Melatonin and Indole-3-Propionic Acid Reduce Oxidative Damage to Membrane Lipids Induced by High Iron Concentrations in Porcine Skin
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Assay of Lipid Peroxidation
2.4. Measurement of Lipid Peroxidation Products
2.5. Statistical Analyses
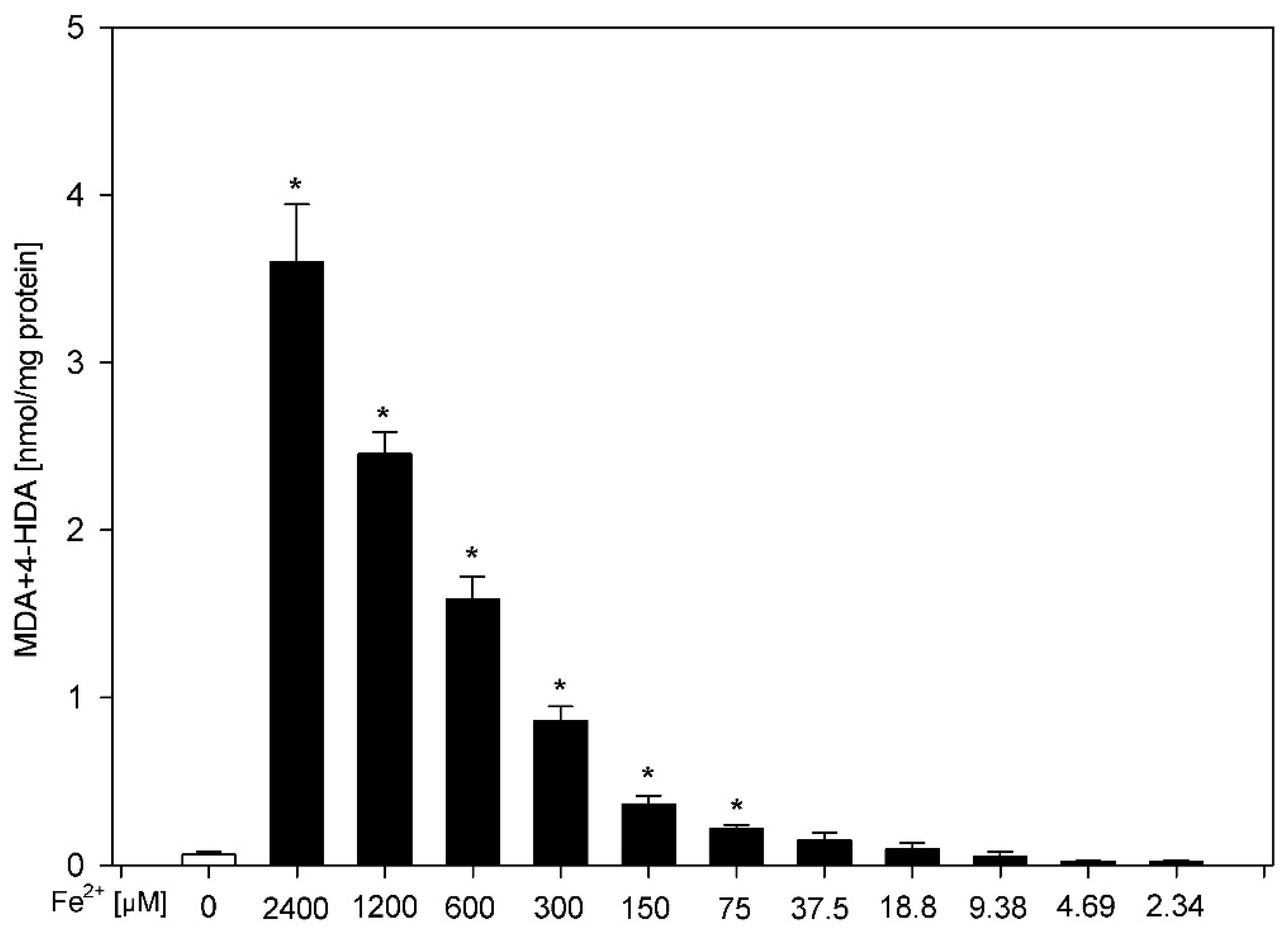
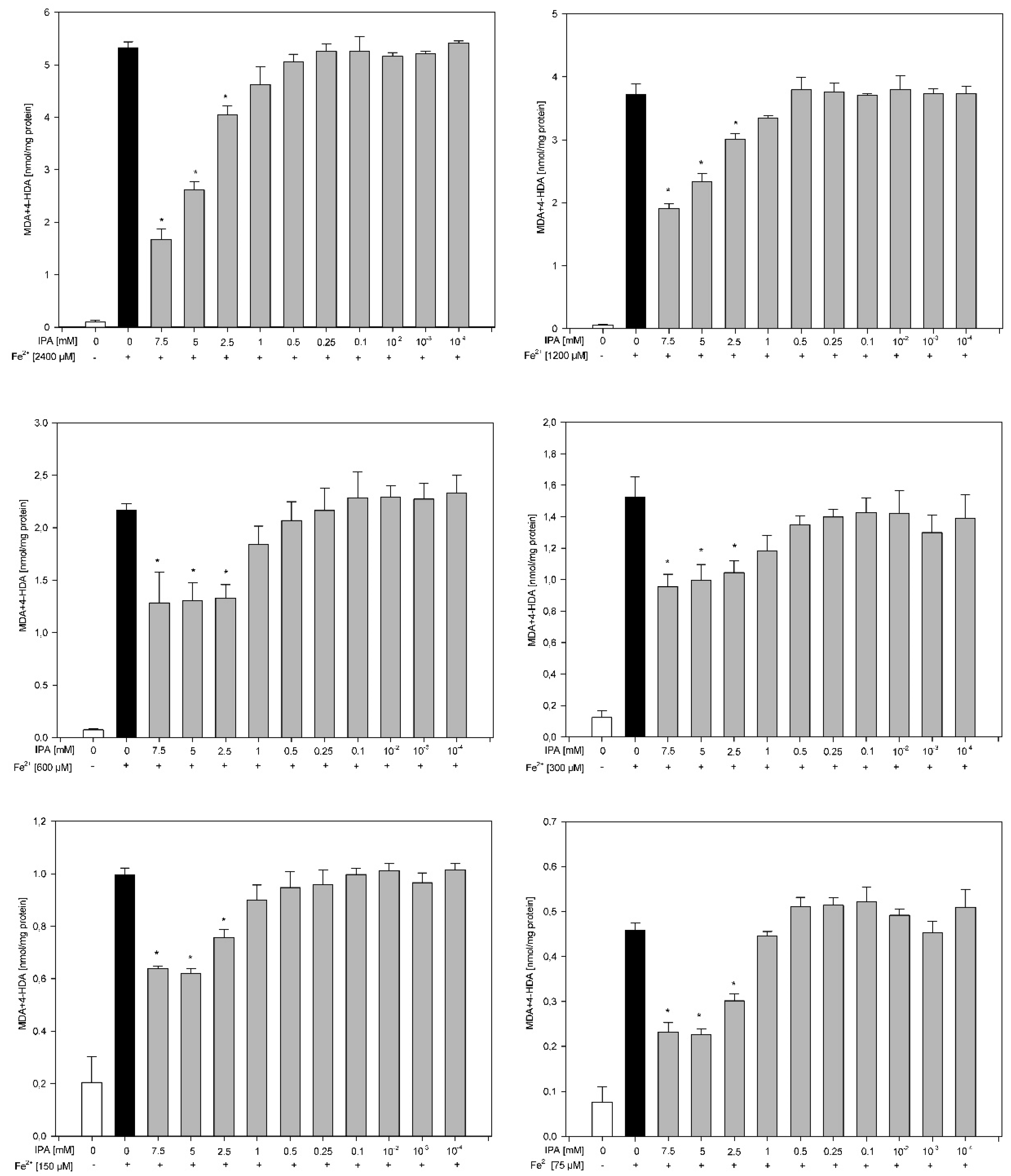
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bentsen, H. Dietary polyunsaturated fatty acids, brain function and mental health. Microb. Ecol. Health Dis. 2017, 28, 1281916. [Google Scholar] [CrossRef]
- Nam, T.G. Lipid peroxidation and its toxicological implications. Toxicol. Res. 2011, 27, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Trouba, K.J.; Hamadeh, H.K.; Amin, R.P.; Germolec, D.R. Oxidative stress and its role in skin disease. Antioxid. Redox Signal. 2002, 4, 665–673. [Google Scholar] [CrossRef]
- Dizen-Namdar, N.; Emel Kocak, F.; Kidir, M.; Sarici, G.; Tak, H.; Altuntas, I. Evaluation of serum paraoxonase, arylesterase, prolidase activities and oxidative stress in patients with alopecia areata. Skin Pharmacol. Physiol. 2019, 32, 59–64. [Google Scholar] [CrossRef]
- Wölfle, U.; Seelinger, G.; Bauer, G.; Meinke, M.C.; Lademann, J.; Schempp, C.M. Reactive molecule species and antioxidative mechanisms in normal skin and skin aging. Skin Pharmacol. Physiol. 2014, 27, 316–332. [Google Scholar] [CrossRef] [PubMed]
- Sagan, D.; Stepniak, J.; Gesing, A.; Lewinski, A.; Karbownik-Lewinska, M. Melatonin reverses the enhanced oxidative damage to membrane lipids and improves skin biophysical characteristics in former-smokers—A study in postmenopausal women. Ann. Agric. Environ. Med. 2017, 24, 659–666. [Google Scholar] [CrossRef]
- Szokalska, K.; Stepniak, J.; Karbownik-Lewinska, M. Lipid peroxidation evaluated in epidermis exfoliated during microdermabrasion is a reliable marker of oxidative stress related to obesity. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1429–1431. [Google Scholar] [CrossRef]
