A Composite Membrane System with Gold Nanoparticles, Hydroxyapatite, and Fullerenol for Dual Interaction for Biomedical Purposes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Multilayered Films
2.2. Characterization of Multilayered Shells and Systems of Cells Immobilized within the Shells
2.2.1. Wettability of Membrane Shell
2.2.2. Transmission Electron Microscopy
2.3. Culture of HDF and hFOB Cells
2.4. Fluorescence Staining
2.5. Flow Cytometric Analysis
2.6. Scanning Electron Microscopy Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Multilayered Shells and Systems of Cells Immobilized within the Shells
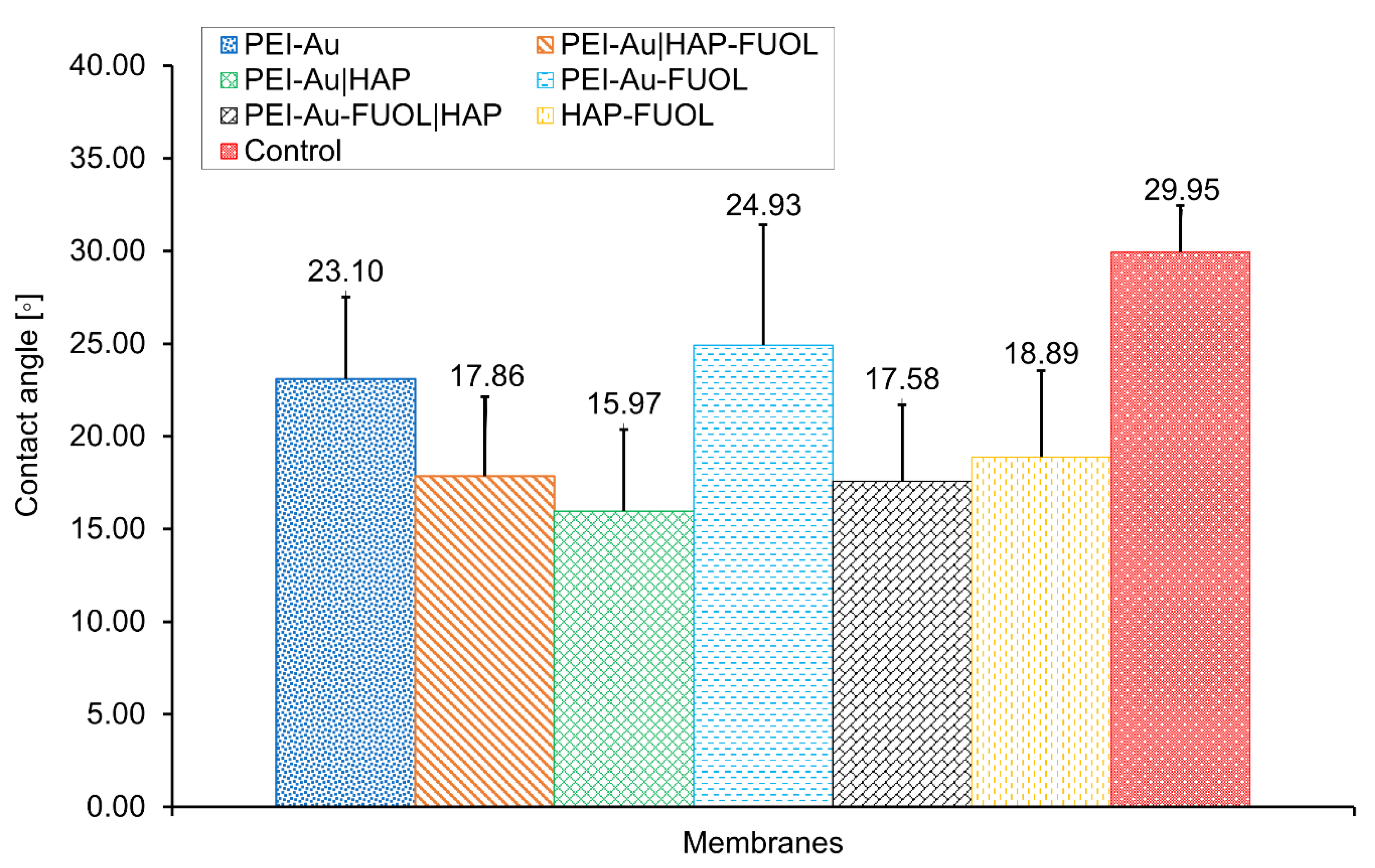
Wettability Assessment of Membrane Shells
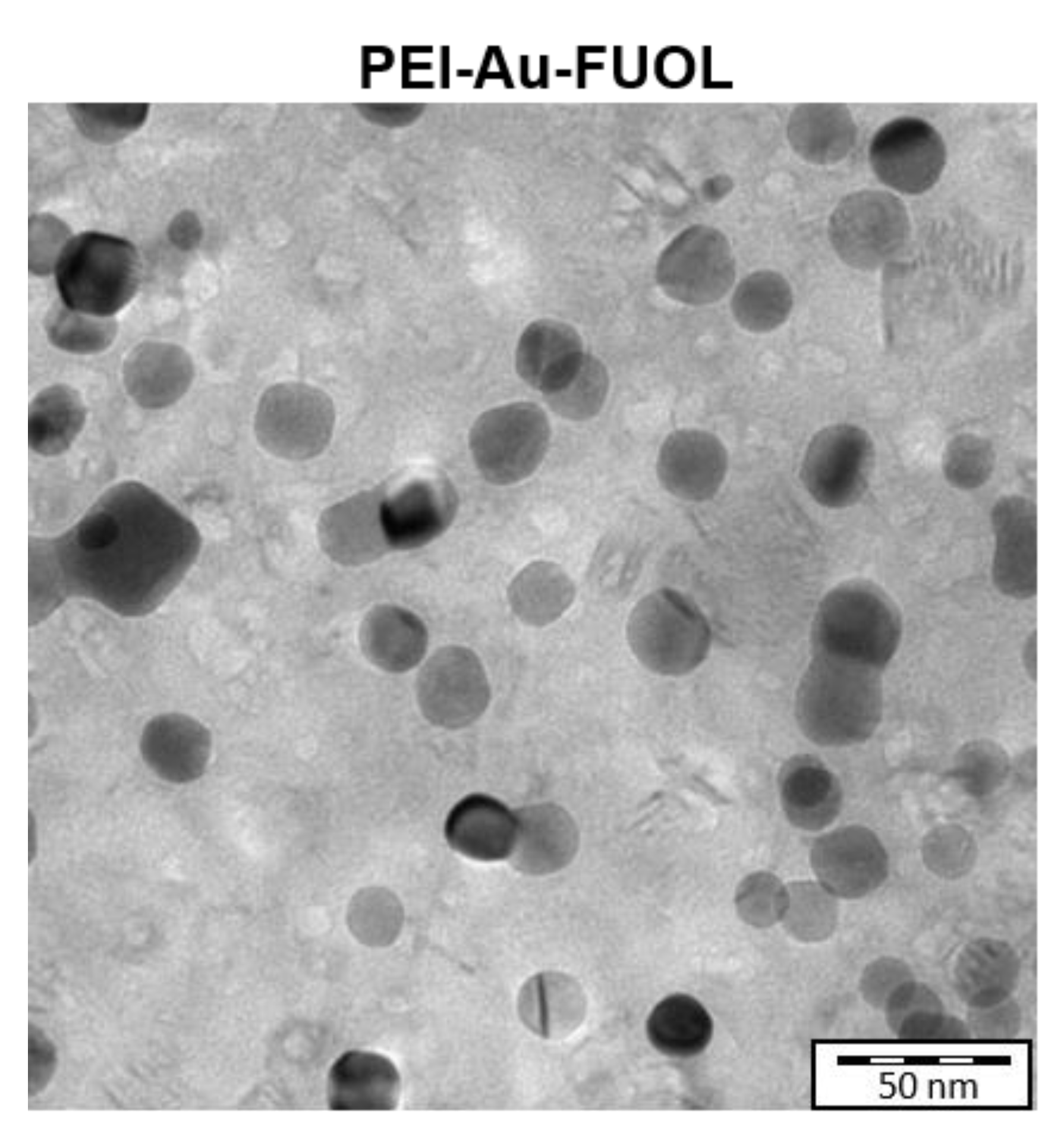
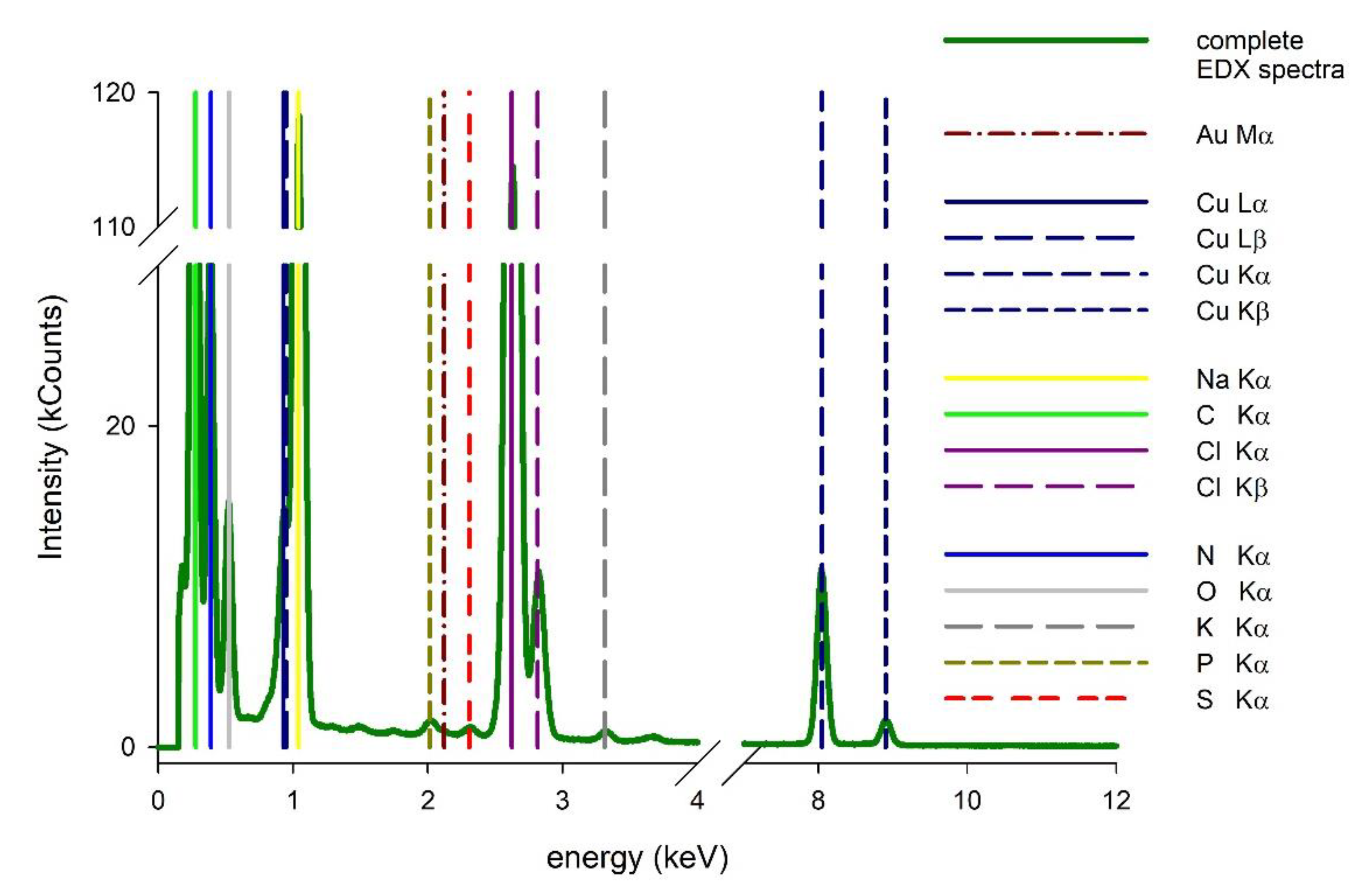
3.2. Study of the Structure of Developed Membrane Shells
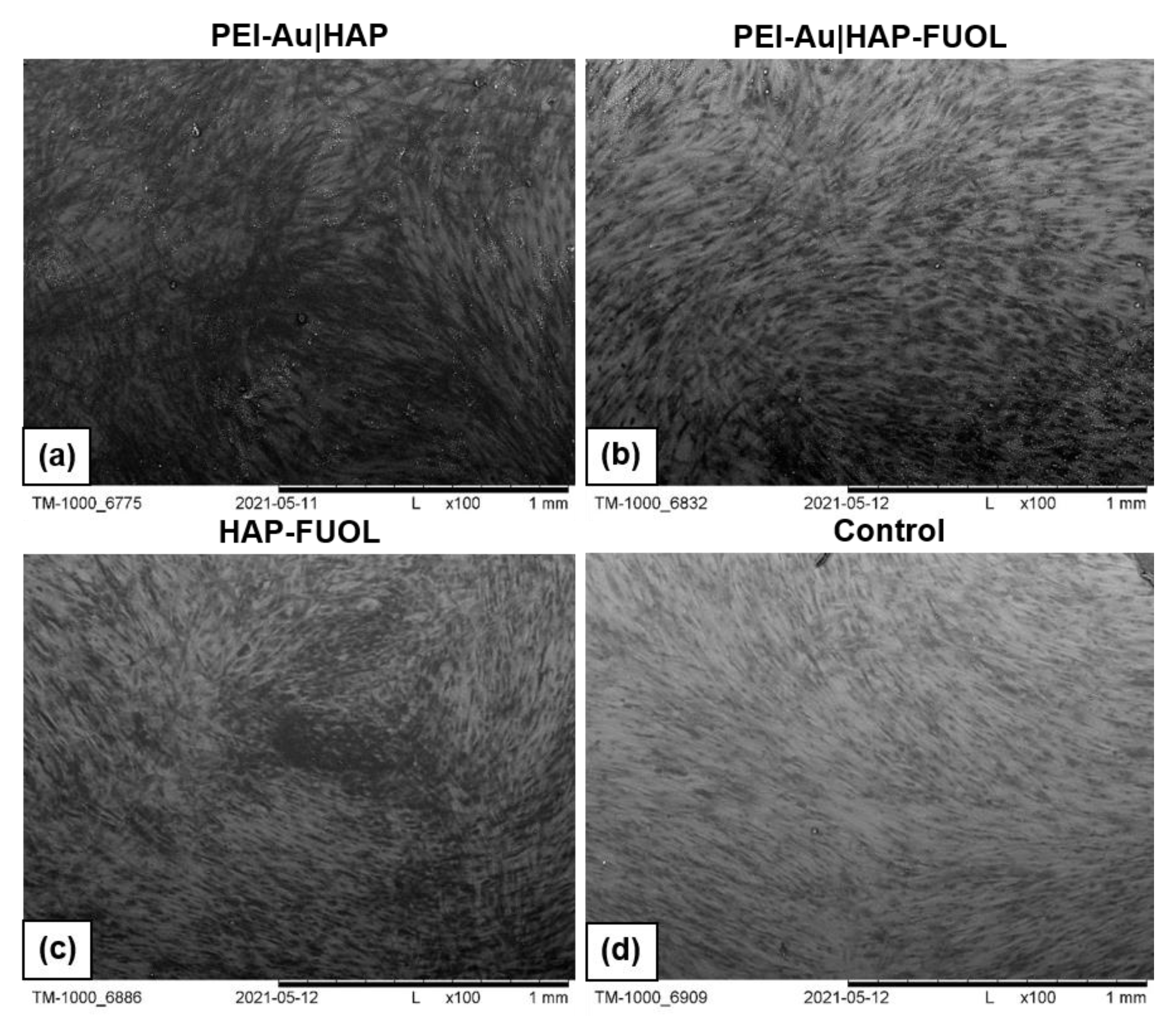
Surface Characterization of the Designed Membrane Shells
3.3. Cytotoxicity of the Membrane Shells
