; Yamanaka, K.; Akiyama, Y.; Sato, M.; Mashimo, T.; Toriumi, T.; Arai, Y.; Kaneko, T.; et al. Periodontal tissue regeneration by transplantation of rat adipose-derived stromal cells in combination with PLGA-based solid scaffolds. Biomed. Res. 2014, 35, 91–103. [Google Scholar] [CrossRef] [Green Version]
- Dziedzic, D.S.M.; Mogharbel, B.F.; Ferreira, P.E.; Irioda, A.C.; de Carvalho, K.A.T. Transplantation of Adipose-derived Cells for Periodontal Regeneration: A Systematic Review. Curr. Stem Cell Res. Ther. 2018. [Google Scholar] [CrossRef]
- Kubo, M.; Sonoda, Y.; Muramatsu, R.; Usui, M. Immunogenicity of human amniotic membrane in experimental xenotransplantation. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1539–1546. [Google Scholar]
- Leal-Marin, S.; Kern, T.; Hofmann, N.; Pogozhykh, O.; Framme, C.; Borgel, M.; Figueiredo, C.; Glasmacher, B.; Gryshkov, O. Human Amniotic Membrane: A review on tissue engineering, application, and storage. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1198–1215. [Google Scholar] [CrossRef]
- Etchebarne, M.; Fricain, J.C.; Kerdjoudj, H.; Di Pietro, R.; Wolbank, S.; Gindraux, F.; Fenelon, M. Use of Amniotic Membrane and Its Derived Products for Bone Regeneration: A Systematic Review. Front. Bioeng. Biotechnol. 2021, 9, 661332. [Google Scholar] [CrossRef]
- Guo, Q.; Lu, X.; Xue, Y.; Zheng, H.; Zhao, X.; Zhao, H. A new candidate substrate for cell-matrix adhesion study: The acellular human amniotic matrix. J. Biomed. Biotechnol. 2012, 2012, 306083. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chung, M.C.; Jane Yao, C.C.; Huang, C.H.; Chang, H.H.; Jeng, J.H.; Young, T.H. The effects of acellular amniotic membrane matrix on osteogenic differentiation and ERK1/2 signaling in human dental apical papilla cells. Biomaterials 2012, 33, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Salah, R.A.; Mohamed, I.K.; El-Badri, N. Development of decellularized amniotic membrane as a bioscaffold for bone marrow-derived mesenchymal stem cells: Ultrastructural study. J. Mol. Histol. 2018, 49, 289–301. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Sameni, M.; Radenkovic, D.; Mozafari, M.; Mossahebi-Mohammadi, M.; Seifalian, A. Decellularized human amniotic membrane: How viable is it as a delivery system for human adipose tissue-derived stromal cells? Cell Prolif. 2016, 49, 115–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Ma, G.; Brazile, B.; Li, N.; Dai, W.; Butler, J.R.; Claude, A.A.; Wertheim, J.A.; Liao, J.; Wang, B. Investigating the Potential of Amnion-Based Scaffolds as a Barrier Membrane for Guided Bone Regeneration. Langmuir 2015, 31, 8642–8653. [Google Scholar] [CrossRef] [PubMed]
- Fenelon, M.; Etchebarne, M.; Siadous, R.; Gremare, A.; Durand, M.; Sentilhes, L.; Torres, Y.; Catros, S.; Gindraux, F.; L’Heureux, N.; et al. Assessment of fresh and preserved amniotic membrane for guided bone regeneration in mice. J. Biomed. Mater. Res. A 2020, 108, 2044–2056. [Google Scholar] [CrossRef]