- Szachowicz-Petelska, B.; Łuczaj, W.; Wroński, A.; Jastrząb, A.; Dobrzyńska, I. The differential effect of cannabidiol on the composition and physicochemical properties of keratinocyte and fibroblast membranes from psoriatic patients and healthy people. Membranes 2021, 11, 111. [Google Scholar] [CrossRef]
- Karbownik, M.; Gitto, E.; Lewinski, A.; Reiter, R.J. Relative efficacies of indole antioxidants in reducing autoxidation and iron-induced lipid peroxidation in hamster testes. J. Cell. Biochem. 2001, 81, 693–699. [Google Scholar] [CrossRef]
- Karbownik, M.; Lewiński, A. Melatonin reduces Fenton reaction-induced lipid peroxidation in porcine thyroid tissue. J. Cell. Biochem. 2003, 90, 806–811. [Google Scholar] [CrossRef]
- Stepniak, J.; Lewinski, A.; Karbownik-Lewinska, M. Oxidative damage to membrane lipids in the thyroid–no differences between sexes. Drug Chem. Toxicol. 2019, 1–6, published online ahead of print. [Google Scholar] [CrossRef]
- Rynkowska, A.; Stępniak, J.; Karbownik-Lewińska, M. Fenton reaction-induced oxidative damage to membrane lipids and protective effects of 17β-estradiol in porcine ovary and thyroid homogenates. Int. J. Environ. Res. Public Health 2020, 17, 6841. [Google Scholar] [CrossRef]
- Sabat, M.J.; Wiśniewska-Becker, A.M.; Markiewicz, M.; Marzec, K.M.; Dybas, J.; Furso, J.; Pabisz, P.; Duda, M.; Pawlak, A.M. Tauroursodeoxycholic acid (TUDCA)-lipid interactions and antioxidant properties of TUDCA studied in model of photoreceptor membranes. Membranes 2021, 11, 327. [Google Scholar] [CrossRef]
- Kokoszko-Bilska, A.; Stepniak, J.; Lewinski, A.; Karbownik-Lewinska, M. Protective antioxidative effects of caffeic acid phenethyl ester (CAPE) in the thyroid and the liver are similar to those caused by melatonin. Thyroid Res. 2014, 7, 5. [Google Scholar] [CrossRef][Green Version]
- Stepniak, J.; Karbownik-Lewinska, M. 17β-estradiol prevents experimentally–induced oxidative damage to membrane lipids and nuclear DNA in porcine ovary. Syst. Biol. Reprod. Med. 2016, 62, 17–21. [Google Scholar] [CrossRef]