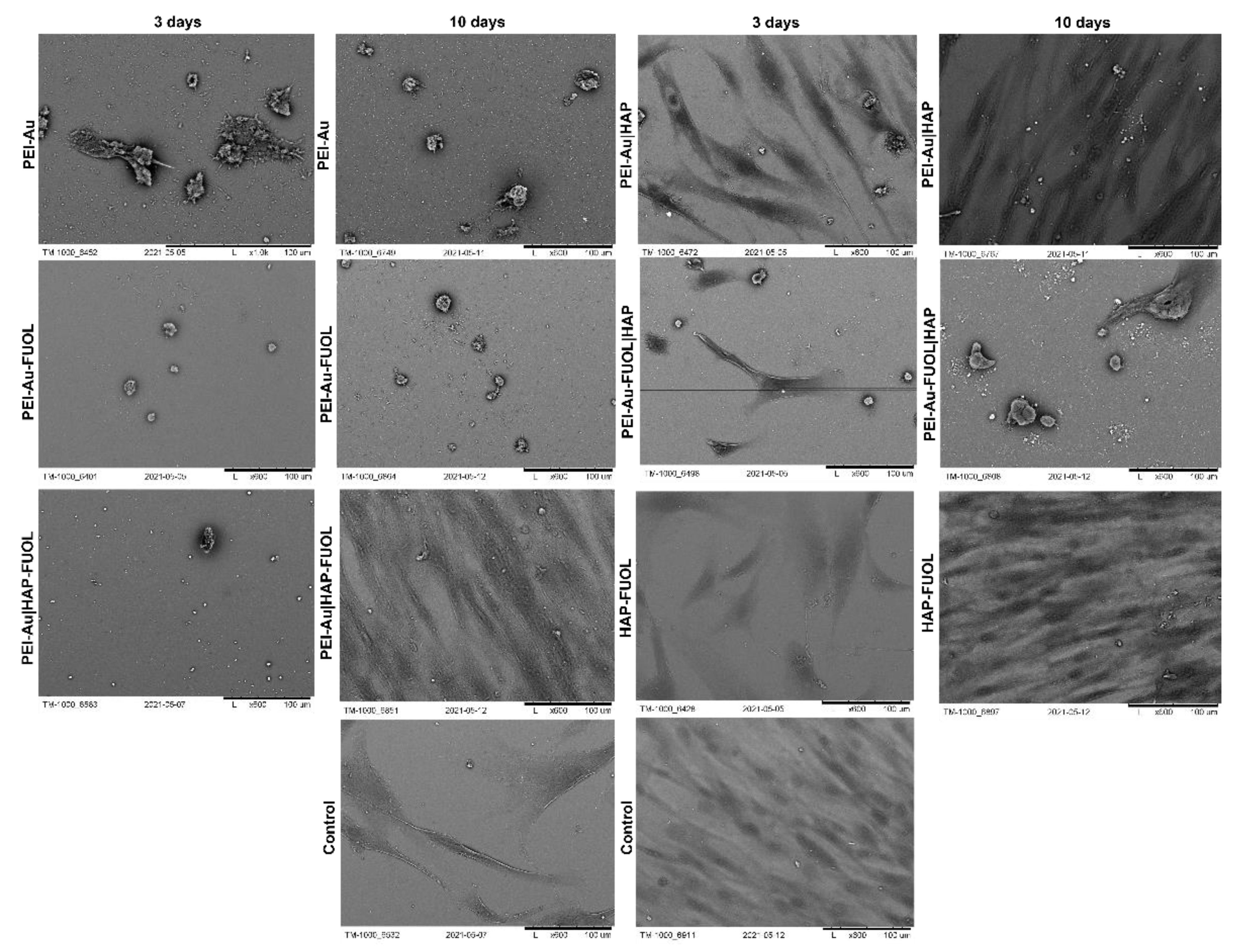
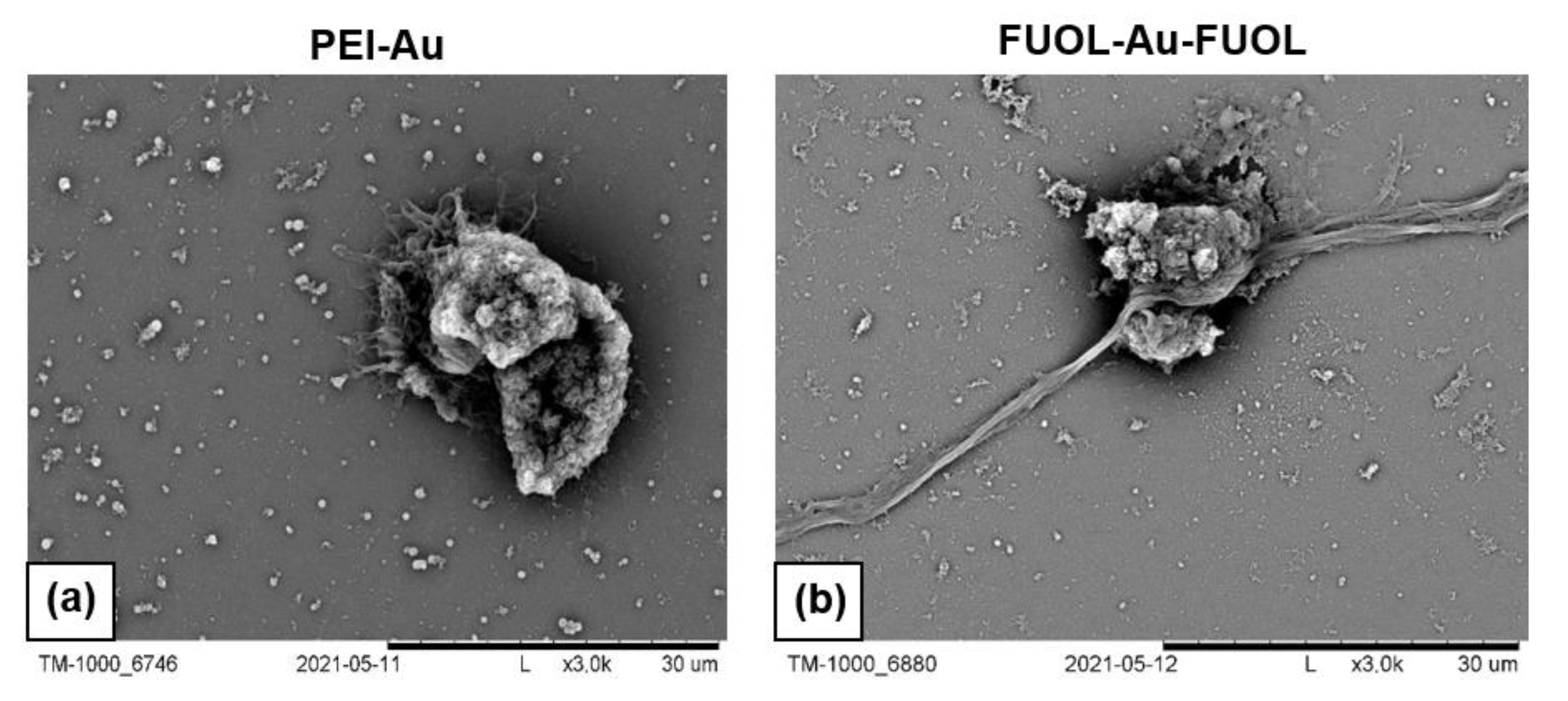
3.3.1. Scanning Electron Microscopy Analysis
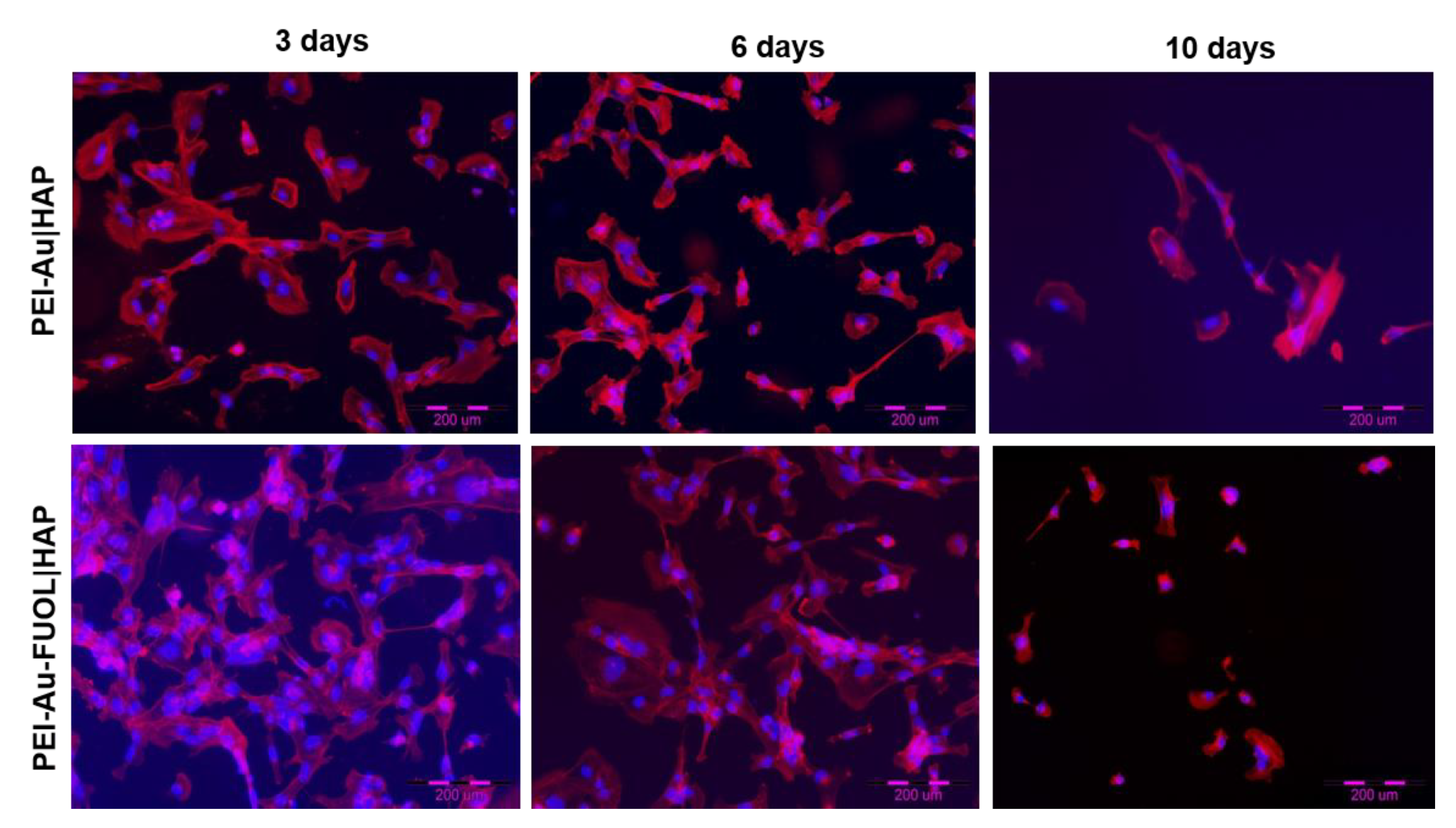
3.3.2. Fluorescence Microscopy Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, K.; Bae, L.; Yew, W. Post-operative wound management. Aust. Fam. Physician 2013, 42, 867. [Google Scholar]
- Rogge, M.N.; Slutsky, J.B.; Council, M.L.; Fosko, S.W. Bovine collagen xenograft repair of extensive surgical