- Akazawa, K.; Iwasaki, K.; Nagata, M.; Yokoyama, N.; Ayame, H.; Yamaki, K.; Tanaka, Y.; Honda, I.; Morioka, C.; Kimura, T. Double-layered cell transfer technology for bone regeneration. Sci. Rep. 2016, 6, 33286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsugawa, J.; Komaki, M.; Yoshida, T.; Nakahama, K.; Amagasa, T.; Morita, I. Cell-printing and transfer technology applications for bone defects in mice. J. Tissue Eng. Regen. Med. 2011, 5, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, D.S.M.; Francisco, J.C.; Mogharbel, B.F.; Irioda, A.C.; Stricker, P.E.F.; Floriano, J.; de Noronha, L.; Abdelwahid, E.; Franco, C.R.C.; de Carvalho, K.A.T. Combined Biomaterials: Amniotic Membrane and Adipose Tissue to Restore Injured Bone as Promoter of Calcification in Bone Regeneration: Preclinical Model. Calcif. Tissue Int. 2021. [Google Scholar] [CrossRef]
- Semyari, H.; Rajipour, M.; Sabetkish, S.; Sabetkish, N.; Abbas, F.M.; Kajbafzadeh, A.M. Evaluating the bone regeneration in calvarial defect using osteoblasts differentiated from adipose-derived mesenchymal stem cells on three different scaffolds: An animal study. Cell Tissue Bank 2016, 17, 69–83. [Google Scholar] [CrossRef]
- Wu, P.H.; Chung, H.Y.; Wang, J.H.; Shih, J.C.; Kuo, M.Y.; Chang, P.C.; Huang, Y.; Wang, P.C.; Chang, C.C. Amniotic membrane and adipose-derived stem cell co-culture system enhances bone regeneration in a rat periodontal defect model. J. Formos. Med. Assoc. 2015. [Google Scholar] [CrossRef] [Green Version]
- Amemiya, T.; Nakamura, T.; Yamamoto, T.; Kinoshita, S.; Kanamura, N. Immunohistochemical study of oral epithelial sheets cultured on amniotic membrane for oral mucosal reconstruction. Biomed. Mater. Eng. 2010, 20, 37–45. [Google Scholar] [CrossRef]
- Iwasaki, K.; Komaki, M.; Yokoyama, N.; Tanaka, Y.; Taki, A.; Honda, I.; Kimura, Y.; Takeda, M.; Akazawa, K.; Oda, S.; et al. Periodontal regeneration using periodontal ligament stem cell-transferred amnion. Tissue Eng. Part A 2014, 20, 693–704. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, K.; Akazawa, K.; Nagata, M.; Komaki, M.; Honda, I.; Morioka, C.; Yokoyama, N.; Ayame, H.; Yamaki, K.; Tanaka, Y.; et al. The Fate of Transplanted Periodontal Ligament Stem Cells in Surgically Created Periodontal Defects in Rats. Int. J. Mol. Sci. 2019, 20, 192. [Google Scholar] [CrossRef] [Green Version]
- Takizawa, S.; Yamamoto, T.; Honjo, K.I.; Sato, Y.; Nakamura, K.; Yamamoto, K.; Adachi, T.; Uenishi, T.; Oseko, F.; Amemiya, T.; et al. Transplantation of dental pulp-derived cell sheets cultured on human amniotic membrane induced to differentiate into bone. Oral Dis. 2019, 25, 1352–1362. [Google Scholar] [CrossRef]
- Nejad, A.R.; Hamidieh, A.A.; Amirkhani, M.A.; Sisakht, M.M. Update review on five top clinical applications of human amniotic membrane in regenerative medicine. Placenta 2020, 103, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Lafzi, A.; Abolfazli, N.; Faramarzi, M.; Eyvazi, M.; Eskandari, A.; Salehsaber, F. Clinical comparison of coronally-advanced flap plus amniotic membrane or subepithelial connective tissue in the treatment of Miller’s class I and II gingival recessions: A split-mouth study. J. Dent. Res. Dent. Clin. Dent. Prospects 2016, 10, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Amemiya, T.; Nakamura, T.; Yamamoto, T.; Kinoshita, S.; Kanamura, N. Autologous transplantation of oral mucosal epithelial cell sheets cultured on an amniotic membrane substrate for intraoral mucosal defects. PLoS ONE 2015, 10, e0125391. [Google Scholar] [CrossRef] [Green Version]