- Toyokuni, S. Iron and carcinogenesis: From Fenton reaction to target genes. Redox Rep. 2002, 7, 189–197. [Google Scholar] [CrossRef]
- Halliwell, B. Free radicals and antioxdants: Updating a personal viev. Nutr. Rev. 2012, 70, 257–265. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Dong, K.; Goyarts, E.; Rella, A.; Pelle, E.; Wong, Y.H.; Pernodet, N. Age associated decrease of MT−1 melatonin receptor in human dermal skin fibroblasts impairs protection against UV-induced DNA damage. Int. J. Mol. Sci. 2020, 21, 326. [Google Scholar] [CrossRef]
- Young, S.N.; Anderson, G.M.; Gauthier, S.; Purdy, W.C. The origin of indoleacetic acid and indolepropionic acid in rat and human cerebrospinal fluid. J. Neurochem. 1980, 34, 1087–1092. [Google Scholar] [CrossRef]
- Ren, S.; Rutto, L.; Katuuramu, D. Melatonin acts synergistically with auxin to promote lateral root development through fine tuning auxin transport in Arabidopsis thaliana. PLoS ONE 2019, 14, e0221687. [Google Scholar] [CrossRef]
- Karbownik, M.; Lewinski, A.; Reiter, R.J. Anticarcinogenic actions of melatonin which involve antioxidative processes: Comparison with other antioxidants. Int. J. Biochem. Cell Biol. 2001, 33, 735–753. [Google Scholar] [CrossRef]
- Iwan, P.; Stepniak, J.; Karbownik-Lewinska, M. Melatonin reduces high levels of lipid peroxidation induced by potassium iodate in porcine thyroid. Int. J. Vitam. Nutr. Res. 2021, 91, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.W.; Slominski, A.; Zmijewski, M.A.; Reiter, R.J.; Paus, R. Melatonin as a major skin protectant: From free radical scavenging to DNA damage repair. Exp. Dermatol. 2008, 17, 713–730. [Google Scholar] [CrossRef] [PubMed]