scalp wounds with exposed calvarium in the elderly: Increased rates of wound healing. Dermatol. Surg. 2015, 41, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Beecher, S.M.; O’Leary, D.P.; McLaughlin, R.; Kerin, M.J. The impact of surgical complications on cancer recurrence rates: A literature review. Oncol. Res. Treat. 2018, 41, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Garajei, A.; Arabkheradmand, A.; Motamedi, M.H.K.; Pazoki, A.E.; Rashad, A. Reconstruction of the face following cancer ablation. In A Textbook of Advanced Oral and Maxillofacial Surgery; InTechOpen: Rijeka, Croatia, 2015; Volume 2, pp. 643–713. [Google Scholar]
- Ferraro, N.; August, M. Reconstruction following resection for maxillofacial tumors. Oral Maxillofac. Surg. Clin. N. Am. 1993, 5, 355–383. [Google Scholar] [CrossRef]
- Shockley, W.; Weissler, M. Reconstructive alternatives following segmental mandibulectomy. Am. J. Otolaryngol. 1992, 13, 156–167. [Google Scholar] [CrossRef]
- Walter, C.J.; Dumville, J.C.; Sharp, C.A.; Page, T. Systematic review and meta-analysis of wound dressings in the prevention of surgical-site infections in surgical wounds healing by primary intention. Br. J. Surg. 2012, 99, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Ruszczak, Z.; Schwartz, R.; Joss-Wichman, E.; Wichman, R.; Zalewska, A. Surgical Dressings. Available online: Emedicine.medscape.com/article/1127868-overview#showall (accessed on 6 June 2021).