- Lopez, M.J.; Spencer, N.D. In vitro adult rat adipose tissue-derived stromal cell isolation and differentiation. Methods Mol. Biol. 2011, 702, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P. Adipose-Derived Stem Cells in Tissue Regeneration: A Review. ISRN Stem Cells 2013, 2013, 35. [Google Scholar] [CrossRef] [Green Version]
- Susin, C.; Fiorini, T.; Lee, J.; De Stefano, J.A.; Dickinson, D.P.; Wikesjo, U.M. Wound healing following surgical and regenerative periodontal therapy. Periodontol. 2000 2015, 68, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Fenelon, M.; Maurel, D.B.; Siadous, R.; Gremare, A.; Delmond, S.; Durand, M.; Brun, S.; Catros, S.; Gindraux, F.; L’Heureux, N.; et al. Comparison of the impact of preservation methods on amniotic membrane properties for tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109903. [Google Scholar] [CrossRef]
- Iwata, T.; Washio, K.; Yoshida, T.; Ishikawa, I.; Ando, T.; Yamato, M.; Okano, T. Cell sheet engineering and its application for periodontal regeneration. J. Tissue Eng. Regen. Med. 2015, 9, 343–356. [Google Scholar] [CrossRef]
- Davies, J.E. Bone bonding at natural and biomaterial surfaces. Biomaterials 2007, 28, 5058–5067. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Y.; Li, J.; Guo, Q.; Peng, J.; Liu, S.; Yang, J.; Wang, Y. Cell-Derived Extracellular Matrix: Basic Characteristics and Current Applications in Orthopedic Tissue Engineering. Tissue Eng. Part B Rev. 2016, 22, 193–207. [Google Scholar] [CrossRef]
- Sabouri, L.; Farzin, A.; Kabiri, A.; Milan, P.B.; Farahbakhsh, M.; Mehdizadehkashi, A.; Kajbafzadeh, A.; Samadikuchaksaraei, A.; Yousefbeyk, F.; Azami, M.; et al. Mineralized Human Amniotic Membrane as a Biomimetic Scaffold for Hard Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2020, 6, 6285–6298. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xing, H.; Zhang, G.; Wu, X.; Zou, X.; Feng, L.; Wang, D.; Li, M.; Zhao, J.; Du, J.; et al. Restoration of a Critical Mandibular Bone Defect Using Human Alveolar Bone-Derived Stem Cells and Porous Nano-HA/Collagen/PLA Scaffold. Stem Cells Int. 2016, 2016, 8741641. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Van Manh, N.; Wang, H.; Zhong, X.; Zhang, X.; Li, C. Synergistic intrafibrillar/extrafibrillar mineralization of collagen scaffolds based on a biomimetic strategy to promote the regeneration of bone defects. Int. J. Nanomed. 2016, 11, 2053–2067. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Li, W.; Lu, Z.; Chen, R.; Ling, J.; Ran, Q.; Jilka, R.L.; Chen, X.D. Rescuing replication and osteogenesis of aged mesenchymal stem cells by exposure to a young extracellular matrix. FASEB J. 2011, 25, 1474–1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thibault, R.A.; Scott Baggett, L.; Mikos, A.G.; Kasper, F.K. Osteogenic differentiation of mesenchymal stem cells on pregenerated extracellular matrix scaffolds in the absence of osteogenic cell culture supplements. Tissue Eng. Part A 2010, 16, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Nunez, J.; Sanz-Blasco, S.; Vignoletti, F.; Munoz, F.; Arzate, H.; Villalobos, C.; Nunez, L.; Caffesse, R.G.; Sanz, M. Periodontal regeneration following implantation of cementum and periodontal ligament-derived cells. J. Periodontal Res. 2012, 47, 33–44. [Google Scholar] [CrossRef]