- Rusanova, I.; Martínez-Ruiz, L.; Florido, J.; Rodríguez-Santana, C.; Guerra-Librero, A.; Acuña-Castroviejo, D.; Escames, G. Protective effects of melatonin on the skin: Future perspectives. Int. J. Mol. Sci. 2019, 20, 4948. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Pinheiro, T.; Silva, R.; Fleming, R.; Gonçalves, A.; Barreiros, M.A.; Silva, J.N.; Morlière, P.; Santus, R.; Filipe, P. Distribution and quantitation of skin iron in primary haemochromatosis: Correlation with total body iron stores in patients undergoing phlebotomy. Acta Derm. Venereol. 2014, 94, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Giménez García, R.M.; Carrasco Molina, S. Drug–induced hyperpigmentation: Review and case series. J. Am. Board Fam. Med. 2019, 32, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Herrling, T.; Jung, K.; Fuchs, J. The role of melanin as protector against free radicals in skin and its role as free radical indicator in hair. Spectrochim Acta A Mol. Biomol. Spectrosc. 2008, 69, 1429–1435. [Google Scholar] [CrossRef]
- Yamashita, Y.; Okano, Y.; Ngo, T.; Buche, P.; Sirvent, A.; Girard, F.; Masaki, H. Differences in susceptibility to oxidative stress in the skin of Japanese and French subjects and physiological characteristics of their skin. Skin Pharmacol. Physiol. 2012, 25, 78–85. [Google Scholar] [CrossRef]
- Shi, M.H.; Wu, Y.; Li, L.; Cai, Y.F.; Liu, M.; Gao, X.H.; Chen, H.D. Meta-analysis of the association between vitiligo and the level of superoxide dismutase or malondialdehyde. Clin. Exp. Dermatol. 2017, 42, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.N.; Liza, K.F.; Sarwar, M.S.; Ahmed, J.; Adnan, M.T.; Chowdhury, M.I.; Hossain, M.Z.; Islam, M.S. Effect of lipid peroxidation, antioxidants, macro minerals and trace elements on eczema. Arch. Dermatol. Res. 2015, 307, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Chyan, Y.J.; Poeggeler, B.; Omar, R.A.; Chain, D.G.; Frangione, B.; Ghiso, J.; Pappolla, M.A. Potent neuroprotective properties against the Alzheimer beta-amyloid by an endogenous melatonin-related indole structure, indole-3-propionic acid. J. Biol. Chem. 1999, 274, 21937–21942. [Google Scholar] [CrossRef] [PubMed]