- Grzeczkowicz, A.; Gruszczynska-Biegala, J.; Czeredys, M.; Kwiatkowska, A.; Strawski, M.; Szklarczyk, M.; Koźbiał, M.; Kuźnicki, J.; Granicka, L.H. Polyelectrolyte membrane scaffold sustains growth of neuronal cells. J. Biomed. Mater. Res. Part A 2019, 107, 839–850. [Google Scholar] [CrossRef]
- Murphy, P.S.; Evans, G.R.D. Review article advances in wound healing: A review of current wound healing products. Plast. Surg. Int. 2012, 2012, 190436. [Google Scholar] [CrossRef]
- Becker, G.D.; Adams, L.A.; Levin, B.C. Secondary intention healing of exposed scalp and forehead bone after Mohs surgery. Otolaryngol. Head Neck Surg. 1999, 121, 751–754. [Google Scholar] [CrossRef]
- Hurvitz, K.; Kobayashi, M.; Evans, G. Current options in head and neck reconstruction. Plast. Reconstr. Surg. 2006, 118, 122e–133e. [Google Scholar] [CrossRef]
- Kinsella, C., Jr.; Grunwaldt, L.; Cooper, G.; Mills, M.; Losee, J. Scalp reconstruction: Regeneration with acellular dermal matrix. J. Craniofac. Surg. 2010, 21, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, B.; Tenna, S.; Segreto, F.; Persichetti, P. The use of acellular dermal matrix in reconstruction of complex scalp defects. Dermatol. Surg. 2011, 37, 527–529. [Google Scholar] [CrossRef]
- Bickels, J.; Kollender, Y.; Wittig, J.C.; Cohen, N.; Meller, I.; Malawer, M.M. Vacuum-assisted wound closure after resection of musculoskeletal tumors. Clin. Orthop. Relat. Res. 2005, 441, 346–350. [Google Scholar] [CrossRef]
- Schintler, M.; Prandl, E.; Wittguber, G.; Zink, B.; Spendel, S.; Hellbom, B.; Scharnagl, E. The impact of the VAC-treatment for locally advanced malignancy of the scalp. Zentralbl. Chir. 2004, 129, S141–S146. [Google Scholar]
- Molnar, J.; DeFranzo, A.; Marks, M. Single-stage approach to skin grafting the exposed skull. Plast. Reconstr. Surg. 2000, 105, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Läuchli, S.; Hafner, J.; Wehrmann, C.; French, L.; Hunziker, T. Post-surgical scalp wounds with exposed bone treated with a plantderived wound therapeutic. Management of Patients With Venous Leg Ulcers View project Molecules expressed in putative cancer stem cells: Possible biomarkers? View project. J. Wound Care 2012, 21, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMed 2015, 5, 24–28. [Google Scholar] [CrossRef]
- Moshakis, V.; Fordyce, M.; Griffiths, J.; McKinna, J. Tegadern versus gauze dressing in breast surgery—PubMed. Br. J. Clin. Pract. 1984, 38, 149–152. [Google Scholar] [PubMed]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef]
- Lee, I.C.; Wu, Y.C. Facilitating neural stem/progenitor cell niche calibration for neural lineage differentiation by polyelectrolyte multilayer films. Colloids Surf. B Biointerfaces 2014, 121, 54–65. [Google Scholar] [CrossRef]
- Yamanlar, S.; Sant, S.; Boudou, T.; Picart, C.; Khademhosseini, A. Surface functionalization of hyaluronic acid hydrogels by polyelectrolyte multilayer films. Biomaterials 2011, 32, 5590–5599. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Lu, Y.; Gao, Y.; Cheng, S.; Tian, F.; Xiao, Y.; Li, F. Chitosan/alginate/hyaluronic acid polyelectrolyte composite sponges cross-linked with genipin for wound dressing application. Int. J. Biol. Macromol. 2021, 182, 512–523. [Google Scholar] [CrossRef]
- Drabik, M.; Kazimierczak, B.; Grzeczkowicz, A.; Antosiak-Iwanska, M.; Kwiatkowska, A.; Granicka, L. The membrane composite with silver nanoparticles for fibroblastic cell growth sustaining. Desalin. Water Treat. 2018, 101, 70–76. [Google Scholar] [CrossRef]
- Teow, S.-Y.; Wong, M.M.-T.; Yap, H.-Y.; Peh, S.-C.; Shameli, K. Bactericidal properties of plants-derived metal and metal oxide nanoparticles (NPs). Molecules 2018, 23, 1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Z.; Li, J.; Qu, J.; Sheng, W.; Yang, J.; Li, M. Cationized Bombyx mori silk fibroin as a delivery carrier of the VEGF165–Ang-1 coexpression plasmid for dermal tissue regeneration. J. Mater. Chem. B 2018, 7, 80–94. [Google Scholar] [CrossRef]