- Reynolds, M.A.; Aichelmann-Reidy, M.E.; Branch-Mays, G.L. Regeneration of periodontal tissue: Bone replacement grafts. Dent. Clin. N. Am. 2010, 54, 55–71. [Google Scholar] [CrossRef]
- Ivanovski, S.; Vaquette, C.; Gronthos, S.; Hutmacher, D.W.; Bartold, P.M. Multiphasic scaffolds for periodontal tissue engineering. J. Dent. Res. 2014, 93, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Padial-Molina, M.; Marchesan, J.T.; Taut, A.D.; Jin, Q.; Giannobile, W.V.; Rios, H.F. Methods to validate tooth-supporting regenerative therapies. Methods Mol. Biol. 2012, 887, 135–148. [Google Scholar] [CrossRef] [Green Version]
| Treatments | ||||||
|---|---|---|---|---|---|---|
| Level | T0 | T1 | T2 | T3 | T4 | p * |
| (n = 20) | (n = 5) | (n = 6) | (n = 4) | (n = 5) | ||
| A Gingival | 19.4 a ± 17.2 | 49.1 b ± 27.4 | 49.8 b ± 27.8 | 27.4 a,b ± 33.7 | 24.7 a,b ± 17.0 | 0.006 |
| B | 29.5 ± 27.6 | 53.4 ± 24.5 | 58.0 ± 28.6 | 39.0 ± 37.3 | 32.4 ± 14.6 | 0.088 |
| C | 38.2 ± 28.9 | 67.9 ± 21.5 | 66.5 ± 27.5 | 50.3 ± 33.8 | 32.6 ± 16.5 | 0.077 |
| D | 45.4 a ± 29.1 | 77.5 a ± 15.5 | 72.0 a ± 22.8 | 58.3 a,b ± 29.4 | 33.1 b ± 13.9 | 0.039 |
| E Apical | 52.3 ± 27.7 | 82.8 ± 11.0 | 75.8 ± 19.4 | 69.3 ± 26.8 | 38.5 ± 19.0 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziedzic, D.S.M.; Mogharbel, B.F.; Irioda, A.C.; Stricker, P.E.F.; Perussolo, M.C.; Franco, C.R.C.; Chang, H.-W.; Abdelwahid, E.; de Carvalho, K.A.T. Adipose-Derived Stromal Cells and Mineralized Extracellular Matrix Delivery by a Human Decellularized Amniotic Membrane in Periodontal Tissue Engineering. Membranes 2021, 11, 606. https://doi.org/10.3390/membranes11080606
Dziedzic DSM, Mogharbel BF, Irioda AC, Stricker PEF, Perussolo MC, Franco CRC, Chang H-W, Abdelwahid E, de Carvalho KAT. Adipose-Derived Stromal Cells and Mineralized Extracellular Matrix Delivery by a Human Decellularized Amniotic Membrane in Periodontal Tissue Engineering. Membranes. 2021; 11(8):606. https://doi.org/10.3390/membranes11080606
Chicago/Turabian StyleDziedzic, Dilcele Silva Moreira, Bassam Felipe Mogharbel, Ana Carolina Irioda, Priscila Elias Ferreira Stricker, Maiara Carolina Perussolo, Célia Regina Cavichiolo Franco, Hsueh-Wen Chang, Eltyeb Abdelwahid, and Katherine Athayde Teixeira de Carvalho. 2021. "Adipose-Derived Stromal Cells and Mineralized Extracellular Matrix Delivery by a Human Decellularized Amniotic Membrane in Periodontal Tissue Engineering" Membranes 11, no. 8: 606. https://doi.org/10.3390/membranes11080606
APA StyleDziedzic, D. S. M., Mogharbel, B. F., Irioda, A. C., Stricker, P. E. F., Perussolo, M. C., Franco, C. R. C., Chang, H.-W., Abdelwahid, E., & de Carvalho, K. A. T. (2021). Adipose-Derived Stromal Cells and Mineralized Extracellular Matrix Delivery by a Human Decellularized Amniotic Membrane in Periodontal Tissue Engineering. Membranes, 11(8), 606. https://doi.org/10.3390/membranes11080606