- Iwan, P.; Stepniak, J.; Karbownik-Lewinska, M. Cumulative protective effect of melatonin and indole-3-propionic acid against KIO3-induced lipid peroxidation in porcine thyroid. Toxics 2021, 9, 89. [Google Scholar] [CrossRef]
- Park, E.K.; Lee, H.J.; Lee, H.; Kim, J.H.; Hwang, J.; Koo, J.I.; Kim, S.H. The anti-wrinkle mechanism of melatonin in UVB treated HaCaT keratinocytes and hairless mice via inhibition of ROS and sonic hedgehog mediated inflammatory proteins. Int. J. Mol. Sci. 2018, 19, 1995. [Google Scholar] [CrossRef] [PubMed]
- Gérard-Monnier, D.; Erdelmeier, I.; Régnard, K.; Moze-Henry, N.; Yadan, J.C.; Chaudière, J. Reactions of 1-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals. Analytical applications to a colorimetric assay of lipid peroxidation. Chem. Res. Toxicol. 1998, 11, 1176–1183. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rynkowska, A.; Stępniak, J.; Karbownik-Lewińska, M. Melatonin and Indole-3-Propionic Acid Reduce Oxidative Damage to Membrane Lipids Induced by High Iron Concentrations in Porcine Skin. Membranes 2021, 11, 571. https://doi.org/10.3390/membranes11080571
Rynkowska A, Stępniak J, Karbownik-Lewińska M. Melatonin and Indole-3-Propionic Acid Reduce Oxidative Damage to Membrane Lipids Induced by High Iron Concentrations in Porcine Skin. Membranes. 2021; 11(8):571. https://doi.org/10.3390/membranes11080571
Chicago/Turabian StyleRynkowska, Aleksandra, Jan Stępniak, and Małgorzata Karbownik-Lewińska. 2021. "Melatonin and Indole-3-Propionic Acid Reduce Oxidative Damage to Membrane Lipids Induced by High Iron Concentrations in Porcine Skin" Membranes 11, no. 8: 571. https://doi.org/10.3390/membranes11080571
APA StyleRynkowska, A., Stępniak, J., & Karbownik-Lewińska, M. (2021). Melatonin and Indole-3-Propionic Acid Reduce Oxidative Damage to Membrane Lipids Induced by High Iron Concentrations in Porcine Skin. Membranes, 11(8), 571. https://doi.org/10.3390/membranes11080571