- David, G.; Clima, L.; Calin, M.; Constantinescu, C.; Balan-Porcarasu, M.; Uritu, C.; Simionescu, B. Squalene/polyethylenimine based non-viral vectors: Synthesis and use in systems for sustained gene release. Polym. Chem. 2018, 9, 1072–1081. [Google Scholar] [CrossRef]
- Zhou, B.; Yang, J.; Peng, C.; Zhu, J.; Tang, Y.; Zhu, X.; Shen, M.; Zhang, G.; Shi, X. PEGylated polyethylenimine-entrapped gold nanoparticles modified with folic acid for targeted tumor CT imaging. Colloids Surf. B Biointerfaces 2016, 140, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhao, L.; Shen, M.; Zhao, J.; Shi, X. A multifunctional polyethylenimine-based nanoplatform for targeted anticancer drug delivery to tumors in vivo. J. Mater. Chem. B 2017, 5, 1542–1550. [Google Scholar] [CrossRef]
- De Almeida, A.T.; Figueredo, V.; da Cunha, A.L.G.; Casabona, G.; Costa de Faria, J.R.; Alves, E.V.; Sato, M.; Branco, A.; Guarnieri, C.; Palermo, E. Consensus recommendations for the use of hyperdiluted calcium hydroxyapatite (Radiesse) as a face and body biostimulatory agent. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2160. [Google Scholar] [CrossRef]
- Tripathi, G.; Basu, B. A porous hydroxyapatite scaffold for bone tissue engineering: Physico-mechanical and biological evaluations. Ceram. Int. 2012, 38, 341–349. [Google Scholar] [CrossRef]
- Granicka, L.H.; Antosiak-Iwańska, M.; Godlewska, E.; Strawski, M.; Szklarczyk, M.; Maranowski, B.; Kowalewski, C.; Wiśniewsk, J. Conformal nano-thin modified polyelectrolyte coatings for encapsulation of cells. Artif. Cells Blood Substit. Biotechnol. 2011, 39, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Granicka, L.H.; Antosiak-Iwáńska, M.; Godlewska, E.; Hoser, G.; Strawski, M.; Szklarczyk, M.; Dudziński, K. The experimental study of polyelectrolyte coatings suitability for encapsulation of cells. Artif. Cells Blood Substit. Biotechnol. 2009, 37, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Drabik, M.; Grzeczkowicz, A.; Bącal, P.; Kwiatkowska, A.; Strawski, M.; Antosiak-Iwańska, M.; Kazimierczak, B.; Godlewska, E.; Granicka, L.H. Nanocomposite membrane scaffolds for cell function maintaining for biomedical purposes. Nanomaterials 2021, 11, 1094. [Google Scholar] [CrossRef]
- Shah, J.V. Cells in tight spaces: The role of cell shape in cell function. J. Cell Biol. 2010, 191, 233–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzeczkowicz, A.; Drabik, M.; Lipko, A.; Bącal, P.; Kwiatkowska, A.; Kazimierczak, B.; Granicka, L.H. A Composite Membrane System with Gold Nanoparticles, Hydroxyapatite, and Fullerenol for Dual Interaction for Biomedical Purposes. Membranes 2021, 11, 565. https://doi.org/10.3390/membranes11080565
Grzeczkowicz A, Drabik M, Lipko A, Bącal P, Kwiatkowska A, Kazimierczak B, Granicka LH. A Composite Membrane System with Gold Nanoparticles, Hydroxyapatite, and Fullerenol for Dual Interaction for Biomedical Purposes. Membranes. 2021; 11(8):565. https://doi.org/10.3390/membranes11080565
Chicago/Turabian StyleGrzeczkowicz, Anna, Monika Drabik, Agata Lipko, Paweł Bącal, Angelika Kwiatkowska, Beata Kazimierczak, and Ludomira H. Granicka. 2021. "A Composite Membrane System with Gold Nanoparticles, Hydroxyapatite, and Fullerenol for Dual Interaction for Biomedical Purposes" Membranes 11, no. 8: 565. https://doi.org/10.3390/membranes11080565
APA StyleGrzeczkowicz, A., Drabik, M., Lipko, A., Bącal, P., Kwiatkowska, A., Kazimierczak, B., & Granicka, L. H. (2021). A Composite Membrane System with Gold Nanoparticles, Hydroxyapatite, and Fullerenol for Dual Interaction for Biomedical Purposes. Membranes, 11(8), 565. https://doi.org/10.3390/membranes11080565






